Moringa oleifera Lam. Seed Extracts Improve the Growth, Essential Minerals, and Phytochemical Constituents of Lessertia frutescens L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Materials
2.3. Moringa Seed Extract (MSE) Preparation and Treatments
2.4. Experimental Design and Plant Management
2.5. Plant Growth and Biomass Yield Measurement
2.6. Chlorophyll Content
2.7. Inorganic Nutrient Contents
2.8. Ascorbic Acid
2.9. Total Phenolic Content
2.10. Total Flavonoid Content
2.11. Statistical Analysis
3. Results and Discussion
3.1. Plant Growth Attributes
3.2. Chlorophyll Content
3.3. Inorganic Nutrient Contents
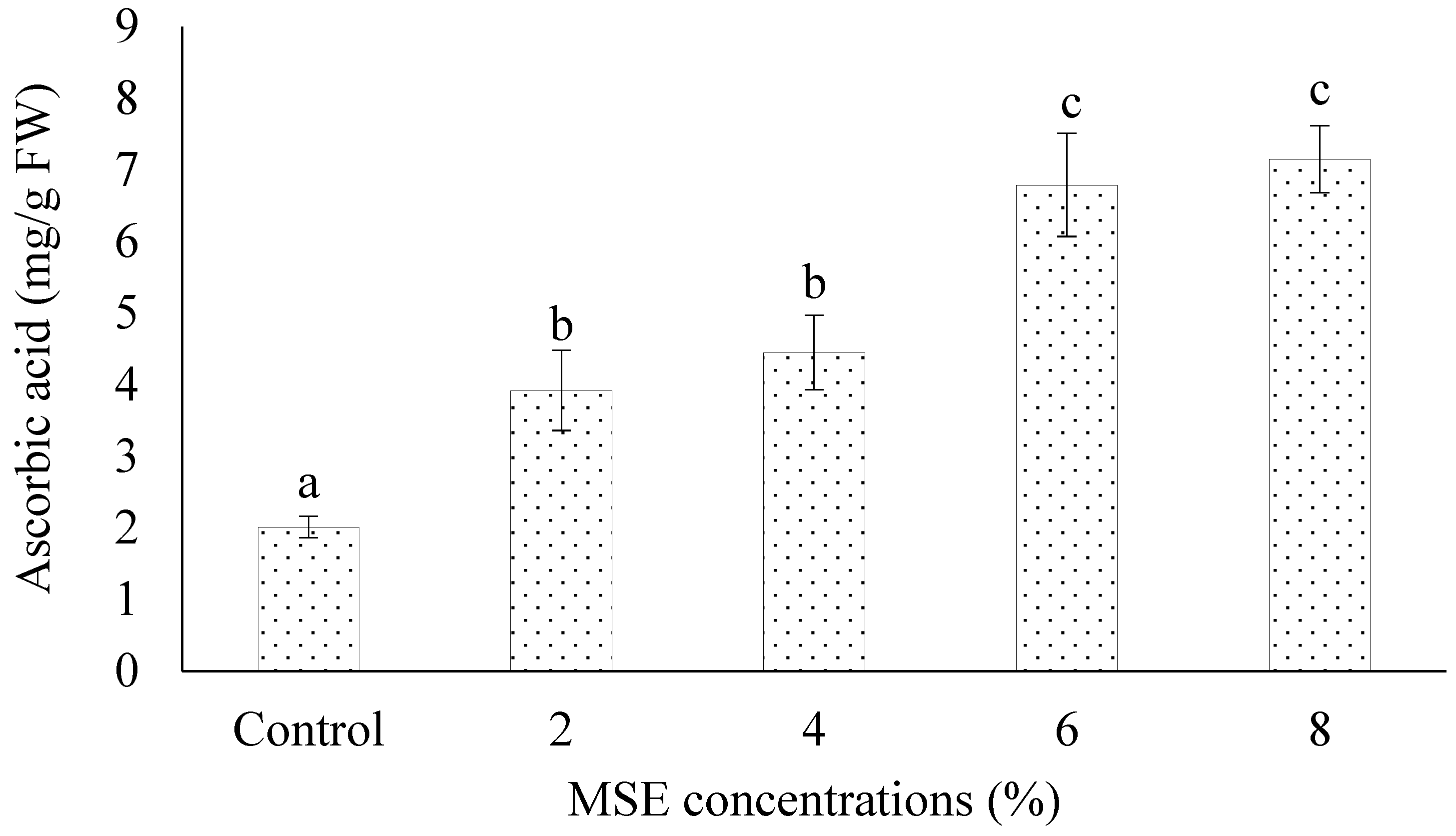
3.4. Ascorbic Acid
3.5. Total Phenolic Content
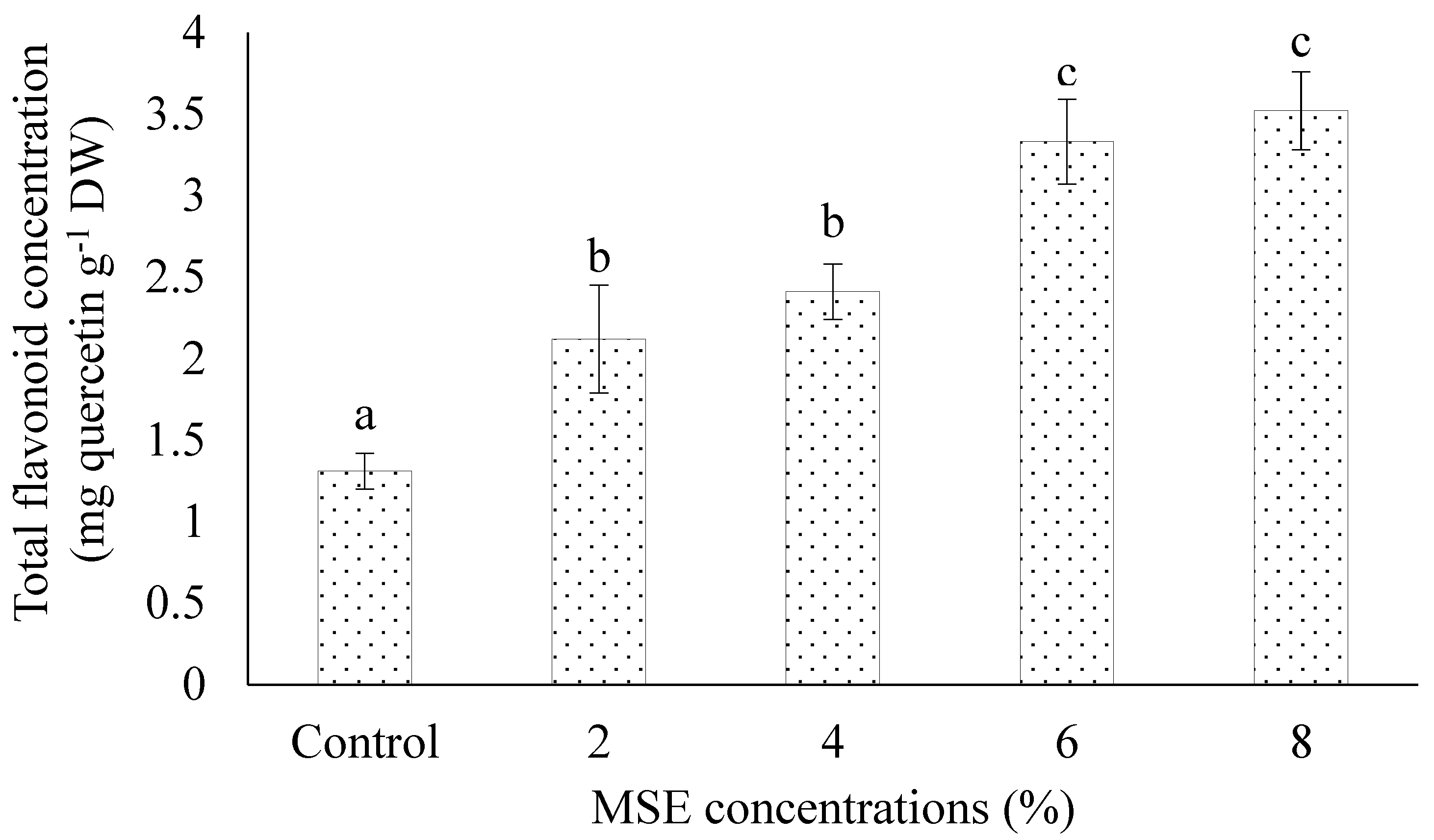
3.6. Total Flavonoid Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gouws, C.; Smit, T.; Willers, C.; Svitina, H.; Calitz, C.; Wrzesinski, K. Anticancer Potential of Sutherlandia frutescens and Xysmalobium undulatum in LS180 Colorectal Cancer Mini-Tumors. Molecules 2021, 26, 605. [Google Scholar] [CrossRef]
- Mncwangi, N.P.; Viljoen, A.M. Quantitative variation of amino acids in Sutherlandia frutescens (Cancer bush)—Towards setting parameters for quality control. S. Afr. J. Bot. 2012, 82, 46–52. [Google Scholar] [CrossRef]
- Zonyane, S.; Fawole, O.A.; La Grange, C.; Stander, M.A.; Opara, U.L.; Makunga, N.P. The implication of chemotypic variation on the anti-oxidant and anti-cancer activities of sutherlandia frutescens (L.) R.Br. (Fabaceae) from different geographic locations. Antioxidants 2020, 9, 152. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Chouchane, H.; Krol, M.S.; Hoekstra, A.Y. Expected increase in staple crop imports in water-scarce countries in 2050. Water Res. 2018, 1, 100001. [Google Scholar] [CrossRef]
- Vasa, T.N.; Chacko, S.P. Recovery of struvite from wastewaters as an eco-friendly fertilizer: Review of the art and perspective for a sustainable agriculture practice in India. Sustain. Energy Technol. Assess. 2021, 48, 101573. [Google Scholar] [CrossRef]
