Identification of Apple Varieties Resistant to Fire Blight (Erwinia amylovora) Using Molecular Markers
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collection
2.2. DNA Isolation
2.3. SNP Genotyping
2.4. SCAR Markers
3. Results
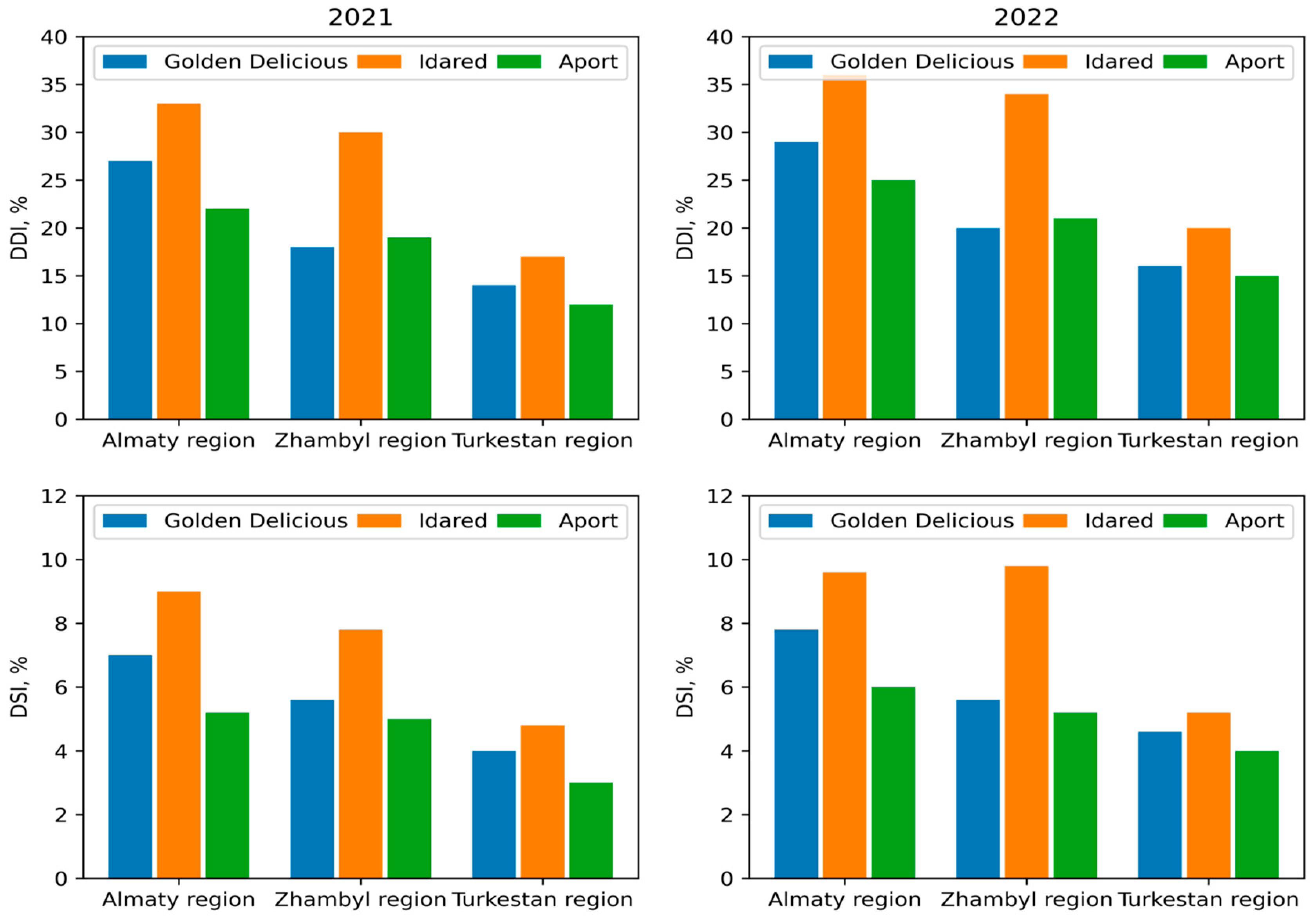
3.1. Monitoring
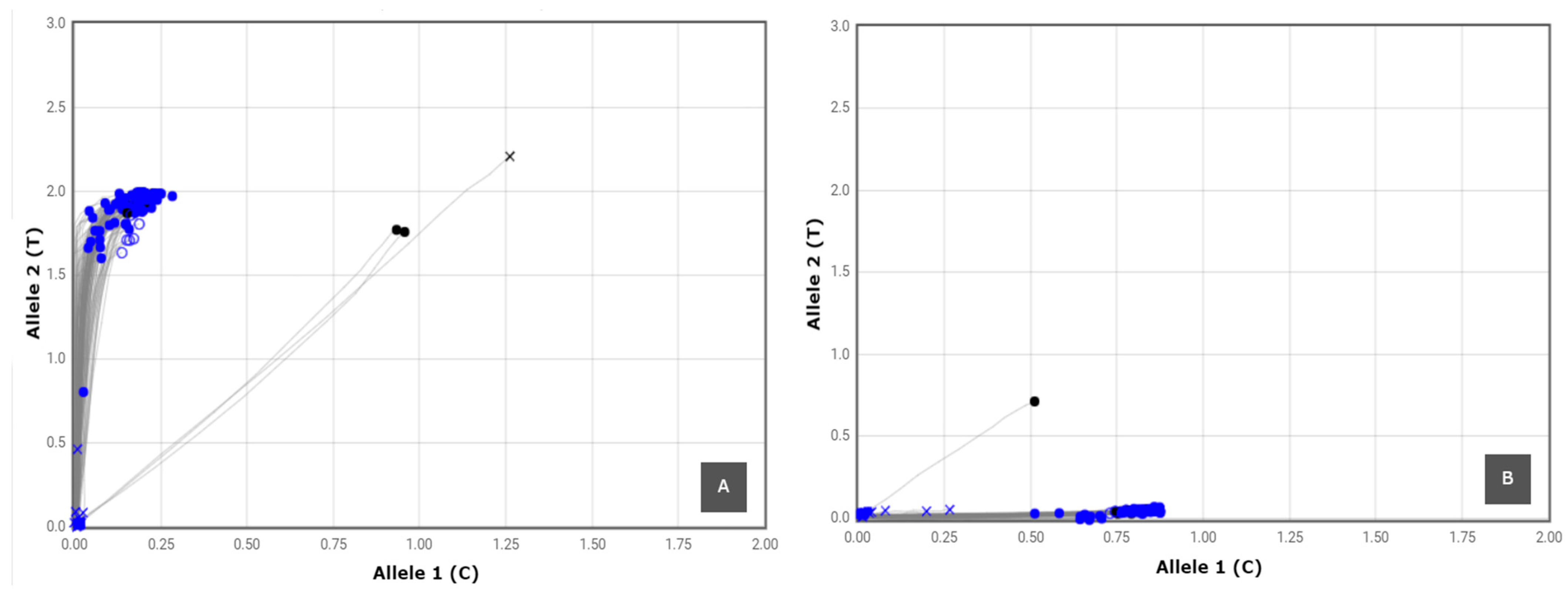
