Abstract
The application of organic amendments, biochar, and wood distillate (WD), as well as the exposure to UV-B radiation, are two sustainable ways to enhance soil fertility and increase plant nutraceutical quality, respectively. However, they have always been studied separately, without testing the eventual synergistic or antagonistic effect when applied together. The present study investigated the effects of biochar (2% w/w), WD (1:100), and their combination (BWD) on some biometric and biochemical parameters of basil plants (Ocimum basilicum L.) exposed to different doses of UV-B radiation (0, 1, 2 h d−1; UV-B irradiance of 1.36 W m−2) in controlled conditions. Root and stem length and weight were not affected by soil amendments, while 1 h d−1 UV-B increased the length (+28%) and weight (+62%) of the aerial part. When combining the above- and below-ground factors, a decrease in root length was observed in the 2 h d−1 UV-B-treated plants in both WD (−36%) and BWD (−39%) treatments. The co-application of below- and above-ground treatments generally decreased phenolic and flavonoid concentration in both fully expanded leaves and vegetative shoot apices. This preliminary study highlights an antagonistic action of the combination of the investigated factors, at these doses, on the plant growth and metabolism that should be considered.
1. Introduction
In recent years, the awareness of sustainable agriculture practices has led to ever-increasing research towards eco-friendly solutions to ensure sufficient and high-quality production. Among them, the modulation of the light spectrum has gained popularity among growers since it was found that the exposure of plants to different visible and UV wavelengths can differentially stimulate the production of specific protective and antioxidant compounds [1,2,3,4].
In light of this, ultraviolet (UV)-B radiation (280–315 nm) has attracted attention among researchers as a green technology to increase the nutraceutical value in fruits and plants of food interest [4,5,6,7]. Several studies have proven the ability of UV-B treatment to induce the accumulation of health-promoting phytochemicals, such as phenolic compounds, flavonoids, carotenoids, and ascorbic acid, consequently increasing the antioxidant capacity of the UV-B-irradiated plants, mainly as an acclimation response to such high-energy light conditions [7,8,9,10,11,12,13,14,15,16]. Indeed, depending on the irradiance and duration of exposure, UV-B radiation might induce completely different morpho-physiologic and biochemical responses, which can be either positive (e.g., accumulation of defensive compounds and increase in the resistance against biotic and abiotic stresses) when the UV-B exposure is ecologically relevant and able to trigger specific adaptation mechanisms, or negative (e.g., decrease in biomass content, biochemical and physiological impairments) when the UV-B treatment is applied at high doses and/or for prolonged time [17,18].
It is known that biochar is a multifunctional soil fertilizer due to its physical and chemical properties [19,20,21,22,23], such as high porosity, surface functional groups, carbon–Crecalcitrant structure, and adsorption capacity [21,22,23]. Many studies reported the ability of biochar to create pore spaces in the soil, increasing its porosity and allowing for better water infiltration, root penetration, and air exchange [19,20,22,23]. Biochar also improves both water retention and drainage properties of soil. Moreover, biochar alleviates soil compaction through the creation of channels for root penetration, the increase in the movement of air and water, and the reduction in compaction-induced constraints on plant growth [19,20,22,23]. Biochar enhances nutrient retention and availability through its high cation exchange capacity (CEC), which allows it to attract and retain positively charged nutrients by adsorbing these nutrients and preventing their leaching [19,20,22,23]. Furthermore, the pH-modifying capabilities of biochar are beneficial for adjusting soil pH to optimal levels for plant growth and nutrient availability [19,20,22,23].
Wood distillate (WD), also known as pyroligneous acid or wood vinegar, is a liquid product obtained through the distillation or pyrolysis of wood. Destructive distillation involves heating wood in the absence of oxygen, resulting in the decomposition of its organic compounds [24]. The wood distillate is a complex mixture of various organic compounds, including phenols, acids, aldehydes, ketones, and other volatile substances. Its composition can vary depending on the wood species, pyrolysis conditions, and collection methods employed [25]. The primary components found in wood distillate include acetic acid, methanol, acetone, formaldehyde, and a wide range of phenolic compounds [26]. WD on soil and plants can vary depending on its composition, concentration, and application method [25,27]. WD compounds can provide a carbon source for beneficial soil microorganisms, promoting nutrient cycling and organic matter decomposition. It has been observed that the application of WD can make Ca, Mg, and K more bioavailable [28], likely due to soil acidification. Other authors reported an indirect effect of WD on soil nutrient fertility through the stimulation of microorganism metabolism and soil enzyme activities [29,30]. WD may also have plant growth-promoting effects. Some components, such as phenolic compounds, can stimulate root growth and nutrient uptake in plants [25,31]. Additionally, WD may enhance seed germination, improve root development, and increase biomass production in certain plant species [25]. It has also been observed that WD can increase or decrease chlorophyll content [32] depending on dilution treatment.
Although it has been demonstrated that biochar and WD have the ability to improve soil characteristics and crop productivity [24,25], few researchers have reported the effect of both single and co-application of these byproducts on the secondary metabolism of plants. The specific effects of biochar and WD on plant secondary metabolism can depend on various factors, including amendment properties, soil type, plant species, and environmental conditions; these different variables can generate different results [31,33,34,35,36,37].
Basil (genus Ocimum L., family Lamiaceae) represents a worldwide renowned herb of food interest. The most cultivated basil cultivars, for both ornamental and culinary applications, belong to the sweet basil (O. basilicum) species. Basil is also recognized for its large pool of antioxidants, especially phenolic compounds, and greatly appreciated aromatic compounds and essential oils [38]. Indeed, basil extracts have shown potential health-promoting properties for human health, thanks to their antibacterial, anthelmintic, anti-inflammatory, and anticancerogenic effects [38,39,40,41,42]. Several studies underlined the strong impact of environmental factors and cultivation techniques on the content of basil secondary metabolites [43,44], and accordingly, multiple attempts have been made to find the best conditions to increase the concentration of beneficial compounds in basil leaves. The modulation of the electromagnetic spectrum, in terms of both visible and UV radiation wavelengths, was found to greatly influences both the productivity and the accumulation of phenolic compounds, affecting the antioxidant capacity of the plant [45,46,47,48,49]. In addition, below-ground factors, such as the inoculation of arbuscular mycorrhizal fungi, the application of salt stress, and the addition of organic amendments, impact many physiological and biochemical aspects of basil plants, e.g., by affecting the plant yield and enhancing the content of many health-promoting phytochemicals [35,50,51].
This study aimed to investigate the effects of two UV-B doses (an above-ground factor) and the application of biochar and wood distillate (below-ground factors), alone or in combination, on some biometric and biochemical parameters of basil plants (O. basilicum var. Genovese) cultivated in controlled conditions. The influence of upper- and below-ground treatments on plant physiology and biochemistry is not easily predictable based on the responses to the single factor, likely due to synergistic or antagonistic outcomes. To our knowledge, this is the first time that soil amendments and UV-B radiation are studied in combination to verify if such factors that are known to positively alter plant growth (soil amendments) or stimulate the plant’s secondary metabolism (UV-B radiation) might behave differently from what is expected.