- Muurinen, J.; Stedtfeld, R.; Karkman, A.; Parnanen, K.; Tiedje, J.; Virta, M. Influence of manure application on the environmental resistome under Finnish agricultural practice with restricted antibiotic use. Environ. Sci. Technol. 2017, 51, 5989–5999. [Google Scholar] [CrossRef]
- Xie, W.Y.; Yuan, S.T.; Xu, M.G.; Yang, X.P.; Shen, Q.R.; Zhang, W.W.; Su, J.Q.; Zhao, F.J. Long-term effects of manure and chemical fertilizers on soil antibiotic resistome. Soil Biol. Biochem. 2018, 122, 111–119. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.M.A.; Nawaz, M.; Hussain, I.; Foidl, N. Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.). S. Afr. J. Bot. 2020, 129, 74–81. [Google Scholar] [CrossRef]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- Abdalla, M.M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int. J. Plant Physiol. Biochem. 2023, 5, 42–49. [Google Scholar]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Xuan, T.D. Enhancing growth, yield, biochemical, and hormonal contents of snap bean (Phaseolus vulgaris L.) sprayed with moringa leaf extract. Arch. Agron. Soil Sci. 2017, 63, 687–699. [Google Scholar] [CrossRef]
- Mazrou, R.M. Moringa leaf extract application as a natural biostimulant improves the volatile oil content, radical scavenging activity and total phenolics of coriander. J. Med. Plants 2019, 7, 45–51. [Google Scholar]
- Buthelezi, N.M.D.; Gololo, S.S.; Mugivhisa, L.L. An assessment of moringa (Moringa oleifera L.) seed extract on crop water productivity and physico-biochemical properties of cancer bush (Sutherlandia frutescens L.) under deficit irrigation. Horticulturae 2022, 8, 938. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physiobiochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Fu, S.F.; Sun, P.F.; Lu, H.Y.; Wei, J.Y.; Xiao, H.S.; Fang, W.T.; Cheng, B.Y.; Chou, J.Y. Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata Lab. Fungal Biol. 2016, 120, 433–448. [Google Scholar] [CrossRef]
