Aroma Components Analysis and Origin Differentiation of Black Tea Based on ATD-GC-MS and E-Nose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pharmaceuticals and Instruments
2.3. Method
2.3.1. Electronic Nose Detection Method
2.3.2. ATD-GC–MS Detection Method
2.3.3. Qualitative and Quantitative Analyses of the Aroma Substances
2.3.4. Single-Factor Experimental Optimizations
Adsorption Grams of Tea Samples
Optimization of the Adsorption Time
Optimization of the Adsorption Temperature
Optimization of the Cold Trap Temperature
2.3.5. Response Surface Optimization Experiment
2.4. Statistical Analyses
3. Results and Discussion
3.1. One-Factor Optimization of the ATD Conditions
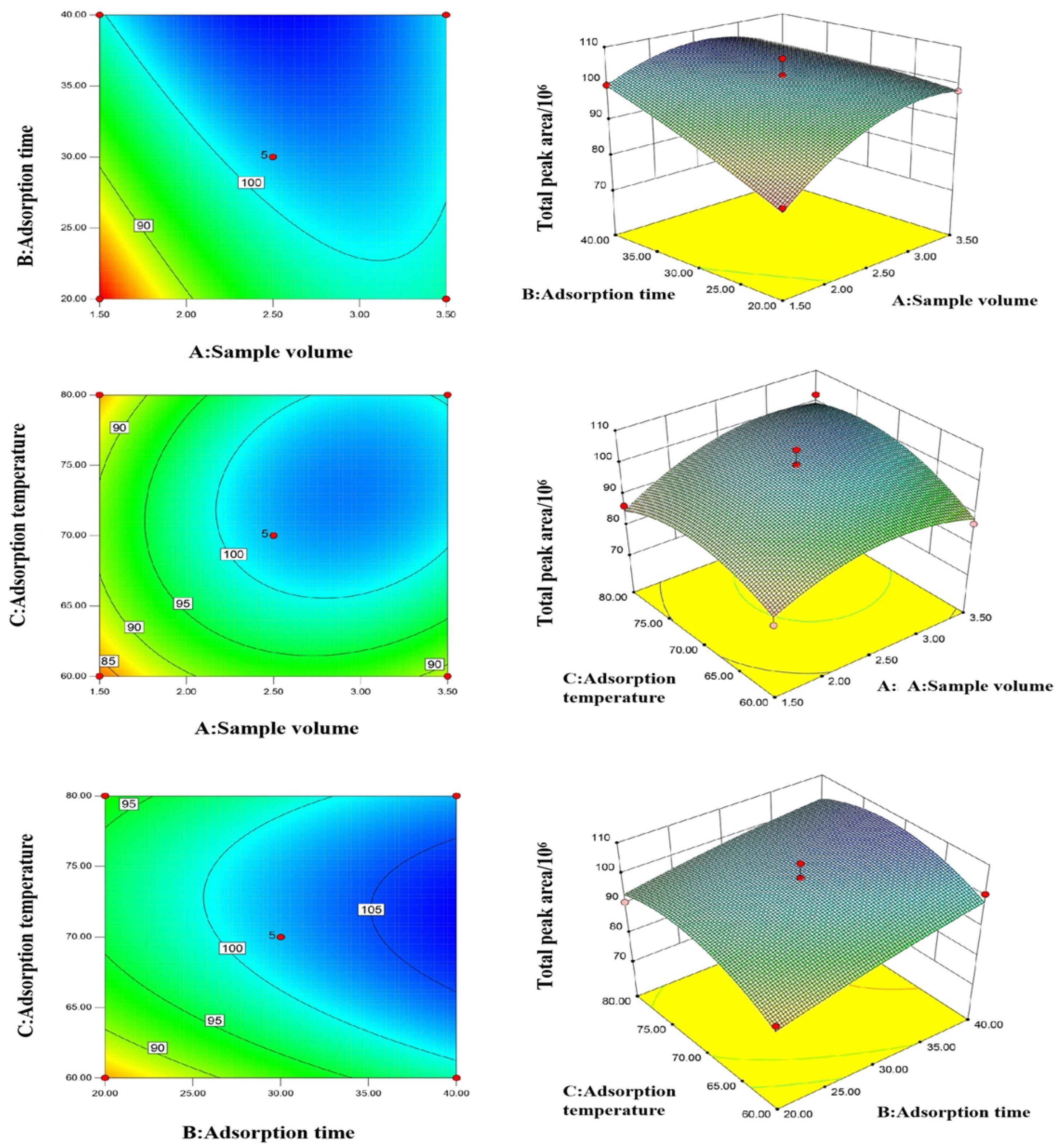
3.2. Response Surface Optimization of the ATD Conditions
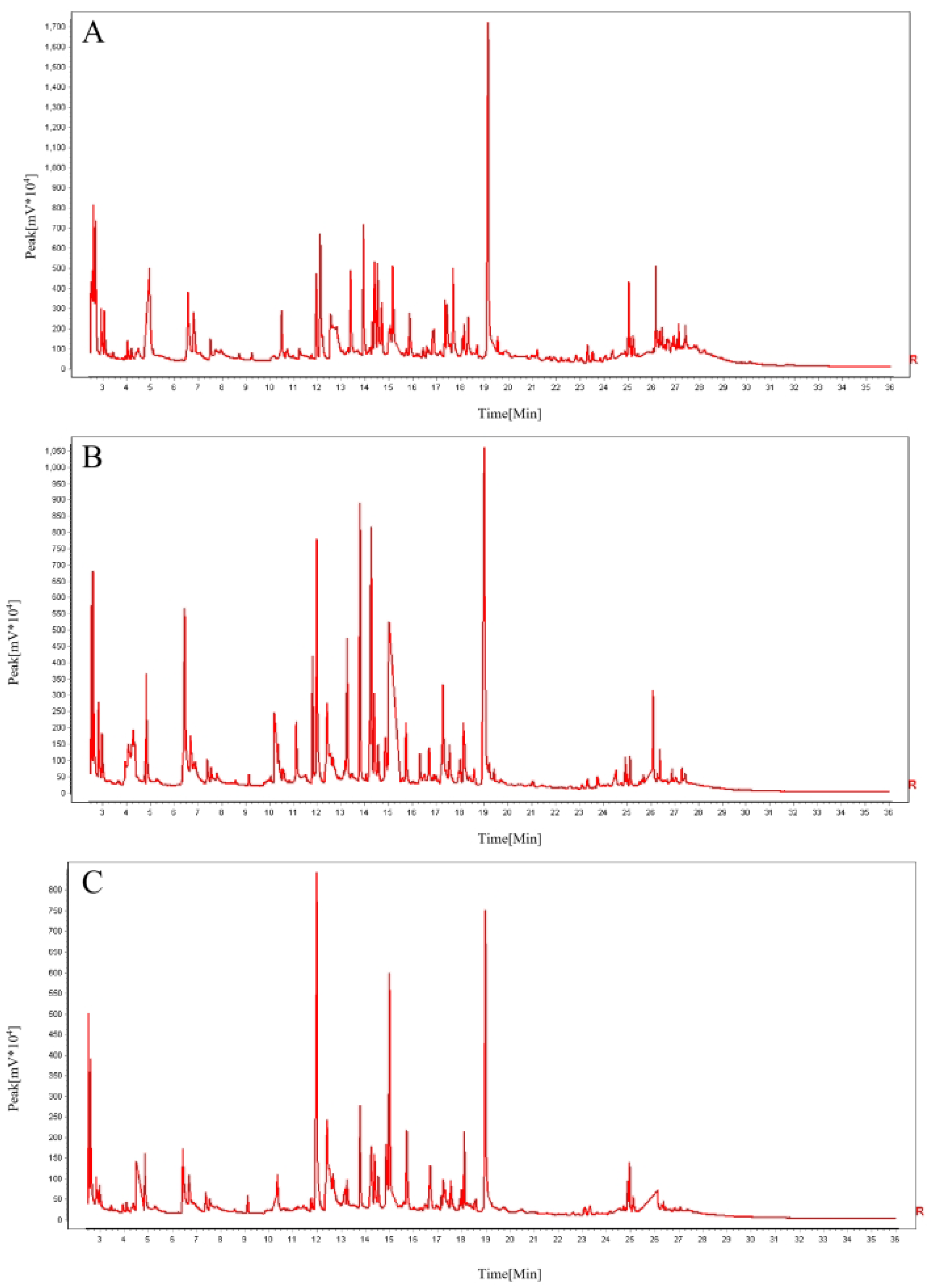
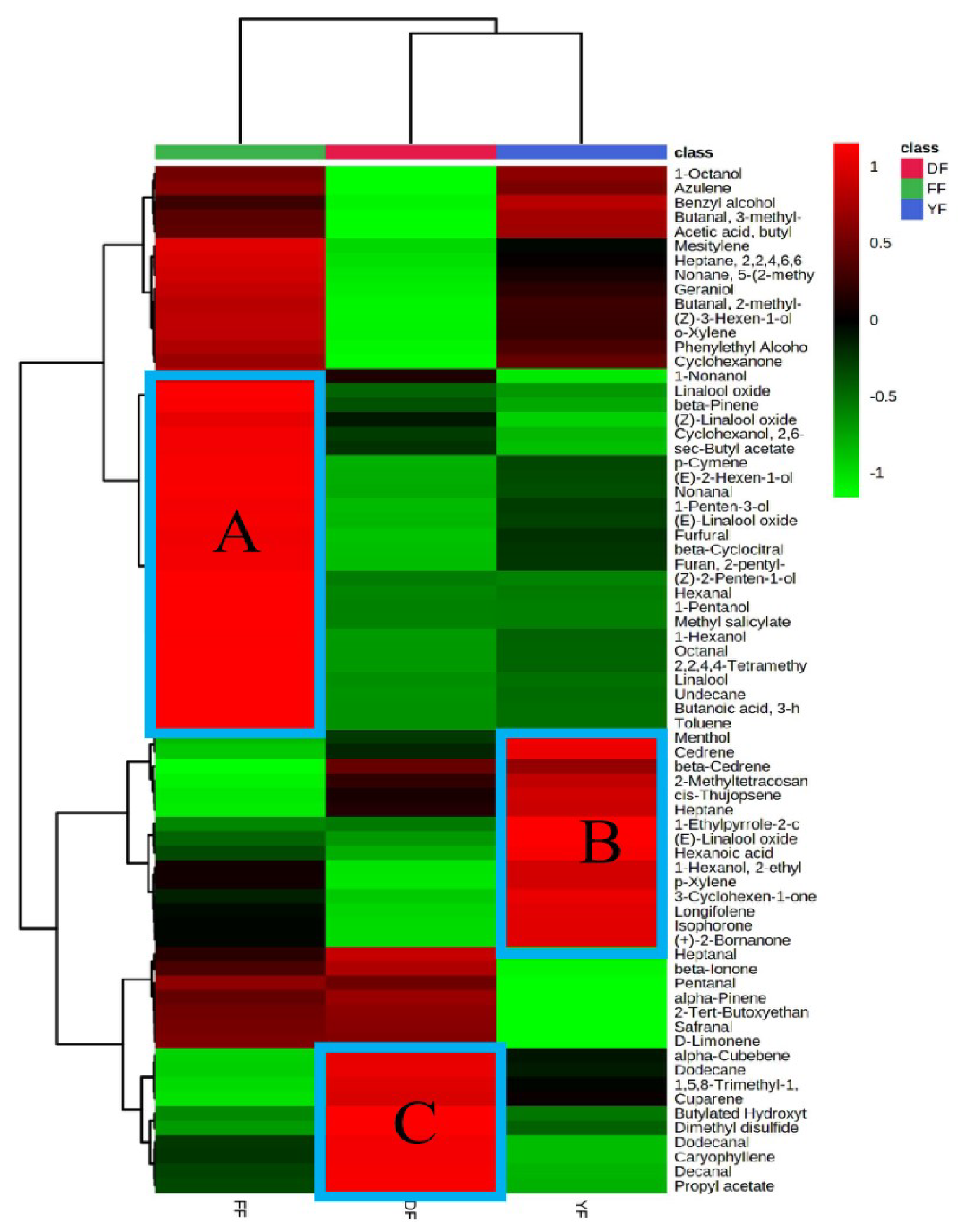
3.3. Analysis of Aroma Characteristics of Black Tea from Different Regions
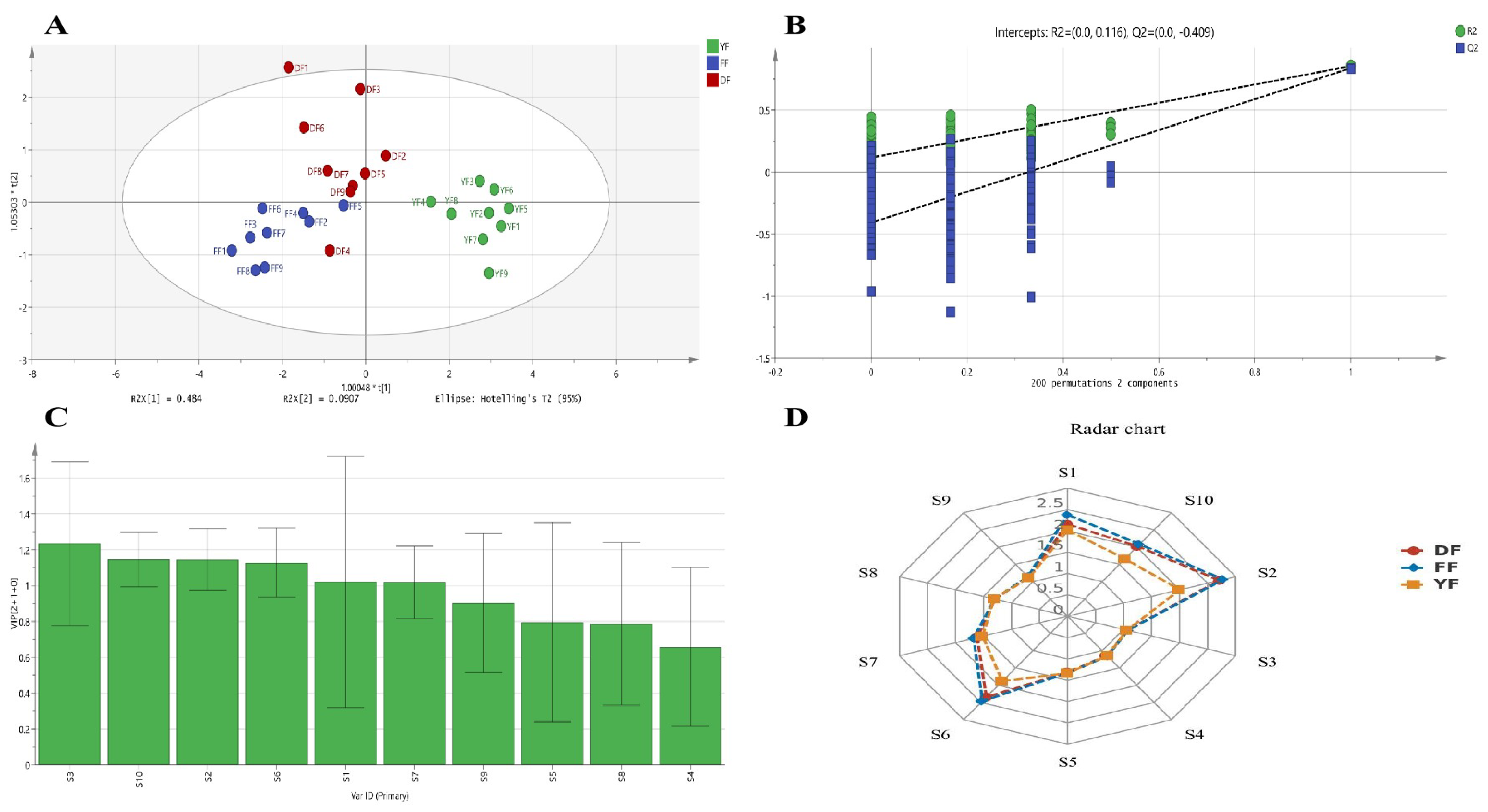
3.4. Analyses with the Electronic Nose for Black Teas from Different Origins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, K.; Ouyang, J.; Huang, J.; Liu, Z. Research progress of black tea thearubigins: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tai, L.-L.; Wan, X.-C.; Li, D.-X.; Zhao, Y.-Q.; Xu, Y. Comparative effects of green and black tea extracts on lowering serum uric acid in hyperuricemic mice. Pharm. Biol. 2017, 55, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Q.; Wang, C.; Li, C.-W.; Liu, S.-H.; Zhang, C.-X.; Li, L.-W.; Jiang, D.-H. Characterization of Aroma-Active Compounds of Pu-erh Tea by Headspace Solid-Phase Microextraction (HS-SPME) and Simultaneous Distillation-Extraction (SDE) Coupled with GC-Olfactometry and GC-MS. Food Anal. Methods 2015, 9, 1188–1198. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.; Liu, Y.; Liu, Y.; Dong, C.; Lin, Z.; Teng, J. Black tea aroma formation during the fermentation period. Food Chem. 2021, 374, 131640. [Google Scholar] [CrossRef]
- Yener, S.; Sánchez-López, J.A.; Granitto, P.M.; Cappellin, L.; Märk, T.D.; Zimmermann, R.; Bonn, G.K.; Yeretzian, C.; Biasioli, F. Rapid and Direct Volatile Com-pound Profiling of Black and Green Teas (Camellia sinensis) from Different Countries with Ptr-Tof-Ms. Talanta 2016, 152, 45–53. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wu, J.; Wen, J.; Yu, Y.; An, K.; Zou, B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021, 150, 110784. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Lin, J.; Zhu, J.; Ye, N.; Huang, J.; Wang, P.; Jin, S.; Zheng, D.; Yang, J. Aroma analysis of Fuyun 6 and Jinguanyin black tea in the Fu’an area based on E-nose and GC–MS. Eur. Food Res. Technol. 2022, 248, 947–961. [Google Scholar] [CrossRef]
- Sharma, P.; Ghosh, A.; Tudu, B.; Sabhapondit, S.; Baruah, B.D.; Tamuly, P.; Bhattacharyya, N.; Bandyopadhyay, R. Monitoring the fermentation process of black tea using QCM sensor based electronic nose. Sens. Actuators B Chem. 2015, 219, 146–157. [Google Scholar] [CrossRef]
