Establishment and Optimization of Micropropagation System for Southern Highbush Blueberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Micropropagation Steps
2.2. Explant Sterilization
2.3. Optimization of Basal Culture Media
2.4. Screening of Growth Regulator Combinations
2.5. Substrate Screening for Rooting
2.6. Screening of Growth Regulators for Root Induction
2.7. Statistical Analysis
3. Results
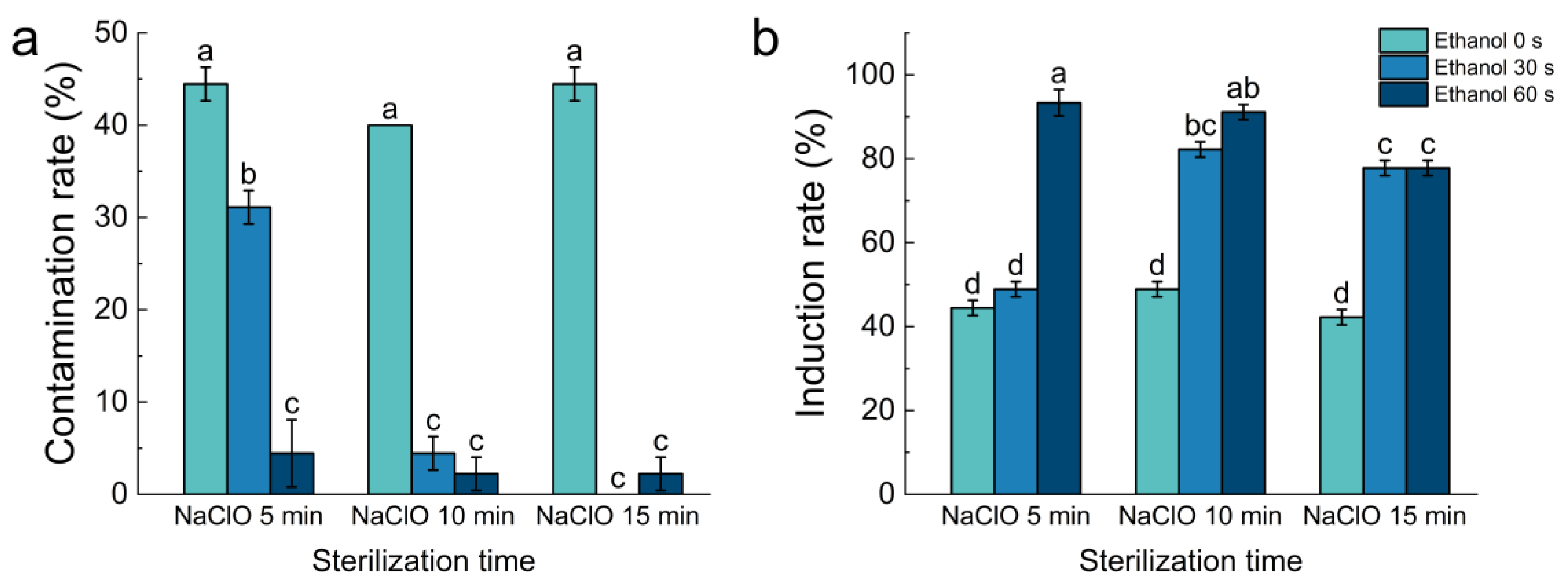
3.1. Effect of Different Ethanol and NaClO Sterilization Times on the Contamination and Induction Rates of Explants
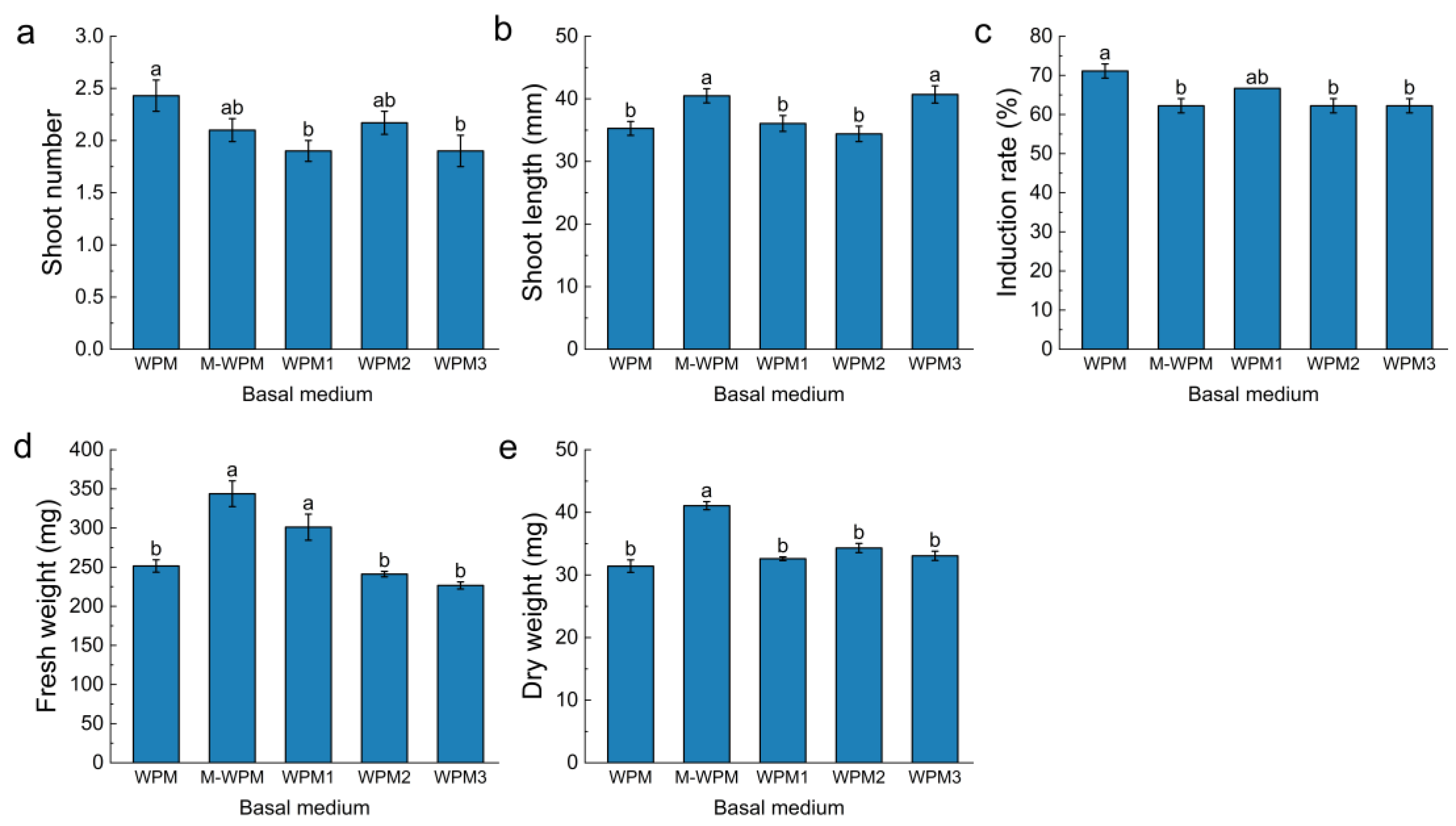
3.2. Effects of Different Basal Medium Formulations on Blueberry In Vitro Establishment
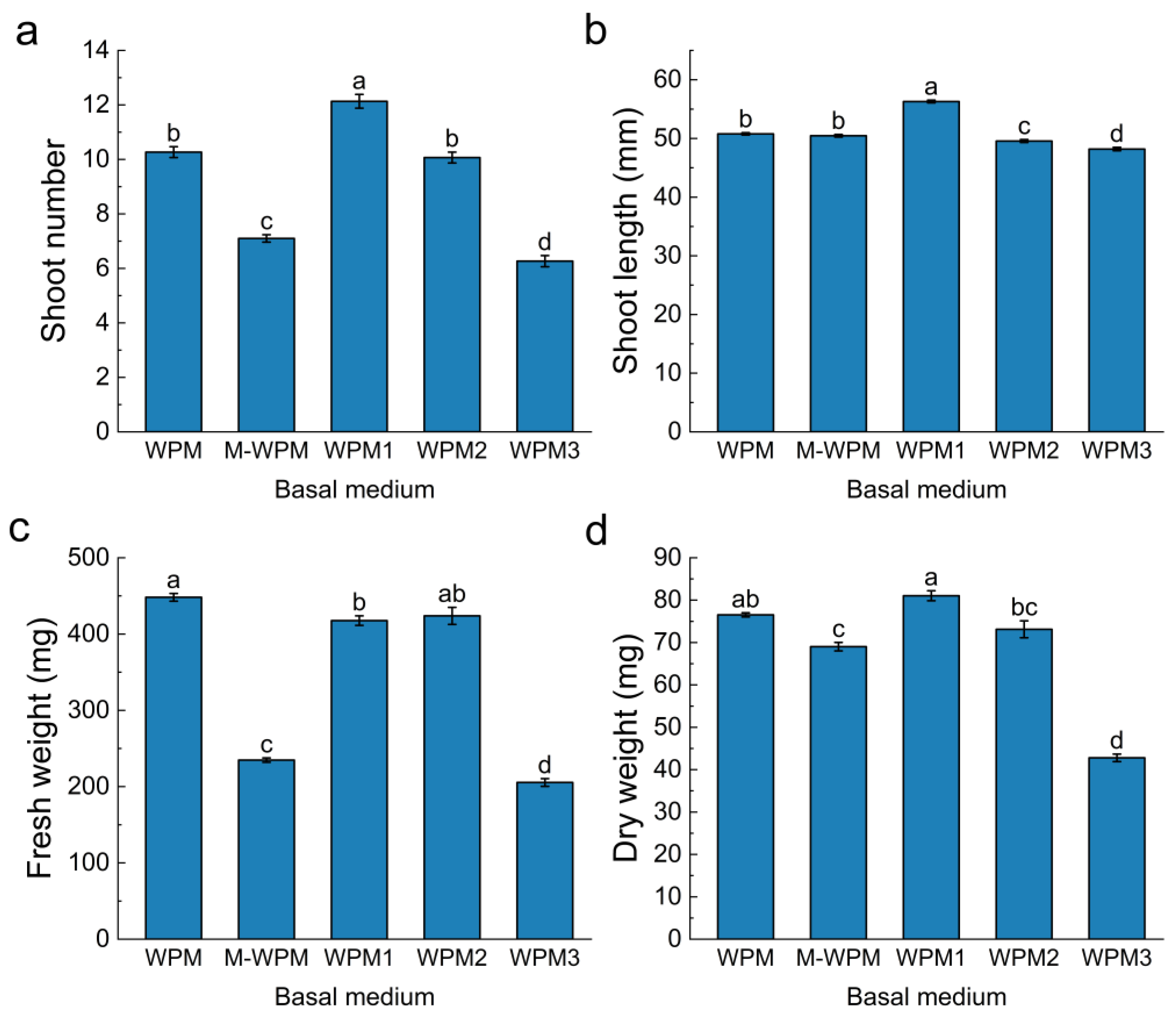
3.3. Effects of Different Basal Medium Formulations on In Vitro Blueberry Proliferation
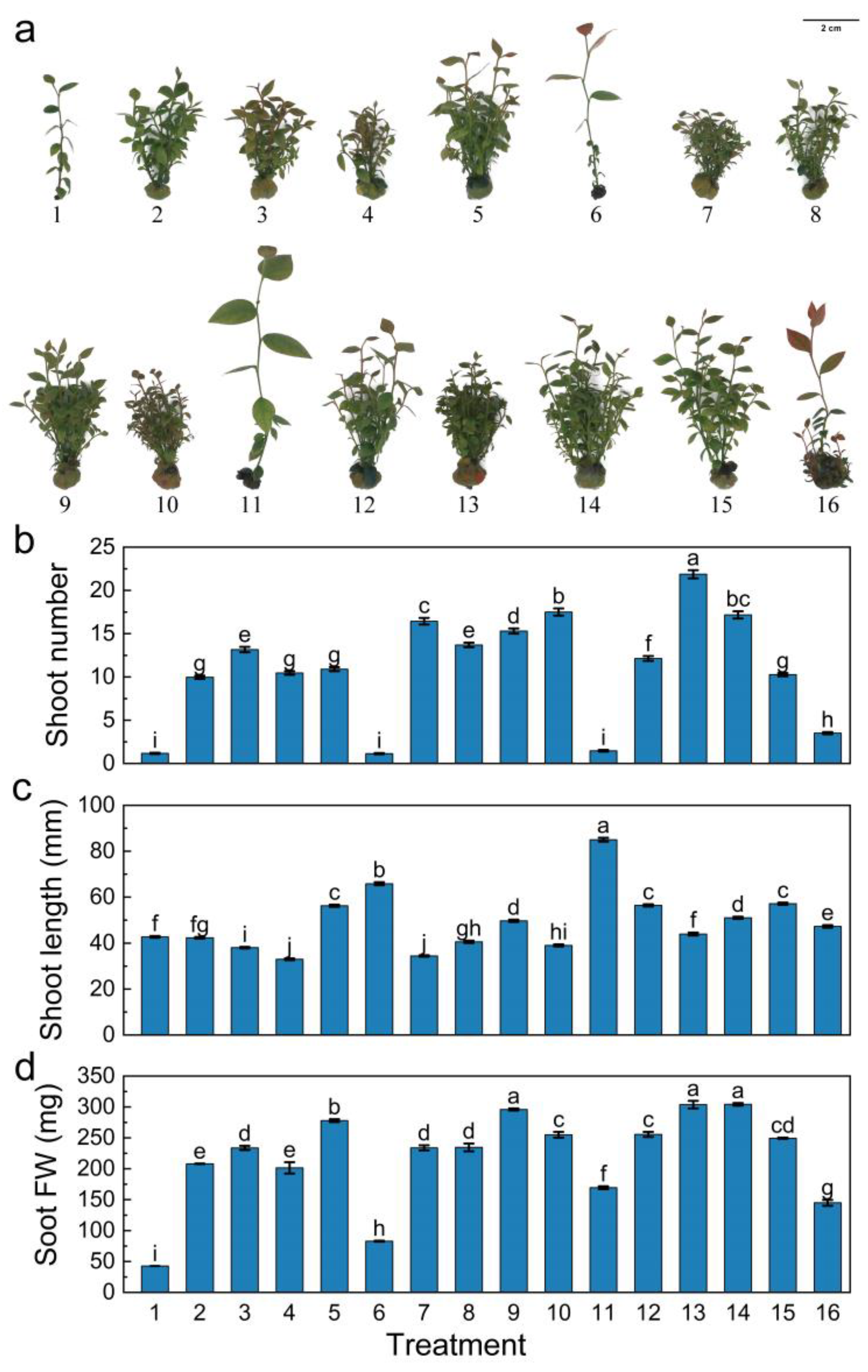
3.4. Effects of Different Growth Regulator Combinations on In Vitro Proliferation of Blueberry
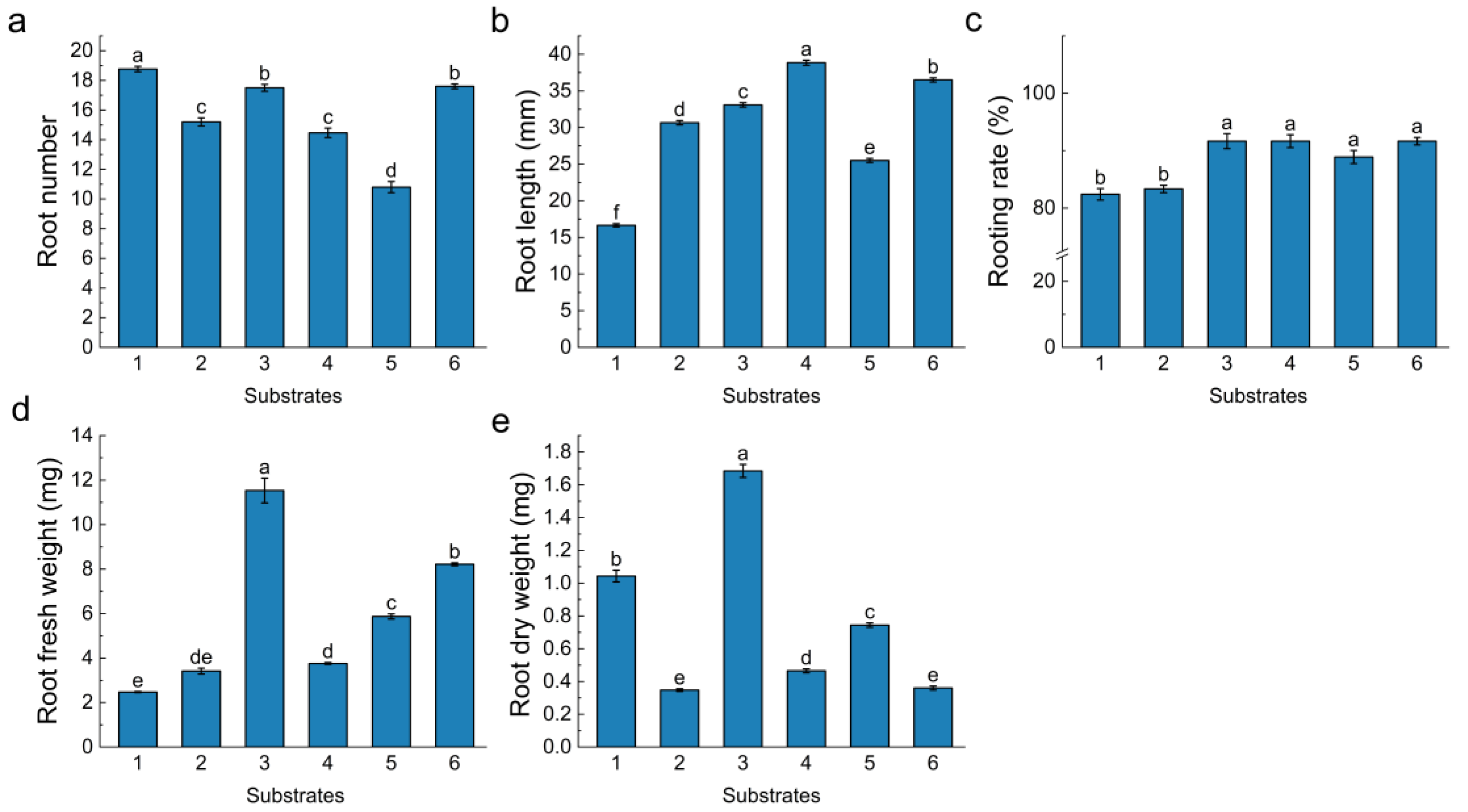
3.5. Effects of Different Substrates on Ex Vitro Rooting of Blueberry
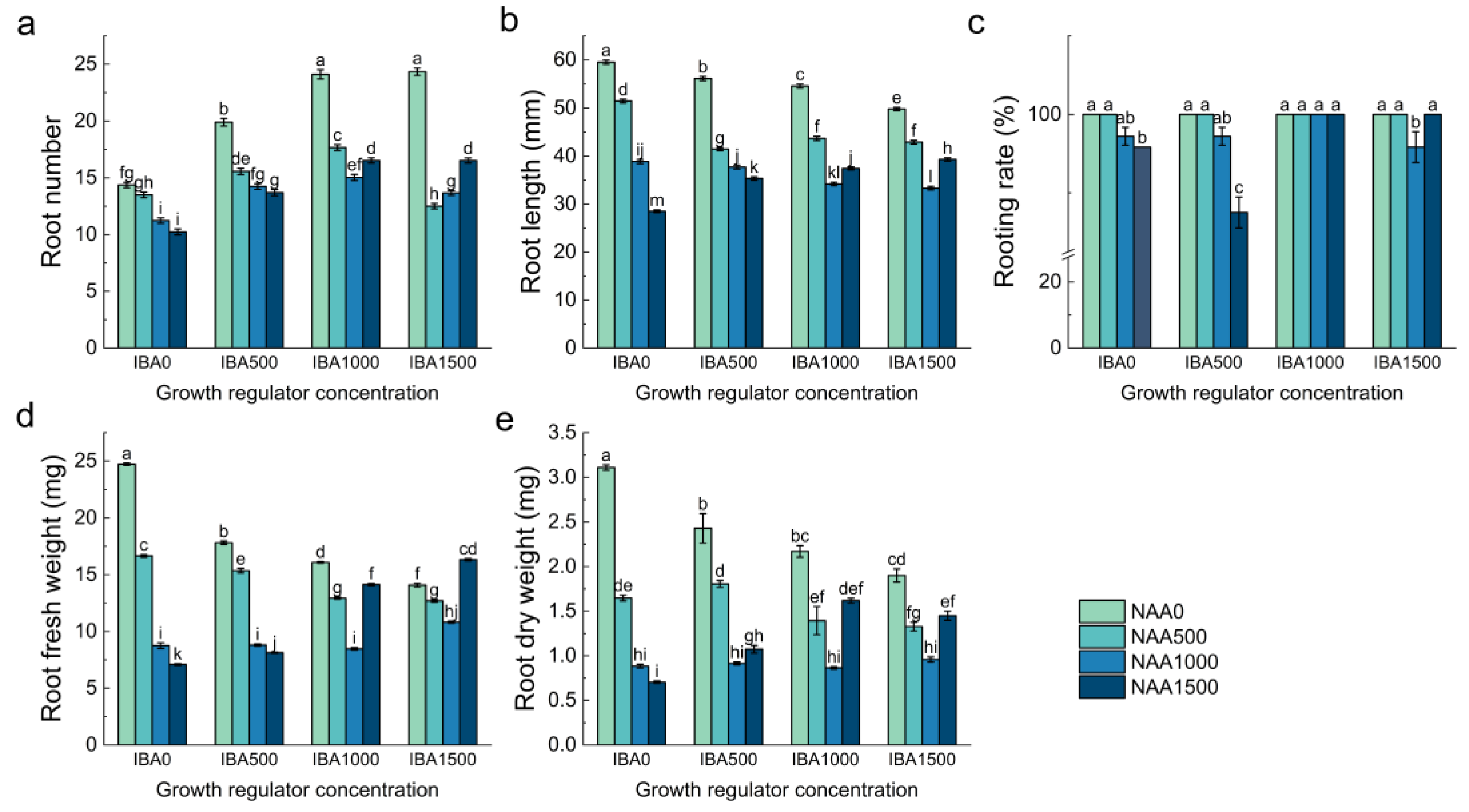
3.6. Effects of Different Growth Regulators and Their Combinations on Ex Vitro Rooting
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry Anthocyanins in Health Promotion: A Metabolic Overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry Fruit Valorization and Valuable Constituents: A Review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef]
- Nishiyama, S.; Fujikawa, M.; Yamane, H.; Shirasawa, K.; Babiker, E.; Tao, R. Genomic Insight into the Developmental History of Southern Highbush Blueberry Populations. Heredity 2021, 126, 194–205. [Google Scholar] [CrossRef]