- Terceros, G.C.; Resentini, F.; Cucinotta, M.; Manrique, S.; Colombo, L.; Mendes, M.A. The importance of cytokinins during reproductive development in Arabidopsis and beyond. Int. J. Mol. Sci. 2020, 21, 8161. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Ucar, E.; Ozyigit, Y.; Demirbas, A.; Yasin Guven, D.; Turgut, K. Effect of different nitrogen doses on dry matter ratio, chlorophyll and macro/micro nutrient content in sweet herb (Stevia rebaudiana Bertoni). Commun. Soil Sci. Plant Anal. 2017, 48, 1231–1239. [Google Scholar] [CrossRef]
- Black, C.A.; Evans, D.D.; Ensminger, L.E. Methods of soil analysis. Am. Soc. Agron. 1965, 15, 122–130. [Google Scholar]
- Murphy, L.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Anjum, M.A.; Akram, H.; Zaidi, M.; Ali, S. Effect of gum arabic and Aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’guava fruits. Sci. Hortic. 2020, 271, 109506. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.S.A.; Abdel-Kader, A.A.S. Response of Fennel (Foeniculum vulgare, Mill) plants to foliar application of moringa leaf extract and benzyladenine (BA). S. Afri. J. Bot. 2020, 129, 113–122. [Google Scholar] [CrossRef]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Rashid, N.; Khan, S.; Wahid, A.; Ibrar, D.; Irshad, S.; Bakhsh, A.; Hasnain, Z.; Alkahtani, J.; Alwahibi, M.S.; Gawwad, M.R.A.; et al. Exogenous application of moringa leaf extract improves growth, biochemical attributes, and productivity of late-sown quinoa. PLoS ONE 2021, 16, e0259214. [Google Scholar] [CrossRef]
- Gholamin, R.; Khayatnezhad, M. Assessment of the correlation between chlorophyll content and drought resistance in corn cultivars (Zea Mays). Helix Sci. Explor. Peer Rev. Bimon. Int. J. 2020, 10, 93–97. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cozzolino, E.; Ottaiano, L.; Petropoulos, S.A.; Nocerino, S.; Pelosi, M.E.; Rouphael, Y.; Mori, M.; Di Mola, I. Effect of biostimulant application on plant growth, chlorophylls and hydrophilic antioxidant activity of spinach (Spinacia oleracea L.) grown under saline stress. Horticulturae 2022, 8, 971. [Google Scholar] [CrossRef]
