Saffron Stigmas Apocarotenoid Contents from Saffron Latent Virus (SaLV)-Infected Plants with Different Origins and Dehydration Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction
2.3. HPLC-DAD Analysis
2.4. Identification and Quantification of Crocetin Esters, Picrocrocin, Safranal, Kaempferol Glycosides, and HTCC
2.5. Nomenclature for Crocetin Esters
2.6. Serological and Molecular Assays
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grilli Caiola, M.; Canini, A. Ultrastructure of Chromoplasts and Other Plastids in Crocus sativus L. (Iridaceae). Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2004, 138, 43–52. [Google Scholar] [CrossRef]
- Dewan, R. Bronze Age Flower Power: The Minoan Use and Social Significance of Saffron and Crocus Flowers; Institute for European and Mediterranean Archaeology: New York, NY, USA, 2015; Volume 5, pp. 42–55. [Google Scholar]
- Shahnoushi, N.; Abolhassani, L.; Kavakebi, V.; Reed, M.; Saghaian, S. Economic Analysis of Saffron Production. In Saffron; Elsevier: Amsterdam, The Netherlands, 2020; pp. 337–356. ISBN 978-0-12-818638-1. [Google Scholar]
- Bazrafshan, O.; Ramezani Etedali, H.; Gerkani Nezhad Moshizi, Z.; Shamili, M. Virtual Water Trade and Water Footprint Accounting of Saffron Production in Iran. Agric. Water Manag. 2019, 213, 368–374. [Google Scholar] [CrossRef]
- Ramezani, M.; Dourandish, A.; Jamali Jaghdani, T.; Aminizadeh, M. The Influence of Dense Planting System on the Technical Efficiency of Saffron Production and Land Use Sustainability: Empirical Evidence from Gonabad County, Iran. Agriculture 2022, 12, 92. [Google Scholar] [CrossRef]
- Wyeth, P.; Malik, N. A Strategy for Promoting Afghan Saffron Exports; ICARDA and Washington State University: Pullman, WA, USA, 2008. [Google Scholar]
- Mollafilabi, A.; Aslami, M.H.; Shoorideh, H. Replacement of Saffron (Crocus sativus L.) with Poppy (Papaver somniferum L.) and Its Socio-Economic Results. Acta Hortic. 2010, 850, 299–302. [Google Scholar] [CrossRef]
- Bagur, M.J.; Alonso Salinas, G.; Jiménez-Monreal, A.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2018, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.; Zalacain, A.; Alonso, G.L. The Chemical Composition of Saffron: Color, Taste and Aroma; Bomarzo SL: Albacete, Spain, 2006. [Google Scholar]
- Molina, R.V.; García-Luis, A.; Coll, V.; Ferrer, C.; Valero, M.; Navarro, Y.; Guardiola, J.L. Flower Formation in the Saffron Crous (Crocus sativus L.) the Role of Temperature. Acta Hortic. 2004, 39–47. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Pardo, J.E.; López, E.; Alvarruiz, A.; Alonso, G.L. Influence of Different Drying and Aging Conditions on Saffron Constituents. J. Agric. Food Chem. 2005, 53, 3974–3979. [Google Scholar] [CrossRef]
- Moratalla-López, N.; Parizad, S.; Habibi, M.K.; Winter, S.; Kalantari, S.; Bera, S.; Lorenzo, C.; García-Rodríguez, M.V.; Dizadji, A.; Alonso, G.L. Impact of Two Different Dehydration Methods on Saffron Quality, Concerning the Prevalence of Saffron Latent Virus (SaLV) in Iran. Food Chem. 2021, 337, 127786. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin Esters, Picrocrocin and Its Related Compounds Present in Crocus sativus Stigmas and Gardenia Jasminoides Fruits. Tentative Identification of Seven New Compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef]
- D’Archivio, A.; Di Donato, F.; Foschi, M.; Maggi, M.; Ruggieri, F. UHPLC Analysis of Saffron (Crocus sativus L.): Optimization of Separation Using Chemometrics and Detection of Minor Crocetin Esters. Molecules 2018, 23, 1851. [Google Scholar] [CrossRef]
- del Campo, C.P.; Carmona, M.; Maggi, L.; Kanakis, C.D.; Anastasaki, E.G.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Picrocrocin Content and Quality Categories in Different (345) Worldwide Samples of Saffron (Crocus sativus L.). J. Agric. Food Chem. 2010, 58, 1305–1312. [Google Scholar] [CrossRef]
- García-Rodríguez, M.V.; López-Córcoles, H.; Alonso, G.L.; Pappas, C.S.; Polissiou, M.G.; Tarantilis, P.A. Comparative Evaluation of an ISO 3632 Method and an HPLC-DAD Method for Safranal Quantity Determination in Saffron. Food Chem. 2017, 221, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Himeno, H.; Sano, K. Synthesis of Crocin, Picrocrocin and Safranal by Saffron Stigma-like Structures Proliferated in vitro. Agric. Biol. Chem. 1987, 51, 2395–2400. [Google Scholar] [CrossRef]