2. Materials and Methods
2.1. Experimental Setup
Basil seeds (O. basilicum var. Genovese) were bought from Gargini Sementi snc. (Capannori, Italy, https://www.gargisementi.it/, accessed on 10 January 2020), sterilized (20 min in 5% sodium hypochlorite), and carefully rinsed multiple times with deionized sterile water [52]. The seeds were germinated in Petri dishes covered with filter paper at room temperature. Once the cotyledons were fully developed, seedlings were transplanted into blond peat-filled pots and moved to a controlled chamber (24 ± 2 °C, 16 h light/8 h dark photoperiod, 228 mol m−2 s−1 photosynthetic photon flux density (PPFD) provided by a combination of blue/red/green (3/6/1 ratio) LEDs (C-LED, Imola, Italy)), and whetted weekly with half-strength Hoagland solution (pH~6). The plants were gently explanted once the second pair of true leaves had fully developed, and the roots were mildly rinsed with water to eliminate peat residues. Lastly, they were relocated separately into 576 cm3 pots with soil and treated as described in the following paragraph.
2.2. Organic Treatments Characteristics
Biochar and wood distillate (WD) were produced from woodchips (30–50 G g) of selected virgin wood tree species (Abies sp., Alnus sp., Castanea sativa, Fraxinus sp., Quercus sp., and Robinia pseudoacacia) by pyrogasification process (Bio-Esperia S.r.l., Arezzo, Italy; https://bioesperia.com/, accessed 2 January 2020). Biochar production was characterized by an average heating rate before reaching a peak of 1100 °C was 75–80 °C min−1. The distillation process of WD had different parameters: the average temperature was 50–70 °C hour−1 for 10 h, with a final peak of 1200 °C for about half an hour.
The parameters for the biochar characterization were analyzed through certified methods approved by Italian regulations (D.lgs. 75/2010 and D.M. 6793/2018): 9.8 pH, 400% water holding capacity, 87% organic carbon (OC), <0.5% total nitrogen (N), 0.34‰ total phosphorus (P), and 0.3% total potassium (K). The main characteristics of WD used in this study were as follows: pH, 2.8; density, 1.037 g mL−1; total organic carbon (TOC), 33.8 g L−1; total N, 0.43 g L−1; organic acid, 32.3 g kg−1; phenolic compounds, 13.0 g L−1; and methanol, 13.4 g L−1. Polychlorobiphenyls and polycyclic aromatic hydrocarbons (PAHs) were determined by using solid-phase microextraction prior to their analysis by gas chromatography coupled to tandem mass spectrometry, but none of these toxic compounds was present in relevant concentrations. Among PAHs, only acenaphthylene and phenanthrene reached 0.09 ng L−1, well below the most restrictive legislative limits.
2.3. Potting Soil Mixture Application and UV-B Treatment
The plants (108 in total) were divided into four groups: 27 plants transplanted in soil and not subjected to any additional treatment (S), 27 plants transplanted in soil amended with 2% w/w biochar (S_B), 27 plants transplanted in soil and fertirrigated once a week with 1:100 (v/v) wood distillate (S_WD), and 27 plants subjected to a combination of biochar and WD treatments (S_BWD).
The S potting soil was collected in January 2022 at Pontasserchio (Pisa, Italy; Latitude 43°39′38.96″ N; Longitude 10°18′22.17″ E) in an agricultural area. It was a loamy sand soil (Typic Xerorthent, USDA Soil Taxonomy; for soil properties, see Table 1) moderately fertile according to the “Agrelan” method developed by ARPAV [53]. S_B potting soil was a mixture of the same soil of S and biochar (for the properties, see Table 1). The parameters for soil characterization, such as particle size distribution, pH in water, total CaCO3, total N, available P, exchangeable K, and Cation Exchange Capacity (CEC), were measured following standard methods (n = 3) and are reported in Table 1 [54].

Table 1.
Physical and chemical characteristics of soil (S) and soil/biochar mixture (S_B) used for the experiment. Data represent the mean ± SD of three replicates. For each parameter, asterisks (*) indicate significant differences between S and S_B groups (** p < 0.01, *** p < 0.001) according to t-Student.
To prevent concealing the effects of the amendments, soil and soil/biochar samples were not fertilized but only irrigated with the same volume of water (60% of the water holding capacity of the soil, WHC) for all the experimental groups. For each group, two sub-groups of 9 plants each were exposed to an additional 1 h (4.90 kJ m−2) or 2 h (9.79 kJ m−2) per day of UV-B radiation (Philips Ultraviolet-B Narrowband, TL 20W/01—RS, Koninklijke Philips Electronics, Eindhoven, The Netherlands; irradiance 1.36 W m−2), respectively. The sub-group consisting of the remaining 9 plants was exposed only to the 16 h PAR with the conditions described above.
The experimental conditions previously described were maintained for the duration of the experiment (34 days).
2.4. Determination of Biometric Parameters
At the end of the 34 days, the plants were gently rinsed under running water to remove the soil, and both length (cm) and weight (g FW) of the root and the aerial parts were measured. For each plant, the pool of fully developed leaves and the vegetative shoot apices of young unexpanded leaves were sampled separately using liquid nitrogen and stored at −80 °C before being freeze-dried. The fresh/dry weight (FW/DW) ratio was then calculated.
2.5. Extraction and Determination of Total Phenolics, Flavonoids, and Antioxidant Activity
Extraction of total phenolics was conducted on 50 mg freeze-dried material from both fully developed leaves and vegetative shoot apices. The extraction procedure was performed in 80% methanol following the method described by [55], with slight modifications. Determination of total phenolics was performed with the Folin–Ciocalteau method [56] by reading the absorbance at 750 nm using an Ultrospec 2100 pro-UV–vis spectrophotometer (Amersham Biosciences). The total phenolic content was reported as mg of gallic acid equivalents (GAE) g−1 FW.
Measurement of total flavonoid concentration was conducted following the method by Kim et al. [57]. The absorbance was read at 510 nm, and the results were expressed as mg of catechin equivalents (CAE) g−1 FW.
Total antioxidant capacity was evaluated using the 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) assay by measuring the absorbance at 734 nm [58]. Antioxidant capacity was reported as μmol of Trolox equivalent antioxidant capacity (TEAC) g−1 FW.
The concentration of total phenolics, flavonoids, and antioxidant capacity were calculated using proper standard curves with the respective commercial standards.