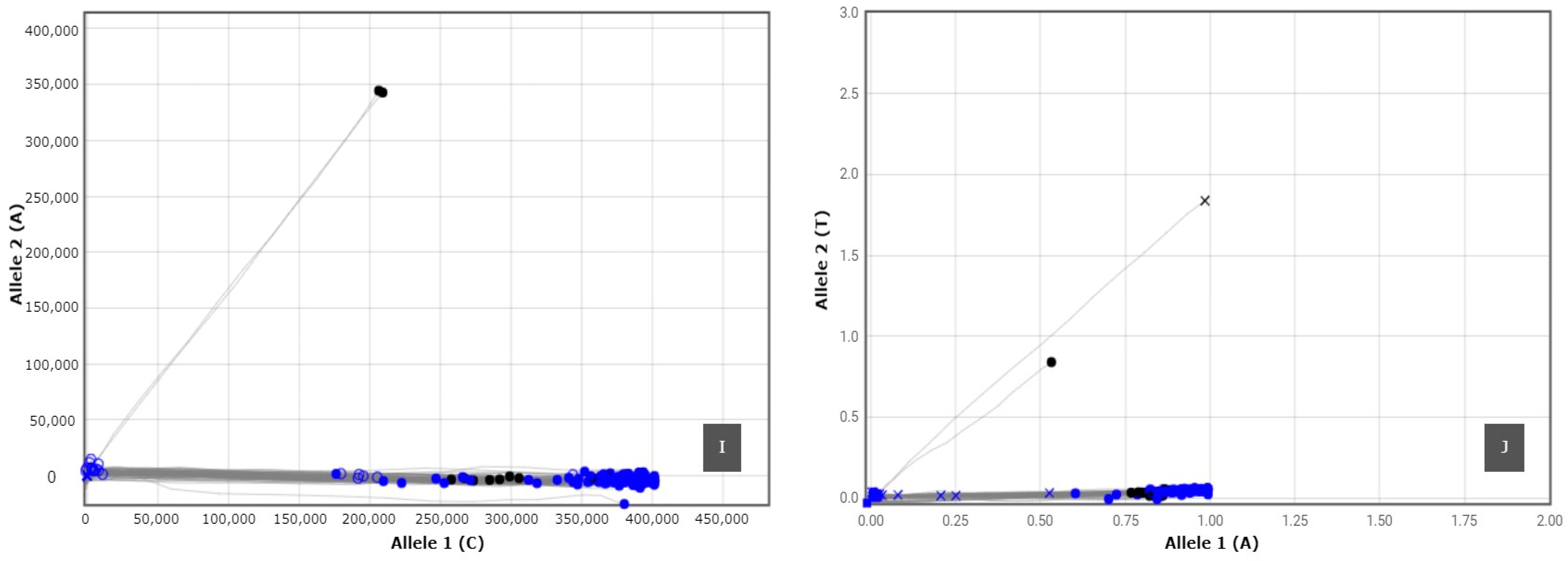
3.2. SNP Genotyping
3.3. Molecular Screening Using SCAR Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Strategic Planning and Reforms Agency of the Republic of Kazakhstan. Home Page. Available online: https://stat.gov.kz/official/industry/14/statistic/7 (accessed on 15 June 2023).
