Plant Responses to Global Climate Change and Urbanization: Implications for Sustainable Urban Landscapes
Abstract
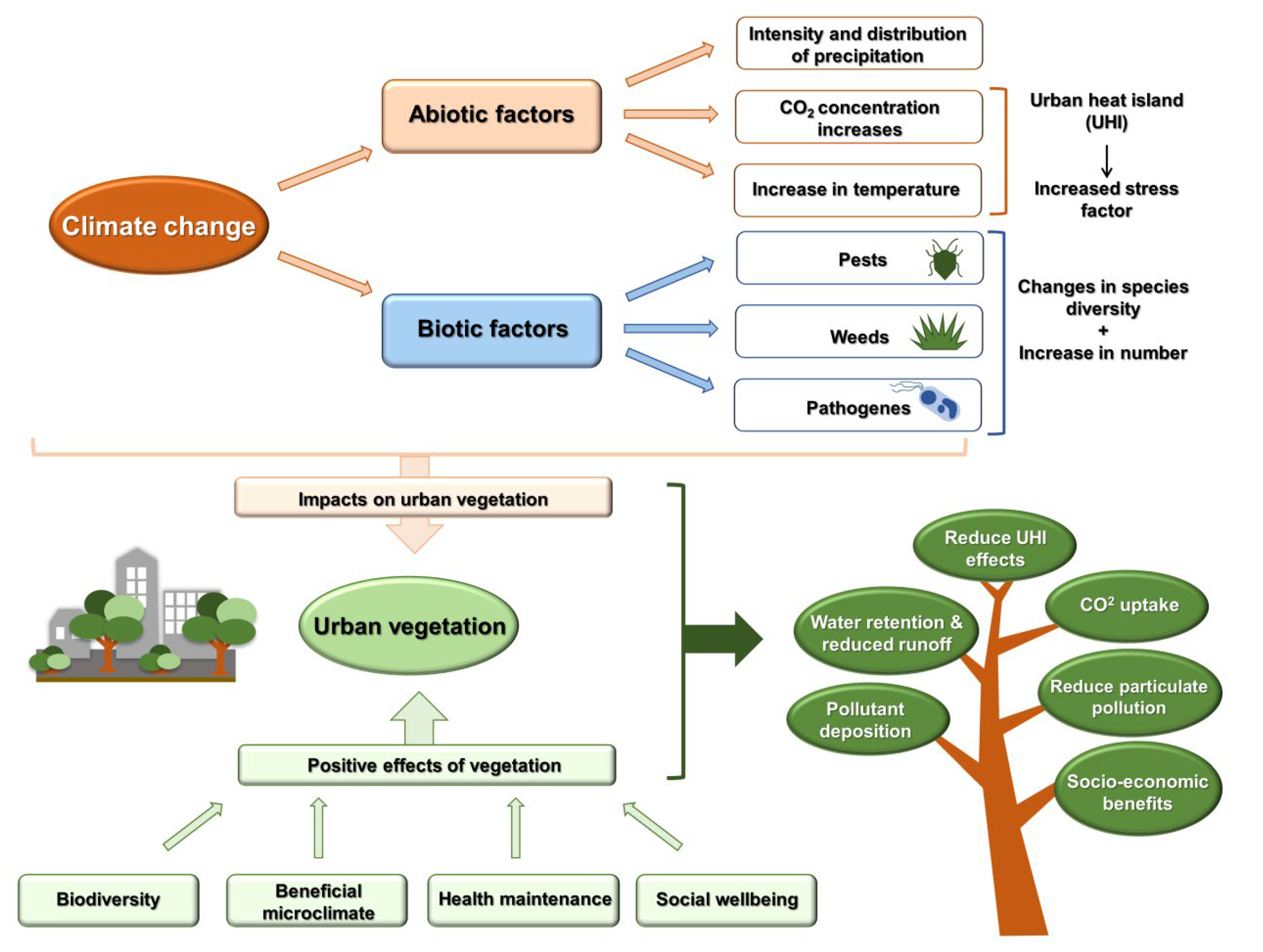
:1. Introduction
2. Effects of Climate Change on Plant Development
3. Stress Effects Caused by Climate Change
3.1. Air Pollution
3.2. Drought
3.3. High Temperature
3.4. High Salt Concentration
3.5. High Heavy Metal Concentration
4. Urban Biodiversity in the Light of Climate Change
5. Stress Resistance Breeding for Urban Climate
6. In Addition to Breeding, There Are Other Possible Solutions for Increasing Urban Tolerance
7. Conclusions and Future Prospectus
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhary, J.R.; Tripathi, A.; Kaldate, R.; Rana, M.; Mehta, S.; Ahlawat, J.; Wani, S.H. Breeding efforts for crop productivity in abiotic stress environment. In Augmenting Crop Productivity in Stress Environment; Springer Nature: Singapore, 2022; pp. 63–103. [Google Scholar]
- Calfapietra, C.; Peñuelas, J.; Niinemets, Ü. Urban plant physiology: Adaptation-mitigation strategies under permanent stress. Trends Plant Sci. 2015, 20, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, G.W.; Berglann, H.; Jenssen, E.; Kårstad, S.; Rodriguez, D.G.P. The Value of Urban Farming in Oslo, Norway: Community Gardens, Aquaponics and Vertical Farming. Int. J. Food Syst. Dyn. 2022, 13, 17–29. [Google Scholar]
- Ciftcioglu, G.C.; Ebedi, S.; Abak, K. Evaluation of the relationship between ornamental plants–based ecosystem services and human wellbeing: A case study from Lefke Region of North Cyprus. Ecol. Indic. 2019, 102, 278–288. [Google Scholar] [CrossRef]
- Gabellini, S.; Scaramuzzi, S. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A grey literature review. Horticulturae 2022, 8, 234. [Google Scholar] [CrossRef]
- Wani, M.A.; Nazki, I.T.; Din, A.; Iqbal, S. Floriculture Sustainability Initiative: The Dawn of New Era. Sustain. Agric. Rev. 2018, 27, 91. [Google Scholar]
- Wani, M.A.; Din, A.; Nazki, I.T.; Rehman, T.U.; Al-Khayri, J.M.; Jain, S.M.; Lone, R.A.; Bhat, Z.A.; Mushtaq, M. Navigating the future: Exploring technological advancements and emerging trends in the sustainable ornamental industry. Front. Environ. Sci. 2023, 11, 1188643. [Google Scholar] [CrossRef]
- Ilie, D.; Cosmulescu, S. Spontaneous Plant Diversity in Urban Contexts: A Review of Its Impact and Importance. Diversity 2023, 15, 277. [Google Scholar] [CrossRef]
- Salmond, J.A.; Tadaki, M.; Vardoulakis, S.; Arbuthnott, K.; Coutts, A.; Demuzere, M.; Dirks, K.N.; Heaviside, C.; Lim, S.; Macintyre, H.; et al. Health and climate related ecosystem services provided by street trees in the urban environment. Environ Health 2016, 15 (Suppl. S1), 95–111. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Hoehn, R.E.; Lapoint, E. Carbon storage and sequestration by trees in urban and community areas of the United States. Environ. Pollut. 2013, 178, 229–236. [Google Scholar] [CrossRef]
- Leal Filho, W.; Icaza, L.E.; Neht, A.; Klavins, M.; Morgan, E.A. Coping with the impacts of urban heat islands: A literature based study on understanding urban heat vulnerability and the need for resilience in cities in a global climate change context. J. Clean. Prod. 2018, 171, 1140–1149. [Google Scholar] [CrossRef]
- Birkmann, J.; Bach, C.; Vollmer, M. Tools for Resilience Building and Adaptive Spatial Governance. Raumforsch. Raumordn. 2012, 70, 293–308. [Google Scholar] [CrossRef]
- Wang, X.; Dallimer, M.; Scott, C.E.; Shi, W.; Gao, J. Tree species richness and diversity predicts the magnitude of urban heat island mitigation effects of greenspaces. Sci. Total. Environ. 2021, 770, 145211. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, K.M.; Nelson, F.E.; Klene, A.E.; Bell, J.H. The urban heat island in winter at Barrow, Alaska. Int. J. Climatol. 2003, 23, 1889–1905. [Google Scholar] [CrossRef]
- Meineke, E.K.; Holmquist, A.J.; Wimp, G.M.; Frank, S.D. Changes in spider community composition are associated with urban temperature, not herbivore abundance. J. Urban Ecol. 2017, 3, juw010. [Google Scholar] [CrossRef]
- Fried, G.G.; Wichrowski, M.J. Horticultural therapy: A psychosocial treatment option at the Stephen D. Hassenfeld children’s center for cancer and blood disorders. Prim. Psychiatry 2008, 15, 73–77. [Google Scholar]
- Shoemaker, C.A.; Randall, K.; Relf, P.D.; Geller, E.S. Relationships between Plants, Behavior, and Attitudes in an Office Environment. HortTechnology 1992, 2, 205–206. [Google Scholar] [CrossRef]
- Barwise, Y.; Kumar, P. Designing vegetation barriers for urban air pollution abatement: A practical review for appropriate plant species selection. npj Clim. Atmos. Sci. 2020, 3, 12. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-H.; Lee, D.K.; Park, C.Y.; Jeong, S.G. The influence of small green space type and structure at the street level on urban heat island mitigation. Urban For. Urban Green. 2017, 21, 203–212. [Google Scholar] [CrossRef]
- Kończak, B.; Cempa, M.; Deska, M. Assessment of the ability of roadside vegetation to remove particulate matter from the urban air. Environ. Pollut. 2020, 268, 115465. [Google Scholar] [CrossRef]
