In Vitro Propagation of Peumus boldus Mol, a Woody Medicinal Plant Endemic to the Sclerophyllous Forest of Central Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material under Study
2.2. Disinfection of Plant Material
2.3. In Vitro Establishment
2.4. In Vitro Shoot Multiplication
2.5. In Vitro Rooting and Acclimation
3. Results
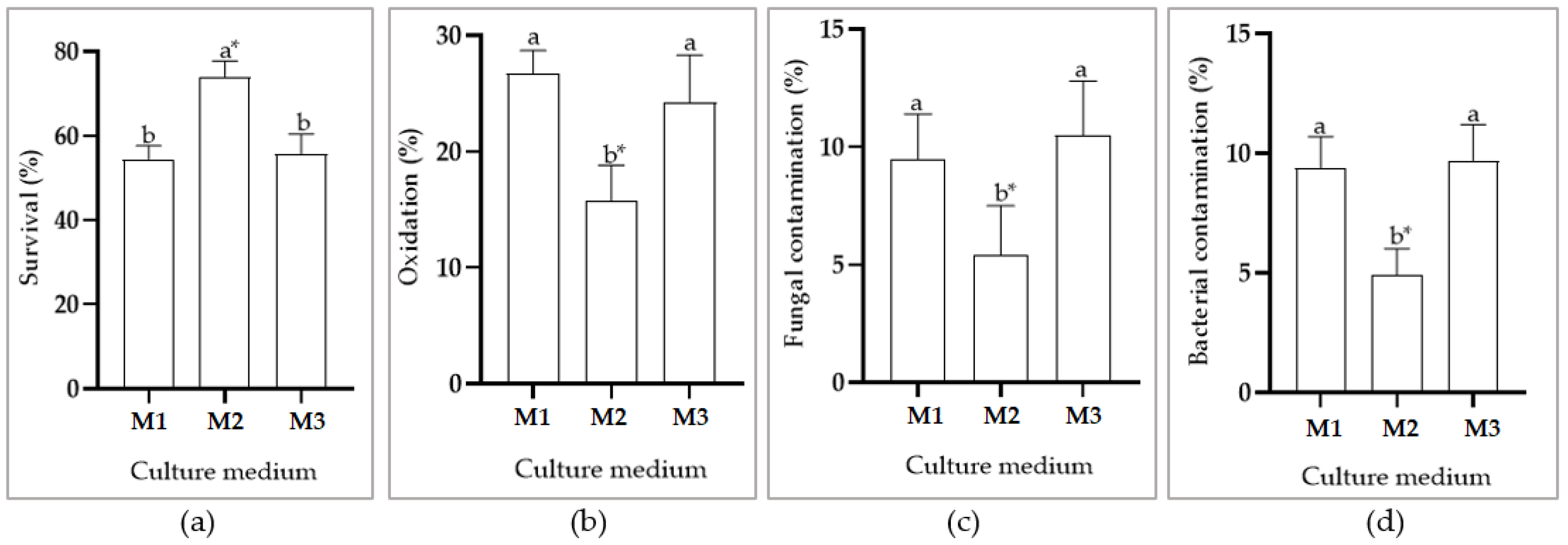
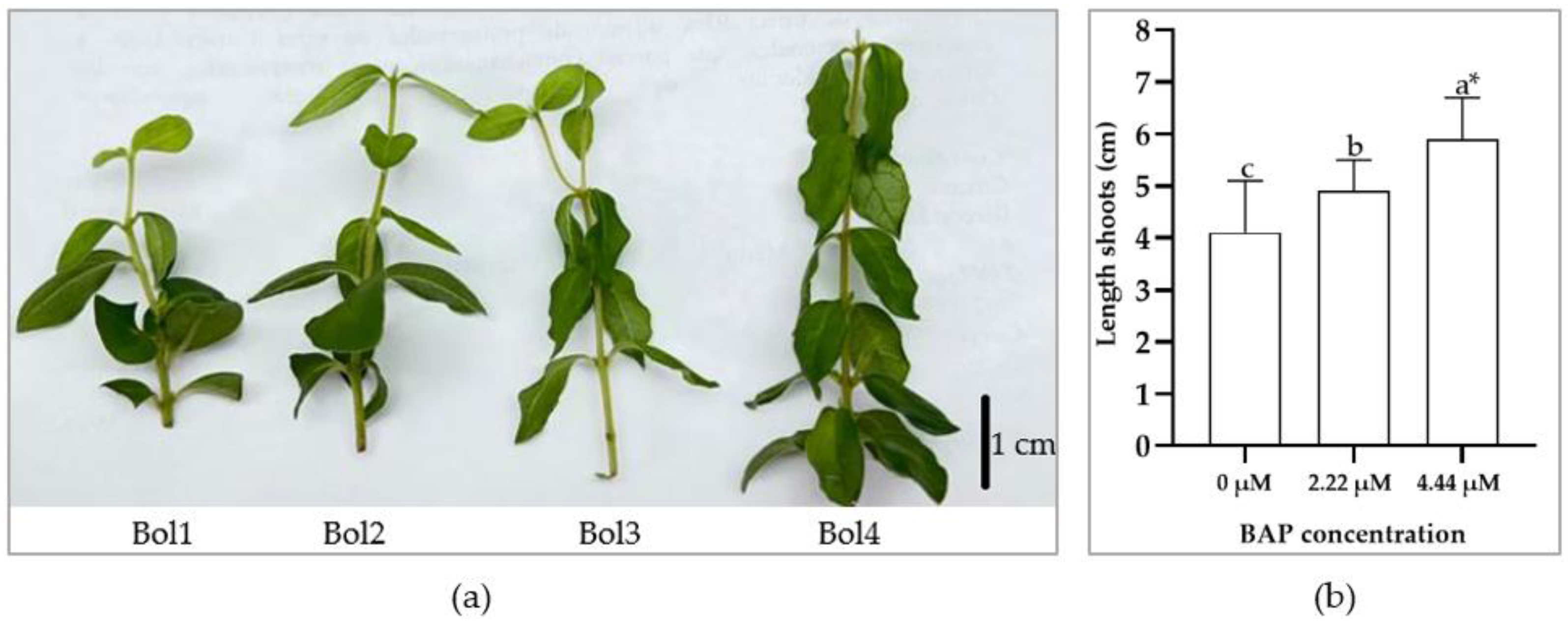
3.1. In Vitro Establishment
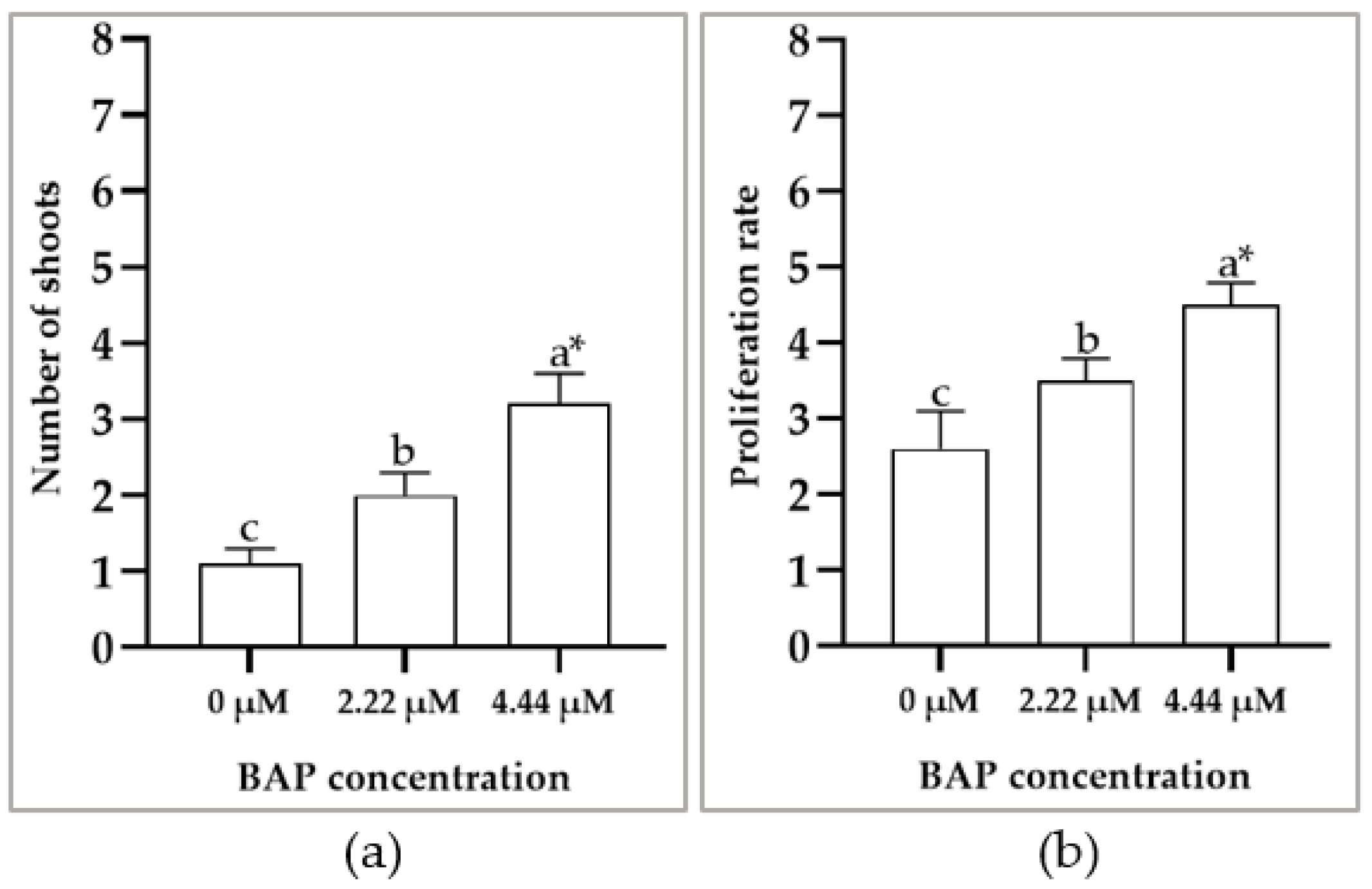
3.2. In Vitro Shoot Multiplication
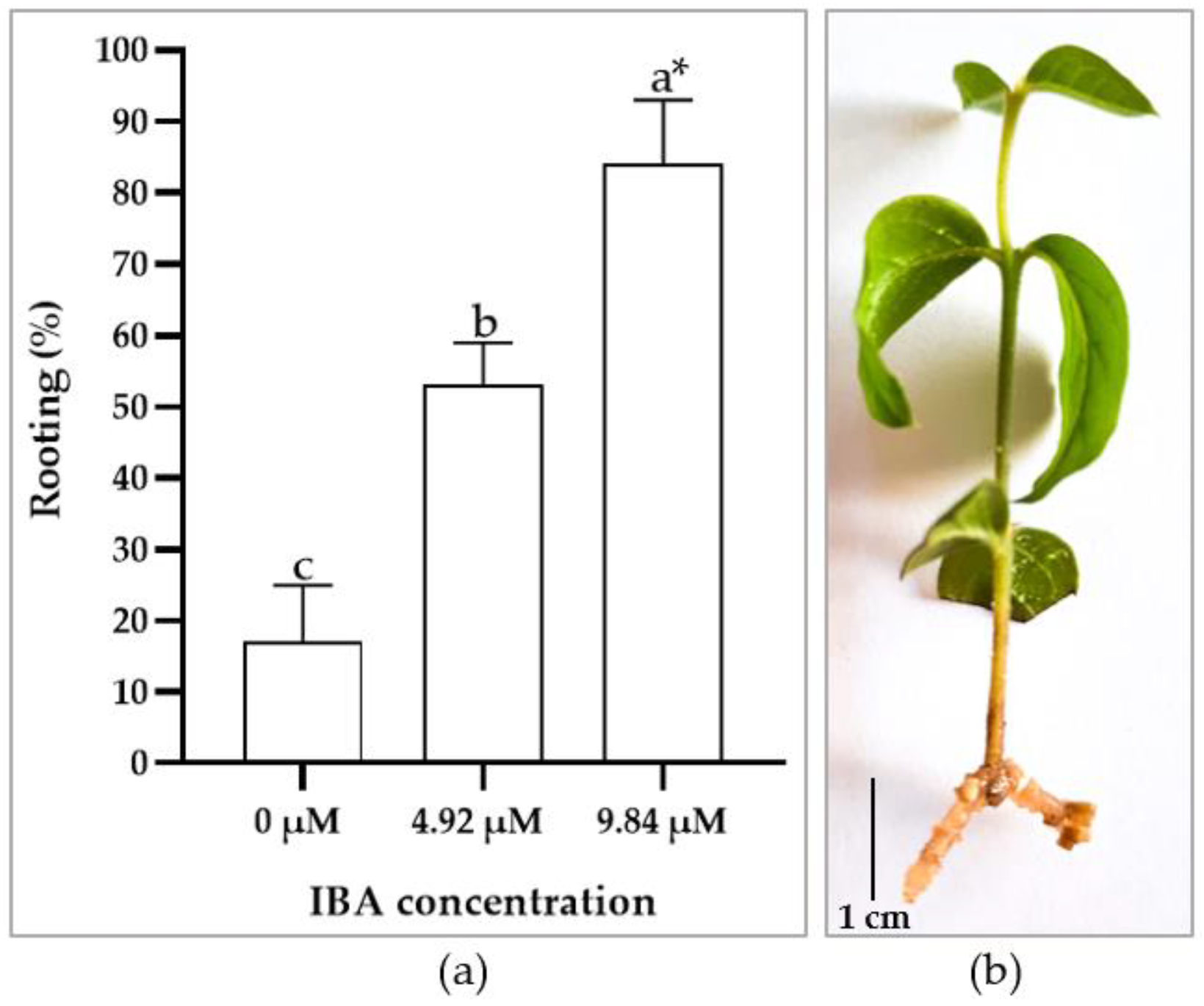
3.3. In Vitro Rooting and Acclimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grau, J.; Zizka, G. Chiles Pflanzenwelt; Palmengarten Sonderheft 19: Fráncfort del Meno, Germany, 1992; p. 42. [Google Scholar]
- Montenegro, G. Chile Nuestra Flora útil. Guía Para Uso Apícola, Medicinal, Folclórica, Artesanal y Ornamental; Universidad Católica de Chile: Santiago, Chile, 2000; p. 267. [Google Scholar]
- Doll, U.; Aedo, D.; Lopez, P. Caracterización morfológica de tres procedencias de boldo (Peumus boldus) en una plantación joven de 6 años. Bosque 2005, 26, 45–54. [Google Scholar] [CrossRef]
- Hoffmann, A.; Farga, C.; Lastra, J.; Veghazi, E. Plantas Medicinales de Uso Común en Chile, 3rd ed.; Fundación Claudio Gray: Santiago, Chile, 2003; p. 275. [Google Scholar]
- Barros, S.; Benedetti, S. Boldo (Peumus boldus Mol.) Rescate de Un Patrimonio Forestal Chileno. Manejo Sustentable y Valorización de Sus Productos; Instituto Forestal: Santiago, Chile, 2011; p. 235. [Google Scholar]
