Determination of the Permanent Wilting Point of Physalis peruviana L.
Abstract
1. Introduction
2. Materials and Methods
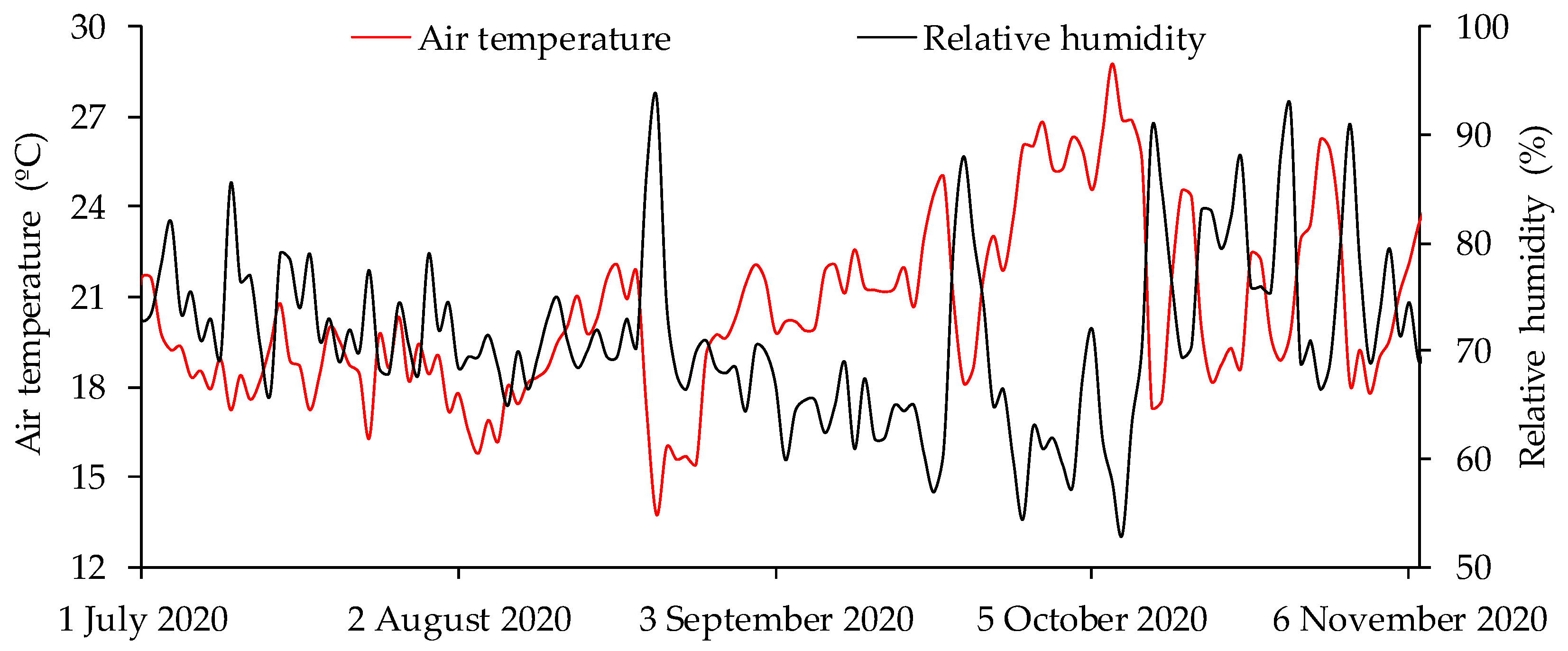
2.1. Characterization of the Experiment and Experimental Design
2.2. Preparation of Pots
2.3. Plant Material
2.4. Soil–Water Availability
2.5. Determination of Permanent Wilting Point
2.6. Statistical Analyses
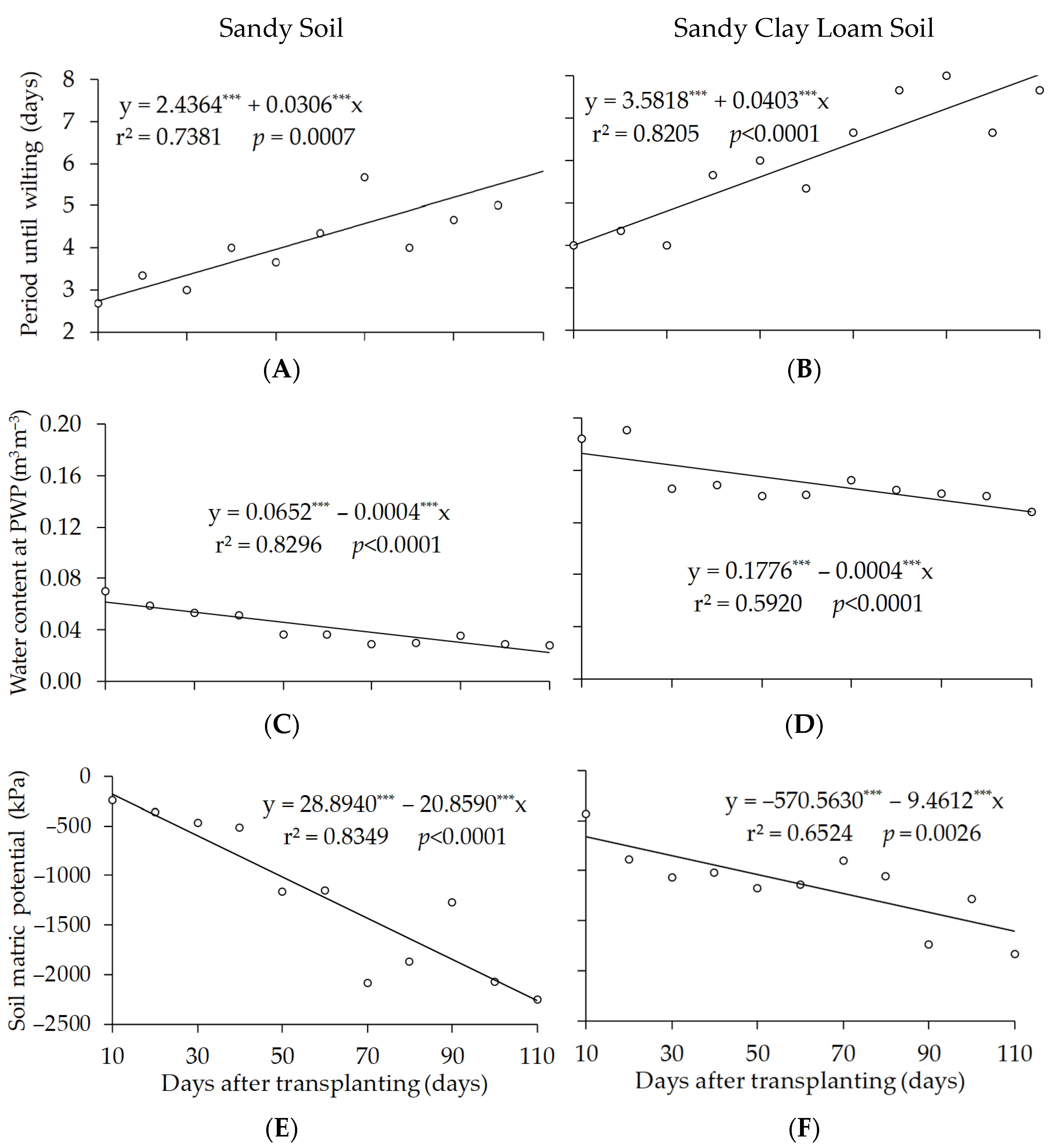
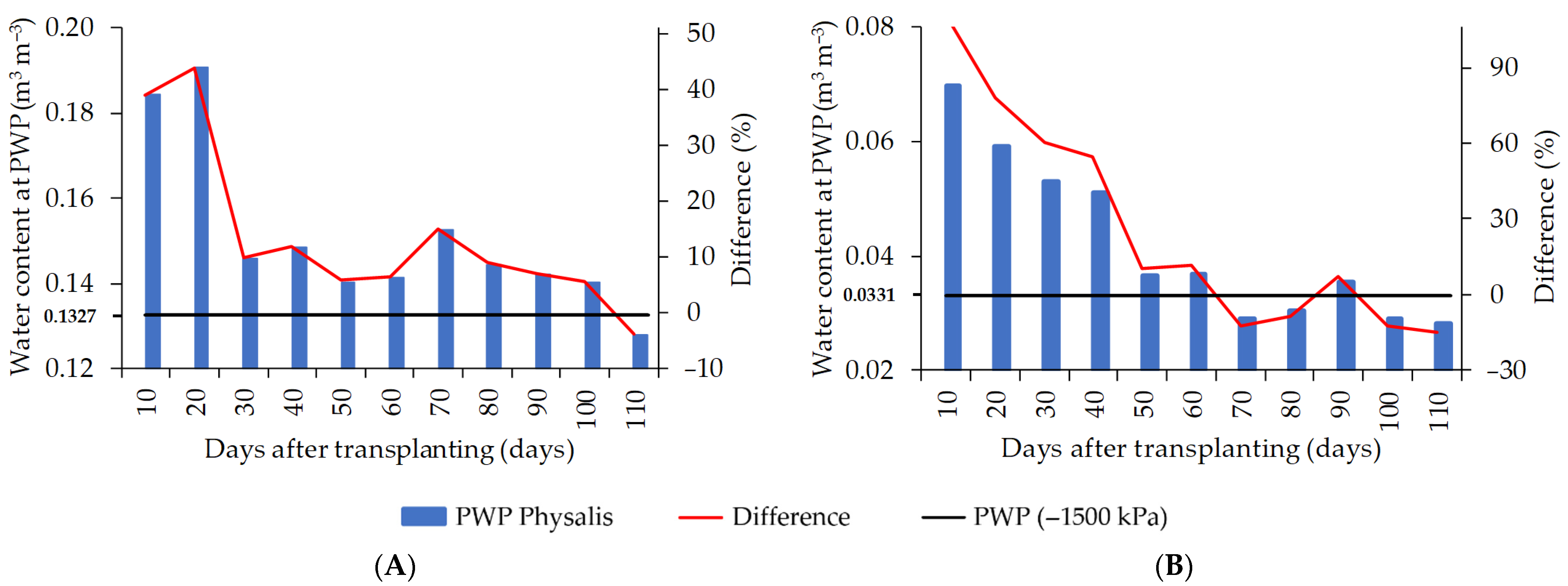
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, A.C.; Silva, G.Z.; Carbonari, M.E.E. Sustentabilidade, Responsabilidade Social e Meio Ambiente, 1st ed.; Editora Saraiva: São Paulo, Brazil, 2017; p. 216. [Google Scholar]
- Kümmerer, K.; Dionysiou, D.D.; Olsson, O.; Kassinos, D.F. Reducing aquatic micropollutants–Increasing the focus on input prevention and integrated emission management. Sci. Total Environ. 2019, 652, 836–850. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Cadore, J.S.; Tochetto, M. Water resources: Overview of the sector and prospects for servicing the 2030 Agenda. Braz. J. Environ. 2021, 9, 122–136. [Google Scholar] [CrossRef]
- Deihimfard, R.; Moghaddam, S.R.; Collins, B.; Azizi, K. Future climate change could reduce irrigated and rainfed wheat water footprint in arid environments. Sci. Total Environ. 2022, 807, 150991. [Google Scholar] [CrossRef]
- Lyu, S.; Wu, L.; Wen, X.; Wang, J.; Chen, W. Effects of reclaimed wastewater irrigation on soil-crop systems in China: A review. Sci. Total Environ. 2021, 813, 152531. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, J.J.; Rezaei, E.E.; Remenyi, T.A.; Webb, M.A.; Webber, H.A.; Kamali, B.; Harris, R.M.B.; Brown, J.N.; Kidd, D.B.; Mohammed, C.L.; et al. Effects of soil-and climate data aggregation on simulated potato yield and irrigation water requirement. Sci. Total Environ. 2020, 710, 135589. [Google Scholar] [CrossRef] [PubMed]
- Tolk, J.A. Soils, permanent wilting points. Water Sci. 2003, 92, 927–929. [Google Scholar]