- Kumazawa, K.; Wada, Y.; Masuda, H. Characterization of Epoxydecenal Isomers as Potent Odorants in Black Tea (Dimbula) Infusion. J. Agric. Food Chem. 2006, 54, 4795–4801. [Google Scholar] [CrossRef]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.-P.; Zhang, Y.; Dai, W.-D.; Guo, L.; Tan, J.-F.; Peng, Q.-H.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, X.; Su, X.; Yang, Z. Chinese oolong tea: An aromatic beverage produced under multiple stresses. Trends Food Sci. Technol. 2020, 106, 242–253. [Google Scholar] [CrossRef]
- Wei, B.; Cui, Y.; Fang, X.; Ouyang, J. Analysis on Volatile Profiles of Chestnut Flowers by GC-MS Coupled with Automatic Thermal Desorption. Food Ferment. Ind. 2014, 40, 192–195. [Google Scholar]
- He, C.; Li, Y.; Zhou, J.; Yu, X.; Zhang, D.; Chen, Y.; Ni, D.; Yu, Z. Study on the Suitability of Tea Cultivars for Processing Oolong Tea from the Perspective of Aroma Based on Olfactory Sensory, Electronic Nose, and GC-MS Data Correlation Analysis. Foods 2022, 11, 2880. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Xiang, H.; Li, Z.; Li, J.; Li, L.; Song, C.F. Monitoring the baking quality of Tieguanyin via electronic nose combined with GC–MS. Food Res. Int. 2023, 165, 112513. [Google Scholar] [CrossRef]
- Villaseor, M.J.; Valero, E.; Sanz, J.; Castro, I. Analysis of Volatile Components of Manchego Cheese by Dynamic Headspace Followed by Automatic Thermal Desorption-GC-MS. Milchwissenschaft 2000, 55, 78–82. [Google Scholar]
- Cui, Y.; Wei, B.; Fang, X.; Ou Yang, J. Study on Volatile Components of Chestnut Mushroom. Sci. Technol. Food Ind. 2013, 34, 88–90. [Google Scholar]
- Yang, Y.-Q.; Yin, H.-X.; Yuan, H.-B.; Jiang, Y.-W.; Dong, C.-W.; Deng, Y.-L. Characterization of the volatile components in green tea by IRAE-HS-SPME/GC-MS combined with multivariate analysis. PLoS ONE 2018, 13, e0193393. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Hamid, N.; Bekhit, A.; Robertson, J.; Law, T. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC–MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem. J. 2013, 111, 16–24. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Zhang, S.; Luo, L.; Zeng, L. Effect of brewing conditions on phytochemicals and sensory profiles of black tea infusions: A primary study on the effects of geraniol and β-ionone on taste perception of black tea infusions. Food Chem. 2021, 354, 129504. [Google Scholar] [CrossRef]
- GB/T 35810-2018; Technical Specification for Black Tea Processing. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China Standardization Administration of China: Beijing, China, 2018.
- Wang, S.; Zhao, F.; Wu, W.; Wang, P.; Ye, N. Comparison of Volatiles in Different Jasmine Tea Grade Samples Using Electronic Nose and Automatic Thermal Desorption-Gas Chromatography-Mass Spectrometry Followed by Multivariate Statistical Analysis. Molecules 2020, 25, 380. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Liu, C.; Yang, R.-J.; Zheng, T.-T.; Zhao, M.-M.; Ma, L.; Yan, L. Comparison of volatile profiles and bioactive components of sun-dried Pu-erh tea leaves from ancient tea plants on Bulang Mountain measured by GC-MS and HPLC. J. Zhejiang Univ. B 2019, 20, 563–575. [Google Scholar] [CrossRef]