- Khan, S.; Basit, A.; Hafeez, M.B.; Irshad, S.; Bashir, S.; Bashir, S.; Maqbool, M.M.; Saddiq, M.S.; Hasnain, Z.; Aljuaid, B.S.; et al. Moringa leaf extract improves biochemical attributes, yield and grain quality of rice (Oryza sativa L.) under drought stress. PLoS ONE 2021, 16, e0254452. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, A.A.; Takacs-Hajos, M. Evaluation of moringa (Moringa oleifera Lam.) leaf extract on bioactive compounds of lettuce (Lactuca sativa L.) grown under glasshouse environment. J. King Saud Univ. Sci. 2022, 34, 101916. [Google Scholar] [CrossRef]
- Hoque, T.S.; Jahan, I.; Ferdous, G.; Abedin, M.A. Foliar application of moringa leaf extract as a biostimulant on growth, yield and nutritional quality of brinjal. J. Agric. Food Environ. 2020, 1, 94–99. [Google Scholar] [CrossRef]
- Sardar, H.; Nisar, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Ali, S.; Javed, M.S.; Ahmad, R. Foliar spray of moringa leaf extract improves growth and concentration of pigment, minerals and stevioside in stevia (Stevia rebaudiana Bertoni). Ind. Crops Prod. 2021, 166, 113485. [Google Scholar] [CrossRef]
- Nasir, M.; Khan, A.S.; Basra, S.M.A.; Malik, A.U. Improvement in growth, productivity and quality of ‘Kinnow’mandarin fruit after exogenous application of Moringa olifera leaf extract. S. Afr. J. Bot. 2020, 129, 263–271. [Google Scholar] [CrossRef]
- Mashamaite, C.V.; Ngcobo, B.L.; Manyevere, A.; Bertling, I.; Fawole, O.A. Assessing the usefulness of Moringa oleifera leaf extract as a biostimulant to supplement synthetic fertilizers: A Review. Plants 2022, 11, 2214. [Google Scholar] [CrossRef]
- Hassan, A.; Amjad, S.F.; Saleem, M.H.; Yasmin, H.; Imran, M.; Riaz, M.; Ali, Q.; Joyia, F.A.; Ahmed, S.; Ali, S.; et al. Foliar application of ascorbic acid enhances salinity stress tolerance in barley (Hordeum vulgare L.) through modulation of morpho-physio-biochemical attributes, ions uptake, osmo-protectants and stress response genes expression. Saudi J. Biol. Sci. 2021, 28, 4276–4290. [Google Scholar] [CrossRef]

| Component | Unit | Value |
|---|---|---|
| Protein | g 100 g−1 | 30.44 ± 1.11 |
| Total free amino acids | 28.11 ± 0.56 | |
| Free proline | 0.25 ± 0.03 | |
| Soluble sugars | 10.11 ± 0.37 | |
| Vitamin B1 | mg 100 g−1 | 0.12 ± 0.04 |