- Vanneste, J.L. What is Fire Blight? Who is Erwinia amylovora? How to Control It. In Fire Blight. The Disease and Its Causative Agent, Erwinia Amylovora; CABI Publishing: Wallingford, UK, 2000; pp. 1–6. [Google Scholar] [CrossRef]
- Norelli, J.L.; Jones, A.L.; Aldwinckle, H.S. Fire Blight Management in The Twenty First Century: Using New Techniques That Enhance Host Resistance in Apple. Plant Dis. 2003, 87, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Oktem, Y.E.; Benlioğlu, K. Yumuşak Çekirdekli Meyve Ağaçlarında Görülen Ateş Yanıklığı Hastalık Etmeni (Erwinia amylovora (Burr.)). Üzerinde Çalışmalar 1988, 4, 69–74. (In Turkish) [Google Scholar]
- Öztürk, G.; Basım, E.; Basım, H.; Emre, R.A.; Karamürsel, Ö.F.; Eren, İ.; İşçi, M.; Kaçal, E. Kontrollü Melezleme Yoluyla Ateş Yanıklığı (Erwinia amylovora) Hastalığına Karşı Dayanıklı Yeni Armut Çeşitlerinin Geliştirilmesi: İlk Meyve Gözlemleri. In Proceedings of the 6th Bahçe Bitkileri Congress, Şanlıurfa, Turkey, 4–8 October 2011. [Google Scholar]
- Drenova, N.V.; Isin, M.M.; Dzhaymurzina, A.A.; Zharmukhamedova, G.A.; Aitkulov, A.K. Bacterial burn of fruit crops in the Republic of Kazakhstan. J. Plant Quar. Sci. Pract. 2013, 1, 39–43. (In Russian) [Google Scholar]
- Azhimakhan, M.A.; Varitsev, Y.A.; Drenova, N.V.; Khasanov, V.T.; Dzhaymurzina, A.A.; Tuleeva, A.K.; Umiralieva, Z.Z. Development of enzyme-linked immunosorbent diagnostics of a test system to detect the causative agent of bacterial burn of fruit crops (Erwinia amylovora). Biotechnol. Theory Pract. 2016, 28–34. [Google Scholar]
- Gritsenko, D.A.; Nizamedinova, G.K.; Khamdieva, O.K.; Dinasilov, A.S. Identification of a bacterial burn by molecular gene-tic methods. News Natl. Acad. Sci. Repub. Kazakhstan Agric. Sci. Ser. 2017, 6, 109–115. [Google Scholar]
- Saveliev, N.N.; Savelyeva, N.I. Application of the achievements of genetics in the breeding of fruit crops: The contribution of the Michurin branch of the Vavilov Society of Geneticists and Breeders. Plant Genet. Breed. 2016, 20, 555–562. [Google Scholar] [CrossRef]
- Lee, S.A.; Ngugi, H.K.; Halbrendt, N.O.; O’Keefe, G.; Lehman, B.; Travis, J.W.; Sinn, J.P.; Mc Nellis, T.W. Virulence characte-ristics accounting for fire blight disease severity in apple trees and seedlings. Phytopathology 2010, 100, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Emeriewen, O.F.; Wöhner, T.; Flachowsky, H.; Peil, A. Malus hosts—Erwinia amylovora interactions: Strain pathogenicity and resistance mechanisms. Front. Plant Sci. 2019, 10, 551. [Google Scholar] [CrossRef]
- Norelli, J.L.; Aldwinckle, H.S. Differential susceptibility of Malus spp. Cultivars Robusta 5, Novole, and Ottawa 523 to Erwinia amylovora. Plant Dis. 1986, 70, 1017–1019. [Google Scholar] [CrossRef]
- Norelli, J.L.; Holleran, H.T.; Johnson, W.C.; Robinson, T.L.; Aldwinckle, H.S. Resistance of Geneva and other apple rootstocks to Erwinia amylovora. Plant Dis. 2003, 87, 26–32. [Google Scholar] [CrossRef]
- Peil, A.; Wöhner, T.; Hanke, M.V.; Flachowsky, H.; Richter, K.; Wensing, A.; Kilian, A. Comparative mapping of fire blight resistance in Malus. Acta Hortic. 2013, 1056, 47–517. [Google Scholar] [CrossRef]
- Fazio, G.; Aldwinckle, H.; Robinson, T. Unique characteristics of Geneva® apple rootstocks. Encontro Nac. Sobre Frutic. Clima Temperado 2013, 1, 23. [Google Scholar]
- Kellerhals, M.; Schütz, S.; Patpcchi, A. Breeding for host resistance to the fire blight. J. Plant Pathol. 2017, 99, 37–43. Available online: https://www.jstor.org/stable/45156717 (accessed on 15 June 2023).
- Peil, A.; Emeriewen, O.F.; Khan, A.; Kostick, S.; Malnoy, M. Status of fire blight resistance breeding in Malus. J. Plant Pathol. 2021, 103, 3–12. [Google Scholar] [CrossRef]
- Kostick, S.A.; Teh, S.L.; Evans, K.M. Contributions of Reduced Susceptibility Alleles in Breeding Apple Cultivars with Durable Resistance to Fire Blight. Plants 2021, 10, 409. [Google Scholar] [CrossRef]
- Khan, M.A.; Durel, C.E.; Duffy, B.; Drouet, D.; Kellerhals, M.; Gessler, C.; Patocchi, A. Development of molecular markers linked to the ‘Fiesta’ linkage group 7 major QTL for fire blight resistance and their application for marker-assisted selection. Genome 2007, 50, 568–577. [Google Scholar] [CrossRef]
- Omasheva, M.Y.; Pozharskiy, A.S.; Maulenbay, A.D.; Ryabushkina, N.A.; Galiakparov, N.N. SSR genotyping of Kazakhstan apple varieties and identification of alleles associated with resistance to the most dangerous pathogens. Biotechnol. Theory Pract. 2016, 2, 168. [Google Scholar] [CrossRef]
- Lyzhin, A.; Saveleva, N. Identification of QTL FBF7 fire blight resistance in apple varieties germplasm. BIO Web Conf. 2021, 34, 02002. [Google Scholar] [CrossRef]
- International Standard GOST 33829-2016; Plant Protection. Requirements for the Production of Products of Plant Origin at Risk of Developing a Phytosanitary Condition. Standardinform: Moscow, Russian, 2016.
- Interstate Standard for Phytosanitary Measures (ISPM 27); Diagnostic Protocols for Regulated Pests. International Plant Protection Convention (IPPC). Food and Agriculture Organization of the United Nations: Rome, Italy, 2016.
- Garden Focused. Home Page. Available online: https://www.gardenfocused.co.uk/contactus.php (accessed on 15 June 2021).
- Keepers Nursery. Home Page. Available online: http://surl.li/hrfcb (accessed on 22 June 2023).
- Rennie Orchards. Home Page. Available online: https://rennieorchards.com/gala-apple-tree/ (accessed on 20 June 2023).
- Livingasia. Home Page. Available online: https://livingasia.online/amp/2016/09/10/apples-almaty/# (accessed on 26 June 2023).
- The Arbor Day Foundation. Home Page. Available online: https://www.arborday.org/trees/treeguide/TreeDetail.cfm?itemID=2514 (accessed on 20 June 2023).
- Plant Me Green. Home Page. Available online: https://www.plantmegreen.com/products/golden-delicious-apple-tree (accessed on 20 June 2023).
- Orange Pippin. Home Page. Available online: https://www.orangepippin.com/varieties/apples/granny-smith (accessed on 22 June 2023).