- Wang, M.; Ma, W.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H. Characterization of the Key Aroma Compounds in Longjing Tea Using Stir Bar Sorptive Extraction (SBSE) Combined with Gas Chromatography-Mass Spectrometry (GC–MS), Gas Chromatography-Olfactometry (GC-O), Odor Activity Value (OAV), and Aroma Recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Zhang, Z.; Jiang, J.; Gao, X.; Yue, P. Multiple responses optimization of instant dark tea production by submerged fermentation using response surface methodology. J. Food Sci. Technol. 2018, 55, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Qiao, Y.; Du, L.; Li, Y.; Huang, S.; Liu, F.; Xiao, D. Evaluation and Optimization of a Superior Extraction Method for the Characterization of the Volatile Profile of Black Tea by HS-SPME/GC-MS. Food Anal. Methods 2017, 10, 2481–2489. [Google Scholar] [CrossRef]
- Pu, B.; Zhang, Y.; Liu, Y.; Liu, X. Optimization of Aroma Components Extraction from Loquat Wine by HP-SPME. Food Ferment. Ind. 2011, 37, 114–119. [Google Scholar]
- Huang, Y.; Du, L.; Li, J.; Xiao, D.; Li, C.; Xu, Y. Analysis of Wood Aroma Characteristic Compounds in Pu-Erh Tea by HS-SPME-GC-MS. Beverage Ind. 2015, 18, 6. [Google Scholar]
- Ning, P.; Liu, T. Analysis of Volatiles from Garlic Oil by Headspace-Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry. Sci. Technol. Food Ind. 2010, 31, 172–176. [Google Scholar]
- Zhang, Z.; Janusz, P. Headspace Solid-Phase Microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Sin, H.; Yusof, S.; Hamid, S.A.; Rahman, A. Optimization of Hot Water Extraction for Sapodilla Juice Using Response Surface Methodology. J. Food Eng. 2006, 74, 352–358. [Google Scholar] [CrossRef]
- Mahawar, M.K.; Samuel, D.V.K.; Sinha, J.P.; Jalgaonkar, K. Optimization of Pea (Pisum sativum) Seeds Hydro-priming by Application of Response Surface Methodology. Acta Physiol. Plant. 2016, 38, 212. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Wang, G.; Sun, S.; Liu, R.; Hong, B.; Gao, R.; Bai, K. Processing Optimization and Characterization of Angiotensin-Ι-Converting Enzyme Inhibitory Peptides from Lizardfish (Synodus macrops) Scale Gelatin. Mar. Drugs 2018, 16, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, D.; Ren, H. Bioremediation of Oil Contaminated Soil Using Agricultural Wastes Via Microbial Consortium. Sci. Rep. 2020, 10, 9188. [Google Scholar]
- Wu, Y.; Lv, S.; Wang, C.; Gao, X.; Li, J.; Meng, Q. Comparative analysis of volatiles difference of Yunnan sun-dried Pu-erh green tea from different tea mountains: Jingmai and Wuliang mountain by chemical fingerprint similarity combined with principal component analysis and cluster analysis. Chem. Cent. J. 2016, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Schieberle, P. New Developments in Methods for Analysis of Volatile Flavor Compounds and Their Precursors. Charact. Food 1995, 403–431. [Google Scholar]