- Diretto, G.; Ahrazem, O.; Rubio-Moraga, Á.; Fiore, A.; Sevi, F.; Argandoña, J.; Gómez-Gómez, L. UGT709G1: A Novel Uridine Diphosphate Glycosyltransferase Involved in the Biosynthesis of Picrocrocin, the Precursor of Safranal in Saffron (Crocus sativus). New Phytol. 2019, 224, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Rubio-Moraga, A.; Berman, J.; Capell, T.; Christou, P.; Zhu, C.; Gómez-Gómez, L. The Carotenoid Cleavage Dioxygenase CCD 2 Catalysing the Synthesis of Crocetin in Spring Crocuses and Saffron Is a Plastidial Enzyme. New Phytol. 2016, 209, 650–663. [Google Scholar] [CrossRef]
- Sharma, M.; Kaul, S.; Dhar, M.K. Transcript Profiling of Carotenoid/Apocarotenoid Biosynthesis Genes during Corm Development of Saffron (Crocus sativus L.). Protoplasma 2019, 256, 249–260. [Google Scholar] [CrossRef]
- Acar, B.; Sadikoglu, H.; Ozkaymak, M. Freeze Drying of Saffron (Crocus sativus L.). Dry. Technol. 2011, 29, 1622–1627. [Google Scholar] [CrossRef]
- Chaouqi, S.; Moratalla-López, N.; Lage, M.; Lorenzo, C.; Alonso, G.L.; Guedira, T. Effect of Drying and Storage Process on Moroccan Saffron Quality. Food Biosci. 2018, 22, 146–153. [Google Scholar] [CrossRef]
- del Campo, C.P.; Carmona, M.; Maggi, L.; Kanakis, C.D.; Anastasaki, E.G.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Effects of Mild Temperature Conditions during Dehydration Procedures on Saffron Quality Parameters. J. Sci. Food Agric. 2010, 90, 719–725. [Google Scholar] [CrossRef]
- Tong, Y.; Zhu, X.; Yan, Y.; Liu, R.; Gong, F.; Zhang, L.; Hu, J.; Fang, L.; Wang, R.; Wang, P. The Influence of Different Drying Methods on Constituents and Antioxidant Activity of Saffron from China. Int. J. Anal. Chem. 2015, 2015, 953164. [Google Scholar] [CrossRef]
- Parizad, S.; Dizadji, A.; Koohi Habibi, M.; Winter, S.; Kalantari, S.; Movi, S.; García-Arenal, F.; Ayllón, M.A. Description and Genetic Variation of a Distinct Species of Potyvirus Infecting Saffron (Crocus sativus L.) Plants in Major Production Regions in Iran. Ann. Appl. Biol. 2018, 173, 233–242. [Google Scholar] [CrossRef]
- Parizad, S.; Dizadji, A.; Habibi, M.K.; Winter, S.; Kalantari, S.; Garcıa, F. Prevalence of Saffron Latent Virus (SaLV), a New Potyvirus Species, in Saffron Fields of Iran. J. Plant Pathol. 2017, 99, 802. [Google Scholar]
- Movi, S.; Dizadji, A.; Parizad, S.; Zarghani, S.N. Biological Characteristics and Genetic Variation Analyses of Saffron Latent Virus (SaLV) Based on Genomic P1-Pro and P3 Regions. Eur. J. Plant Pathol. 2022, 164, 299–312. [Google Scholar] [CrossRef]
- Parizad, S.; Dizadji, A.; Habibi, M.K.; Winter, S.; Kalantari, S.; Movi, S.; Lorenzo Tendero, C.; Alonso, G.L.; Moratalla-Lopez, N. The Effects of Geographical Origin and Virus Infection on the Saffron (Crocus sativus L.). Qual. Food Chem. 2019, 295, 387–394. [Google Scholar] [CrossRef]
- Zhi, L.; Xianmei, G.; Jian, Y.; Duoyong, Z.; Bin, L.; Zihong, Z.; Piao, C.; Dongguang, W. Quality Evaluation and Origin Traceability of the Imported and Domestic Saffron Spice (Crocus sativus L.) Products in China Market Using Chemical Composition and Stable Isotope Analysis. J. Food Compos. Anal. 2023, 118, 105202. [Google Scholar] [CrossRef]
- El Hani, O.; García-Guzmán, J.J.; Palacios-Santander, J.M.; Digua, K.; Amine, A.; Gharby, S.; Cubillana-Aguilera, L. Geographical Classification of Saffron (Crocus sativus L.) Using Total and Synchronous Fluorescence Combined with Chemometric Approaches. Foods 2023, 12, 1747. [Google Scholar] [CrossRef]
- Nie, J.; Yang, J.; Liu, C.; Li, C.; Shao, S.; Yao, C.; Chen, B.; Tao, Y.; Wang, F.; Zhang, Y.; et al. Stable Isotope and Elemental Profiles Determine Geographical Origin of Saffron from China and Iran. Food Chem. 2023, 405, 134733. [Google Scholar] [CrossRef]
- ISO 3632; Saffron (Crocus sativus L.). Part 1 (Specification) and Part 2 (Test Methods). International Organization for Standardization (ISO): Geneva, Switzerland, 2011.