- Briggs, L.J.; Shantz, H.L. The wilting coefficient and its indirect determination. Bot. Gaz. 1912, 53, 20–37. [Google Scholar] [CrossRef]
- Richards, L.A.; Weaver, L.E. Fifteen-atmosphere percentage as related to the permanent wilting percentage. Soil Sci. 1943, 56, 331–340. [Google Scholar] [CrossRef]
- Veihmeyer, F.J.; Hendrickson, A.H. Soil moisture at permanent wilting of plants. Plant Physiol. 1928, 3, 355–357. [Google Scholar] [CrossRef][Green Version]
- Furr, J.R.; Reeve, J.O. The range of soil moisture percentages through which plants undergo permanent wilting point in some soils from semi-arid irrigated areas. J. Agric. Res. 1945, 71, 149–170. [Google Scholar]
- Gee, G.W.; Ward, A.L.; Zhang, Z.F.; Campbell, G.S.; Mathison, J. The influence of hydraulic nonequilibrium on pressure plate data. Vadose Zone J. 2002, 1, 172–178. [Google Scholar] [CrossRef]
- Coelho, J.; Barros, M.D.F.; Bezerra, N.E.; Souza, E.R.D. Physiological permanent wilting point and osmotic potential of cowpea grown. Rev. Bras. Eng. Agric. Ambient. 2015, 18, 708–713. [Google Scholar] [CrossRef]
- Ferreira, R.O.; Souza, L.D.S.; Nascimento, M.N.D.; Silveira, F.G.F.D. Permanent wilt point from two methods for different combinations of citrus rootstock. Cienc. Rural 2020, 50, e20190074. [Google Scholar] [CrossRef]
- Silvestro, P.C.; Pignatti, S.; Yang, H.; Yang, G.; Pascucci, S.; Castaldi, F.; Casa, R. Sensitivity analysis of the Aquacrop and SAFYE crop models for the assessment of water limited winter wheat yield in regional scale applications. PLoS ONE 2017, 12, e0187485. [Google Scholar] [CrossRef]
- Van Lier, Q.J.; Gubiani, P.I. Beyond the “least limiting water range”: Rethinking soil physics research in Brazil. Rev. Bras. Cienc. Solo 2015, 39, 925–939. [Google Scholar] [CrossRef]
- Garg, A.; Bordoloi, S.; Ganesan, S.P.; Sekharan, S.; Sahoo, L. A relook into plant wilting: Observational evidence based on unsaturated soil–plant-photosynthesis interaction. Sci. Rep. 2020, 10, 22064. [Google Scholar] [CrossRef]