- Fang, Y.; Nunez, G.H.; Silva, M.N.D.; Phillips, D.A.; Munoz, P.R. A Review for Southern Highbush Blueberry Alternative Production Systems. Agronomy 2020, 10, 1531. [Google Scholar] [CrossRef]
- Meiners, J.; Schwab, M.; Szankowski, I. Efficient in Vitro Regeneration Systems for Vaccinium Species. Plant Cell Tissue Organ Cult. 2007, 89, 169–176. [Google Scholar] [CrossRef]
- Fan, S.; Jian, D.; Wei, X.; Chen, J.; Beeson, R.C.; Zhou, Z.; Wang, X. Micropropagation of Blueberry ‘Bluejay’ and ‘Pink Lemonade’ through in Vitro Shoot Culture. Sci. Hortic. 2017, 226, 277–284. [Google Scholar] [CrossRef]
- El-Shiekh, A.; Wildung, D.K.; Luby, J.J.; Sargent, K.L.; Read, P.E. Long-Term Effects of Propagation by Tissue Culture or Softwood Single-Node Cuttings on Growth Habit, Yield, and Berry Weight of `Northblue’ Blueberry. J. Am. Soc. Hortic. Sci. 1996, 121, 339–342. [Google Scholar] [CrossRef]
- Liu, C.; Callow, P.; Rowland, L.J.; Hancock, J.F.; Song, G. Adventitious Shoot Regeneration from Leaf Explants of Southern Highbush Blueberry Cultivars. Plant Cell Tissue Organ Cult. 2010, 103, 137–144. [Google Scholar] [CrossRef]
- Brevis, P.A.; Bassil, N.V.; Ballington, J.R.; Hancock, J.F. Impact of Wide Hybridization on Highbush Blueberry Breeding. J. Amer. Soc. Hort. Sci. 2008, 133, 427–437. [Google Scholar] [CrossRef]
- Thorpe, T.A. History of Plant Tissue Culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Jayusman; Hakim, L.; Dalimunthe, A. Season, Basal Media and Plant Growth Regulators Effect in Wood Plant in Vitro Propagation: A Comprehensive Review. IOP Conf. Ser. Earth Environ. Sci. 2022, 1115, 012051. [Google Scholar] [CrossRef]