2.6. Statistical Analysis
Differences in the biometric and biochemical parameters among the different experimental groups were assessed with a two-way ANOVA followed by post hoc Tukey–Kramer test (p < 0.05) to evaluate the impact of the UV-B exposure and the application of organic amendments alone and in combination. Biochemical data (total phenolic and flavonoid content and antioxidant capacity) from both vegetative shoot apices and fully developed leaves were used to perform a canonical discriminant analysis (CDA) to find a linear combination of the aforementioned multiple original variables that maximally discriminate between the groups. The robustness of this statistical analysis was assessed with four MANOVA tests (Wilk’s Lambda, Roy’s Largest Root Test, Hotelling–Lawley Trace, and Pillai–Bartlett Trace). JMP software (JMP®, Version 16. SAS Institute Inc., Cary, NC, USA, 1989–2021) was used to perform the statistical analysis.
3. Results
3.1. Biometric Parameters
Table 2 shows the effects of the different treatments on the biometric parameters. Although organic amendments did not affect all the biometric parameters mentioned with respect to S, biochar (S_B) treatment increased the root length by 22.6% compared to wood distillate (S_WD). Moreover, shoot/root length was increased by WD and BWD amendments compared to S_B (15% both).

Table 2.
Biometric parameters of shoot apices and fully expanded leaves of basil plants cultivated with different soil amendments (biochar, S_B; wood distillate, S_WD; and a combination of biochar and wood distillate, S_BWD) and/or treated with UV-B radiation (0, 1, 2 h d−1 for 34 days). Data represent the mean ± SE (n = 6). Different letters indicate statistically significant differences among the different treatments applied, according to two-way ANOVA followed by Tukey–Kramer test (p < 0.05).
Regarding the effect of the UV-B irradiation, 1 h d−1 exposure positively affected the growth of the plants, increasing the length of the stem (+28%), the root (+24%), and the total plant (+29%) compared to 0 h d−1 (no supplemental UV-B radiation, control). Similarly, 1 h d−1 of UV-B exposure induced a positive effect on the weight of the aerial part (+62%) and total plant (+28%) compared to the control, while the root weight underwent a decrease following this UV-B treatment (−6.3%), leading to an increase in the shoot/root fresh biomass ratio (+63%). On the other hand, the highest exposure dose instead had a negative impact on the plant length (−18%, −20%, and −17% for stem, roots, and whole plant, respectively) and weight (−32%, −26%, and −29% for aerial part, roots, and whole plant, respectively) compared to the control. Finally, when comparing the exposure doses to each other, the negative effect of 2 h d−1 was still evident for all parameters except for root weight, which was similar between the two groups.
Only root length and the shoot/root length ratio were significantly influenced by the interaction between the upper- and below-ground treatments. Specifically, for root length, plants exposed to 2 h d−1 of UV-B radiation and receiving WD amendment, either alone or in combination with biochar, underwent a reduction in root length (−36% and −39%, respectively) as compared to the control plants (S). When observing the S_B treatment group, root length was increased by S_B and 1 h d−1 of UV-B radiation (+53.8%) compared to S_B and 2 h d−1 of UV-B. Similarly, this parameter was also increased by the interaction between S_BWD treatment and 1 h d−1 of UV-B radiation compared to S_BWD and 0 h d−1 and 2 h d−1 of UV-B (+50.7% and +117.0%, respectively).
The shoot/root length significantly increased over the control in plants treated with WD but not irradiated (S_WD 0 h d−1 UV-B; +36%) and in those receiving both amendments and the highest UV-B dose (S_BWD 2 h d−1 UV-B; +54%).
3.2. Phenols, Flavonoid, and Antioxidant Activity in Vegetative Shoot Apices
The changes in phenolics concentration and antioxidant activity in vegetative shoot apices of basil plants are reported in Figure 1. Regarding the effects given by the amendments, soil treated with biochar (S_B) enhanced the content of phenols (+28.1% and +19.6%, respectively) and the antioxidant activity (+29.9%, +59.1%, and 44.7%, respectively) if compared to S, S_WD, and S_BWD. Moreover, flavonoid (Figure 1) concentration was increased (+26.5%) in basil vegetative shoots treated with biochar amendment (S_B) compared to the wood distillate (S_WD).
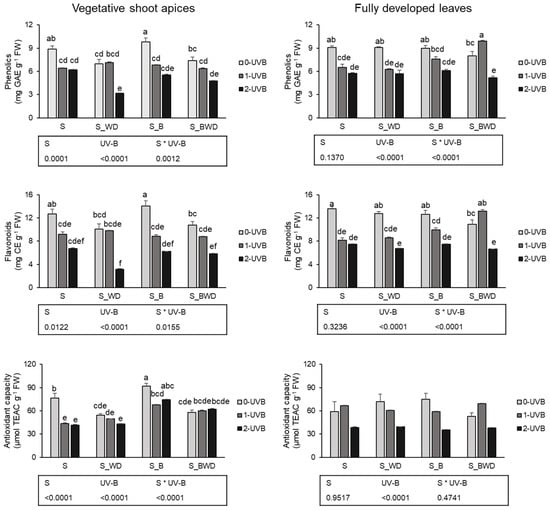
Figure 1.
Total phenolics, flavonoids, and antioxidant capacity of vegetative shoot apices and fully developed leaves of basil plants cultivated with different soil amendments (biochar, S_B, wood distillate, S_WD, a combination of biochar and wood distillate, S_BWD), and/or treated with UV-B radiation (0, 1, 2 h d−1) for 34 days. Data represent the mean ± SE (n = 6). Different letters indicate statistically significant differences, according to two-way ANOVA, followed by Tukey–Kramer post hoc test (p < 0.05). The p values (S, soil treatments; UV-B, ultraviolet-B treatments, S*UV-B, interaction of soil and UV-B treatments) are reported in the box below each histogram.
When analyzing the effects due to the UV-B treatments, 1 h d−1 and 2 h UV-B d−1 negatively impact the phenolic (−19.5% and −40.5%, respectively) and flavonoid (−23.1% and −54.0%, respectively) concentrations, and the antioxidant capacity (−21.0% and −21.2%, respectively) compared to the control (0 h d−1).
The influence of upper- and below-ground treatments compared to the control plants (S, 0 h d−1) was mainly negative with both UV-B doses. Total phenols, flavonoids, and antioxidant activity were decreased in plants grown in soil without amendments (S) and treated with UV-B radiation (−27.9%, −27.6%, and −42.9% for 1 h d−1, respectively; −30.0%, −46.9%, and 45.5% for 2 h d−1, respectively). Similarly, S_WD, S_B, and S_BWD, in combination with UV-B foliar treatment, at both intensities, generally decreased total phenolic content compared to the control plants (S and 0 h d−1), with S_D-treated plants irradiated with 2 h d−1 of UV-B showing the lowest concentration. Flavonoids followed the same trend, and S_WD 2 h d−1 of UV-B determined the lowest concentration of these compounds. Interestingly, S_B amended plants receiving no UV-B radiation showed higher antioxidant activity compared to the control plants (+20%). Finally, when comparing the different exposure doses within each amendment treatment, it was possible to notice that 2 h d−1 of UV-B reduced phenols and flavonoids compared to amended plants receiving no UV-B (−55.5% and −68.0% for S_WD; −35.8% and −45.6% for S_BWD). Instead, the S_B group was negatively affected by both UV-B doses.