- García-Rodríguez, M.V.; Serrano-Díaz, J.; Tarantilis, P.A.; López-Córcoles, H.; Carmona, M.; Alonso, G.L. Determination of Saffron Quality by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2014, 62, 8068–8074. [Google Scholar] [CrossRef]
- Sánchez, A.M.; Carmona, M.; del Campo, C.P.; Alonso, G.L. Solid-Phase Extraction for Picrocrocin Determination in the Quality Control of Saffron Spice (Crocus sativus L.). Food Chem. 2009, 116, 792–798. [Google Scholar] [CrossRef]

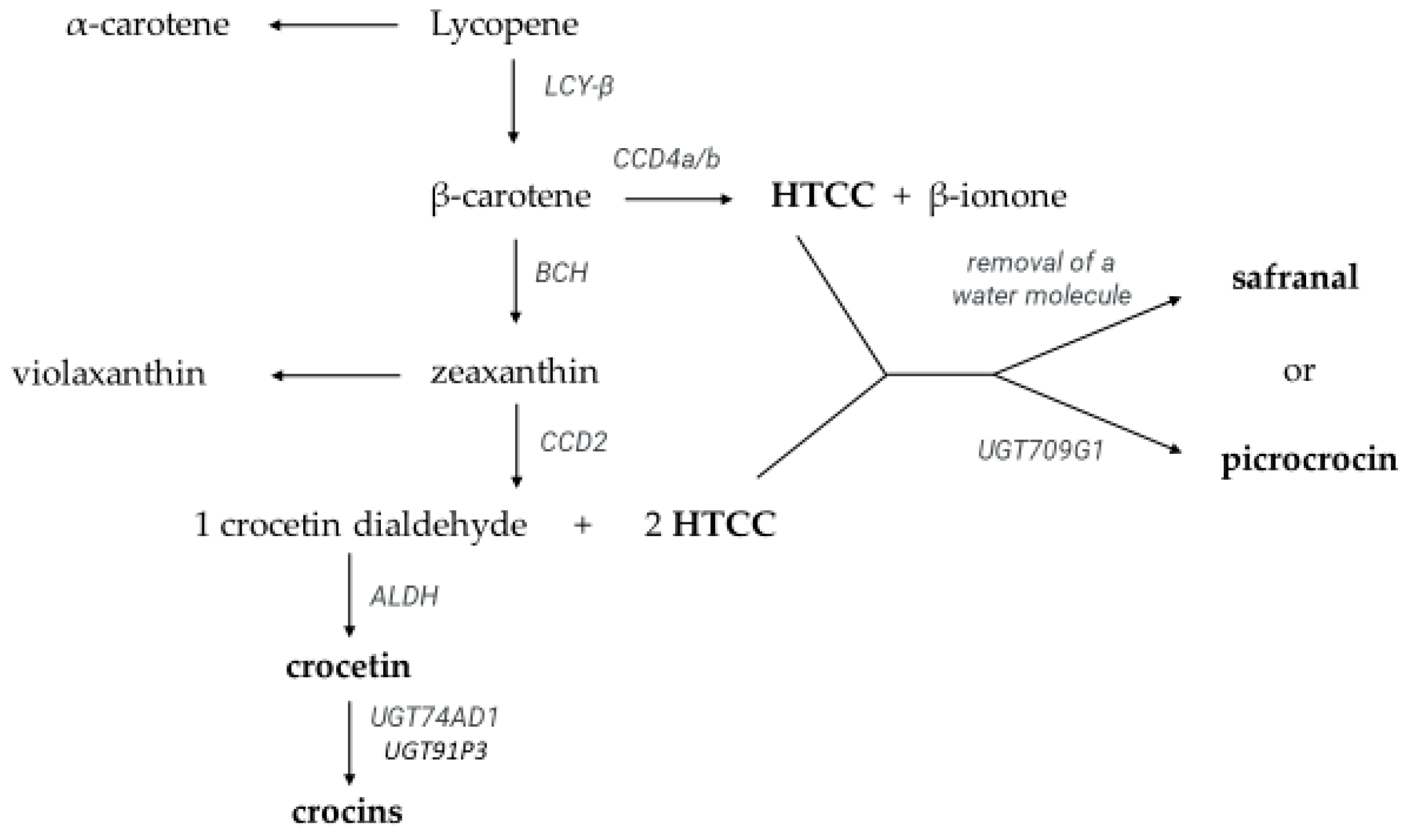
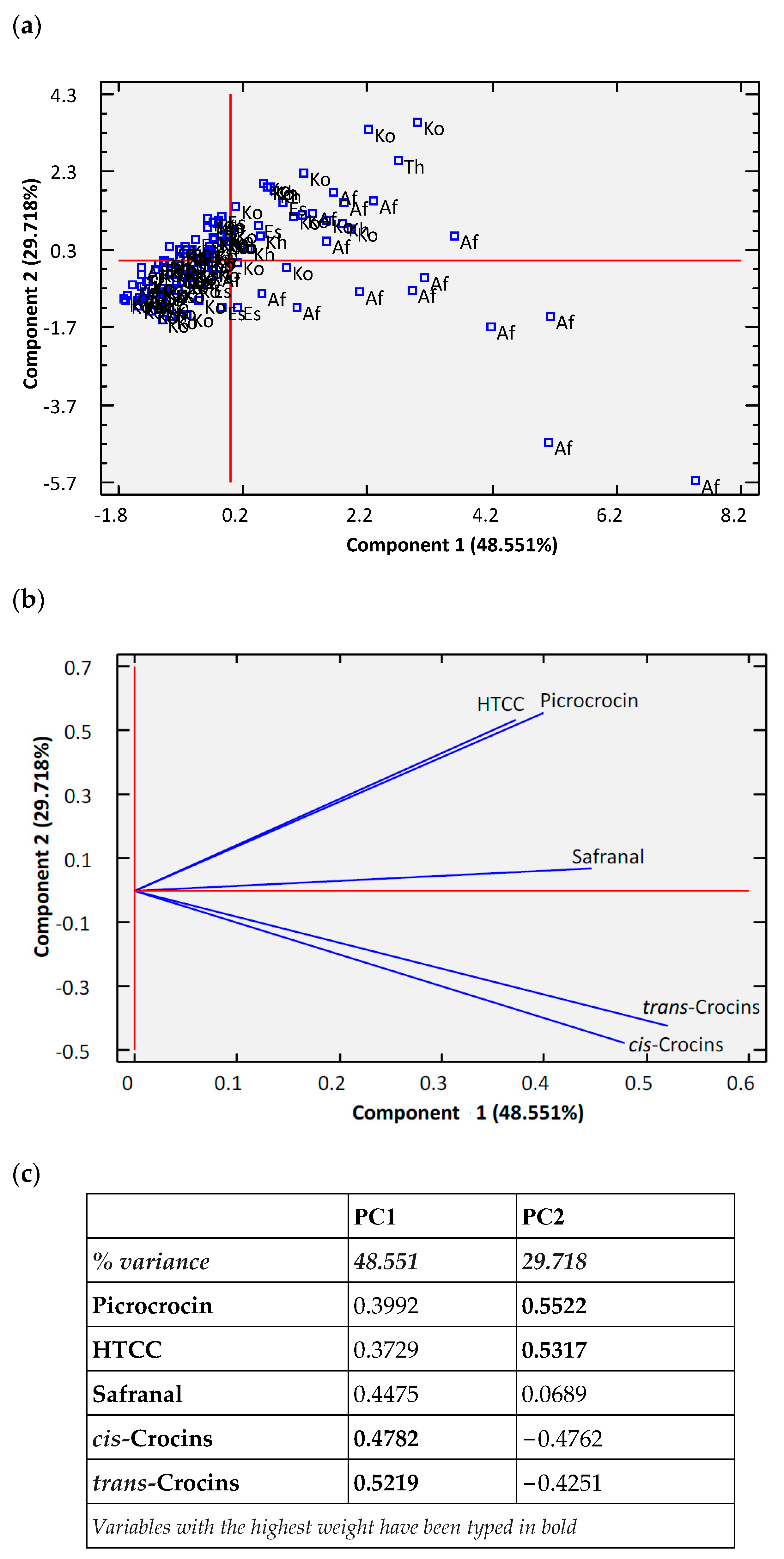
| Number of Subplots | Af | Sh | Kh | Th | Es | Ko |
|---|---|---|---|---|---|---|
| corms from the same farmer in that area | 26 | 10 | 15 | 3 | 14 | 78 |
| stigmas were dehydrated at 40 °C for 120 min | 18 | 8 | 11 | 3 | 14 | 67 |
| stigmas were dehydrated at 55 °C for 45 min | 8 | 2 | 4 | 0 | 0 | 11 |
| Zones | Compound (mmol/kg Saffron) * | |||||||
|---|---|---|---|---|---|---|---|---|
| Af | Sh | Kh | Ko | |||||
| Dehydration Temperature (°C) | 40 | 55 | 40 | 55 | 40 | 55 | 40 | 55 |
| Picrocrocin | 194.41 a | 216.20 a | 169.07 a | 94.72 a | 183.23 a | 174.40 a | 111.08 a | 108.63 a |
| Safranal | 13.09 b | 5.03 a,b | 0 | 0 | 5.75 a,b | 0 | 0 | 5.09 a,b |
| HTCC | 278.42 a | 263.08 a | 159.92 a | 120.91 a | 240.78 a | 224.15 a | 216.69 a | 258.09 a |
| ∑ HTCC derv. | 485.92 a | 484.31 a | 328.99 a | 215.63 a | 429.76 a | 398.55 a | 327.77 a | 371.81 a |