- Pozo Menéndez, E. Greenery urban design for good mental health: Analysis of a vulnerable district of Madrid. In Urban Design and Planning for Age-Friendly Environments Across Europe: North and South: Developing Healthy and Therapeutic Living Spaces for Local Contexts; Springer International Publishing: Cham, Switzerland, 2022; pp. 291–309. [Google Scholar]
- Hoyle, H.; Jorgensen, A.; Hitchmough, J.D. What determines how we see nature? Perceptions of naturalness in designed urban green spaces. People Nat. 2019, 1, 167–180. [Google Scholar] [CrossRef]
- Tresch, S.; Frey, D.; Le Bayon, R.-C.; Zanetta, A.; Rasche, F.; Fliessbach, A.; Moretti, M. Litter decomposition driven by soil fauna, plant diversity and soil management in urban gardens. Sci. Total. Environ. 2019, 658, 1614–1629. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Li, D.; Jane, H.-A. Wild or tended nature? The effects of landscape location and vegetation density on physiological and psychological responses. Landsc. Urban Plan. 2017, 167, 72–83. [Google Scholar] [CrossRef]
- Simkin, J.; Ojala, A.; Tyrväinen, L. Restorative effects of mature and young commercial forests, pristine old-growth forest and urban recreation forest—A field experiment. Urban For. Urban Green 2020, 48, 126567. [Google Scholar] [CrossRef]
- Colléony, A.; White, R.; Shwartz, A. The influence of spending time outside on experience of nature and environmental attitudes. Landsc. Urban Plan. 2019, 187, 96–104. [Google Scholar] [CrossRef]
- Gong, P.; Liang, S.; Carlton, E.J.; Jiang, Q.; Wu, J.; Wang, L.; Remais, J.V. Urbanisation and health in China. Lancet 2012, 379, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Rahnema, S.; Sedaghathoor, S.; Allahyari, M.S.; Damalas, C.A.; El Bilali, H. Preferences and emotion perceptions of ornamental plant species for green space designing among urban park users in Iran. Urban For. Urban Green. 2019, 39, 98–108. [Google Scholar] [CrossRef]
- Lewis, E.; Phoenix, G.K.; Alexander, P.; David, J.; Cameron, R.W.F. Rewilding in the Garden: Are garden hybrid plants (cultivars) less resilient to the effects of hydrological extremes than their parent species? A case study with Primula. Urban Ecosyst. 2019, 22, 841–854. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Q.; Zhu, Y. Urban park system on public health: Underlying driving mechanism and planning thinking. Front. Public Health 2023, 11, 1193604. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Cui, J.; Wang, L.; Liu, H.; Liu, H. Chinese young people’s perceptions and preferences with regard to various edible urban plants. J. Zhejiang Univ. B 2023, 24, 359–365. [Google Scholar] [CrossRef]
- Luo, Z.; Sun, O.J.; Ge, Q.; Xu, W.; Zheng, J. Phenological responses of plants to climate change in an urban environment. Ecol. Res. 2007, 22, 507–514. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dahlhausen, J.; Schütze, G.; Perkins, D.; Rötzer, T.; Caldentey, J.; Koike, T.; Con, T.V.; et al. Climate change accelerates growth of urban trees in metropolises worldwide. Sci. Rep. 2017, 7, 15403. [Google Scholar] [CrossRef]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root Phenotyping for Drought Tolerance: A Review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- Franco, J.A.; Banón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Root development in horticultural plants grown under abiotic stress conditions—A review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, pH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Cirillo, C.; Rouphael, Y. Biochemical, Physiological, and Molecular Aspects of Ornamental Plants Adaptation to Deficit Irrigation. Horticulturae 2021, 7, 107. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, R.D.; Kumar, A.; Singh, S.; Singh, S. Understanding the effect of different abiotic stresses on wild marigold (Tagetes minuta L.) and role of breeding strategies for developing tolerant lines. Front. Plant Sci. 2022, 12, 754457. [Google Scholar] [CrossRef] [PubMed]
- Darvizheh, H.; Zahedi, M.; Abbaszadeh, B.; Razmjoo, J. Changes in some antioxidant enzymes and physiological indices of purple coneflower (Echinacea purpurea L.) in response to water deficit and foliar application of salicylic acid and spermine under field condition. Sci. Hortic. 2019, 247, 390–399. [Google Scholar] [CrossRef]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef]
- Raza, A.; Mehmood, S.S.; Tabassum, J.; Batool, R. Targeting plant hormones to develop abiotic stress resistance in wheat. In Wheat Production in Changing Environments: Responses, Adaptation and Tolerance; Springer: Singapore, 2019; pp. 557–577. [Google Scholar]
- Bhattacharya, A. Physiological Processes in Plants under Low Temperature Stress; Springer: Singapore, 2022; p. 734. [Google Scholar]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Rai, A.; Kumar, R.G.; Dubey, R.S. Heat stress and its effects on plant growth and metabolism. In Abitic Stress Tolerance Mechanisms in Plants; Rai, G.K., Kumar, R.R., Bagati, S., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 203–235. [Google Scholar]