- FGBNU VNIISPK. Home Page. Available online: https://vniispk.ru/varieties/suislepskoe-suisleper-malinovka (accessed on 5 July 2023).
- Anig Lider Innovation. Home Page. Available online: http://anig.kz/ru/catalog/yablonya-renet-burhardta-limonka_151 (accessed on 13 July 2023).
- Yastrebov, I.I. Brief Description of Apple Varieties. Available online: https://www.dachacha.ru/u/245.html (accessed on 20 July 2023).
- The Arbor Day Foundation. Home Page. Available online: https://www.arborday.org/trees/treeguide/TreeDetail.cfm?ItemID=721 (accessed on 26 June 2023).
- Nurmuratuly, T.; Madenov, E.D.; Nurtazina, N.Y. Genofond of local and Old varieties of apple, pear, apricot, and grapes in the South and Southeast of Kazakhstan. Russ. J. Genet. 2012, 54, 176–187. [Google Scholar]
- Anig Lider Innovation. Home Page. Available online: https://www.anig.kz/ru/catalog/yablonya-saltanat_199 (accessed on 15 June 2023).
- Sadovnik Ingo. Home Page. Available online: https://sadovnik.info/sort-yabloni-zarya-alatau.html (accessed on 15 June 2023).
- Specialty Produce. Home Page. Available online: https://specialtyproduce.com/produce/Modi_Apples_14446.php (accessed on 15 June 2023).
- Garden Focused. Home Page. Available online: https://www.gardenfocused.co.uk/fruitarticles/apples/variety-braeburn.php (accessed on 15 June 2023).
- Tsesmelis. Home Page. Available online: https://tsesmelis.gr/en/portfolio/jeromin-apple-variety-2/ (accessed on 20 June 2023).
- Salt Spring Apple Company. Home Page. Available online: https://www.saltspringapplecompany.com/quinte (accessed on 15 June 2023).
- Cummins Nursery. Home Page. Available online: https://www.cumminsnursery.com/buy-trees/product-detail.php?type=tree&id=2886 (accessed on 15 June 2023).
- Minneopa Orchards. Home Page. Available online: https://minnetonkaorchards.com/mutsu-apple-tree/ (accessed on 15 June 2023).
- Soldatov, I.V. The Best Local/Old-Time Varieties of Apple Trees in Kyrgyzstan. Available online: http://centralasia.bioversityinternational.org/fileadmin/templates/centralasia.net/upload/Resources/TRG/2614-0020.pdf (accessed on 15 June 2023).
- Orange Pippin. Home Page. Available online: https://www.orangepippin.com/varieties/apples/pinklady (accessed on 15 June 2023).
- Victoriana Nursery Gardens. Home Page. Available online: https://www.victoriananursery.co.uk/Apple-Tree-Starks-Earliest/ (accessed on 15 June 2023).
- Dalival. Home Page. Available online: https://www.dalival.com/pommes/scarlet-spur-evasni-ru/ (accessed on 15 June 2023).
- Seedlings Maryanivka. Home Page. Available online: https://sadzhanciderev.com.ua/p722676977-sazhentsy-yablon-samared.html (accessed on 15 June 2023).
- Roots to Fruits Nursery. Home Page. Available online: https://rootstofruitsnursery.com/products/redfree (accessed on 15 June 2023).
- Orange Pipin. Home Page. Available online: https://www.orangepippintrees.com/trees/apple-trees/williams-pride (accessed on 15 June 2023).
- Garden Web. Home Page. Available online: http://gardenweb.ru/diana-sort-yabloni (accessed on 15 June 2023).
- Jparker’s. Home Page. Available online: https://www.jparkers.co.uk/apple-golden-spur-0001160c (accessed on 15 June 2023).
- Stark Bros. Home Page. Available online: https://www.starkbros.com/products/fruit-trees/apple-trees/honeycrisp-apple (accessed on 15 June 2023).
- B2B.Trade. Home Page. Available online: https://b2b.trade/product/agrofirma-doneckaya-dolina-yablonya-red-chif-kamspur (accessed on 15 June 2023).
- Sad Sezon. Home Page. Available online: https://sadsezon.com/sad/plodovie/yabloni/sorta/k/korej.html (accessed on 15 June 2023).
- Nature Hills. Home Page. Available online: https://www.naturehills.com/anna-apple-tree (accessed on 15 June 2023).
- FGBNU VNIISPK. Home Page. Available online: https://vniispk.ru/varieties/iulskoe-chernenko (accessed on 15 June 2023).
- Orange Pippin. Home Page. Available online: https://www.orangepippin.com/varieties/apples/elstar (accessed on 15 June 2023).
- Sortover.ru. Home Page. Available online: https://sortoved.ru/yablonya/sort-yabloni-dzhonagold.html (accessed on 15 June 2023).
- Johan Nicolai. Home Page. Available online: https://johan-nicolai.com/en/varieties/d/detail/elstar-elrosa (accessed on 10 June 2023).
- Mary, E.E. Gardening Know How. Home Page. Available online: https://www.gardeningknowhow.com/edible/fruits/apples/prima-apple-information.htm (accessed on 10 June 2023).
- Celtic Orchards. Home Page. Available online: https://www.theapplefarm.com/celtic/redelstar.htm (accessed on 10 June 2023).
- OOO Firma Ltd. Home Page. Available online: https://ltdsad.ru/catalog/brebur-marija-red/ (accessed on 10 June 2023).
- New England Apple. Home Page. Available online: https://newenglandapples.org/apples/jonaprince/ (accessed on 10 June 2023).
- Alma Global. Home Page. Available online: https://www.almaglobal.kz/sinap-kandil-almatinskij (accessed on 15 June 2023).
- Johan Nicolai. Home Page. Available online: https://johan-nicolai.com/en/varieties/d/detail/wilton-s-star-red-jonaprince-select (accessed on 10 June 2023).
- Fresh Plaza. Home Page. Available online: https://www.freshplaza.com/north-america/article/9156688/overview-global-apple-market/ (accessed on 10 June 2023).