3.3. Phenols, Flavonoid, and Antioxidant Activity in Fully Developed Leaves
The changes in total phenolic content and antioxidant activity in fully developed leaves of basil plants are reported in Figure 1. Differently from young shoot apices, no significant differences were detected according to the soil amendments used in this study.
Conversely, 1 h d−1 and 2 h d−1 UV-B exposure had negative effects on phenolics (−13.8% and −35.3%, respectively) and flavonoids (−20.1% and −43.3%, respectively) compared to the control (0 h d−1). Moreover, the antioxidant capacity was decreased by −41.5% and −40.8%, respectively, after 2 h d−1 of UV-B exposure compared to 0 h and 1 h d−1 of UV-B.
Concerning the vegetative shoots apices, soil treatments and their combination with UV-B radiation significantly affected the concentrations of bioactive compounds in fully developed leaves. Leaves of plants grown in soil (S) but irradiated with UV-B (1 h d−1 and 2 h d−1) decreased both total phenols (−27.5% and −36.2%, respectively) and flavonoids (−39.9% and 44.8%, respectively) compared with plants grown with 0 h d−1 of UV-B. Additionally, S_WD treatment negatively impacts the phenolic (−31% for 1 h d−1; −37% for 2 h d−1) and flavonoid (−36% for 1 h d−1; −50% for 2 h d−1) levels compared to the control plants (S and 0 h d−1 UV-B). S_B treatment followed the same trend considering the flavonoid content (−27% for 1 h d−1; −45% for 2 h d−1), while phenolics were lowered by 32% only by 2 h UV-B d−1 compared to the control plants. Interestingly, S_BWD treated with 1 h d−1 showed similar levels of phenols and flavonoids if compared to the control plants, while the higher UV-B dose determined a reduction in both metabolic classes (−42% and −51% for phenols and flavonoids, respectively).
Finally, when comparing the different exposure doses within each soil treatment, wood distillate (S_WD) and UV-B, both 1 h d−1 and 2 h d−1, resulted in a reduction in the phenolic content (−30.8% and −37.4%, respectively) and flavonoids (−32.3% and 47.2%, respectively), compared with S_WD and 0 h d−1 UV-B. The same trend was caused by the combination of biochar (S_B) and 2 h d−1 of UVB irradiation, which negatively impacted the phenolic (−31.8%) and flavonoid (−41.3%) contents compared to S_B and 0 h d−1 UV-B. Differently, S_BWD and 1 h d−1 UV-B increased both total phenolic content (+25.3% and +98.4%, respectively), compared to leaves of plants cultivated with S_BWD and both 0 h d−1 and 2 h d−1 UV-B, and flavonoids (+98.9%), compared to S_BWD and 2 h d−1 UV-B.
3.4. Canonical Discriminant Analysis (CDA) on Vegetative Shoot Apices and Fully Developed Leaves
To better understand the key factors that statistically differentiate the groups according to the biochemical data, the multivariate CDA (Figure 2) was performed based on the results from the total phenolic and flavonoid content and the antioxidant capacity. All the MANOVA tests (Wilk’s Lambda, Roy’s Largest Root Test, Hotelling–Lawley Trace, and Pillai–Bartlett Trace) gave highly significant p-values (<0.0001), confirming the robustness of this statistical analysis. Regarding both the CDA on the vegetative shoot apices (Figure 2a) and the one on the fully developed leaves (Figure 2b), the canonical function 1 explained most of the group segregation (95.69% and 83.91%, respectively), while the canonical function 2 accounted for only 2.69% and 12.87%, respectively. Based on the projection of the different replicates on the canonical function 1, it is possible to see a separation of the groups based on the UV-B exposure time, which is detectable for both the vegetative shoot apices and the one on the fully developed leaves. Indeed, the 0 h-, 1 h-, and 2 h-UV-B-treated replicates were located on the right, central, and left parts of both the scatterplots, respectively. Regarding the vegetative shoot apices (Figure 2a), although the 0 h- and the 2 h-UV-B-treated samples were well separated within the hyperspace considered the canonical 1, the 1 h group resulted in partially overlapping with the other two experimental groups, indicating a UV-B-dose-dependent response in terms of the biochemical variables considered. A similar consideration can be performed regarding the CDA of the fully developed leaves (Figure 2b), although the 1 h-UV-B-treated replicates were more smeared within the plot. Regarding the soil treatment, a good separation among the different experimental groups on the canonical variable 1 was observed in the CDA referred to the vegetative shoot apices, particularly in the 2 h UV-B exposed samples, where, from left to right, it is possible to distinguish the S_WD, S, S_BWD, and S_WD treatments. A slightly worse but still detectable separation can be seen among the 0 h-UV-B-treated groups, although the S_WD and the S_BWD replicates were partially overlapping. Regarding the fully developed leaves, however, no clear segregation among groups of the different soil treatments can be observed.
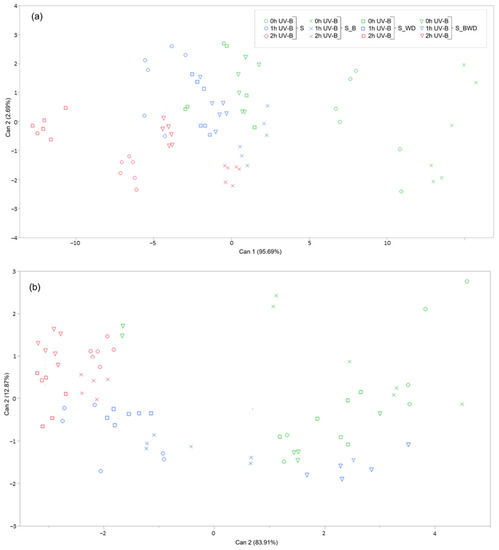
Figure 2.
Two-dimensional scatterplot of canonical discriminant analysis of the vegetative shoot apices (a) and the fully developed leaves (b) considering the different soil amendments (biochar, S_B, wood distillate, S_WD, and a combination of biochar and wood distillate, S_BWD) and/or treated with UV-B radiation (0, 1, 2 h d−1) for 34 days. Can 1 and 2 refer to canonical functions 1 and 2.
4. Discussion
Other researchers have already investigated the plant responses to the application of natural amendments, such as wood distillate and biochar [31,59,60,61,62,63,64], but no studies examined the effects of the combination of WD and biochar in terms of stem and root length and weight of basil plants, as well as the impact on phenolics metabolism and antioxidant capacity.