- Salehi, B.; Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.; Delporte, C.; Barra, G.; Cazar, M.E.; López, M.; Ramírez-Alarcón, K.; Cruz-Martins, N.; et al. Ethnopharmacology, phytochemistry and biological activities of native chilean plants. Curr. Pharm. Des. 2020, 27, 953–970. [Google Scholar]
- Speisky, H.; Cassels, B. Boldo and boldine: An emerging case of natural drug development. Pharmacol. Res. 1994, 29, 1–12. [Google Scholar]
- Kubinova, R.; Machala, M.; Minksova, K.; Neca, J.; Suchý, V. Chemoprotective activity of boldine: Modulation of drug-metabolizing enzymes. Pharmazie 2001, 56, 242–243. [Google Scholar]
- O’Brien, P.; Carrasco-Pozo, C.; Speisky, H. Boldine and its antioxidant or health-promoting properties. Chem. Biol. Interact. 2006, 159, 1–17. [Google Scholar]
- Verdeguer, M.; García-Rellán, D.; Boira, h.; Pérez, E.; Gandolfo, S.; Blázquez, M. Herbicidal activity of Peumus boldus and Drimys winteri essential oils from Chile. Molecules 2011, 16, 403–411. [Google Scholar] [CrossRef]
- Uquiche, E.; Huerta, E.; Sandoval, A.; Del Valle, J.M. Effect of boldo (Peumus boldus M.) pretreatment on kinetics of supercritical CO2 extraction of essential oil. J. Food Eng. 2012, 109, 230–237. [Google Scholar]
- Soto, C.; Caballero, E.; Pérez, E.; Zúñiga, M.E. Effect of extraction conditions on total phenolic content and antioxidant capacity of pretreated wild Peumus boldus leaves from Chile. Food Bioprod. Process. 2014, 92, 328–333. [Google Scholar] [CrossRef]