- Orange Pippin. Home Page. Available online: https://www.orangepippin.com/varieties/apples/santana (accessed on 10 June 2023).
- Minneopa Orchards. Home Page. Available online: https://minnetonkaorchards.com/pinova-apple-tree/ (accessed on 10 June 2023).
- Orange Pippin. Home Page. Available online: https://www.orangepippin.com/varieties/apples/topaz (accessed on 10 June 2023).
- Fresh Plaza. Home Page. Available online: https://www.fruit-inform.com/ru/technology/experience/175413 (accessed on 10 June 2023).
- Beer, S.V.; Schwager, S.J.; Norelli, J.L.; Aldwinckle, H.S.; Burr, T.J. Development of a Risk-Assessment System for Fire Blight. In Proceedings of the Sixth International Conference on Plant Pathogenic Bacteria, College Park, MA, USA, 2–7 June 1985; p. 4. [Google Scholar]
- Jansch, M.; Broggini, G.A.L.; Weger, J.; Bus, V.G.M.; Gardiner, S.E.; Bassett, H.; Patocchi, A. Identification of SNPs linked to eight apple disease resistance loci. Mol. Breed. 2015, 35, 45. [Google Scholar] [CrossRef]
- Gardiner, S.E.; Norelli, J.L.; de Silva, N.; Fazio, G.; Peil, A.; Malnoy, M.; Horner, M.; Bowatte, D.; Carlisle, C.; Wiedow, C.; et al. Putative resistance gene markers associated with quantitative trait loci for fire blight resistance in Malus ‘Robusta 5’ accessions. BMC Genet. 2012, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Chagne, D.; Vanderzande, S.; Kirk, C.; Profitt, N.; Weskett, R.; Gardiner, S.; Peace, C.; Volz, R.; Bassil, N. Validation of SNP markers for fruit quality and disease resistance loci in apple (Malus × domestica Borkh.) using the OpenArray® platform. Hortic. Res. 2019, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Meteorological and Hydrological Databases. Home Page. Available online: https://www.kazhydromet.kz/en/meteo_db (accessed on 10 June 2023).
- Van der Zwet, T.; Halbrendt, O.N.; Zeller, W. Fire Blight: History, Biology and Management; APS Press: Saint Paul, MI, USA, 2016. [Google Scholar] [CrossRef]
- Brown, S. Apple. In Fruit Breeding, Handbook of Plant Breeding; Springer: Berlin/Heidelberg, Germany, 2012; pp. 329–367. [Google Scholar]
- DuPont, S.T.; Munir, M.; Cox, K.; Johnson, K.; Peter, K.; Baro, A. Evaluation of pruning therapies in apple trees with fire blight. J. Plant Pathol. 2023. [Google Scholar] [CrossRef]
- Kostick, S.A.; Norelli, J.L.; Evans, K.M. Novel metrics to classify fire blight resistance of 94 apple cultivars. Plant Pathol. 2019, 68, 985–996. [Google Scholar] [CrossRef]
- Baumgartner, I.; Franck, L.; Silvestri, G.; Patocchi, A.; Duffy, B.; Frey, J.; Kellerhals, M. Advanced strategies for breeding fire blight resistant high-quality apples. In Proceedings of the 14th International Conference on Organic Fruit-Growing, Hohenheim, Germany, 22–24 February 2010. [Google Scholar]
- Peace, C.P. DNA-informed breeding of rosaceous crops: Promises, progress and prospects. Hortic. Res. 2017, 4, 17006. [Google Scholar] [CrossRef]
- Kostick, S.A.; Teh, S.; Norelli, J.L.; Vanderzande, S.; Peace, C.; Evans, K.M. Fire blight QTL analysis in a multi-family popula-tion identifies a reduced-susceptibility allele in ‘Honeycrisp’. Hortic. Res. 2021, 8, 28. [Google Scholar] [CrossRef]
- Sehic, J.; Nybom, H.; Garkava-Gustavsson, L.; Patocchi, A.; Kellerhals, M.; Duffy, B. Fire blight (Erwinia amylovora) resistance in apple varieties associated with molecular markers. Int. J. Hortic. Sci. 2009, 15, 53–57. [Google Scholar] [CrossRef]
- Quamme, H.A.; van der Zwet, T.; Dirks, V. Relationship of fire blight resistance of young pear seedlings inoculated in the greenhouse to mature seedling trees naturally infected in the field. Plant Dis. Report. 1976, 60, 660–664. Available online: https://www.jstor.org/stable/41992429 (accessed on 15 June 2023).