| Vitamin B2 | 0.04 ± 0.01 | |
| Vitamin B3 | 0.12 ± 0.04 | |
| Vitamin C | 2.92 ± 0.16 | |
| Vitamin E | 490.35 ± 5.47 | |
| Calcium (Ca) | 35.08 ± 0.13 | |
| Magnesium (Mg) | 509.12 ± 0.64 | |
| Phosphorus (P) | 59.96 ± 0.27 | |
| Copper (Cu) | 4.19 ± 0.59 | |
| Sulphur (S) | 0.02 ± 0.00 | |
| DPPH (antioxidant activity) Cytokinins (CKs) | % µg g−1 | 70.28 ± 0.66 0.94 ± 0.04 |
| Gibberellins (GAs) Indol acetic acid (IAA) | 0.85 ± 0.01 0.72 ± 0.11 |
| MSE (%) | Plant Height (cm) | Stem Diameter (mm) | Shoot Dry Weight (g) | Root Dry Weight (g) | Biomass Yield/Plant (g) | Chl Content (SPAD Value) Seedling Stage | Chl Content (SPAD Value) Vegetative Stage | Chl Content (SPAD Value) Reproductive Stage |
|---|---|---|---|---|---|---|---|---|
| Control | 38.95 ± 0.74 a | 20.03 ± 0.73 a | 14.98 ± 0.39 a | 3.74 ± 0.15 a | 592.42 ± 39.21 a | 37.74 ± 2.98 a | 36.16 ± 0.48 a | 37.10 ± 0.61 a |
| 2 | 50.22 ± 0.57 b | 20.05 ± 1.09 b | 20.40 ± 0.56 bc | 5.10 ± 0.59 ab | 720.27 ± 23.54 b | 40.18 ± 1.07 ab | 38.95 ± 0.94 b | 39.40 ± 0.48 b |
| 4 | 51.22 ± 0.46 b | 25.05 ± 0.54 b | 19.99 ± 0.62 b | 5.71 ± 0.81 b | 738.71 ± 39.04 b | 41.08 ± 0.69 abc | 40.05 ± 0.90 bc | 40.03 ± 0.70 b |
| 6 | 60.22 ± 0.67 c | 30.01 ± 0.74 c | 21.40 ± 0.26 bc | 6.43 ± 0.87 b | 789.16 ± 11.42 b | 44.96 ± 2.06 bc | 42.45 ± 0.91 cd | 42.13 ± 0.59 c |
| 8 | 61.03 ± 1.04 c | 35.37 ± 1.20 d | 21.91 ± 0.15 c | 6.48 ± 0.27 b | 801.08 ± 14.72 b | 45.88 ± 1.75 c | 42.98 ± 0.57 d | 43.93 ± 0.71 d |
| LSD = 1.762 p < 0.001 | LSD = 2.659 p < 0.001 | LSD = 1.541 p < 0.001 | LSD = 1.565 p = 0.019 | LSD = 88.30 p = 0.004 | LSD = 5.522 p = 0.044 | LSD = 2.512 p = 0.001 | LSD = 1.787 p < 0.001 |
| Macronutrients | N | P | K | Mg | Ca |
|---|---|---|---|---|---|
| MSE (%) | (mg/g DW) | ||||
| Control | 33.34 ± 0.46 a | 5.91 ± 0.58 a | 10.81 ± 0.52 a | 4.56 ± 0.62 a | 30.08 ± 0.58 a |
| 2 | 39.44 ± 1.30 b | 6.94 ± 0.57 ab | 15.24 ± 0.61 b | 6.10 ± 0.49 b | 40.26 ± 0.46 b |
| 4 | 44.12 ± 0.99 c | 7.42 ± 0.69 bc | 20.25 ± 1.04 c | 6.68 ± 0.36 bc | 42.95 ± 0.61 c |
| 6 | 48.01 ± 1.06 d | 8.30 ± 0.42 bc | 20.94 ± 0.56 c | 7.12 ± 0.48 cd | 54.99 ± 0.49 d |