- Francini, A.; Sebastiani, L. Abiotic Stress Effects on Performance of Horticultural Crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Meineke, E.; Youngsteadt, E.; Dunn, R.R.; Frank, S.D. Urban warming reduces aboveground carbon storage. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161574. [Google Scholar] [CrossRef] [PubMed]
- Monder, M.J. Trends in the Phenology of Climber Roses under Changing Climate Conditions in the Mazovia Lowland in Central Europe. Appl. Sci. 2022, 12, 4259. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dugo, V.; Durand, J.L.; Gastal, F.; Bariac, T.; Poincheval, J. Restricted Root-to-Shoot Translocation and Decreased Sink Size are Responsible for Limited Nitrogen Uptake in Three Grass Species under Water Deficit. Environ. Exp. Bot. 2012, 75, 258–267. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.R.; Mach, K.J.; Mastrandrea, M.D.; Van Aalst, M.; Adger, W.N.; Yohe, G.W. Technical Summary Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; pp. 35–94. [Google Scholar]
- Balfagón, D.; Zandalinas, S.I.; Mittler, R.; Gómez-Cadenas, A. High temperatures modify plant responses to abiotic stress conditions. Physiol. Plant. 2020, 170, 335–344. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The Contribution of Ornamental Plants to Urban Ecosystem Services. Earth 2022, 3, 1258–1274. [Google Scholar] [CrossRef]
- Leotta, L.; Toscano, S.; Ferrante, A.; Romano, D.; Francini, A. New Strategies to Increase the Abiotic Stress Tolerance in Woody Ornamental Plants in Mediterranean Climate. Plants 2023, 12, 2022. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, N.V.; Battisti, D.S.; Beachy, R.N.; Cooper, P.J.M.; Fischhoff, D.A.; Hodges, C.N.; Knauf, V.C.; Lobell, D.; Mazur, B.J.; Molden, D.; et al. Radically Rethinking Agriculture for the 21st Century. Science 2010, 327, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Fahad, S.; Aqeel, M.; Ali, U.; Amanullah; Anwar, S.; Baloch, S.K.; Zainab, M. miRNAs: Major modulators for crop growth and development under abiotic stresses. Biotechnol. Lett. 2017, 39, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Maitra, S.; Pramanick, B.; Bhutia, K.L.; Ahmad, Z.; Moulik, D.; Aftab, T. Wild relatives of plants as sources for the development of abiotic stress tolerance in plants. In Plant Perspectives to Global Climate Changes; Academic Press: Cambridge, MA, USA, 2022; pp. 471–518. [Google Scholar]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar-Mathur, P.; Vadez, V.; Sharma, K.K. Transgenic approaches for abiotic stress tolerance in plants: Retrospect and prospects. Plant Cell Rep. 2008, 27, 411–424. [Google Scholar] [CrossRef]
- Chauhan, H.; Khurana, N.; Agarwal, P.; Khurana, P. Heat shock factors in rice (Oryza sativa L.): Genome-wide expression analysis during reproductive development and abiotic stress. Mol. Genet. Genom. 2011, 286, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Jaishankar, J.; Muthamilarasan, M.; Shweta, S.; Dangi, A.; Prasad, M. Genome-wide analysis of heat shock proteins in C4 model, foxtail millet identifies potential candidates for crop improvement under abiotic stress. Sci. Rep. 2016, 6, 32641. [Google Scholar] [CrossRef] [PubMed]
- Zinta, G.; Khan, A.; AbdElgawad, H.; Verma, V.; Srivastava, A.K. Unveiling the Redox Control of Plant Reproductive Development during Abiotic Stress. Front. Plant Sci. 2016, 7, 700. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A.; Khan, Z.H.; Quddusi, S.; Mohammad, F. Occurrence, biosynthesis and potentialities of ascorbic acid in plants. Int. J. Plant Anim. Environ. Sci. 2011, 1, 167–184. [Google Scholar]
- Mann, A.; Nehra, K.; Rana, J.; Dahiya, T. Antibiotic resistance in agriculture: Perspectives on upcoming strategies to overcome upsurge in resistance. Curr. Res. Microb. Sci. 2021, 2, 100030. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Gurney, K.R.; Romero-Lankao, P.; Seto, K.C.; Hutyra, L.R.; Duren, R.; Kennedy, C.; Grimm, N.B.; Ehleringer, J.R.; Marcotullio, P.; Hughes, S.; et al. Climate change: Track urban emissions on a human scale. Nature 2015, 525, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Przybysz, A.; Popek, R.; Stankiewicz-Kosyl, M.; Zhu, C.; Małecka-Przybysz, M.; Maulidyawati, T.; Mikowska, K.; Deluga, D.; Griżuk, K.; Sokalski-Wieczorek, J.; et al. Where trees cannot grow—Particulate matter accumulation by urban meadows. Sci. Total. Environ. 2021, 785, 147310. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, E.; Kontunen-Soppela, S. Plants have different strategies to defend against air pollutants. Curr. Opin. Environ. Sci. Health 2021, 19, 100222. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, L.; McNulty, S. An investigation on the leaf accumulation-removal efficiency of atmospheric particulate matter for five urban plant species under different rainfall regimes. Atmos. Environ. 2019, 208, 123–132. [Google Scholar] [CrossRef]