- van der Zwet, T.; van der Zwet, K.T.; Keil, H.L. Fire blight, a bacterial disease of rosaceous plants. In Agriculture Handbook; U.S. Department of Agriculture: Washington, DC, USA, 1979. [Google Scholar]
- van der Zwet, T.; Beer, S.V. Fire blight—Its Nature, Prevention, and Control: A Practical Guide to Integrated Disease Management; USDA: Washington, DC, USA, 1999. [Google Scholar] [CrossRef]
- Brisset, M.N.; Paulin, J.P. Plant mechanisms interfering with fire blight infection. Acta Hortic. 2006, 704, 483–487. [Google Scholar] [CrossRef]
- van der Zwet, J.; Hanssen, V.G.; Zwietering, P.J.; Muijtjens, P.J.; van der Vleuten, A.M.; Metsemakers, C.P. Workplace learning in general practice: Supervision, patient mix and independence emerge from the black box once again. Med. Teach. 2010, 32, e294–e299. [Google Scholar] [CrossRef]
- Aldwinckle, H.S.; Gustafson, H.L.; Forsline, P.L. Evaluation of the core subset of the USDA apple germplasm collection for resistance to fire blight. Acta Hortic. 1999, 489, 269–272. [Google Scholar] [CrossRef]
- Le Lezek, M.; Paulin, J.P.; Lecomte, P. Shoot and blossom susceptibility to fireblight of apple cultivars. Acta Hortic. 1987, 217, 311–315. [Google Scholar] [CrossRef]
- Mohan, S.K.; Fallahi, E.; Bijman, V.P. Evaluation of apple varieties for susceptibility to Erwinia amylovora by artificial inocula-tion under field conditions. Acta Hortic. 2002, 590, 373–375. [Google Scholar] [CrossRef]
- Hampson, C.R.; Sholberg, P.L. Estimating combining ability for fire blight resistance in apple progenies. Acta Hortic. 2008, 793, 337–343. [Google Scholar] [CrossRef]
- Nurtaza, A.; Pozharskiy, A.; Dyussembekova, D. Conservation of Malus Niedzwetzkyana Dieck Ex Koehne Genotypes from Kazakhstan Resistant to Scab and Fire Blight Diseases. Res. Sq. 2022, 1–18. [Google Scholar] [CrossRef]
- Shamshin, I.N.; Maslova, M.V.; Drenova, N.V.; Dubrovsky, M.L.; Parusova, O.V. Assessment of fire blight resistance in apple clonal rootstocks using molecular markers. Proc. Appl. Bot. Genet. Breed. 2021, 181, 185–191. [Google Scholar] [CrossRef]
- Nybom, H.; Mikicinski, A.; Garkava-Gustavsson, L.; Sehic, J.; Lewandowski, M.; Sobiczewski, P. Assessment of fire blight tole-rance in apple based on plant inoculations with Erwinia amylovora and DNA markers. Trees 2012, 26, 199–213. [Google Scholar] [CrossRef]
- Sätra, S.A.J.; Troggio, M.; Odilbekov, F.; Sehic, J.; Mattisson, H.; Hjalmarsson, I.; Ingvarsson, P.K.; Garkava-Gustavsson, L. Genetic Status of the Swedish Central collection of heirloom apple cultivars. Sci. Hortic. 2020, 272, 109599. [Google Scholar] [CrossRef]





| # | Genotype | Origin | Location * | Reference |
|---|---|---|---|---|
| 1 | Idared | Cross between Jonathan and Wagener varieties, first raised in Idaho, USA. It was first released to the public in 1942 after a ten-year breeding program. Included in the State Register of the Republic of Kazakhstan since 1998. | 1; 4; 6 | [24] |
| 2 | Starkrimson | Pollination: Starkrimson is self-sterile and requires a pollinator to produce a crop. Origin: USA, 1870. Included in the State Register of the Republic of Kazakhstan since 1988. | 6; 8 | [25] |
| 3 | Gala | Developed in New Zealand, it is the result of breeding among some of the most historically important varieties such as Cox’s Orange Pippin, Golden Delicious, and Kidd’s Orange Red. Included in the State Register of the Republic of Kazakhstan since 2011. | 4; 6 | [26] |
| 4 | Aport | An ancient variety from Russia. The originator is not registered. Crossing them with the local wild Sievers apple tree made the new variety famous. Included in the State Register of the Republic of Kazakhstan since 1965. | 4; 7 | [27] |
| 5 | Fuji | Bred by crossing Red Delicious × Rale Jennet. Originator: Tohoku Science Station, Marioka, Japan. Included in the State Register of the Republic of Kazakhstan since 2011. | 1; 6 | [28] |
| 6 | Golden Delicious | Bred by crossing Grimes Golden × Golden Reinette. The originator is not registered. One of the symbols of West Virginia, USA. Included in the State Register of the Republic of Kazakhstan since 1965. | 1; 6 | [29] |
| 7 | Granny Smith | Bred by crossing a French wild apple tree with a local Australian apple tree. Originator: Maria Ann Smith, Australia. Included in the State Register of the Republic of Kazakhstan since 2011. | 6 | [30] |
| 8 | Suislepper | From Baltic countries, it is an old variety of folk selection. Included in the State Register of the Republic of Kazakhstan since 1965. | 7 | [31] |
| 9 | Renet Burhardta | A variety of Crimean origin. Originator: State Nikitsky Botanical Garden, Ukraine. Included in the State Register of the Republic of Kazakhstan since 1965. | 4; 7 | [32] |
| 10 | Pestrushka | An ancient South Kazakhstan variety. The originator is not registered. Included in the State Register of the Republic of Kazakhstan since 1965. | 4 | [33] |
| 11 | Red Delicious (5) | Red-fruited clone of the variety Delicious. Originator: private nursery in Wilsburg, USA. Included in the State Register of the Republic of Kazakhstan since 2011. | 5 | [34] |
| 12 | Tulpan (8) | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Autumn maturity. Passes the State variety test. | 8 | [35] |
| 13 | Ainur | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Winter maturity. Included in the State Register of the Republic of Kazakhstan since 2011. | 1; 7 | [35] |
| 14 | Aigul | Bred by crossing Aport × Golden Delicious. Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. | 7 | [35] |
| 15 | Saltanat | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Bred by selection of seedlings Renette Burchardt from free pollination. Included in the State Register of the Republic of Kazakhstan since 1980. | 7 | [36] |
| 16 | Voskhod | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Winter maturity. Included in the State Register of the Republic of Kazakhstan since 2011. | 1 | [35] |
| 17 | Egemen | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Winter maturity. Included in the State Register of the Republic of Kazakhstan since 2019. | 1 | [35] |
| 18 | Zarya Alatau | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Obtained from seedlings of the variety Renet Orleans from free pollination. Included in the State Register of the Republic of Kazakhstan since 1974. | 1 | [37] |