- Jaakola, L.; Tolvanen, A.; Laine, K.; Hohtola, A. Effect of N6-Isopentenyladenine Concentration on Growth Initiation in Vitro and Rooting of Bilberry and Lingonberry Microshoots. Plant Cell Tissue Organ Cult. 2001, 66, 73–77. [Google Scholar] [CrossRef]
- Teixeira Da Silva, J.A. Disinfection of Explants for Saffron (Crocus Sativus) Tissue Culture. Environ. Exp. Bot. 2016, 14, 183–198. [Google Scholar] [CrossRef]
- Mihaljevic, I.; Dugalic, K.; Tomas, V.; Viljevac, M.; Pranjic, A.; Cmelik, Z.; Puskar, B.; Jurkovic, Z. In Vitro Sterilization Procedures for Micropropagation of ‘Oblacinska’ Sour Cherry. J. Agric. Sci. 2013, 58, 117–126. [Google Scholar] [CrossRef]
- Alizadeh Arimi, F.; Yadollahi, A.; Imani, A.; Fakoor-Aryan, M. Optimization of the Sterilization and Establishment Steps for Almonds 2-22 Genotype. J. Nuts 2020, 11, 279–290. [Google Scholar] [CrossRef]
- Hesami, M.; Naderi, R.; Yoosefzadeh-Najafabadi, M. Optimizing Sterilization Conditions and Growth Regulator Effects on in Vitro Shoot Regeneration through Direct Organogenesis in Chenopodium Quinoa. Bio Technol. 2018, 99, 49–57. [Google Scholar] [CrossRef]
- Wang, G.; Liu, M.; Liu, G.; Bao, Z.; Ma, F. Establishment and Optimization of Agrobacterium-Mediated Transformation in Blueberry (Vaccinium Species). Sci. Hortic. 2022, 304, 111258. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant Tissue Culture Media and Practices: An Overview. Vitr. Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Schuchovski, C.; Biasi, L. In Vitro Establishment of ‘Delite’ Rabbiteye Blueberry Microshoots. Horticulturae 2019, 5, 24. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L. Use of a Cytokinin Conjugate for Efficient Shoot Regeneration from Leaf Sections of Highbush Blueberry. HortScience 1992, 27, 1127–1129. [Google Scholar] [CrossRef]