- Dadkhah-Aghdash, H.; Rasouli, M.; Rasouli, K.; Salimi, A. Detection of urban trees sensitivity to air pollution using physiological and biochemical leaf traits in Tehran, Iran. Sci. Rep. 2022, 12, 15398. [Google Scholar] [CrossRef] [PubMed]
- Miliauskienė, J.; Sakalauskienė, S.; Lazauskas, S.; Povilaitis, V.; Brazaitytė, A.; Duchovskis, P. The competition between winter rape (C3) and maize (C4) plants in response to elevated carbon dioxide and temperature, and drought stress. Zemdirb. Agric. 2016, 103, 21–28. [Google Scholar] [CrossRef]
- Compant, S.; Van Der Heijden, M.G.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Rathore, D. Dust pollution: Its removal and effect on foliage physiology of urban trees. Sustain. Cities Soc. 2019, 51, 101696. [Google Scholar] [CrossRef]
- Hubai, K.; Kováts, N.; Teke, G. Effects of urban atmospheric particulate matter on higher plants using Lycopersicon esculentum as model species. SN Appl. Sci. 2021, 3, 770. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Bozca, F.D.; Leblebici, S. Interactive effect of boric acid and temperature stress on phenological characteristics and antioxidant system in Helianthus annuus L. South Afr. J. Bot. 2022, 147, 391–399. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2018, 135, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Kholova, J.; Medina, S.; Kakkera, A.; Anderberg, H. Transpiration efficiency: New insights into an old story. J. Exp. Bot. 2014, 65, 6141–6153. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- McClung, T.; Ibáñez, I. Quantifying the synergistic effects of impervious surface and drought on radial tree growth. Urban Ecosyst. 2018, 21, 147–155. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Tordoni, E.; Di Bonaventura, A.; Tomasella, M.; Natale, S.; Panepinto, F.; Bacaro, G.; Nardini, A. Turgor loss point and vulnerability to xylem embolism predict species-specific risk of drought-induced decline of urban trees. Plant Biol. 2022, 24, 1198–1207. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, L.D.L.; Huang, C. Testing early warning metrics for drought-induced tree physiological stress and mortality. Glob. Chang. Biol. 2019, 25, 2459–2469. [Google Scholar] [CrossRef]
- Burley, H.; Beaumont, L.J.; Ossola, A.; Baumgartner, J.B.; Gallagher, R.; Laffan, S.; Esperon-Rodriguez, M.; Manea, A.; Leishman, M.R. Substantial declines in urban tree habitat predicted under climate change. Sci. Total. Environ. 2019, 685, 451–462. [Google Scholar] [CrossRef]
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; Haque, E.U.; et al. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020, 10, 18013. [Google Scholar] [CrossRef]
- Brune, M. Urban Trees Under Climate Change. In Potential Impacts of Dry Spells and Heat Waves in Three German Regions in the 2050s; Climate Service Center Germany: Hamburg, Germany, 2016. [Google Scholar]
- Hassan, M.U.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.M.; Habib-Ur-Rahman, M.; Noor, M.A.; Sher, A.; Fahad, S. Linking Plants Functioning to Adaptive Responses Under Heat Stress Conditions: A Mechanistic Review. J. Plant Growth Regul. 2022, 41, 2596–2613. [Google Scholar] [CrossRef]
- Álvarez, S.; Rodríguez, P.; Broetto, F.; Sánchez-Blanco, M.J. Long term responses and adaptive strategies of Pistacia lentiscus under moderate and severe deficit irrigation and salinity: Osmotic and elastic adjustment, growth, ion uptake and photosynthetic activity. Agric. Water Manag. 2018, 202, 253–262. [Google Scholar] [CrossRef]
- Vinod, K.K. Stress in plantation crops: Adaptation and management. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 45–137. [Google Scholar]
- Chen, J.-W.; Zhang, Q.; Li, X.-S.; Cao, K.-F. Independence of stem and leaf hydraulic traits in six Euphorbiaceae tree species with contrasting leaf phenology. Planta 2009, 230, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Driesen, E.; Van den Ende, W.; De Proft, M.; Saeys, W. Influence of Environmental Factors Light, CO2, Temperature, and Relative Humidity on Stomatal Opening and Development: A Review. Agronomy 2020, 10, 1975. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Fujita, M. Calcium-mediated growth regulation and abiotic stress tolerance in plants. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Cham, Switzerland, 2019; pp. 291–331. [Google Scholar]
- Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Maurino, V.G.; Flugge, U.I. Experimental Systems to Assess the Effects of Reactive Oxygen Species in Plant Tissues. Plant Signal. Behav. 2008, 3, 923. [Google Scholar] [CrossRef]
- Kamakura, R.P.; DeWald, L.E.; Sniezko, R.A.; Elliott, M.; Chastagner, G.A. Using differences in abiotic factors between seed origin and common garden sites to predict performance of Pacific madrone (Arbutus menziesii Pursh). For. Ecol. Manag. 2021, 497, 119487. [Google Scholar] [CrossRef]
- Toscano, S.; Scuderi, D.; Giuffrida, F.; Romano, D. Responses of Mediterranean ornamental shrubs to drought stress and recovery. Sci. Hortic. 2014, 178, 145–153. [Google Scholar] [CrossRef]
- Sæbø, A.; Benedikz, T.; Randrup, T.B. Selection of trees for urban forestry in the Nordic countries. Urban For. Urban Green. 2003, 2, 101–114. [Google Scholar] [CrossRef]