| trans-5-tG | 1.33 b | 0.23 a | 0.30 a | 0.36 a | 0.29 a | 0.67 a | 0.21 a | 0.19 a |
| trans-5-nG | 3.50 a | 1.27 a | 0.80 a | 2.41 a | 8.56 a | 9.33 a | 5.15 a | 1.10 a |
| trans-4-GG | 71.45 b | 15.50 a | 15.32 a | 18.26 a | 11.02 a | 2.43 a | 6.04 a | 10.01 a |
| trans-4-ng | 4.04 a | 0.38 a | 0.34 a | 0.83 a | 2.89 a | 12.15 b | 4.31 a | 0.94 a |
| trans-3-Gg | 73.53 b | 17.59 a | 12.24 a | 16.87 a | 14.82 a | 3.63 a | 15.03 a | 11.96 a |
| trans-2-gg | 19.96 b | 6.21 a | 3.32 a | 6.91 a | 5.90 a | 0.39 a | 8.74 a | 4.37 a |
| cis-4-GG | 5.84 b | 1.16 a | 1.27 a | 1.75 a | 1.50 a | 1.02 a | 1.12 a | 0.96 a |
| trans-2-G | 34.80 b | 0.71 a | 0.83 a | 1.43 a | 1.43 a | 1.19 a | 0.66 a | 0.56 a |
| cis-3-Gg | 4.94 b | 1.41 a | 1.01 a | 2.61 a,b | 2.64 a,b | 1.50 a | 3.22 a,b | 1.64 a |
| trans-1-g | 16.58 b | 0.25 a | 0.38 a | 0.45 a | 0.36 a | 0.59 a | 0.43 a | 0.46 |
| cis-2-gg | 3.35 b | 0.49 a | 0.30 a | 1.39 a | 1.02 a | 0.49 a | 0.83 a | 0.31 a |
| ∑ Crocins | 239.32 b | 38.82 a | 32.60 a | 45.04 a | 44.53 a | 29.24 a | 35.78 a | 27.30 a |
| Zone | Dehydration Temperature (°C) | |
|---|---|---|
| Picrocrocin | 4.82 *** | 0.11 |
| HTCC | 1.62 | 1.08 |
| Safranal | 8.63 *** | 1.29 |
| trans-5-tG | 9.25 *** | 4.22 * |
| trans-5-nG | 9.56 *** | 0.30 |
| trans-4-GG | 14.50 *** | 5.48 * |
| trans-4-ng | 4.73 *** | 2.41 |
| trans-3-Gg | 17.72 *** | 10.56 ** |