- Wolfe, D.E.; Eck, P.; Chin, C.-K. Evaluation of Seven Media for Micropropagation of Highbush Blueberry. HortScience 1983, 18, 703–705. [Google Scholar] [CrossRef]
- Yue, Y.; Gai, W.; Boustras, G. Exploration of the Causes of Ammonium Nitrate Explosions: Statistics and Analysis of Accidents over the Past 100 Years. Saf. Sci. 2023, 158, 105954. [Google Scholar] [CrossRef]
- Liang, K.; Feng, F.; Rui, D.; Liang, Z.; Xi, Y. Effects of Ammonium Sulfate as Substitute of Ammonium Nitrateon Proliferation of Hydrocotyle Sibthorpioides Lam Adventitious Buds. Southeast Hortic. 2021, 9, 19–24. (In Chinese) [Google Scholar]
- Agudelo-Morales, C.E.; Lerma, T.A.; Martínez, J.M.; Palencia, M.; Combatt, E.M. Phytohormones and Plant Growth Regulators—A Review. J. Sci. Technol. Appl. 2021, 10, 27–65. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Aftab, T. Phytohormones, Plant Growth Regulators and Signaling Molecules: Cross-Talk and Stress Responses. Plant Cell Rep. 2021, 40, 1301–1303. [Google Scholar] [CrossRef]
- Pelizza, T.R.; Nascimento, D.C.; Affonso, L.B.; Camargo, S.S.; Carra, B.; Schuch, M.W. Rooting of blueberry seedlings under ex vitro conditions and different substrates. Rev. Bras. Frutic. 2012, 34, 255–261. [Google Scholar] [CrossRef][Green Version]
- Kingston, P.H.; Scagel, C.F.; Bryla, D.R.; Strik, B. Suitability of Sphagnum Moss, Coir, and Douglas Fir Bark as Soilless Substrates for Container Production of Highbush Blueberry. HortScience 2017, 52, 1692–1699. [Google Scholar] [CrossRef]