- Rejeb Ruett, M.; Junker-Frohn, L.V.; Siegmann, B.; Ellenberger, J.; Jaenicke, H.; Whitney, C.; Luedeling, E.; Tiede-Arlt, P.; Rascher, U. Hyperspectral imaging for high-throughput vitality monitoring in ornamental plant production. Sci. Hortic. 2022, 291, 110546. [Google Scholar] [CrossRef]
- Saunier, A.; Ormeño, E.; Moja, S.; Fernandez, C.; Robert, E.; Dupouyet, S.; Despinasse, Y.; Baudino, S.; Nicolè, F.; Bousquet-Mélou, A. Lavender sensitivity to water stress: Comparison between eleven varieties across two phenological stages. Ind. Crop. Prod. 2022, 177, 114531. [Google Scholar] [CrossRef]
- Zahir, A.; Abbasi, B.H.; Adil, M.; Anjum, S.; Zia, M.; Haq, I.U. Synergistic Effects of Drought Stress and Photoperiods on Phenology and Secondary Metabolism of Silybum marianum. Appl. Biochem. Biotechnol. 2014, 174, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Asrar, A.A.; Abdel-Fattah, G.M.; Elhindi, K.M. Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 2012, 50, 305–316. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Miles, L.S.; Breitbart, S.T.; Wagner, H.H.; Johnson, M.T.J. Urbanization Shapes the Ecology and Evolution of Plant-Arthropod Herbivore Interactions. Front. Ecol. Evol. 2019, 7, 310. [Google Scholar] [CrossRef]
- Gao, S.; Chen, Y.; Li, K.; He, B.; Hou, P.; Guo, Z. Frequent heatwaves limit the indirect growth effect of urban vegetation in China. Sustain. Cities Soc. 2023, 96, 104662. [Google Scholar] [CrossRef]
- Masouleh, S.S.S.; Aldine, N.J.; Sassine, Y.N. The role of organic solutes in the osmotic adjustment of chilling-stressed plants (vegetable, ornamental and crop plants). Ornam. Hortic. 2019, 25, 434–442. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, R.; Kumar, M. Urban Tree Carbon Density and CO2 equivalent of National Zoological Park, Delhi. Environ. Monit. Assess. 2018, 193, 841. [Google Scholar]
- Ashkiani, A.; Sayfzadeh, S.; Shirani Rad, A.H.; Valadabadi, A.; Hadidi Masouleh, E. Effects of Foliar Zinc Application on Yield and Oil Quality of Rapeseed Genotypes under Drought Stress. J. Plant Nutr. 2020, 43, 1594–1603. [Google Scholar] [CrossRef]
- Feyisa, G.L.; Dons, K.; Meilby, H. Efficiency of parks in mitigating urban heat island effect: An example from Addis Ababa. Landsc. Urban Plan. 2014, 123, 87–95. [Google Scholar] [CrossRef]
- Zhang, Z.; Lan, M.; Han, X.; Wu, J.; Wang-Pruski, G. Response of Ornamental Pepper to High-Temperature Stress and Role of Exogenous Salicylic Acid in Mitigating High Temperature. J. Plant Growth Regul. 2020, 39, 133–146. [Google Scholar] [CrossRef]
- Jiang, C.; Bi, Y.; Zhang, R.; Feng, S. Expression of RcHSP70, heat shock protein 70 gene from Chinese rose, enhances host resistance to abiotic stresses. Sci. Rep. 2020, 10, 2445. [Google Scholar] [CrossRef]
- Du, X.; Li, W.; Sheng, L.; Deng, Y.; Wang, Y.; Zhang, W.; Yu, K.; Jiang, J.; Fang, W.; Guan, Z.; et al. Over-expression of chrysanthemum CmDREB6 enhanced tolerance of chrysanthemum to heat stress. BMC Plant Biol. 2018, 18, 178. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Liu, J.; Yang, Z.; Huang, B. Molecular regulation and physiological functions of a novel FaHsfA2c cloned from tall fescue conferring plant tolerance to heat stress. Plant Biotechnol. J. 2017, 15, 237–248. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z. Salinity: A major agricultural problem—Causes, impacts on crop productivity and management strategies. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Springer: Cham, Switzerland, 2019; pp. 83–99. [Google Scholar]
- Guo, J.; Shan, C.; Zhang, Y.; Wang, X.; Tian, H.; Han, G.; Zhang, Y.; Wang, B. Mechanisms of Salt Tolerance and Molecular Breeding of Salt-Tolerant Ornamental Plants. Front. Plant Sci. 2022, 13, 854116. [Google Scholar] [CrossRef]
- Ahsan, M.; Zulfiqar, H.; Farooq, M.A.; Ali, S.; Tufail, A.; Kanwal, S.; Radicetti, E. Strigolactone (GR24) Application positively regulates photosynthetic attributes, stress-related metabolites and antioxidant enzymatic activities of ornamental sunflower (Helianthus annuus cv. Vincent’s Choice) under salinity stress. Agriculture 2022, 13, 50. [Google Scholar] [CrossRef]
- Gill, R.A.; Ahmar, S.; Ali, B.; Saleem, M.H.; Khan, M.U.; Zhou, W.; Liu, S. The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 12792. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ke, Y.; Qin, J.; Huang, Y.; Zhao, Y.; Liu, Y.; Wei, H.; Liu, G.; Lian, B.; Chen, Y.; et al. Genome-wide identification of calcineurin B-like protein-interacting protein kinase gene family reveals members participating in abiotic stress in the ornamental woody plant Lagerstroemia indica. Front. Plant Sci. 2022, 13, 942217. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ghneim-Herrera, T.; Ben Romdhane, W.; Dabbous, A.; Ben Saad, R.; Brini, F.; Abdelly, C.; Ben Hamed, K. Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima. Funct. Plant Biol. 2020, 47, 912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Niu, G.; Deng, C.; Wang, Y.; Gardea-Torresdey, J. Growth, Gas Exchange, and Mineral Nutrients of Ornamental Grasses Irrigated with Saline Water. HortScience 2019, 54, 1840–1846. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Long-term effect of salinity on plant quality, water relations, photosynthetic parameters and ion distribution in Callistemon citrinus. Plant Biol. 2014, 16, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Breś, W.; Bandurska, H.; Kupska, A.; Niedziela, J.; Frąszczak, B. Responses of pelargonium (Pelargonium × hortorum LH bailey) to long-term salinity stress induced by treatment with different NaCl doses. Acta Physiol. Plant. 2016, 38, 26. [Google Scholar] [CrossRef]
