Photosynthesis, Biochemical and Yield Performance of Grapevine Hybrids in Two Rootstock and Trellis Height
Abstract
1. Introduction
2. Materials and Methods
2.1. Localization and Climate Conditions
2.2. Treatments
2.3. Sampling
2.3.1. Photosynthesis Assay
2.3.2. Biochemical Assay
2.4. Harvest, Yield, and Must Quality
2.5. Statistical Analyses
3. Results
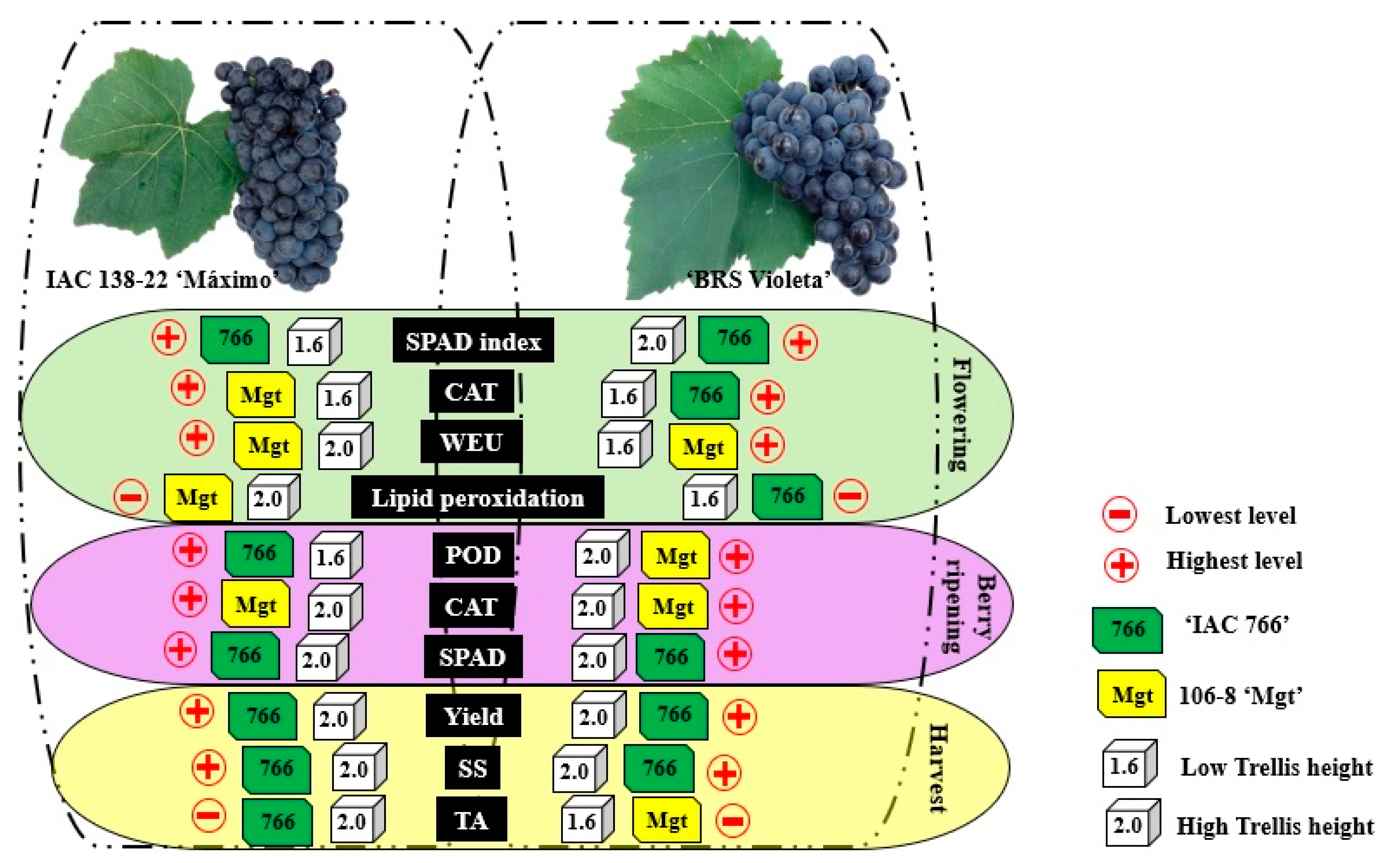
3.1. Impact of Rootstock and Trellis Height on Variety (‘IAC 138-22 Maximo’): Physiological, Biochemical Parameters and Yield
3.2. Impact of Rootstock and Trellis Height on Variety (‘BRS Violeta’): Physiological, Biochemical Parameters and Yield
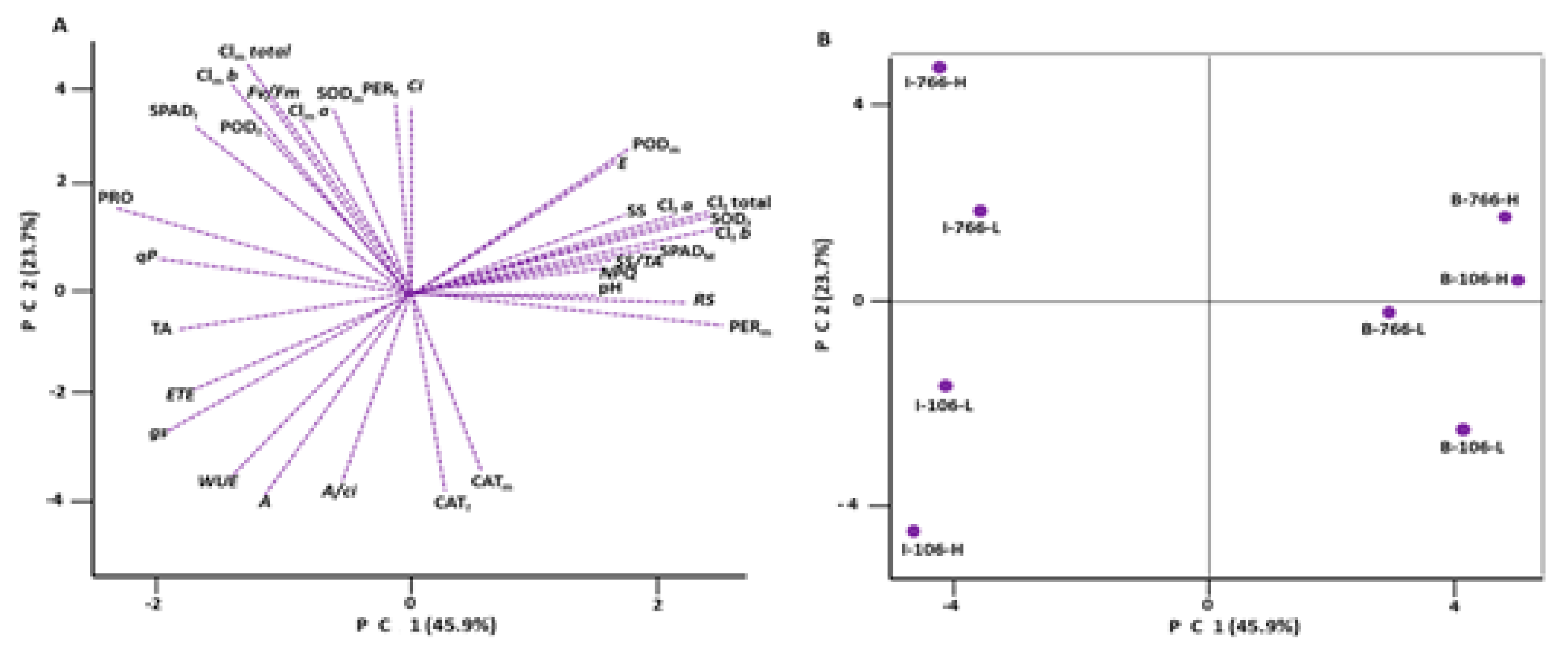
3.3. Principal Component Analysis (PCA)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bellée, A.; Comont, G.; Nivault, A.; Abou-Mansour, E.; Coppin, C.; Dufour, M.C.; Corio-Costet, M.F. Life traits of four Botryosphaeriaceae species and molecular responses of different grapevine cultivars or hybrids. Plant Pathol. 2017, 66, 763–776. [Google Scholar] [CrossRef]
- Nikolić, D.; Ranković-Vasić, Z.; Jovanović-Cvetković, T.; Petrović, A.; Radojević, I.; Sivčev, B. Effect of genotype x environment interactions of grapevine hybrids characteristics. BIO Web Conf. 2017, 9, 1018. [Google Scholar] [CrossRef]
- Marín, D.; Armengol, J.; Carbonell-Bejerano, P.; Escalona, J.; Gramaje, D.; Hernández-Montes, E.; Intrigliolo, D.; Martínez-Zapater, J.; Medrano, H.; Mirás-Avalos, J.; et al. Challenges of viticulture adaptation to global change: Tackling the issue from the roots. Aust. J. Grape Wine Res. 2021, 27, 8–25. [Google Scholar] [CrossRef]