- Vogel, H.; Rasmilic, I.; San Martín, J.; Doll, U.; González, B. Plantas Medicinales Chilenas. Experiencias de Domesticación y Cultivo de Boldo, Matico, Bailahuén, Canelo, Peumo y Maqui; Universidad de Talca: Talca, Chile, 2005; p. 194. [Google Scholar]
- Lara, A.; Little, C.; Urrutia, R.; Mcphee, J.; Alvarez-Garretón, C.; Oyarzún, C.; Soto, D.; Donoso, P.; Nahuelhual, L.; Pino, M.; et al. Assessment of ecosystem services as an opportunity for the conservation and management of native forests in Chile. For. Ecol. Manag. 2009, 258, 415–424. [Google Scholar]
- Castañeda, L.E.; Godoy, K.; Manzano, M.; Marquet, P.A.; Barbosa, O. Comparison of soil microbial communities inhabiting vineyards and native sclerophyllous forests in central Chile. Ecol. Evol. 2015, 5, 3857–3868. [Google Scholar] [CrossRef] [PubMed]
- Santelices, R.; Bobadilla, S. Arraigamiento de estacas de Quillaja saponaria Mol. y Peumus boldus Mol. Rev. Bosque 1997, 18, 77–85. [Google Scholar] [CrossRef]
- Figueroa, J.; Jaksic, F. Latencia y banco de semillas en plantas de la región mediterránea de Chile central. Rev. Chil. Hist. Nat. 2004, 77, 201–215. [Google Scholar] [CrossRef]
- Ríos, D.; Sandoval, D.; Gómez, D. In vitro culture of Peumus boldus Molina via direct organogenesis. J. Med. Chem. 2010, 2, 70–72. [Google Scholar]
- Koch, Z.; González, J.; Benedetti, S. Regeneración de plantas in vitro de Peumus boldus Mol. (Boldo) mediante organogénesis de brotes epicórmicos de árboles maduros. Cienc. Investig. For. 2018, 24, 57–74. [Google Scholar] [CrossRef]