| trans-2-gg | 9.18 *** | 10.72 * |
| cis-4-GG | 5.60 *** | 3.83 |
| trans-2-G | 14.66 *** | 11.16 ** |
| cis-3-Gg | 3.88 ** | 8.18 ** |
| trans-1-g | 15.79 *** | 13.19 *** |
| cis-2-gg | 2.15 | 8.20 ** |
| Compound (mmol/kg Saffron) * | Zones | |||||
|---|---|---|---|---|---|---|
| Af | Sh | Kh | Te | Es | Ko | |
| Picrocrocin | 190.18 a,b | 125.18 a | 145.03 a | 282.84 b | 117.11 a | 105.73 a |
| Safranal | 12.23 b | 1.38 a | 3.82 a | 6.65 a | 1.83 a | 1.67 a |
| HTCC | 255.21 a | 130.03 a | 219.35 a | 190.75 a | 193.96 a | 189.13 a |
| ∑ derv. HTCC | 457.62 a | 256.59 a | 368.20 a | 480.24 a | 312.90 a | 296.53 a |
| trans-5-tG | 1.57 b | 0.23 a | 0.32 a | 1.03 a,b | 0.58 a | 0.22 a |
| trans-5-nG | 3.86 a | 0.98 a | 12.22 a | 5.12 a | 30.14 b | 4.07 a |
| trans-4-GG | 85.42 b | 13.12 a | 5.51 a | 5.28 a | 0.48 a | 4.94 a |
| trans-3-Gg | 76.15 b | 13.26 a | 10.09 a | 3.60 a | 7.41 a | 12.56 a |
| trans-2-gg | 19.46 b | 4.95 a | 4.34 a | 0.41 a | 0.36 a | 10.28 a,b |
| cis-4-GG | 10.33 b | 1.52 a | 0.89 a | 1.13 a | 0.84 a | 1.04 a |
| trans-2-G | 28.76 b | 0.94 a | 1.52 a | 0.63 a | 1.37 a | 0.65 a |
| cis-3-Gg | 6.39 b | 1.65 a | 2.18 a | 1.93 a | 2.47 a | 2.52 a |
| cis-2-gg | 3.46 b | 0.36 a,b | 1.11 a,b | 0.47 a | 1.80 a,b | 1.41 a,b |