- Procópio, S.O.; Santos, J.B.; Silva, A.A.; Donagemma, G.K.; Mendonça, E.S. Permanent wilting point of soybean, bean and weeds. Planta Daninha 2004, 22, 35–41. [Google Scholar] [CrossRef]
- Torres, L.C.; Keller, T.; Lima, R.P.; Tormena, C.A.; Lima, H.V.; Giarola, N.F.B. Impacts of soil type and crop species on permanent wilting of plants. Geoderma 2021, 384, 114798. [Google Scholar] [CrossRef]
- Wiecheteck, L.H.; Giarola, N.F.; Lima, R.P.; Tormena, C.A.; Torres, L.C.; Paula, A.L. Comparing the classical permanent wilting point concept of soil (−15,000 hPa) to biological wilting of wheat and barley plants under contrasting soil textures. Agric. Water Manag. 2020, 230, 105965. [Google Scholar] [CrossRef]
- Yao, N.; Li, Y.; Xu, F.; Liu, J.; Chen, S.; Ma, H.; Chau, H.W.; Liu, D.L.; Li, M.; Feng, H.; et al. Permanent wilting point plays an important role in simulating winter wheat growth under water deficit conditions. Agric. Water Manag. 2020, 229, 105954. [Google Scholar] [CrossRef]
- Aparecido, L.E.O.; Batista, R.M.; Moraes, J.R.S.C.; Costa, C.T.S.; Oliveira, A.F.M. Agricultural zoning of climate risk for Physalis peruviana cultivation in Southeastern Brazil. Pesqui. Agropecu. Bras. 2019, 54, e00057. [Google Scholar] [CrossRef]
- Marchioretto, L.R.; Rossi, A.; Conte, E.D. Chemical root pruning improves quality and nutrient uptake of cape gooseberry (Physalis peruviana) seedlings. Sci. Hortic. 2020, 261, 108948. [Google Scholar] [CrossRef]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by HPLC-DAD-APCI-MSn. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Santos, L.E.; Martínez-Girón, J.; Arias-Jaramillo, M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017, 233, 96–100. [Google Scholar] [CrossRef]
- Pittock, J.; Corbett, S.; Colloff, M.J.; Wyrwoll, P.; Alexandra, J.; Beavis, S.; Chipperfield, K.; Croke, B.; Lane, P.; Ross, A.; et al. A review of the risks to shared water resources in the Murray–Darling Basin. Australas. J. Water Resour. 2023, 27, 1–17. [Google Scholar] [CrossRef]