- Erst, A.A.; Gorbunov, A.B.; Erst, A.S. Effect of Concentration, Method of Auxin Application and Cultivation Conditions on in Vitro Rooting of Bog Blueberry (Vaccinium Uliginosum L.). J. Berry Res. 2018, 8, 41–53. [Google Scholar] [CrossRef]
- Debnath, S.C.; McRae, K.B. An Efficient in Vitro Shoot Propagation of Cranberry (Vaccinium Macrocarpon Ait.) by Axillary Bud Proliferation. Vitr. Cell. Dev. Biol. Plant 2001, 37, 243–249. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-Feasible Micropropagation of Mountain Laurel, Kalmia Latifolia, by Use of Shoot-Tip Culture. Int. Plant Propagators Soc. Comb. Proc 1980, 30, 421–427. [Google Scholar]
- Ranil, R.H.G.; Niran, H.M.L.; Plazas, M.; Fonseka, R.M.; Fonseka, H.H.; Vilanova, S.; Andújar, I.; Gramazio, P.; Fita, A.; Prohens, J. Improving Seed Germination of the Eggplant Rootstock Solanum Torvum by Testing Multiple Factors Using an Orthogonal Array Design. Sci. Hortic. 2015, 193, 174–181. [Google Scholar] [CrossRef]
- Sedlak, J.; Paprstein, F. In Vitro Multiplication of Highbush Blueberry (Vaccinium Corymbosum L.) Cultivars. Acta Hortic. 2009, 810, 575–580. [Google Scholar] [CrossRef]
- WANG, W.; QI, J.-H.; FAN, Y.-Z.; JIN, W.-M. Effects of MS Medium Containing Ammonium Sulfate or Urea Instead of Ammonium Nitrate on Subculture of Malus, Strawberry and Grape. Plant Physiol. 2009, 45, 702–704. (In Chinese) [Google Scholar]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant Callus: Mechanisms of Induction and Repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Cüce, M.; Sökmen, A. In Vitro Production Protocol of Vaccinium Uliginosum L. (Bog Bilberry)Growing in the Turkish Flora. Turk J Agric 2017, 41, 294–304. [Google Scholar] [CrossRef]