- Wang, Z.; Su, D.; Ren, H.; Lv, Q.; Ren, L.; Li, Y.; Zhou, H. Effect of different drying methods after fermentation on the aroma of Pu-erh tea (ripe tea). LWT 2022, 171, 114129. [Google Scholar] [CrossRef]
- Ni, H.; Jiang, Q.; Lin, Q.; Ma, Q.; Wang, L.; Weng, S.; Huang, G.; Li, L.; Chen, F. Enzymatic hydrolysis and auto-isomerization during β-glucosidase treatment improve the aroma of instant white tea infusion. Food Chem. 2020, 342, 128565. [Google Scholar] [CrossRef]
- Supriyadi, S.; Nareswari, A.R.; Fitriani, A.; Gunadi, R. Enhancement of Black Tea Aroma by Adding the β-Glucosidase Enzyme During Fermentation on Black Tea Processing. Int. J. Food Sci. 2021, 2021, 5542109. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, J.; Yu, X.; Lv, S.; Wu, Y.; Wang, C.; Gao, X.; Li, J.; Zhang, W.; Zhao, P.; et al. Aroma formation in Dianhong black tea: Effects of baking. Int. J. Food Prop. 2017, 20, 2724–2735. [Google Scholar] [CrossRef]
- Daenen, L.; Sterckx, F.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G. Evaluation of the Glycoside Hy-drolase Activity of a Brettanomyces Strain on Glycosides from Sour Cherry (Prunus cerasus L.) Used in the Production of Special Fruit Beers. FEMS Yeast Res. 2008, 8, 1103–1114. [Google Scholar] [CrossRef]
- Daenen, L.; Sterckx, F.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G. Tea Aroma Formation from Six Model Manufacturing Processes. Food Chem. 2019, 285, 347–354. [Google Scholar]
- Xu, M.; Shao, S.; Chen, J.; Tian, H.; Xie, W.; Chen, X.; Ye, N.; Gao, S. Analysis of Volatile and Key Aroma Components in Congou Black Tea Varieties. Mod. Food Sci. Technol. 2023, 39, 281–290. [Google Scholar]
- Zheng, X.-Q.; Li, Q.-S.; Xiang, L.-P.; Liang, Y.-R. Recent Advances in Volatiles of Teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.-N.; Gao, Y.; Du, X.; Bian, J.-L.; Li, H.; Zhang, X.; Wang, C.-M.; Li, S.-Y. Characterization of the effect of cis-3-hexen-1-ol on green tea aroma. Sci. Rep. 2020, 10, 15506. [Google Scholar] [CrossRef]
- Yuan, G.; Ren, J.; Ouyang, X.; Wang, L.; Wang, M.; Shen, X.; Zhang, B.; Zhu, B. Effect of Raw Material, Pressing and Glycosidase on the Volatile Compound Composition of Wine Made from Goji Berries. Molecules 2016, 21, 1324. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, G.; Lin, S.; Liu, Z.; Wang, P.; Li, J.; Zhang, Q.; He, H. Digital Evaluation of Aroma Intensity and Odor Characteristics of Tea with Different Types-Based on Oav-Splitting Method. Foods 2022, 11, 2204. [Google Scholar] [CrossRef]
- Deng, W.; Wang, R.; Yang, T.; Jiang, L.; Zhang, Z. Functional Characterization of Salicylic Acid Carboxyl Methyltransferase from Camellia Sinensis, Providing the Aroma Compound of Methyl Salicylate During Withering Process of White Tea. J. Agric. Food Chem. 2017, 50, 11036–11045. [Google Scholar] [CrossRef]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
- Joshi, R.; Gulati, A. Fractionation and Identification of Minor and Aroma-Active Constituents in Kangra Orthodox Black Tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef]



| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A/Tea samples (g) | 1.5 | 2.5 | 3.5 |