In this study, the application of both soil amendments did not cause any significant changes to the length and weight of both the aerial part and root basil when compared to plants grown in soil alone. Similarly, Borchard et al. [60], studying the effect of biochar (15 g kg−1) on the growth of Zea mays L. in sandy and silty soils, evidenced no modification of the crop dry biomass. On the other hand, the same authors, testing 100 g kg−1 biochar, found a reduction in the plant dry biomass probably due to nutrient imbalances and N-immobilization, while root mass fractions and shoot-to-root ratios remained unchanged. In our study, S_WD treatment negatively affected the root length compared to S_B, and, therefore, an increased shoot/root ratio, suggesting an inhibition effect of wood distillate when applied at 1% dilution. This is in line with other studies [65,66] that reported similar results, suggesting that the heterogeneous composition of WD can lead to negative effects on plant growth.
Becagli et al. [31] described a positive effect of biochar treatment on the dry biomass of basil leaves compared to wood distillate treatment and control plants. Fedeli et al. [61] showed increased dry biomass in lettuce plants sprayed with 0.25% wood distillate at a foliar level compared to the control plants, while 0.5% WD caused no modification compared to the controls. These studies suggest the importance of choosing the right dose of WD or biochar in relation to the plant species and cultivar and the soil conditions.
Interestingly, in this study, we found an increase in the root length of plants treated with biochar compared to the ones treated with wood distillate. Our results are aligned with the ones by Jabborova et al. [35], who observed a 46% increase in the total root length of basil-cropped plants by applying black cherry wood biochar (2% w/w) on a loamy sand soil. Also, Bu et al. [67] reported an increase in Robinia pseudoacacia root length by rice husk and woodchips biochar on calcareous soil. Brunn et al. [59] described an increased root density of barley and an enhanced soil ability to store water available to the plant, with an optimal biochar application of around 1–2% by mass. This may be due to the ability of biochar to modify the soil texture and structure, leading to minor resistance and friction to root growth.
When observing the UV-B effects on plant growth, 2 h d−1 UV-B caused a general reduction in the plant growth, at both the above- and below-ground organs, compared to the control plants. This suggests a slowdown of growth, which is a typical plant response to stressing UV conditions. In this regard, Biever et al. [68] found that the reduction in the hypocotyl length of UV-B-exposed Arabidopsis thaliana plants might be due to several critical factors, but mainly the disruption in the normal cell cycle caused by UV-B-mediated DNA photodimer accumulation. As these photodimers accumulate, they interfere with the DNA replication and repair processes, leading to cell cycle arrest. This, in turn, hampers the normal growth and elongation of the hypocotyls, resulting in the observed reduction in length. Therefore, in this study, it might be that the 2 h d−1 UV-B dose was able to induce DNA mutations and interfere with the cell cycle, leading to physiological impairments and reduced plant growth.
However, when UV treatments are applied at low ecological doses, plant growth is not necessarily reduced. Coffey et al. [69] showed that A. thaliana morphology was negatively influenced by UV-B only in summer conditions (high intensities of UV-B), likely due to the activation of some stressing response pathways, which are independent of the specific UVR8-related pathway. Accordingly, 1 h d−1 UV-B treatment determined an increase in the plant biomass, except for root FW, suggesting that this dose was not only well tolerated by basil plants but also stimulated their growth.
The interaction of amendments and UV-B radiation modified the root length in the case of S_B and S_BWD. In particular, 1 h d−1 UV-B positively affected UV-B-exposed plants compared to 2 h d−1 UV-B treated plants. This fact, in our opinion, once again highlights that two hours of UV-B irradiation acted as a stressing factor for the basil plants. Interestingly, the S_BWD treatment in combination with 1 h d−1 of irradiation determined a 50% increase in the root length compared to plants grown in S_BWD but not irradiated (0 h d−1 UV-B). This result suggests that the effect of the combination of WD and biochar on root growth can be improved by the presence of the right UV-B doses. This also indicates that UV-B perceived by leaves and stem is able to modify the metabolism of this organ not directly reached by the radiation. Indeed it is likely that molecules such as hormones or other types of signaling compounds (nitric oxide, reactive oxygen species, etc.) are able to move from the irradiated shoot to the roots and mediate metabolic changes needed by the plants to adapt to the environmental conditions [52,70]. In the present study, we also noticed a reduction in the root growth in plants irradiated with 2 h d−1 UV-B grown in S_BWD compared to not irradiated plants grown in soil or S_BWD. The more compact root habit found in these groups might be linked to an alteration of the hormone balance in this organ, and the relevance of this feature should be evaluated from a commercial point of view.
Phenolics are secondary metabolites with multiple roles within the plant, usually produced for adaptation and defense mechanisms against biotic or abiotic stresses [71]. In our study, we screened the modification of phenols and flavonoids in basil leaves at two different stages and fully expanded leaves and vegetative shoot apices, which indeed displayed a different response. A positive effect on phenols concentration and antioxidant activity was detected in shoot apices of basil plants cultivated with biochar compared to the control plants and the other amendments. The scientific literature regarding the influence of biochar on plant secondary metabolism is scanty [33,34]. Saha et al. [34] found that the total phenols and antioxidant capacity were higher in kalmegh plants (Andrographis paniculate) cropped in a loam sandy soil amended with 5 t ha−1 lemongrass biochar. Petruccelli et al. [33] partially supported our results since different trends related to different biochar feedstocks were found: wheat-straw and olive residues’ biochar increased the parameters, while poplar biochar decreased them. They suggested that the increase in secondary metabolites and antioxidant capacity was due to an increase in soil fertility (physical and chemical properties) but also to a hormesis effect derived from low concentrations of phytotoxic compounds in biochar. Although the applied biochar of this experiment was produced by softwood feedstock similar to the poplar biochar of Petruccelli et al. [33], the different process parameters could have led to a slight accumulation of these chemicals. No significant differences were detected in fully expanded leaves of plants grown with different amendments, but we cannot exclude modifications in the early days.
A negative effect on the phenol and flavonoid content was caused by UV-B treatments compared to non-irradiated plants in both vegetative apices and fully expanded leaves. This trend might be due to the phenolics consumption in order to counteract the potentially harmful reactive oxygen species (ROS), whose production was likely enhanced by UV-B exposure. A similar reduction was also found by Mannucci et al. [52], who studied the effects of a mild UV-B treatment (15 min d−1, corresponding to 1.19 kJ m−2) for 11 days on tomato (Micro-Tom cultivar) leaves. Csepregi et al. [72] also found a transient reduction in flavonoid (particularly flavonol) content in UV-B-exposed grape (Vitis vinifera L., cv Emperor; 15 W UV broad-band lamp), and the authors suggested that this might be due to the UV-B-triggered oxidation of such compounds in the grape skin, followed by the replacement of the oxidized compounds later during the storage period. Similarly, in our work, it might be that the decrease in such antioxidant molecules was transient and that their level would have been restored to a physiological level after the end of the UV-B irradiation. In addition, Csepregi et al. [72] stated that the observed decrease in flavonoid content is not only an unspecific UV-B-induced oxidation response to a higher ROS concentration, but the authors rather suggested the implication of the enzymatic antioxidant system, particularly the peroxidases enzymes. Indeed, flavonoid compounds, e.g., leaf quercetin, also act as substrates for peroxidases in the light of their electron-donor nature, contributing to the detoxification of the UV-B-induced H2O2 molecules [73]. Therefore, the lower flavonoid content detected in our study could also be due to their consumption as a result of the increased peroxidase activity.