- Moura, L.O.; Silva, M.F.; Cunha, F.F.; Picoli, E.A.T.; Silva, F.C.S.; Silva, F.L. Water deficit as a trigger to immature soybean pod opening. J. Agron. Crop Sci. 2023, 209, 12634. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Penoni, E.S.; Soares, J.D.R.; Silva, R.A.L.; Pasqual, M. Phenological characterization and productivity of Physalis peruviana cultivated in greenhouse. Biosci. J. 2013, 29, 1771–1777. [Google Scholar] [CrossRef]
- Mayes, S.; Massawe, F.J.; Alderson, P.G.; Roberts, J.A.; Azam-Ali, S.N.; Hermann, M. The potential for underutilized crops to improve security of food production. J. Exp. Bot. 2011, 63, 1075–1079. [Google Scholar] [CrossRef]
- Vincent, H.; Wiersema, J.; KelL, S.; Fielder, H.; Dobbie, S.; Álvarez, N.P.C.; Guarino, L.; Eastwood, R.; Leόn, B.; Maxted, N. A prioritized crop wild relative inventory to help underpin global food security. Biol. Conserv. 2013, 167, 265–275. [Google Scholar] [CrossRef]
- Embrapa-Empresa Brasileira de Pesquisa Agropecuária. Centro Nacional de Pesquisa de Solos. In Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasilia, Brazil, 2018; p. 356. [Google Scholar]
- Muniz, J.; Kretzschmar, A.A.; Rufato, L.; Pelizza, T.R.; Rufato, A.R.; Macedo, T.A. General aspects of physalis cultivation. Cienc. Rural 2014, 44, 964–970. [Google Scholar] [CrossRef]
- Brasil Ministério da Agricultura e Reforma Agrária. Regras para Análise de Sementes, 1st ed.; SNDA/DNDV/CLAV: Brasilia, Brazil, 2009; p. 395.
- Silva, E.M.B.; Silva, T.J.; Cabral, C.E.; Valadares, E.; Goldoni, G. Morphological and structural characteristics of the maradu grass fertilized with vegetable ash in oxissol of the cerrado. Enciclop. Biosf. 2011, 7, 1–9. [Google Scholar]
- Cassel, D.K.; Nielsen, D.R. Field capacity and available water capacity. Soil Sci. Soc. Agron. 1986, 1, 901–926. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Richards, L.A. Methods of measuring soil moisture tension. Soil Sci. Soc. Am. J. 1949, 68, 95–112. [Google Scholar] [CrossRef]