| B/Adsorption time (min) | 20 | 30 | 40 |
| C/Adsorption temperature (°C) | 60 | 70 | 80 |
| Test Number | Factor | Total Peak Area (Y1) | Number of Aroma Compounds (Y2) | ||
|---|---|---|---|---|---|
| Tea Samples | Adsorption Time | Adsorption Temperature | |||
| (A)/g | (B)/min | (C)°C | |||
| 1 | 1.5 | 20 | 70 | 80.98 | 80 |
| 2 | 3.5 | 20 | 70 | 98.09 | 86 |
| 3 | 1.5 | 40 | 70 | 99.67 | 84 |
| 4 | 3.5 | 40 | 70 | 99.97 | 92 |
| 5 | 1.5 | 30 | 60 | 80.65 | 82 |
| 6 | 3.5 | 30 | 60 | 86.9 | 84 |
| 7 | 1.5 | 30 | 80 | 86.5 | 84 |
| 8 | 3.5 | 30 | 80 | 102.18 | 92 |
| 9 | 2.5 | 20 | 60 | 86.48 | 85 |
| 10 | 2.5 | 40 | 60 | 100.79 | 85 |
| 11 | 2.5 | 20 | 80 | 90.75 | 87 |
| 12 | 2.5 | 40 | 80 | 100.58 | 93 |
| 13 | 2.5 | 30 | 70 | 100.97 | 88 |
| 14 | 2.5 | 30 | 70 | 98.08 | 90 |
| 15 | 2.5 | 30 | 70 | 102.34 | 95 |
| 16 | 2.5 | 30 | 70 | 102.34 | 93 |
| 17 | 2.5 | 30 | 70 | 106.9 | 93 |
| Source | Degrees of Freedom | Sum of Squares | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 1011.65 | 9 | 112.41 | 9.7 | 0.0034 | ** |
| A | 193.45 | 1 | 193.45 | 16.69 | 0.0047 | ** |
| B | 249.87 | 1 | 249.87 | 21.55 | 0.0024 | ** |
| C | 79.32 | 1 | 79.32 | 6.84 | 0.0346 | * |
| AB | 70.64 | 1 | 70.64 | 6.09 | 0.0429 | * |
| AC | 22.23 | 1 | 22.23 | 1.92 | 0.2087 | |
| BC | 5.02 | 1 | 5.02 | 0.43 | 0.5317 | |
| A2 | 179.02 | 1 | 179.02 | 15.44 | 0.0057 | ** |
| B2 | 3.63 | 1 | 3.63 | 0.31 | 0.5934 | |
| C2 | 180.53 | 1 | 180.53 | 4.24 | 0.0056 | ** |
| Residual | 81.16 | 7 | 11.59 | |||
| Lack of fit | 40.57 | 3 | 13.52 | 1.33 | 0.3814 | |
| Pure error | 40.59 | 4 | 10.15 | |||
| Total dispersion | 1092.81 | 16 | ||||
| R2 | 0.925 | R2Adj | 0.8302 |
| Source | Degrees of Freedom | Sum of Squares | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 295.67 | 9 | 32.85 | 6.61 | 0.0105 | * |
| A | 72 | 1 | 72 | 14.48 | 0.0067 | ** |
| B | 32 | 1 | 32 | 6.44 | 0.0388 | * |
| C | 50 | 1 | 50 | 10.06 | 0.0157 | * |
| AB | 1 | 1 | 1 | 0.2 | 0.6673 | |
| AC | 9 | 1 | 9 | 1.81 | 0.2204 | |
| BC | 9 | 1 | 9 | 1.81 | 0.2204 | |
| A2 | 72.52 | 1 | 72.52 | 14.59 | 0.0065 | ** |
| B2 | 19.46 | 1 | 19.46 | 3.92 | 0.0884 | |
| C2 | 19.46 | 1 | 19.46 | 3.92 | 0.0884 | |
| Residual | 34.8 | 7 | 4.97 | |||
| Lack of fit | 4 | 3 | 1.33 | 0.17 | 0.9093 | |
| Pure error | 30.8 | 4 | 7.7 | |||
| Total dispersion | 330.47 | 16 | ||||
| R2 | 0.8947 | R2Adj | 0.7593 |
| No | Time | CAS | Compound | Aroma Substance Content (10 μg/kg) | VIP | ||
|---|---|---|---|---|---|---|---|
| FF | YF | DF | |||||
| 1 | 2.55 | 590-86-3 | Butanal, 3-methyl- | 77.18 ± 35.75 b | 84.03 ± 36.6 ab | 45.31 ± 17.29 a | 1.18 |
| 2 | 2.63 | 96-17-3 | Butanal, 2-methyl- | 103.24 ± 54.57 b | 87.38 ± 37.43 ab | 47.69 ± 11.62 a | 1.03 |
| 3 | 2.85 | 616-25-1 | 1-Penten-3-ol | 39.34 ± 11.44 b | 20.9 ± 14.83 a | 13.33 ± 4.47 a | 1.31 |
| 4 | 2.93 | 142-82-5 | Heptane | 1.76 ± 2.95 a | 6.39 ± 6.26 a | 4.73 ± 2.84 a | 0.81 |
| 5 | 3 | 110-62-3 | Pentanal | 24.99 ± 15.61 a | 17.15 ± 12.25 a | 24.42 ± 19.92 a | 0.61 |
| 6 | 3.25 | 109-60-4 | Propyl acetate | 0.71 ± 1.11 a | 0.03 ± 0.08 a | 2.75 ± 7.42 a | 1.06 |