- Ostrolucká, M.G.; Libiaková, G.; Ondrußková, E.; Gajdoßová, A. In Vitro Propagation of Vaccinium Species. Acta Univ. Latv. 2004, 676, 207–212. [Google Scholar]
- Arigundam, U.; Variyath, A.M.; Siow, Y.L.; Marshall, D.; Debnath, S.C. Liquid Culture for Efficient in Vitro Propagation of Adventitious Shoots in Wild Vaccinium Vitis-Idaea Ssp. Minus (Lingonberry) Using Temporary Immersion and Stationary Bioreactors. Sci. Hortic. 2020, 264, 109199. [Google Scholar] [CrossRef]
- Kreen, S.; Svensson, M.; Rumpunen, K. Rooting of Clematis Microshoots and Stem Cuttings in Different Substrates. Sci. Hortic. 2002, 96, 351–357. [Google Scholar] [CrossRef]
- Pacholczak, A.; Nowakowska, K. The Ex Vitro Rooting of Blueberry (Vaccinium Corymbosum L.) Microcuttings. Folia Hortic. 2015, 27, 145–150. [Google Scholar] [CrossRef]
- Zolman, B.K.; Yoder, A.; Bartel, B. Genetic Analysis of Indole-3-Butyric Acid Responses in Arabidopsis Thaliana Reveals Four Mutant Classes. Genetics 2000, 156, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- King, J.J.; Stimart, D.P. Genetic Analysis of Variation for Auxin-Induced Adventitious Root Formation among Eighteen Ecotypes of Arabidopsis Thaliana L. Heynh. J. Hered. 1998, 89, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Enders, T.A.; Strader, L.C. Auxin Activity: Past, Present, and Future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Time of Ethanol (70%) Sterilization (s) | Time of NaOCl (4%) Sterilization (min) |
|---|---|---|
| 1 | 0 | 5 |
| 2 | 0 | 5 |
| 3 | 0 | 5 |
| 4 | 30 | 10 |
| 5 | 30 | 10 |
| 6 | 30 | 10 |
| 7 | 60 | 15 |
| 8 | 60 | 15 |
| 9 | 60 | 15 |
| Components | WPM | M-WPM | WPM1 | WPM2 | WPM3 |
|---|---|---|---|---|---|