| 19 | Maksat | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Autumn maturity. Included in the State Register of the Republic of Kazakhstan since 2011. | 1 | [35] |
| 20 | Esen | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Bred by crossing varieties Zailiyskoye and Aport. Passes the State variety test. | 1 | [35] |
| 21 | Sarkyt | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Bred by crossing varieties Zarya Alatau and Aport. Passes the State variety test. | 1 | [35] |
| 22 | Talgarskoe | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Winter maturity. Included in the State Register of the Republic of Kazakhstan since 2004. | 1 | [35] |
| 23 | Zharkyn | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Passes the State variety test. | 1 | [35] |
| 24 | Danalyk | Variety of selection of the Kazakh Research Institute of Fruit Growing and Viticulture. Winter maturity. Included in the State Register of the Republic of Kazakhstan since 2020. | 1 | [35] |
| 25 | Modi | Created at the Consorzio Italiano Vivaisti in Italy by researchers in the 2000s and then introduced to the USA in 2014. Bred by crossing varieties Gala and Liberty. | 8 | [38] |
| 26 | Braeburn | The New Zealand variety was obtained via free pollination of Lady Hamilton in 1952. | 1; 3; 8 | [39] |
| 27 | Jeromini | French variety. Derived from varieties Erovan and Early Red. | 4 | [40] |
| 28 | Quinti | Created in Canada as a result of crossing Crimson Beauty × Red Melba in 1964, summer ripening. | 5; 8 | [41] |
| 29 | Kandil Sinap | An old Crimean variety of autumn ripening. Obtained by random mutation when sowing seeds of the related variety “Sary-sinap”. | 8 | [42] |
| 30 | Mutsu | Created in Japan in 1948. Obtained as a result of crossing Golden Delicious and Indo-Japanese varieties of apples. | 8 | [43] |
| 31 | Kandil Kirghiz | An ancient variety in Kyrgyzstan with a winter ripening period. | 8 | [44] |
| 32 | Pink Lady | An Austrian variety, bred by the English gardener John Cripps by crossing the Australian variety Lady Williams and Golden Delicious. | 4; 6 | [45] |
| 33 | Starks Earliest | Developed in Idaho, USA, in 1938 by the Stark brothers of Missouri. | 8 | [46] |
| 34 | Scarlet Spur | American winter variety. Mutant Oregonspur® Trumdor. | 6 | [47] |
| 35 | Summer red | Summer variety of apples of Canadian selection. The maximum height of the tree is 2.5–3.5 m, belonging to the medium height group. | 6 | [48] |
| 36 | Nicole Grain | No data. | - | - |
| 37 | Redfree | Created by the Purdue, Rutgers, and University of Illinois Agricultural Experimental Station in the 1980s, the Redfree apple was designed to be a disease-resistant summer apple. Obtained as a result of crossing a domestic apple tree and a clone of Malus floribunda 821. | 3 | [49] |
| 38 | Williams Pride | Was developed by the well-respected “co-op” breeding program of Purdue, Rutgers, and Illinois (PRI) universities in the 1970s, and originally known as Co-op 23. Its ancestry is similar to that of many of the PRI varieties, including Rome Beauty and Malus floribunda, but its early-season character probably comes from Mollie’s Delicious and Julyred. | 3 | [50] |
| 39 | Diana | Variety of Ukrainian selection, winter ripening. | 3 | [51] |
| 40 | Gold spur | Obtained from the variety Golden Delicious, winter term maturation. | 4 | [52] |
| 41 | Honeycrisp | Originates from Excelsior, Minnesota in 1974. Crossed between Mekaun and Honeygold. | 4 | [53] |
| 42 | Camspur | A Starkimson mutant discovered in 1967 in Washington, DC. Maturing time—Winter. Derevo: average, crown Compact. Power Supporters: Idared, Granny Smith, Everest, Professor Sprenger. | 4 | [54] |
| 43 | Korea | Japanese selection at the Aomori experimental station, obtained by hybridization of Golden Delicious and Indus apple trees. | 4 | [55] |
| 44 | Gala Anna | Produced in Italy as a Gala clone. | 5 | [56] |
| 45 | July Chernenko | An ancient variety of Russian selection. Obtained from crossing varieties Anis velvet and Papirovka. | 1 | [57] |
| 46 | Elstar | Received in 1954–1955 in the Netherlands. Created by crossing varieties Ingrid Maria and Golden Delicious. | 1 | [58] |
| 47 | Jonagold | Bred in the USA at the Geneva Experimental Station by crossing varieties Golden Delicious and Jonathan. | 1 | [59] |
| 48 | Elrosa | Wilfred and Annet Zweeren, Netherlands, 1999. | 1 | [60] |
| 49 | Prima | Early autumn variety of American selection. Created by crossing varieties: M. floribunda 821 and the varieties Welsey, Melba, Rum Beauty, Golden Delicious, and their derivatives. | 1 | [61] |
| 50 | Red Elstar | Dutch selection, winter ripening. | 1 | [62] |
| 51 | Maria Red | Dutch selection, winter ripening. | 1 | [63] |
| 52 | Red JonaPrince (1) | It is one of the clones of Jonagold, isolated in Belgium, winter ripening. | 1 | [64] |
| 53 | Synap Almatinsky | An ancient variety, comes from the Crimean Sinap known since the 19th century. Winter maturity. | 1 | [65] |
| 54 | Wilton Star | The Netherlands. The Wilton’s Star Red Jonaprince Select is a new mutation from Red Jonaprince, which colors darker red. | 2 | [66] |
| 55 | Deljonca | The Deljonca varieties in the German growing areas. Formed by crossing varieties Delbard Florina × Stark Jongrimes. | 2 | [67] |
| 56 | Santana (2) | Bred by the International Plant Research Institute Wageningen (P.R.I. Wageningen), the Netherlands. | 2 | [68] |
| 57 | Pinova | Winter variety of German selection is obtained by crossing varieties Golden Delicious and Clivia. | 2; 7 | [69] |
| 58 | Red Topaz (2) | Winter variety of Czech selection by crossing varieties Vanda and Rubín in 1984. | 2 | [70] |
| 59 | SQ159 | A variety of apples for long storage, suitable for organic production. Developed by PRI Wageningen (Netherlands). | 2 | [71] |
| SNP Marker | Linkage Group | Gene/Locus Name | SNP ID | Taqman Assay ID | SNP Type | Reference |
|---|---|---|---|---|---|---|
| FBE-1_Y320 | 12 | FBE | FBE-1_Y320 | AH704YY | C/T | [73] |
| FBE-2_Y192 | 12 | FBE | FBE-2_Y192 | AH89246 | C/T | [73] |
| FBE-2_Y495 | 12 | FBE | FBE-2_Y495 | AHABIAZ | C/T | [73] |
| FBE-2_Y551 | 12 | FBE | FBE-2_Y551 | AHBKGG7 | C/T | [73] |
| FB-MR5-K35 | 3 | MR5 | FB-MR5-NZsnEH034548_K35 | AH0JFXM | G/T | [73] |
| FB-MR5-R240 | 3 | MR5 | FB-MR5-NZsnEH034548_R240 | AH1SD3U | A/G | [73] |