| 7 | 3.72 | 624-92-0 | Dimethyl disulfide | 0.92 ± 0.85 a | 1.23 ± 0.57 a | 3.05 ± 4.66 a | 1.07 |
| 8 | 3.95 | 105-46-4 | sec-Butyl acetate | 13.84 ± 11.01 a | 6.69 ± 8.48 a | 9.04 ± 7.04 a | 0.64 |
| 9 | 4.11 | 108-88-3 | Toluene | 20.21 ± 13.75 b | 8.05 ± 6.65 a | 6.90 ± 3.95 a | 1 |
| 10 | 4.26 | 71-41-0 | 1-Pentanol | 13.67 ± 4.73 b | 4.56 ± 3.44 a | 4.57 ± 1.91 a | 1.39 |
| 11 | 4.38 | 1576-96-1 | (Z)-2-Penten-1-ol | 25.44 ± 13.84 b | 8.80 ± 6.71 a | 9.16 ± 5.81 a | 1.04 |
| 12 | 4.86 | 66-25-1 | Hexanal | 123.74 ± 80.19 b | 53.1 ± 31.17 a | 51.42 ± 24.88 a | 1.15 |
| 13 | 5.28 | 123-86-4 | Acetic acid, butyl ester | 12.55 ± 11.62 a | 14.43 ± 28.63 a | 1.88 ± 1.44 a | 0.99 |
| 14 | 5.6 | 7580-85-0 | 2-Tert-Butoxyethanol | 2.61 ± 1.10 a | 2.09 ± 1.10 a | 2.65 ± 4.20 a | 0.93 |
| 15 | 6.05 | 98-01-1 | Furfural | 2.15 ± 2.38 a | 1.36 ± 1.35 a | 0.97 ± 0.92 a | 0.48 |
| 16 | 6.45 | 928-96-1 | (Z)-3-Hexen-1-ol | 82.91 ± 97.73 a | 70.31 ± 51.05 a | 41.7 ± 23.43 a | 0.4 |
| 17 | 6.71 | 95-47-6 | o-Xylene | 45.47 ± 18.83 a | 55.17 ± 65.59 a | 32.79 ± 18.10 a | 0.92 |
| 18 | 6.78 | 106-42-3 | p-Xylene | 23.92 ± 8.92 b | 19.35 ± 17.84 ab | 9.13 ± 5.42 a | 1.05 |
| 19 | 6.83 | 928-95-0 | (E)-2-Hexen-1-ol | 10.26 ± 10.88 a | 4.55 ± 4.53 a | 2.82 ± 1.87 a | 0.84 |
| 20 | 6.91 | 111-27-3 | 1-Hexanol | 34.55 ± 16.29 b | 14.14 ± 6.72 a | 10.86 ± 3.71 a | 1.29 |
| 21 | 7.32 | 104-76-7 | 1-Hexanol, 2-ethyl- | 190.48 ± 125.07 ab | 295.21 ± 153.66 b | 57.42 ± 49.37 a | 1.36 |
| 22 | 7.33 | 143-08-8 | 1-Nonanol | 2.69 ± 1.31 b | 0.09 ± 0.19 a | 1.63 ± 2.91 ab | 1 |
| 23 | 7.6 | 108-94-1 | Cyclohexanone | 17.27 ± 6.79 b | 15.62 ± 10.9 b | 4.97 ± 3.30 a | 1.15 |
| 24 | 7.86 | 111-71-7 | Heptanal | 18.52 ± 6.30 a | 12.21 ± 12.31 a | 22.09 ± 26.31 a | 1.06 |
| 25 | 8.61 | 2437-95-8 | alpha-Pinene | 3.26 ± 2.02 a | 2.23 ± 1.02 a | 3.41 ± 2.45 a | 0.91 |
| 26 | 10.07 | 127-91-3 | beta-Pinene | 5.41 ± 5.40 a | 2.49 ± 3.14 a | 3.09 ± 3.73 a | 1.00 |
| 27 | 10.39 | 13475-82-6 | Heptane, 2,2,4,6,6-pentamethyl- | 40.15 ± 18.69 a | 30.96 ± 15.88 a | 20.65 ± 19.18 a | 0.96 |
| 28 | 10.62 | 3777-69-3 | Furan, 2-pentyl- | 14.29 ± 5.22 b | 7.29 ± 4.89 a | 3.78 ± 4.60 a | 1.29 |
| 29 | 10.73 | 108-67-8 | Mesitylene | 4.92 ± 2.7 b | 2.68 ± 1.36 a | 0.66 ± 0.99 a | 1.26 |
| 30 | 10.85 | 1120-21-4 | Undecane | 5.78 ± 2.19 a | 3.93 ± 1.92 a | 3.70 ± 3.70 a | 0.89 |
| 31 | 11.13 | 124-13-0 | Octanal | 17.95 ± 15.67 a | 10.06 ± 6.08 a | 9.00 ± 4.47 a | 1.02 |
| 32 | 11.51 | 142-62-1 | Hexanoic acid | 7.59 ± 10.36 a | 13.73 ± 8.01 a | 5.87 ± 6.46 a | 0.64 |
| 33 | 11.64 | 62183-79-3 | 2,2,4,4-Tetramethyloctane | 5.23 ± 2.37 a | 3.66 ± 1.32 a | 3.43 ± 1.63 a | 0.97 |
| 34 | 11.88 | 5989-27-5 | D-Limonene | 29.06 ± 51.06 a | 14.01 ± 15.64 a | 29.33 ± 52.02 a | 0.41 |
| 35 | 12.5 | 100-51-6 | Benzyl alcohol | 108.98 ± 75.17 a | 133.16 ± 98.66 a | 49.37 ± 33.99 a | 0.85 |
| 36 | 12.69 | 2167-14-8 | 1-Ethylpyrrole-2-carbaldehyde | 30.97 ± 25.21 a | 62.23 ± 31.96 a | 32.13 ± 22.57 a | 1.06 |
| 37 | 12.78 | 62185-53-9 | Nonane, 5-(2-methylpropyl)- | 4.91 ± 7.35 a | 3.69 ± 3.33 a | 1.93 ± 3.36 a | 0.24 |