| Macronutrients | Final Concentration in the Culture Medium (mg·L−1) | ||||
| NH4H2PO4 | - | - | 99.98 | 99.8 | |
| (NH4)2SO4 | - | - | 330.18 | 247.65 | 247.65 |
| NH4NO3 | 400.00 | 400.00 | - | - | - |
| KNO3 | - | 190.00 | 505.8 | 695.8 | 190 |
| K2SO4 | 990.00 | - | 554.57 | - | - |
| KH2PO4 | 170.00 | 170.00 | 170 | - | - |
| CaCl2 | 72.5 | - | 72.5 | - | - |
| Ca(NO3)2·4H2O | 556.00 | 684.00 | 834.00 | 684.00 | 924.42 |
| MgSO4·7H2O | 370.00 | 370.00 | 370.00 | 370.00 | 370.00 |
| Micronutrients | |||||
| FeSO4·7H2O | 27.80 | 55.60 | 27.80 | 55.60 | 55.60 |
| Na2-EDTA | 37.30 | 74.6 | 37.3 | 74.6 | 74.6 |
| H3BO3 | 6.20 | 6.20 | 6.20 | 6.20 | 6.20 |
| MnSO4·H2O | 22.30 | 22.30 | 22.30 | 22.30 | 22.30 |
| ZnSO4·7H2O | 8.60 | 8.60 | 8.60 | 8.60 | 8.60 |
| Na2MoO4·2H2O | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| CuSO4·5H2O | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamins | |||||
| Glycine | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Inositol | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Vitamin B1 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Vitamin B6 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Nicotinic acid | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Hormone Treatment | IBA Concentration (mg·L−1) | TDZ Concentration (mg·L−1) | ZT Concentration (mg·L−1) |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0.0005 | 0.5 |
| 3 | 0 | 0.001 | 1 |
| 4 | 0 | 0.005 | 2 |
| 5 | 0.01 | 0 | 0.5 |
| 6 | 0.01 | 0.0005 | 0 |
| 7 | 0.01 | 0.001 | 2 |
| 8 | 0.01 | 0.005 | 1 |
| 9 | 0.005 | 0 | 1 |
| 10 | 0.005 | 0.0005 | 2 |
| 11 | 0.005 | 0.001 | 0 |
| 12 | 0.005 | 0.005 | 0.5 |
| 13 | 0.1 | 0 | 2 |