Regarding the interaction of the amendments and UV-B treatments, we saw an overall negative effect on both phenolics and flavonoid content in young and old leaves.
Particularly, in vegetative shoot apices, the application of phenolics-rich WD, in combination with 2 h d−1 UV-B, caused the greatest reduction in phenols and flavonoids. This trend might suggest that a likely hyperaccumulation due to the above- and below-ground factors probably led to the sequestration of these compounds or were addressed towards oxidative reactions, as it is known that these molecules have a dual nature that allows them to act as antioxidants and prooxidants under certain conditions [74]. In fully expanded leaves, the interaction of biochar and wood distillate with 1 h d−1 UV-B stimulated the production of phenolics compared to plants cultivated with the same amendments but receiving no UV-B. This enhancement highlights the positive action of UV-B on the secondary metabolism of basil plants grown in S_BWD amended soils, and it suggests a likely improvement of the plant defense towards further biotic and abiotic stressors.
All data presented in this work, related to the general growth of basil plants and the modification of some secondary metabolite classes, highlighted a general antagonistic action during the combination of the investigated treatment factors at these doses. However, we would like to highlight that the variety of plants, soil types, light regimes, and different kinds of feedstocks for the amendment production are variables that should be addressed to eventually find a synergism between upper- and below-ground treatments that was not possible to obtain in this experiment.
5. Conclusions
This study aimed to investigate the combined effect of green technologies, namely UV-B and organic amendments, biochar, and wood distillate, as above- and below-ground factors, respectively, able to affect the growth and nutraceutical quality of basil plants. To our knowledge, this is the first time that these factors have been investigated simultaneously. Although the growth-promoting properties of organic amendments are well described, we did not notice an enhanced growth compared to the control plants (soil), but a positive effect was found on the root length after biochar application compared to WD. When amendments and UV-B were applied in combination, the plant growth was positively affected in S_BWD with 1 h UV-B compared to the same soil condition but with no irradiation. Regarding the secondary metabolism, we highlighted a different response of vegetative apices and fully expanded leaves. Biochar was able to increase the phenolic concentration and the antioxidant activity of young apices, while old leaves did not show a modification of these compounds in response to soil amendments, but we cannot exclude a different behavior in the early days. UV-B, which is a portion of the light spectrum known to be able to stimulate the phenolic biosynthetic pathway, was applied for 1 h or 2 h per day for a total of 34 days. The concentration of antioxidant compounds was generally lowered compared to not-irradiated plants, but it is interesting to highlight the positive interaction on the phenol content of S_BWD with 1 h d−1 UV-B compared to plants cultivated with the same amendments but not receiving UV-B. In conclusion, this preliminary study highlighted the potential effects, either positive or negative, that should be considered when combining above- and below-ground factors, which might not necessarily display a synergistic action on plant growth and metabolism.
Author Contributions
Conceptualization, M.S., A.C. and A.R.; methodology, M.S., A.C. and M.B.; software, M.S.; validation, A.M., M.C.S. and M.S.; formal analysis, M.B., A.M. and M.C.S.; investigation, M.B., A.M. and M.C.S.; data curation, M.S., A.M. and M.C.S.; writing—original draft preparation, M.S., M.B., A.M. and M.C.S.; writing—review and editing, M.S.; visualization, A.C. and A.R; supervision, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
The authors acknowledge Bio-Esperia s.r.l. (Arezzo, Italy) for having produced and provided the biochar and the wood distillate. Funding was provided by the University of Pisa.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Santin, M.; Ranieri, A.; Castagna, A. Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants. Plants 2021, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Santamaria, P. UV Lighting in Horticulture: A Sustainable Tool for Improving Production Quality and Food Safety. Horticulturae 2021, 7, 9. [Google Scholar] [CrossRef]
- Puccinelli, M.; Maggini, R.; Angelini, L.G.; Santin, M.; Landi, M.; Tavarini, S.; Castagna, A.; Incrocci, L. Can Light Spectrum Composition Increase Growth and Nutritional Quality of Linum Usitatissimum L. Sprouts and Microgreens? Horticulturae 2022, 8, 98. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as Eustressors in Horticultural and Agricultural Crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Schreiner, M.; Huyskens-Keil, S. Phytochemicals in Fruit and Vegetables: Health Promotion and Postharvest Elicitors. CRC Crit. Rev. Plant Sci. 2006, 25, 267–278. [Google Scholar] [CrossRef]
- Schreiner, M.; Martínez-Abaigar, J.; Glaab, J.; Jansen, M. UV-B Induced Secondary Plant Metabolites. Opt. Photonik 2014, 9, 34–37. [Google Scholar] [CrossRef]
- Hideg, É.; Strid, Å. The effects of UV-B on the biochemistry and metabolism of plants. In UV-B Radiation and Plant Life: Molecular Biology to Ecology; CABI: Wallingford, UK, 2017; pp. 90–110. [Google Scholar]
- Santin, M.; Calogera Sciampagna, M.; Mannucci, A.; Puccinelli, M.; Angelini, L.G.; Tavarini, S.; Accorsi, M.; Incrocci, L.; Ranieri, A.; Castagna, A. Supplemental UV-B Exposure Influences the Biomass and the Content of Bioactive Compounds in Linum Usitatissimum L. Sprouts and Microgreens. Horticulturae 2022, 8, 213. [Google Scholar] [CrossRef]
- Meyer, P.; Van de Poel, B.; De Coninck, B. UV-B light and its application potential to reduce disease and pest incidence in crops. Hortic. Res. 2021, 8, 194. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Pre-harvest UV-B radiation and photosynthetic photon flux density interactively affect plant photosynthesis, growth, and secondary metabolites accumulation in basil (Ocimum basilicum) plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef]