| 38 | 12.94 | 78-59-1 | Isophorone | 122.25 ± 38.81 b | 201.69 ± 87.86 a | 48.20 ± 23.02 a | 1.59 |
| 39 | 13.28 | 5989-33-3 | (Z)-Linalool oxide (furanoid) | 65.54 ± 40.73 a | 29.73 ± 16.38 a | 45.03 ± 40.41 a | 0.84 |
| 40 | 13.51 | 111-87-5 | 1-Octanol | 7.43 ± 7.86 a | 7.76 ± 9.63 a | 2.10 ± 2.10 a | 0.73 |
| 41 | 13.56 | 471-01-2 | 3-Cyclohexen-1-one, 3,5,5-trimethyl- | 9.47 ± 4.38 a | 9.61 ± 8.11 a | 9.20 ± 4.96 a | 0.75 |
| 42 | 13.82 | 34995-77-2 | (E)-Linalool oxide (furanoid) | 155.8 ± 64.67 b | 86.82 ± 50.34 a | 60.56 ± 35.55 a | 1.15 |
| 43 | 14.28 | 78-70-6 | Linalool | 130.1 ± 73.42 b | 53.94 ± 29.87 a | 48.03 ± 24.44 a | 1.09 |
| 44 | 14.41 | 124-19-6 | Nonanal | 59.29 ± 22.65 a | 49.85 ± 14.86 a | 47.23 ± 24.92 a | 0.91 |
| 45 | 14.58 | 5337-72-4 | Cyclohexanol, 2,6-dimethyl- | 39.08 ± 23.49 a | 35.09 ± 18.39 a | 36.53 ± 17.13 a | 0.9 |
| 46 | 14.71 | 99-87-6 | p-Cymene | 3.52 ± 3.21 a | 1.72 ± 1.31 a | 1.1 ± 0.98 a | 0.96 |
| 47 | 14.87 | 1960/12/8 | Phenylethyl Alcohol | 63.13 ± 31.9 b | 55.44 ± 27.21 ab | 30.66 ± 12.51 a | 0.89 |
| 48 | 15.76 | 464-49-3 | (+)-2-Bornanone | 52.32 ± 55.15 a | 77.60 ± 69.51 a | 29.13 ± 24.17 a | 0.79 |
| 49 | 16.48 | 112-54-9 | Dodecanal | 3.90 ± 3.71 a | 2.65 ± 1.91 a | 6.89 ± 4.94 a | 1.15 |
| 50 | 16.54 | 39028-58-5 | (E)-Linalool oxide (pyranoid) | 9.94 ± 9.06 ab | 28.36 ± 29.04 b | 7.27 ± 4.31 a | 1.06 |
| 51 | 16.71 | 39028-58-5 | Linalool oxide | 29.27 ± 21.08 b | 2.88 ± 8.63 a | 6.71 ± 6.98 a | 1.1 |
| 52 | 16.76 | 15356-70-4 | Menthol | 6.97 ± 13.83 a | 26.6 ± 29.22 a | 13.01 ± 15.25 a | 0.7 |
| 53 | 16.92 | 53398-84-8 | Butanoic acid, 3-hexenyl ester, (E)- | 7.45 ± 4.77 b | 1.35 ± 2.26 a | 0.77 ± 1.67 a | 1.17 |
| 54 | 17.02 | 275-51-4 | Azulene | 10.92 ± 5.54 b | 10.69 ± 3.65 b | 1.63 ± 3.84 a | 1.27 |
| 55 | 17.24 | 12-40-3 | Dodecane | 13.19 ± 6.90 a | 16.67 ± 5.88 a | 21.58 ± 9.14 a | 0.94 |
| 56 | 17.29 | 119-36-8 | Methyl salicylate | 87.8 ± 51.2 b | 38.01 ± 18 a | 37.91 ± 39.09 a | 1.05 |
| 57 | 17.44 | 116-26-7 | Safranal | 10.60 ± 7.48 a | 9.20 ± 4.27 a | 10.99 ± 5.50 a | 0.77 |
| 58 | 17.57 | 112-31-2 | Decanal | 36.46 ± 25.77 a | 29.67 ± 15.70 a | 55.78 ± 36.06 a | 1.13 |
| 59 | 18.03 | 432-25-7 | beta-Cyclocitral | 19.89 ± 9.46 a | 16.67 ± 7.12 a | 15.08 ± 8.58 a | 0.9 |
| 60 | 19.04 | 106-24-1 | Geraniol | 294.94 ± 269.96 a | 260.02 ± 117.84 a | 194.93 ± 135.83 a | 0.58 |
| 61 | 22.65 | 4506-36-9 | 1,5,8-Trimethyl-1,2-dihydronaphthalene | 0.59 ± 1.77 a | 2.80 ± 2.37 ab | 5.04 ± 1.87 b | 1.15 |
| 62 | 23.52 | 17699-14-8 | alpha-Cubebene | 0.50 ± 0.54 a | 0.62 ± 0.87 a | 0.80 ± 0.79 a | 0.12 |
| 63 | 24.79 | 475-20-7 | Longifolene | 5.20 ± 2.56 a | 9.00 ± 4.12 b | 1.93 ± 1.35 a | 1.56 |
| 64 | 24.96 | 469-61-4 | Cedrene | 21.03 ± 10.23 a | 47.08 ± 20.71 b | 30.8 ± 13.45 ab | 1.36 |
| 65 | 25.03 | 87-44-5 | Caryophyllene | 6.80 ± 5.47 a | 6.04 ± 2.12 a | 8.46 ± 5.30 a | 0.83 |
| 66 | 25.26 | 79120-98-2 | beta-Cedrene | 5.64 ± 8.51 a | 15.52 ± 5.95 b | 14.44 ± 4.13 b | 1.11 |