- Machakova, I.; Zazimalova, E.; George, E. Plant growth regulators I. Introduction: Auxins, their analogues and inhibitors. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., Klerk, G.J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 175–204. [Google Scholar]
- Brault, M.; Maldiney, R. Mechanisms of cytokinin action. Plant Physiol. Biochem. 1999, 37, 403–412. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kalia, S.; Sharma, S.; Kalia, R. Recent advances in understanding the role of growth regulators in plant growth and development in vitro-I: Conventional growth regulators. Indian For. 2016, 142, 459–470. [Google Scholar]
- Saxena, A.; Shukla, M.; Saxena, P. Synthetic Seeds: Relevance to Endangered Germplasm Conservation In Vitro. In Synthetic Seeds; Springer: Cham, Switzerland, 2019; pp. 21–60. [Google Scholar]
- Bi, W.; Saxena, A.; Ayyanath, M.-M.; Harpur, C.; Shukla, M.R.; Saxena, P.K. conservation, propagation, and redistribution (CPR) of hill’s thistle: Paradigm for plant species at risk. Plant Cell Tissue Organ Cult. 2021, 145, 75–88. [Google Scholar] [CrossRef]
- Saxena, A.; Bi, W.L.; Shukla, M.R.; Cannings, S.; Bennett, B.; Saxena, P.K. Micropropagation and cryopreservation of Yukon Draba (Draba yukonensis), a Special Concern Plant Species Endemic to Yukon Territory, Canada. Plants 2021, 10, 2093. [Google Scholar] [CrossRef]
- Santibáñez, F. Atlas Agroclimático de Chile. Estado Actual y Tendencias del Clima. Tomo III: Regiones de Valparaíso, Metropolitana, O’Higgins y Maule; Fundación para la Innovación Agraria: Santiago, Chile, 2017; p. 37. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially-feasible micro-propagation of Mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Driver, J.A. In vitro Propagation of paradox walnut rootstock. HortScience 1984, 19, 507–709. [Google Scholar] [CrossRef]
- Ostry, M.; Hackett, W.; Michler, C.; Serres, R.; McCown, B. Influence of regeneration method and tissue source on the frequency of somatic variation in Populus to infection by Septoria musiva. Plant Sci. 1994, 97, 209–215. [Google Scholar] [CrossRef]
- Monier, C.; Ochatt, S.J. Establishing micropropagation conditions for five Cotoneaster genotypes. Plant Cell Tissue Organ Cult. 1995, 42, 275–281. [Google Scholar] [CrossRef]
- Rathwell, R.; Shukla, M.R.; Jones, A.M.P.; Saxena, P.K. In vitro propagation of cherry birch (Betula lenta L.). Can. J. Plant Sci. 2016, 96, 571–578. [Google Scholar] [CrossRef]
- Guerra, F.; Badilla, L.; Cautin, R.; Castro, M. Micropropagation of Citronella mucronata D. Don, a Vulnerable Chilean Endemic Tree Species. Plants 2022, 11, 2425. [Google Scholar] [CrossRef]
- Silva, J.; Winarto, B.; Dobránszki, J.; Zeng, S. Disinfection procedures for in vitro propagation of Anthurium. Folia Hortic. 2015, 27, 3–14. [Google Scholar] [CrossRef]
- Khatri, P.; Rana, J.S.; Sindhu, A.; Jamdagni, P. Effect of additives on enhanced in-vitro shoot multiplication and their functional group identification of Chlorophytum borivilianum Sant. Et Fernand. SN Appl. Sci. 2019, 1, 1105. [Google Scholar] [CrossRef]