- Pourret, O. On the necessity of banning the term “heavy metal” from the scientific literature. Sustainability 2018, 10, 2879. [Google Scholar] [CrossRef]
- El-Naggar, A.; Shaheen, S.M.; Ok, Y.S.; Rinklebe, J. Biochar Affects the Dissolved and Colloidal Concentrations of Cd, Cu, Ni, and Zn and their Phytoavailability and Potential Mobility in a Mining Soil under Dynamic Redox-Conditions. Sci. Total Environ. 2018, 624, 1059–1071. [Google Scholar] [CrossRef]
- Barouchas, P.E.; Akoumianaki-Ioannidou, A.; Liopa-Tsakalidi, A.; Moustakas, N.K. Effects of Vanadium and Nickel on Morphological Characteristics and on Vanadium and Nickel Uptake by Shoots of Mojito (Mentha × villosa) and Lavender (Lavandula anqustifolia). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 487–492. [Google Scholar] [CrossRef]
- Goswami, S.; Das, S. Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotoxicol. Environ. Saf. 2016, 126, 211–218. [Google Scholar] [CrossRef]
- Khan, A.H.A.; Kiyani, A.; Mirza, C.R.; Butt, T.A.; Barros, R.; Ali, B.; Iqbal, M.; Yousaf, S. Ornamental plants for the phytoremediation of heavy metals: Present knowledge and future perspectives. Environ. Res. 2021, 195, 110780. [Google Scholar] [CrossRef] [PubMed]
- Asgari Lajayer, B.; Ghorbanpour, M.; Nikabadi, S. Heavy metals in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecoto.x Environ. Safe 2017, 145, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Nakbanpote, W.; Meesungnoen, O.; Prasad, M. Potential of ornamental plants for phytoremediation of heavy metals and income generation. In Bioremediation and Bioeconomy; Elsevier: Alpharetta, GA, USA, 2016; pp. 179–217. [Google Scholar]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Gladkov, E.A.; Tashlieva, I.I.; Gladkova, O.V. Ornamental plants adapted to urban ecosystem pollution: Lawn grasses and painted daisy tolerating copper. Environ. Sci. Pollut. Res. 2021, 28, 14115–14120. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Stewart, C.N., Jr. Functional markers for precision plant breeding. Int. J. Mol. Sci. 2020, 21, 4792. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Pak, H.; Ri, S.; Kim, K.; Mun, N. Improvement of Water Stress Tolerance of Tuberous Begonia (Begonia × tuberhybrida) by OsmiR393a Gene Transformation. J. Plant Sci. 2021, 9, 257–265. [Google Scholar] [CrossRef]
- Planchuelo, G.; von Der Lippe, M.; Kowarik, I. Untangling the role of urban ecosystems as habitats for endangered plant species. Landsc. Urban Plan. 2019, 189, 320–334. [Google Scholar] [CrossRef]
- Avolio, M.L.; Pataki, D.E.; Trammell, T.L.E.; Endter-Wada, J. Biodiverse cities: The nursery industry, homeowners, and neighborhood differences drive urban tree composition. Ecol. Monogr. 2018, 88, 259–276. [Google Scholar] [CrossRef]
- Schröder, R.; Kiehl, K. Ecological restoration of an urban demolition site through introduction of native forb species. Urban For. Urban Green. 2020, 47, 126509. [Google Scholar] [CrossRef]
- Seaton, K.; Bettin, A.; Grüneberg, H. New ornamental plants for horticulture. In Horticulture: Plants for People and Places; Dixon, G., Aldous, D., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 1, pp. 435–463. [Google Scholar]
- Hart, E.; Miller, F.; Bastian, R. Tree Location and Winter Temperature Influence on Mimosa Webworm Populations in a Northern Urban Environment. Arboric. Urban For. 1986, 12, 237–240. [Google Scholar] [CrossRef]
- Langevelde, F.; Braamburg-Annegarn, M.; Huigens, M.E.; Groendijk, R.; Poitevin, O.; Deijk, J.R.; Ellis, W.N.; Grunsven, R.H.A.; Vos, R.; Vos, R.A.; et al. Declines in moth populations stress the need for conserving dark nights. Glob. Chang. Biol. 2018, 24, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Blanusa, T.; Garratt, M.; Cathcart-James, M.; Hunt, L.; Cameron, R.W. Urban hedges: A review of plant species and cultivars for ecosystem service delivery in north-west Europe. Urban For. Urban Green. 2019, 44, 126391. [Google Scholar] [CrossRef]
- Alizadeh, B.; Hitchmough, J. A review of urban landscape adaptation to the challenge of climate change. Int. J. Clim. Chang. Strat. Manag. 2019, 11, 178–194. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New Insight into Ornamental Applications of Cannabis: Perspectives and Challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef]