| 67 | 25.34 | 470-40-6 | cis-Thujopsene | 1.17 ± 1.52 a | 3.25 ± 1.95 a | 2.38 ± 2.34 a | 1.03 |
| 68 | 26.12 | 14901-07-6 | beta-Ionone | 34.46 ± 19.15 a | 28.14 ± 20.71 a | 36.68 ± 15.08 a | 0.58 |
| 69 | 26.4 | 128-37-0 | Butylated Hydroxytoluene | 5.95 ± 5.26 a | 6.23 ± 4.31 a | 10.99 ± 8.55 a | 0.85 |
| 70 | 26.47 | 16982-00-6 | Cuparene | 0.19 ± 0.58 a | 3.84 ± 2.18 b | 6.86 ± 4.78 b | 1.3 |
| 71 | 26.84 | 1560-78-7 | 2-Methyltetracosane | 1.63 ± 3.09 a | 2.96 ± 2.11 a | 2.51 ± 3.25 a | 0.66 |
| Compound | Aroma Threshold (μg/kg) | OAV | Aroma Description | ||
|---|---|---|---|---|---|
| FF | YF | DF | |||
| 1-Penten-3-ol | 400 | 0.10 ± 0.03 b | 0.05 ± 0.04 a | 0.03 ± 0.01 a | fruity, green |
| Benzyl alcohol | 2.54 | 42.91 ± 29.59 a | 52.43 ± 38.84 a | 19.44 ± 13.38 a | sweet, floral |
| Phenylethyl Alcohol | 390 | 0.16 ± 0.08 b | 0.14 ± 0.07 ab | 0.08 ± 0.03 a | floral |
| Linalool | 3.8 | 34.24 ± 19.32 b | 14.19 ± 7.86 a | 12.64 ± 6.43 a | Lavender |
| Geraniol | 20 | 14.75 ± 13.50 a | 13.00 ± 5.89 a | 9.75 ± 6.79 a | rose scent |
| beta-Cyclocitral | 5 | 3.98 ± 1.89 a | 3.33 ± 1.42 a | 3.02 ± 1.72 a | fruity |
| Pentanal | 12 | 2.08 ± 1.30 a | 1.43 ± 1.02 a | 2.04 ± 1.66 a | Almond, Malt |
| Butanal, 3-methyl- | 9 | 8.58 ± 3.97 b | 9.34 ± 4.07 ab | 5.03 ± 1.92 a | apple like |
| Hexanal | 4.5 | 27.50 ± 17.82 b | 11.80 ± 6.93 a | 11.43 ± 5.53 a | green, herbal |
| Heptanal | 10 | 1.85 ± 0.63 a | 1.22 ± 1.23 a | 2.21 ± 2.63 a | sweet herbal scent |
| Octanal | 0.5 | 35.9 ± 31.34 a | 20.12 ± 12.16 a | 18.00 ± 8.94 a | Sweet orange, honey |
| Nonanal | 1 | 59.29 ± 22.65 a | 49.85 ± 14.86 a | 47.23 ± 24.92 a | rose, citrus scent |
| Decanal | 0.1 | 364.60 ± 257.70 a | 296.70 ± 157.00 a | 557.80 ± 360.60 a | Fruity |
| Methyl salicylate | 40 | 2.20 ± 1.28 b | 0.95 ± 0.45 a | 0.95 ± 0.98 a | Wintergreen like |
| D-Limonene | 10 | 2.91 ± 5.11 a | 1.40 ± 1.56 a | 2.93 ± 5.20 a | Lemony |
| Toluene | 5 | 4.04 ± 2.75 b | 1.61 ± 1.33 a | 1.38 ± 0.79 a | Sweet floral |
| alpha-Pinene | 140 | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.02 ± 0.02 a | Pine oil |
| beta-Ionone | 3.5 | 9.85 ± 5.47 a | 8.04 ± 5.92 a | 10.48 ± 4.31 a | floral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Yan, T.; Yang, J.; Xu, H. Aroma Components Analysis and Origin Differentiation of Black Tea Based on ATD-GC-MS and E-Nose. Horticulturae 2023, 9, 885. https://doi.org/10.3390/horticulturae9080885
Huang J, Yan T, Yang J, Xu H. Aroma Components Analysis and Origin Differentiation of Black Tea Based on ATD-GC-MS and E-Nose. Horticulturae. 2023; 9(8):885. https://doi.org/10.3390/horticulturae9080885
Chicago/Turabian StyleHuang, Jianfeng, Tingyu Yan, Jiangfan Yang, and Hui Xu. 2023. "Aroma Components Analysis and Origin Differentiation of Black Tea Based on ATD-GC-MS and E-Nose" Horticulturae 9, no. 8: 885. https://doi.org/10.3390/horticulturae9080885
APA StyleHuang, J., Yan, T., Yang, J., & Xu, H. (2023). Aroma Components Analysis and Origin Differentiation of Black Tea Based on ATD-GC-MS and E-Nose. Horticulturae, 9(8), 885. https://doi.org/10.3390/horticulturae9080885