| 8 | 48.92 ± 2.05 d | 8.45 ± 1.28 c | 25.54 ± 0.49 d | 7.78 ± 0.29 d | 55.69 ± 0.48 d |
| LSD = 3.706 | LSD = 1.486 | LSD = 1.808 | LSD = 0.978 | LSD = 1.437 | |
| p < 0.001 | p = 0.023 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Micronutrients | Fe | Zn | Cu | Mn | Na |
| MSE | (mg/g DW) | ||||
| Control | 1.19 ± 0.17 a | 1.18 ± 0.05 a | 0.77 ± 0.10 a | 0.92 ± 0.13 a | 3.40 ± 0.30 a |
| 2 | 1.94 ± 0.19 b | 2.01 ± 0.15 b | 2.09 ± 0.21 b | 1.52 ± 0.25 b | 6.44 ± 0.32 b |
| 4 | 2.25 ± 0.41 b | 2.41 ± 0.10 c | 2.20 ± 0.11 b | 2.09 ± 0.06 c | 6.80 ± 0.11 b |
| 6 | 2.52 ± 0.49 bc | 2.73 ± 0.19 c | 2.41 ± 0.12 bc | 2.30 ± 0.14 c | 7.99 ± 0.23 c |
| 8 | 2.94 ± 0.36 c | 2.75 ± 0.20 c | 2.69 ± 0.17 c | 2.39 ± 0.21 c | 8.01 ± 0.64 c |
| LSD = 0.615 | LSD = 0.387 | LSD = 0.473 | LSD = 0.558 | LSD = 1.043 | |
| p = 0.002 | p < 0.001 | p < 0.001 | p = 0.001 | p < 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buthelezi, N.M.D.; Ntuli, N.R.; Mugivhisa, L.L.; Gololo, S.S. Moringa oleifera Lam. Seed Extracts Improve the Growth, Essential Minerals, and Phytochemical Constituents of Lessertia frutescens L. Horticulturae 2023, 9, 886. https://doi.org/10.3390/horticulturae9080886
Buthelezi NMD, Ntuli NR, Mugivhisa LL, Gololo SS. Moringa oleifera Lam. Seed Extracts Improve the Growth, Essential Minerals, and Phytochemical Constituents of Lessertia frutescens L. Horticulturae. 2023; 9(8):886. https://doi.org/10.3390/horticulturae9080886
Chicago/Turabian StyleButhelezi, Nana Millicent Duduzile, Nontuthuko Rosemary Ntuli, Liziwe Lizbeth Mugivhisa, and Sechene Stanley Gololo. 2023. "Moringa oleifera Lam. Seed Extracts Improve the Growth, Essential Minerals, and Phytochemical Constituents of Lessertia frutescens L." Horticulturae 9, no. 8: 886. https://doi.org/10.3390/horticulturae9080886
APA StyleButhelezi, N. M. D., Ntuli, N. R., Mugivhisa, L. L., & Gololo, S. S. (2023). Moringa oleifera Lam. Seed Extracts Improve the Growth, Essential Minerals, and Phytochemical Constituents of Lessertia frutescens L. Horticulturae, 9(8), 886. https://doi.org/10.3390/horticulturae9080886