- Jaiswal, D.; Pandey-Rai, S.; Agrawal, S.B. Untangling the UV-B radiation-induced transcriptional network regulating plant morphogenesis and secondary metabolite production. Environ. Exp. Bot. 2021, 192, 104655. [Google Scholar] [CrossRef]
- Yin, R.; Ulm, R. How Plants Cope with UV-B: From Perception to Response. Curr. Opin. Plant Biol. 2017, 37, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light Signaling and UV-B-Mediated Plant Growth Regulation. J. Integr. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Choudhary, K.K. Revisiting the role of phenylpropanoids in plant defense against UV-B stress. Plant Stress 2023, 7, 100143. [Google Scholar] [CrossRef]
- Dotto, M.; Casati, P. Developmental Reprogramming by UV-B Radiation in Plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef]
- Heijde, M.; Ulm, R. UV-B Photoreceptor-Mediated Signalling in Plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.I. Photomorphogenic Responses to Ultraviolet-B Light. Plant Cell Environ. 2017, 40, 2544–2557. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lin, L.; Zhang, Q.; Lu, M.; Skvortsova, M.Y.; Podolec, R.; Yin, R. Mechanisms of UV-B light-induced photoreceptor UVR8 nuclear localization dynamics. New Phytol. 2022, 236, 1824–1837. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The Effects of Biochar Addition on Soil Physicochemical Properties: A Review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Kapoor, A.; Sharma, R.; Kumar, A.; Sepehya, S. Biochar as a Means to Improve Soil Fertility and Crop Productivity: A Review. J. Plant Nutr. 2022, 45, 2380–2388. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop per-formance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, Prospects and Potential Application of Pyroligneous Acid in Agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Ferrarin, M.; Becagli, M.; Guglielminetti, L.; Cardelli, R. Wood Distillate, a Review over Past Application and Future Perspective on Soil and Plant Research. Agrochimica 2021, 65, 17–24. [Google Scholar] [CrossRef]
- Batista Souza, J.G.; Ré-Poppi, N.; Luiz Raposo, J., Jr. Characterization of Pyroligneous Acid Used in Agriculture by Gas Chroma-tography-Mass Spectrometry. J. Braz. Chem. Soc. 2012, 23, 610–617. [Google Scholar]
- Simma, B.; Polthanee, A.; Goggi, A.S.; Siri, B.; Promkhambut, A.; Caragea, P.C. Wood Vinegar Seed Priming Improves Yield and Suppresses Weeds in Dryland Direct-Seeding Rice under Rainfed Production. Agron. Sustain. Dev. 2017, 37, 56. [Google Scholar] [CrossRef]
- Hideki Togoro, A.; Aparecida dos Santos da Silva, J.; Osvaldo Cazetta, J. Chemical Changes in an Oxisol Treated with Pyro-ligneous Acid. Ciência E Agrotecnologia 2014, 38, 113–121. [Google Scholar] [CrossRef]
- Cardelli, R.; Becagli, M.; Marchini, F.; Saviozzi, A. Soil Biochemical Activities after the Application of Pyroligneous Acid to Soil. Soil Res. 2020, 58, 461–467. [Google Scholar] [CrossRef]
- Zhang, F.; Shao, J.; Yang, H.; Guo, D.; Chen, Z.; Zhang, S.; Chen, H. Effects of Biomass Pyrolysis Derived Wood Vinegar on Microbial Activity and Communities of Activated Sludge. Bioresour. Technol. 2019, 279, 252–261. [Google Scholar] [CrossRef]
- Becagli, M.; Santin, M.; Cardelli, R. Co-Application of Wood Distillate and Biochar Improves Soil Quality and Plant Growth in Basil (Ocimum basilicum)#. J. Plant Nutr. Soil Sci. 2022, 185, 120–131. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Meng, J.; Yang, Q.; Zhang, X.; Kang, Z.; Zhou, G. Effect of the combined action of wood vinegar and sodium naphthaleneacetate on photosynthetic characteristics and yield of peanuts. Agric. Res. Arid. Areas 2017, 1, 185–191. [Google Scholar]
- Petruccelli, R.; Bonetti, A.; Traversi, M.L.; Faraloni, C.; Valagussa, M.; Pozzi, A. Influence of Biochar Application on Nutritional Quality of Tomato (Lycopersicon esculentum). Crop Pasture Sci. 2015, 66, 747–755. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B.; Gajbhiye, N.A.; Kalariya, K.A.; Manivel, P. Sustainable Fertilization through Co-Application of Biochar and Chemical Fertilizers Improves Yield, Quality of Andrographis Paniculata and Soil Health. Ind. Crops Prod. 2019, 140, 111607. [Google Scholar] [CrossRef]
- Jabborova, D.; Ma, H.; Bellingrath-Kimura, S.D.; Wirth, S. Impacts of Biochar on Basil (Ocimum basilicum) Growth, Root Morphological Traits, Plant Biochemical and Physiological Properties and Soil Enzymatic Activities. Sci. Hortic. 2021, 290, 110518. [Google Scholar] [CrossRef]
- Hiyasmin Rose, L.; Benzon, S.C.L. Potential of Wood Vinegar in Enhancing Fruit Yield and Antioxidant Capacity in Tomato. Korean J. Plant Resour. 2016, 29, 704–711. [Google Scholar]
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Sun, K.; Luo, X.; Li, F. Comparative Study of Individual and Co-Application of Biochar and Wood Vinegar on Blueberry Fruit Yield and Nutritional Quality. Chemosphere 2020, 246, 125699. [Google Scholar] [CrossRef] [PubMed]
- Ugbogu, O.C.; Emmanuel, O.; Agi, G.O.; Ibe, C.; Ekweogu, C.N.; Ude, V.C.; Uche, M.E.; Nnanna, R.O.; Ugbogu, E.A. A Review on the Traditional Uses, Phytochemistry, and Pharmacological Activities of Clove Basil (Ocimum gratissimum L.). Heliyon 2021, 7, e08404. [Google Scholar] [CrossRef]
- Perna, S.; Alawadhi, H.; Riva, A.; Allegrini, P.; Petrangolini, G.; Gasparri, C.; Alalwan, T.A.; Rondanelli, M. In Vitro and In Vivo Anticancer Activity of Basil (Ocimum spp.): Current Insights and Future Prospects. Cancers 2022, 14, 2375. [Google Scholar] [CrossRef]
- Osei Akoto, C.; Acheampong, A.; Boakye, Y.D.; Naazo, A.A.; Adomah, D.H. Anti-Inflammatory, Antioxidant, and Anthel-mintic Activities of Ocimum basilicum (Sweet Basil) Fruits. J. Chem. 2020, 2020, 2153534. [Google Scholar] [CrossRef]
- Takeuchi, H.; Takahashi-Muto, C.; Nagase, M.; Kassai, M.; Tanaka-Yachi, R.; Kiyose, C. Anti-Inflammatory Effects of Extracts of Sweet Basil (Ocimum basilicum L.) on a Co-Culture of 3t3-L1 Adipocytes and Raw264.7 Macrophages. J. Oleo Sci. 2020, 69, 487–493. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The Antimicrobial Efficacy of Plant Essential Oil Combinations and Interactions with Food Ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum basilicum) Essential Oils Depends on Seasonal Variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, L.; Novikova, A.; Tokupova, E. Improvement of Phenolic Compounds, Essential Oil Content and Antioxidant Properties of Sweet Basil (Ocimum basilicum L.) Depending on Type and Concentration of Selenium Application. Plants 2019, 8, 458. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED Lighting Enhances Growth Characteristics and Total Phenolic Content of Ocimum basilicum, but Variably Affects Transplant Success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Johnson, C.B.; Kirby, J.; Naxakis, G.; Pearson, S. Substantial UV-B-Mediated Induction of Essential Oils in Sweet Basil (Ocimum basilicum L.). Phytochemistry 1999, 51, 507–510. [Google Scholar] [CrossRef]