| 14 | 0.1 | 0.0005 | 1 |
| 15 | 0.1 | 0.001 | 0.5 |
| 16 | 0.1 | 0.005 | 0 |
| Level | Experimental Factor | |
|---|---|---|
| 1h-Indole-3-Butanoic Acid (mg·L−1) | 1-Naphthylacetic Acid (mg·L−1) | |
| 1 | 0 | 0 |
| 2 | 500 | 500 |
| 3 | 1000 | 1000 |
| 4 | 1500 | 1500 |
| Sources | Shoot Number Lant (n º) | Shoot Length (mm) | Fresh Weight (mg) | |||
|---|---|---|---|---|---|---|
| F-Ratio | Prob. F | F-Ratio | Prob. F | F-Ratio | Prob. F | |
| IBA | 4.557 | 0.122 | 3.883 | 0.147 | 9.834 | 0.046 * |
| TDZ | 1.467 | 0.380 | 1.001 | 0.499 | 0.655 | 0.632 |
| ZT | 55.202 | 0.004 * | 6.492 | 0.079 | 38.948 | 0.007 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, X.; Jiang, Z.; Yang, X.; Liu, X.; Ou, X.; Su, W.; Chen, R. Establishment and Optimization of Micropropagation System for Southern Highbush Blueberry. Horticulturae 2023, 9, 893. https://doi.org/10.3390/horticulturae9080893
Wang Y, Zhang X, Jiang Z, Yang X, Liu X, Ou X, Su W, Chen R. Establishment and Optimization of Micropropagation System for Southern Highbush Blueberry. Horticulturae. 2023; 9(8):893. https://doi.org/10.3390/horticulturae9080893
Chicago/Turabian StyleWang, Yuting, Xiaoyun Zhang, Zhehao Jiang, Xiaolong Yang, Xiaojuan Liu, Xi Ou, Wei Su, and Riyuan Chen. 2023. "Establishment and Optimization of Micropropagation System for Southern Highbush Blueberry" Horticulturae 9, no. 8: 893. https://doi.org/10.3390/horticulturae9080893
APA StyleWang, Y., Zhang, X., Jiang, Z., Yang, X., Liu, X., Ou, X., Su, W., & Chen, R. (2023). Establishment and Optimization of Micropropagation System for Southern Highbush Blueberry. Horticulturae, 9(8), 893. https://doi.org/10.3390/horticulturae9080893