- Moline, E.F.V.; Barboza, E.; Simões, L.P.; Ferreira Filho, G.S.; Souza, F.L.F.; Schlindwein, J.A. Ponto de murcha permanente em solos arenoso e argiloso utilizando o tomateiro como cultura indicadora. Glob. Sci. Technol. 2013, 6, 164–170. [Google Scholar] [CrossRef]
- Leite, R.S.; Nascimento, M.N.; Tanan, T.T.; Gonçalves Neto, L.P.; Ramos, C.A.S.; Silva, A.L. Alleviation of water deficit in Physalis angulata plants by nitric oxide exogenous donor. Agric. Water Manag. 2019, 216, 98–104. [Google Scholar] [CrossRef]
- Jabro, J.D.; Stevens, W.B. Soil-water characteristic curves and their estimated hydraulic parameters in no-tilled and conventionally tilled soils. Soil Tillage Res. 2022, 219, 105342. [Google Scholar] [CrossRef]
- Zhang, L.; Han, J. Improving water retention capacity of an aeolian sandy soil with feldspathic sandstone. Sci. Rep. 2019, 9, 14719. [Google Scholar] [CrossRef]
- Botula, Y.D.; Cornelis, W.M.; Baert, G.; Van Ranst, E. Evaluation of pedotransfer functions for predicting water retention of soils in Lower Congo (D.R. Congo). Agric. Water Manag. 2012, 111, 1–10. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Hajabbasi, M.A.; Sabzalian, M.R. Role of fungal endophyte of tall fescue (Epichloë coenophiala) on water availability, wilting point and integral energy in texturally-different soils. Agric. Water Manag. 2016, 163, 197–211. [Google Scholar] [CrossRef]
- Naveed, M.; Moldrup, P.; Tuller, M.; Ferrè, T.P.A.; Kawamoto, K.; Komatsu, T.; Jonge, L.W. Prediction of the soil water characteristic from soil particle volume fractions. Soil Sci. Soc. Am. J. 2012, 76, 1946–1956. [Google Scholar] [CrossRef]
- Prevedello, C.L.; Armindo, R.A. Física do Solo com Problemas Resolvidos, 2nd ed.; UFPR: Curitiba, Brazil, 2015; p. 474. [Google Scholar]
- Donagemma, G.K.; Freitas, P.L.; Balieiro, F.C.; Fontana, A.; Spera, S.T.; Lumbreras, J.F.; Viana, J.H.M.; Araújo Filho, J.C.; Santos, F.C.; Albuquerque, M.R.; et al. Characterization, agricultural potential, and perspectives for the management of light soils in Brazil. Pesqui. Agropecu. Bras. 2016, 51, 1003–1020. [Google Scholar] [CrossRef]