| ∑ Crocins | 191.03 b | 31.50 a | 38.21 a | 26.12 a | 55.29 a | 32.42 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, C.; Shadmani, G.; Valouzi, H.; Moratalla-López, N.; Bahlolzada, H.; Sánchez-Gómez, R.; Dizadji, A.; Alonso, G.L. Saffron Stigmas Apocarotenoid Contents from Saffron Latent Virus (SaLV)-Infected Plants with Different Origins and Dehydration Temperatures. Horticulturae 2023, 9, 933. https://doi.org/10.3390/horticulturae9080933
Lorenzo C, Shadmani G, Valouzi H, Moratalla-López N, Bahlolzada H, Sánchez-Gómez R, Dizadji A, Alonso GL. Saffron Stigmas Apocarotenoid Contents from Saffron Latent Virus (SaLV)-Infected Plants with Different Origins and Dehydration Temperatures. Horticulturae. 2023; 9(8):933. https://doi.org/10.3390/horticulturae9080933
Chicago/Turabian StyleLorenzo, Cándida, Golnaz Shadmani, Hajar Valouzi, Natalia Moratalla-López, Habibullah Bahlolzada, Rosario Sánchez-Gómez, Akbar Dizadji, and Gonzalo L. Alonso. 2023. "Saffron Stigmas Apocarotenoid Contents from Saffron Latent Virus (SaLV)-Infected Plants with Different Origins and Dehydration Temperatures" Horticulturae 9, no. 8: 933. https://doi.org/10.3390/horticulturae9080933
APA StyleLorenzo, C., Shadmani, G., Valouzi, H., Moratalla-López, N., Bahlolzada, H., Sánchez-Gómez, R., Dizadji, A., & Alonso, G. L. (2023). Saffron Stigmas Apocarotenoid Contents from Saffron Latent Virus (SaLV)-Infected Plants with Different Origins and Dehydration Temperatures. Horticulturae, 9(8), 933. https://doi.org/10.3390/horticulturae9080933