| FB-MR5-R249 | 3 | MR5 | FB-MR5-NZsnEH034548_R249 | AH21B92 | A/G | [73] |
| FB-MR5-rp16k15_M106 | 3 | MR5 | FB-MR5-rp16k15_M106 | AH4AAGA | A/C | [73] |
| RLP1a | 3 | RLP1 | RLP1a | AH5I8MI | C/A | [74] |
| RLP1b | 3 | RLP1 | RLP1b | AH6R6SQ | A/T | [74] |
| Gene, Locus | Marker | Primer Sequence (5′–3′) | PCR Cycling Program |
|---|---|---|---|
| F7 QTL | AE10-375 | CTGAAGCGCACGTTCTCC-F CTGAAGCGCATCATTTCTGATAG-R | 1 cycle95 °C—3 min, 35 cycles (95 °C—40 s; 60 °C—40 s; 72 °C—60 s), 1 cycle 72 °C—10 min. |
| F7 QTL | GE-8019 | TTGAGACCGATTTTCGTGTG-F TCTCTCCCAGAGCTTCATTGT-R | 1 cycle 95 °C—3 min, 35 cycles (95 °C—40 s; 60 °C—40 s; 72 °C—60 s), 1 cycle 72 °C—10 min. |
| Genotypes | SNP ID | SCAR Markers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FBE-1_Y320 | FBE-2_Y192 | FBE-2_Y495 | FBE-2_Y551 | FB-MR5-K35 | FB-MRS-R240 | FB-MR5-R249 | FB-MR5-rp16k15_M106 | RLP1a | RLP1b | AE-375 | GE-8019 | |
| Idared (1, 4, 6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Starkrimson (6, 8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Gala (4, 6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Aport (4, 7) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Fuji (1, 6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Golden Delicious (1, 6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Granny Smith (6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Suislepper (7) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | N/A | N/A |
| Renet Burhardta (4, 7) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Pestrushka (4) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Red Delicious (5) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Tulpan (8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Ainur (1, 7) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Aigul (7) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Saltanat (7) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | N/A | N/A |
| Voskhod (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Egemen (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Zarya Alatau (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Maksat (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Esen (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Sarkyt (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Talgarskoe (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Zharkyn (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Danalyk (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | N/A | N/A |
| Modi (8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Braeburn (1, 3, 8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Jeromini (4) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Quinti (5, 8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 1 |
| Kandil Sinap (8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Mutsu (8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Kandil Kirghiz (8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Pink Lady (4, 6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | N/A | N/A |
| Starks Earliest (8) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Scarlet Spur (6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Summer red (6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 1 |
| Nicole Grain (6) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Redfree (3) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Williams Pride (3) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 1 |
| Diana (3) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Gold spur (4) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Honeycrisp (4) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 1 |
| Camspur (4) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Korea (4) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | N/A | N/A |
| Gala Anna (5) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| July Chernenko (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Elstar (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 1 |
| Jonagold (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Elrosa (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Prima (1) | T/T | T/T | T/T | G/G | G/G | C/C | A/A | 0 | 0 | |||
| Red Elstar (1) | T/T | C/C | No AMP | T/T | T/T | G/G | G/G | C/C | C/C | A/A | N/A | N/A |
| Maria Red (1) | T/T | C/C | No AMP | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Jonaprints (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Synap Almatinsky (1) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Wilton Star (2) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 0 |
| Deljonca (2) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | N/A | N/A |
| Santana (2) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
| Pinova (2,7) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 1 |
| Red Topaz (2) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 1 | 1 |
| SQ159 (2) | T/T | C/C | T/T | T/T | T/T | G/G | G/G | C/C | C/C | A/A | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kairova, G.; Daulet, N.; Solomadin, M.; Sandybayev, N.; Orkara, S.; Beloussov, V.; Kerimbek, N.; Gritsenko, D.; Sapakhova, Z. Identification of Apple Varieties Resistant to Fire Blight (Erwinia amylovora) Using Molecular Markers. Horticulturae 2023, 9, 1000. https://doi.org/10.3390/horticulturae9091000
Kairova G, Daulet N, Solomadin M, Sandybayev N, Orkara S, Beloussov V, Kerimbek N, Gritsenko D, Sapakhova Z. Identification of Apple Varieties Resistant to Fire Blight (Erwinia amylovora) Using Molecular Markers. Horticulturae. 2023; 9(9):1000. https://doi.org/10.3390/horticulturae9091000
Chicago/Turabian StyleKairova, Gulshariya, Nurzhan Daulet, Maxim Solomadin, Nurlan Sandybayev, Shynggys Orkara, Vyacheslav Beloussov, Nazym Kerimbek, Dilyara Gritsenko, and Zagipa Sapakhova. 2023. "Identification of Apple Varieties Resistant to Fire Blight (Erwinia amylovora) Using Molecular Markers" Horticulturae 9, no. 9: 1000. https://doi.org/10.3390/horticulturae9091000
APA StyleKairova, G., Daulet, N., Solomadin, M., Sandybayev, N., Orkara, S., Beloussov, V., Kerimbek, N., Gritsenko, D., & Sapakhova, Z. (2023). Identification of Apple Varieties Resistant to Fire Blight (Erwinia amylovora) Using Molecular Markers. Horticulturae, 9(9), 1000. https://doi.org/10.3390/horticulturae9091000