- Zaffar, M.; Sheng-Gao, L. Pore size distribution of clayey soils and its correlation with soil organic matter. Pedosphere 2015, 25, 240–249. [Google Scholar] [CrossRef]
- Bernardo, S.; Mantovani, E.C.; Silva, D.D.; Soares, A.A. Manual de Irrigação, 9th ed.; Editora UFV: Viçosa, Brazil, 2019; p. 545. [Google Scholar]


| Textural Classification | Sand | Silt | Clay | |||||||
| Sandy (SAN) | 84 | 3 | 13 | |||||||
| Sandy Clay Loam (SCL) | 57 | 13 | 30 | |||||||
| Soils | pH | P | K | H + Al | Al+3 | Ca+2 | Mg+2 | CEC | V | OM |
| (H2O) | -- (mg dm–3) -- | ---------------- (cmolc dm–3) ---------------- | (%) | (dag kg–1) | ||||||
| SAN | 5.6 | 10.9 | 47 | 2.97 | 0 | 0.45 | 1.42 | 8.38 | 51.5 | 1.88 |
| SCL | 6.0 | 191.8 | 230 | 3.96 | 0 | 4.41 | 3.92 | 14.75 | 73.2 | 4.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas, E.M.; Vital, T.N.B.; Guimarães, G.F.C.; da Silveira, F.A.; Gomes, C.N.; da Cunha, F.F. Determination of the Permanent Wilting Point of Physalis peruviana L. Horticulturae 2023, 9, 873. https://doi.org/10.3390/horticulturae9080873
de Freitas EM, Vital TNB, Guimarães GFC, da Silveira FA, Gomes CN, da Cunha FF. Determination of the Permanent Wilting Point of Physalis peruviana L. Horticulturae. 2023; 9(8):873. https://doi.org/10.3390/horticulturae9080873
Chicago/Turabian Stylede Freitas, Elis Marina, Thayne Nárgyle Botelho Vital, Gabriel Fernandes Costa Guimarães, Fernando Augusto da Silveira, Carlos Nick Gomes, and Fernando França da Cunha. 2023. "Determination of the Permanent Wilting Point of Physalis peruviana L." Horticulturae 9, no. 8: 873. https://doi.org/10.3390/horticulturae9080873
APA Stylede Freitas, E. M., Vital, T. N. B., Guimarães, G. F. C., da Silveira, F. A., Gomes, C. N., & da Cunha, F. F. (2023). Determination of the Permanent Wilting Point of Physalis peruviana L. Horticulturae, 9(8), 873. https://doi.org/10.3390/horticulturae9080873