- Silveira, S.S.; Cordeiro-Silva, R.; Degenhardt-Goldbach, J.; Quoirin, M. Micropropagation of Calophyllum brasiliense (Cambess.) from nodal segments. Braz. J. Biol. 2016, 76, 656–663. [Google Scholar] [CrossRef]
- Kirillov, V.; Pathak, A.; Stikhareva, T.; Ercisli, S.; Daulenova, M.; Kazangapova, N.; Rakhimzhanov, A. In vitro propagation and ex vitro rooting of Euonymus verrucosus Scop. (Celastraceae)—A rare species of Kazakhstan flora on the southern border of its areal. J. For. Res. 2022, 27, 289–296. [Google Scholar] [CrossRef]
- Ho, W.J.; Huang, Y.K.; Huang, W.W. Effective in vitro culture using dormant bud of nodal sections from a mature Acacia tree. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 437–446. [Google Scholar] [CrossRef]
- Mao, A.A.; Vijayan, D.; Singha, R.K.N.; Pradhan, S. In vitro propagation of Rhododendron wattii Cowan—A critically endangered and endemic plant from India. In Vitr. Cell. Dev. Biol.-Plant 2018, 54, 45–53. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhao, Y.Y.; Zhang, M.; Zhang, L.Y. Development of a novel in vitro rooting culture system for the micropropagation of highbush blueberry (Vaccinium corymbosum) seedlings. Plant Cell Tissue Organ Cult. 2019, 139, 615–620. [Google Scholar] [CrossRef]
- Damiani, C.R.; Schuch, M.W. Diferentes substratos e ambientes no enraizamento in vitro de mirtilo. Cienc. Rural 2009, 39, 563–566. [Google Scholar] [CrossRef]
- Tzatzani, T.T.; Michail, I.; Boutsika, A.; Sarrou, E.; Ganopoulos, I. Micropropagation of guava (Psidium guajava) seedlings, a plant with interest in cool subtropics, using an innovative BB culture medium. Biotechnol. Biotechnol. Equip. 2023, 37, 139–150. [Google Scholar] [CrossRef]