- Chang, X.; Alderson, P.G.; Wright, C.J. Enhanced UV-B Radiation Alters Basil (Ocimum basilicum L.) Growth and Stimulates the Synthesis of Volatile Oils. J. Hortic. For. 2009, 1, 27–31. [Google Scholar]
- Mosadegh, H.; Trivellini, A.; Ferrante, A.; Lucchesini, M.; Vernieri, P.; Mensuali, A. Applications of UV-B Lighting to Enhance Phenolic Accumulation of Sweet Basil. Sci. Hortic. 2018, 229, 107–116. [Google Scholar] [CrossRef]
- Sakalauskaite, J.; Viskelis, P.; Dambrauskiene, E.; Sakalauskiene, S.; Samuoliene, G.; Brazaityte, A.; Duchovskis, P.; Ur-bonavičiene, D. The Effects of Different UV-B Radiation Intensities on Morphological and Biochemical Characteristics in Ocimum basilicum L. J. Sci. Food Agric. 2013, 93, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Patel, A.; Patra, D.D. Biochar Ameliorates Crop Productivity, Soil Fertility, Essential Oil Yield and Aroma Profiling in Basil (Ocimum basilicum L.). Ecol. Eng. 2016, 90, 361–366. [Google Scholar] [CrossRef]
- Scagel, C.F.; Lee, J. Salinity Sensitivity and Mycorrhizal Responsiveness of Polyphenolics in ‘Siam Queen’ Basil Grown in Soilless Substrate. Sci. Hortic. 2020, 269, 109394. [Google Scholar] [CrossRef]
- Mannucci, A.; Mariotti, L.; Castagna, A.; Santin, M.; Trivellini, A.; Reyes, T.H.; Mensuali-Sodi, A.; Ranieri, A.; Quartacci, M.F. Hormone Profile Changes Occur in Roots and Leaves of Micro-Tom Tomato Plants When Exposing the Aerial Part to Low Doses of UV-B Radiation. Plant Physiol. Biochem. 2020, 148, 291–301. [Google Scholar] [CrossRef] [PubMed]
- ARPAV—Agenzia Regionale per la Prevenzione e Protezione Ambientale del Veneto. L’interpretazione delle Analisi del Terreno—Strumento per la Sostenibilità Ambientale; Arpav: Padova, Italy, 2007; ISBN 88-7504-115-6. [Google Scholar]
- Colombo, C.; Miano, T. Metodi di Analisi Chimica del Suolo; Società Italiana della Scienza del Suolo (SISS): Palermo, Italy, 2015. [Google Scholar]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [PubMed]
- Alonso Borbalán, Á.M.; Zorro, L.; Guillén, D.A.; García Barroso, C. Study of the Polyphenol Content of Red and White Grape Varieties by Liquid Chromatography-Mass Spectrometry and Its Relationship to Antioxidant Power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Pellegrini, N.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoid fruit extracts for antioxidant activities applying 2, 2′-azinobit (3-ethylenebenzothiazoline-6-sulfonic acid) radical cation decolorization assay. Methods Enzymol. 1999, 299, 379–386. [Google Scholar]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.; Holm, J.K.; Hauggaard-Nielsen, H. Biochar Amendment to Coarse Sandy Subsoil Improves Root Growth and Increases Water Retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Borchard, N.; Siemens, J.; Ladd, B.; Möller, A.; Amelung, W. Application of Biochars to Sandy and Silty Soil Failed to Increase Maize Yield under Common Agricultural Practice. Soil Tillage Res. 2014, 144, 184–194. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-Based Solutions for Agriculture: Foliar Application of Wood Distillate Alone and in Combination with Other Plant-Derived Corroborants Results in Different Effects on Lettuce (Lactuca sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Giannini, V.; Moro, G.; Marche, M.G.; Hamze, R.; Ruiu, L. Exploring the bioactivity of a novel pine wood distillate (PWD) for plant growth and protection. J. Plant Dis. Prot. 2023, 130, 725–734. [Google Scholar] [CrossRef]
- Carril, P.; Bianchi, E.; Cicchi, C.; Coppi, A.; Dainelli, M.; Gonnelli, C.; Colzi, I. Effects of Wood Distillate (Pyroligneous Acid) on the Yield Parameters and Mineral Composition of Three Leguminous Crops. Environments 2023, 10, 126. [Google Scholar] [CrossRef]
- Rawat, J.; Saxena, J.; Sanwal, P. Biochar: A sustainable approach for improving plant growth and soil properties. Bi-Ochar-Imp. Amend. Soil Environ. 2019, 1–17. [Google Scholar]
- Chen, J.; Wu, J.H.; Si, H.P.; Lin, K.Y. Effects of adding wood vinegar to nutrient solution on the growth, photosynthesis, and absorption of mineral elements of hydroponic lettuce. J. Plant Nutr. 2016, 39, 456–462. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, J.; He, J.; Sun, K.; Sun, Y. Effect of pyrolysis temperature on the characteristics of wood vinegar derived from Chinese fir waste: A comprehensive study on its growth regulation performance and mechanism. ACS Omega 2019, 4, 19054–19062. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.L.; Xue, J.H.; Wu, Y.B.; Ma, W.B. Effect of Biochar on Seed Germination and Seedling Growth of Robinia pseudoacacia L. In Karst Calcareous Soils. Commun. Soil Sci. Plant Anal. 2020, 51, 352–363. [Google Scholar] [CrossRef]
- Biever, J.J.; Brinkman, D.; Gardner, G. UV-B inhibition of hypocotyl growth in etiolated Arabidopsis thaliana seedlings is a consequence of cell cycle arrest initiated by photodimer accumulation. J. Exp. Bot. 2014, 65, 2949–2961. [Google Scholar] [CrossRef]
- Coffey, A.; Prinsen, E.; Jansen, M.A.K.; Conway, J. The UVB Photoreceptor UVR8 Mediates Accumulation of UV-Absorbing Pigments, but Not Changes in Plant Morphology, under Outdoor Conditions. Plant Cell Environ. 2017, 40, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Huang, G.; Wang, L.; Zhou, Q.; Huang, X. Effects of elevated ultraviolet-B radiation on root growth and chemical signaling molecules in plants. Ecotoxicol. Environ. Saf. 2019, 171, 683–690. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, K.; Kőrösi, L.; Teszlák, P.; Hideg, É. Postharvest UV-A and UV-B treatments may cause a transient decrease in grape berry skin flavonol-glycoside contents and total antioxidant capacities. Phytochem. Lett. 2019, 31, 63–68. [Google Scholar] [CrossRef]
- Czégény, G.; Wu, M.; Dér, A.; Eriksson, L.A.; Strid, Å.; Hideg, É. Hydrogen peroxide contributes to the ultraviolet-B (280–315 nm) induced oxidative stress of plant leaves through multiple pathways. FEBS Lett. 2014, 588, 2255–2261. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).