- Stratópoulos, L.M.F.; Zhang, C.; Häberle, K.-H.; Pauleit, S.; Duthweiler, S.; Pretzsch, H.; Rötzer, T. Effects of Drought on the Phenology, Growth, and Morphological Development of Three Urban Tree Species and Cultivars. Sustainability 2019, 11, 5117. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Dohin, N.; Pornin, D.; Rolland, M. Overview and detectability of the genetic modifications in ornamental plants. Hortic. Res. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Huylenbroeck, J.V.; Bhattarai, K. Ornamental plant breeding: Entering a new era? Ornam. Hortic. 2022, 28, 297–305. [Google Scholar] [CrossRef]
- Suprasanna, P.; Jain, S.M. Biotechnology and induced mutations in ornamental plant improvement. In II International Symposium on Tropical and Subtropical Ornamentals 1344; ISHS: Leuven, Belgium, 2021; pp. 1–12. [Google Scholar]
- Younis, A.; Ramzan, F.; Ramzan, Y.; Zulfiqar, F.; Ahsan, M.; Lim, K.B. Molecular Markers Improve Abiotic Stress Tolerance in Crops: A Review. Plants 2020, 9, 1374. [Google Scholar] [CrossRef]
- Soorni, A.; Fatahi, R.; Haak, D.C.; Salami, S.A.; Bombarely, A. Assessment of Genetic Diversity and Population Structure in Iranian Cannabis Germplasm. Sci. Rep. 2017, 7, 15668. [Google Scholar] [CrossRef]
- Chengru, L.; Na, D.; Junwen, Z. A Review for the Breeding of Orchids: Current Achievements and Prospects. Hortic. Plant J. 2021, 7, 380–392. [Google Scholar] [CrossRef]
- Gahlaut, V.; Kumari, P.; Jaiswal, V.; Kumar, S. Genetics, genomics and breeding in Rosa species. J. Hortic. Sci. Biotechnol. 2021, 96, 545–559. [Google Scholar] [CrossRef]
- Begum, Y. Regulatory role of microRNAs (miRNAs) in the recent development of abiotic stress tolerance of plants. Gene 2022, 821, 146283. [Google Scholar] [CrossRef] [PubMed]
- Van Oost, E.; Leus, L.; De Rybel, B.; Van Laere, K. Determination of genetic distance, genome size and chromosome numbers to support breeding in ornamental Lavandula species. Agronomy 2021, 11, 2173. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Sochacki, D.; Marciniak, P. Breeding Aspects of Selected Ornamental Bulbous Crops. Agronomy 2021, 11, 1709. [Google Scholar] [CrossRef]
- Jafarkhani Kermani, M.; Emadpour, M. Application of polyploidization in breeding of ornamental plants. Flower Ornam. Plants 2019, 3, 77–89. [Google Scholar]
- Niazian, M.; Nalousi, A.M. Artificial polyploidy induction for improvement of ornamental and medicinal plants. Plant Cell Tissue Organ Cult. 2020, 142, 447–469. [Google Scholar] [CrossRef]
- Popova, O.V.; Dinh, H.Q.; Aufsatz, W.; Jonak, C. The RdDM pathway is required for basal heat tolerance in Arabidopsis. Mol. Plant 2013, 6, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Manduzio, S.; Kang, H. Epitranscriptomic RNA Methylation in Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2019, 10, 500. [Google Scholar] [CrossRef]
- Sun, X.; Lin, L.; Sui, N. Regulation mechanism of microRNA in plant response to abiotic stress and breeding. Mol. Biol. Rep. 2019, 46, 1447–1457. [Google Scholar] [CrossRef]
- Vakilian, K.A. Machine learning improves our knowledge about miRNA functions towards plant abiotic stresses. Sci. Rep. 2020, 10, 3041. [Google Scholar] [CrossRef]
- Shu, K.; Zhou, W.; Chen, F.; Luo, X.; Yang, W. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jia, Z.; Pu, Q.; Tian, Y.; Zhu, F.; Liu, Y. ABA Mediates Plant Development and Abiotic Stress via Alternative Splicing. Int. J. Mol. Sci. 2022, 23, 3796. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pérez, C.; Begara-Morales, J.C.; Chaki, M.; Sánchez-Calvo, B.; Valderrama, R.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Protein Tyrosine Nitration during Development and Abiotic Stress Response in Plants. Front. Plant Sci. 2016, 7, 1699. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.M.; Adamski, N.M.; Christensen, C.E.; Stuart, D.B.; Vautrin, S.; Hansson, M.; von Wettstein-Knowles, P. The Cer-cqu gene cluster determines three key players in a β-diketone synthase polyketide pathway synthesizing aliphatics in epicuticular waxes. J. Exp. Bot. 2016, 67, 2715–2730. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.M.; Pandey, A.K.; War, A.R.; Hanumantharao, B.; Shwe, T.; Alam, A.; Pratap, A.; Malik, S.R.; Karimi, R.; Mbeyagala, E.K.; et al. Biotic and Abiotic Constraints in Mungbean Production—Progress in Genetic Improvement. Front. Plant Sci. 2019, 10, 1340. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Hu, Q.; Ma, X.; Zhang, X.; Ye, Y.; Liu, H.; Gao, H.; Zheng, J. Comparative transcriptome analysis of heat stress responses of Clematis lanuginosa and Clematis crassifolia. BMC Plant Biol. 2022, 22, 138. [Google Scholar] [CrossRef]