- Kaviani, B.; Barandan, A.; Tymoszuk, A.; Kulus, D. Optimization of In Vitro Propagation of Pear (Pyrus communis L.) ‘Pyrodwarf®(S)’ Rootstock. Agronomy 2023, 13, 268. [Google Scholar] [CrossRef]
- Kaviani, B.; Deltalab, B.; Kulus, D.; Tymoszuk, A.; Bagheri, H.; Azarinejad, T. In Vitro Propagation of Pyracantha angustifolia (Franch.) C.K. Schneid. Horticulturae 2022, 8, 964. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, F.; Badilla, L.; Cautín, R.; Castro, M. In Vitro Propagation of Peumus boldus Mol, a Woody Medicinal Plant Endemic to the Sclerophyllous Forest of Central Chile. Horticulturae 2023, 9, 1032. https://doi.org/10.3390/horticulturae9091032
Guerra F, Badilla L, Cautín R, Castro M. In Vitro Propagation of Peumus boldus Mol, a Woody Medicinal Plant Endemic to the Sclerophyllous Forest of Central Chile. Horticulturae. 2023; 9(9):1032. https://doi.org/10.3390/horticulturae9091032
Chicago/Turabian StyleGuerra, Francesca, Loreto Badilla, Ricardo Cautín, and Mónica Castro. 2023. "In Vitro Propagation of Peumus boldus Mol, a Woody Medicinal Plant Endemic to the Sclerophyllous Forest of Central Chile" Horticulturae 9, no. 9: 1032. https://doi.org/10.3390/horticulturae9091032
APA StyleGuerra, F., Badilla, L., Cautín, R., & Castro, M. (2023). In Vitro Propagation of Peumus boldus Mol, a Woody Medicinal Plant Endemic to the Sclerophyllous Forest of Central Chile. Horticulturae, 9(9), 1032. https://doi.org/10.3390/horticulturae9091032