- Franco, J.A.; Martínez-Sánchez, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-a rid environment. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C.B. Regulated Water Deficits Improve Phytochemical Concentration in Lettuce. J. Am. Soc. Hortic. Sci. 2019, 135, 223–229. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Baruch, Z.; Liddicoat, C.; Cando-Dumancela, C.; Laws, M.; Morelli, H.; Weinstein, P.; Young, J.M.; Breed, M.F. Increased plant species richness associates with greater soil bacterial diversity in urban green spaces. Environ. Res. 2021, 196, 110425. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.G.; Brookes, J.D.; Gellie NJ, C.; Liddicoat, C.; Lowe, A.J.; Sydnor, H.R. Relating Urban Biodiversity to Human Health With the “Holobiont” Concept. Front. Microbiol. 2019, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Vasconcellos, R.L.; Pereira, A.P.; Stürmer, S.L.; Silva, A.M.; Baretta, D.; Bonfim, J.A.; Cardoso, E.J. Reforestation processes, seasonality and soil characteristics influence arbuscular mycorrhizal fungi dynamics in Araucaria angustifolia forest. For. Ecol. Manag. 2020, 460, 117899. [Google Scholar] [CrossRef]
- Nordstedt, N.P.; Chapin, L.J.; Taylor, C.G.; Jones, M.L. Identification of Pseudomonas spp. That Increase Ornamental Crop Quality During Abiotic Stress. Front. Plant Sci. 2020, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, F.; Zhou, B. The Characters of Non-Coding RNAs and Their Biological Roles in Plant Development and Abiotic Stress Response. Int. J. Mol. Sci. 2022, 23, 4124. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The Role of Biostimulants as Alleviators of Biotic and Abiotic Stresses in Grapevine: A Review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Wani, S.A.; Chand, S.; Wani, M.A.; Ramzan, M.; Hakeem, K.R. Azotobacter chroococcum—A potential biofertilizer in agriculture: An overview. In Soil Science: Agricultural and Environmental Prospectives; Springer: Cham, Switzerland, 2016; pp. 333–348. [Google Scholar]

| Plant Species | Advantages and Disadvantages in Urban Environments | References |
|---|---|---|
| Acer campestre L. | Great urban stress tolerance | Stratópoulos et al. [148] |
| Achillea millefolium L. | Filtering harmful substances from the air | Przybysz et al. [71] |
| Ailanthus altissima (Mill.) Swingle | Low urban stress tolerance | Dadkhah-Agdash et al. [74] |
| Antirrhinum majus L. | Increasing drought stress tolerance with mycorrhizal fungi | Asrar et al. [106] |
| Centaurea scabiosa L. | Filtering harmful substances from the air | Przybysz et al. [71] |
| Cercis chinensis Bunge | Earlier spring and delayed autumn phenophases | Luo et al. [32] |
| Chenopodium album L. | Filtering harmful substances from the air | Przybysz et al. [71] |
| Chrysanthemum carinatum Sch.Bip. | Low copper and heavy metal tolerance | Gladkov et al. [107] |
| Convulvulus arvensis L. | Filtering harmful substances from the air | Przybysz et al. [71] |
| Cosmos spp. | Increased drought stress tolerance using Ascophyllum nodosum extract | Battacharyya et al. [107] |
| Echium vulgare L. | Filtering harmful substances from the air | Przybysz et al. [71] |
| Eucaliptus sp. L’Hér. | High temperature tolerance | Feyisa et al. [114] |
| Hibiscus syriacus L. | Earlier spring and delayed autumn phenophases | Luo et al. [32] |
| Lavendula angustifolia Mill. | Negatively affects the essential oil content | Saunier et al. [104], Zahir et al. [105] |
| Lobularia maritima (L.) Desv. | High salt stress tolerance | Hsouna et al. [124] |
| Morus alba L. | Medium urban stress tolerance | Dadkhah-Agdash et al. [74] |
| Olea europea L. | High temperature tolerance | Feyisa et al. [114] |
| Panicum virgatum L. ‘Northwind’ | High salt stress tolerance | Wang et al. [125] |
| Petunia spp. Juss. | Increased drought stress tolerance using Ascophyllum nodosum extract | Battacharyya et al. [107] |
| Prunus davidiana Carrière | Earlier spring and delayed autumn phenophases | Luo et al. [32] |
| Robinia pseudoacacia L. | High temperature tolerance | Feyisa et al. [114] |
| Salix babylonica L. | Low urban stress tolerance | Dadkhah-Agdash et al. [74] |
| Silybum marianum (L.) Gaertn. | Negatively affects the essential oil content | Saunier et al. [104], Zahir et al. [105] |
| Tagetes patula L. | Deterioration in germination, growth, and the quality of essential oil over 35 °C | Kumar et al. [39] |
| Viola tricolor L. | Increased drought stress tolerance using Ascophyllum nodosum extract | Battacharyya et al. [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisvarga, S.; Horotán, K.; Wani, M.A.; Orlóci, L. Plant Responses to Global Climate Change and Urbanization: Implications for Sustainable Urban Landscapes. Horticulturae 2023, 9, 1051. https://doi.org/10.3390/horticulturae9091051
Kisvarga S, Horotán K, Wani MA, Orlóci L. Plant Responses to Global Climate Change and Urbanization: Implications for Sustainable Urban Landscapes. Horticulturae. 2023; 9(9):1051. https://doi.org/10.3390/horticulturae9091051
Chicago/Turabian StyleKisvarga, Szilvia, Katalin Horotán, Muneeb Ahmad Wani, and László Orlóci. 2023. "Plant Responses to Global Climate Change and Urbanization: Implications for Sustainable Urban Landscapes" Horticulturae 9, no. 9: 1051. https://doi.org/10.3390/horticulturae9091051
APA StyleKisvarga, S., Horotán, K., Wani, M. A., & Orlóci, L. (2023). Plant Responses to Global Climate Change and Urbanization: Implications for Sustainable Urban Landscapes. Horticulturae, 9(9), 1051. https://doi.org/10.3390/horticulturae9091051