- Júnior, M.J.P.; Hernandes, J.L.; Moura, M.F. Performance of juice and wine grape cultivars in different training systems. Rev. Bras. Frutic. 2018, 40, 1–8. [Google Scholar] [CrossRef]
- Mattar, G.S.; Modesto, L.R.; Hernandes, J.L.; Tecchio, M.A.; Moura, M.F. Cycle, physicochemical characterization and climatic adaptation of a white hybrid grape on different rootstocks. Bragantia 2019, 78, 470–478. [Google Scholar] [CrossRef]
- Smith, H.M.; Clarke, C.W.; Smith, B.P.; Carmody, B.M.; Thomas, M.R.; Clingeleffer, P.R.; Powell, K.S. Genetic identification of SNP markers linked to a new grape phylloxera resistant locus in Vitis cinerea for marker-assisted selection. BMC Plant Biol. 2018, 18, 360. [Google Scholar] [CrossRef]
- Divers, M.; Gomes, C.B.; Menezes-Netto, A.C.; Lima-Medina, I.; Nondillo, A.; Bellé, C.; Filho, J.V.D.A. Diversity of plant-parasitic nematodes parasitising grapes in Southern Brazil. Trop. Plant Pathol. 2019, 44, 401–408. [Google Scholar] [CrossRef]
- Zhang, L.; Marguerit, E.; Rossdeutsch, L.; Ollat, N.; Gambetta, G.A. The influence of grapevine rootstocks on scion growth and drought resistance. Theor. Exp. Plant Physiol. 2016, 28, 143–157. [Google Scholar] [CrossRef]
- Tecchio, M.A.; da Silva, M.J.R.; Callili, D.; Hernandes, J.L.; Moura, M. Yield of white and red grapes, in terms of quality, from hybrids and Vitis labrusca grafted on different rootstocks. Sci. Hortic. 2020, 259, 108846. [Google Scholar] [CrossRef]
- Nowicka, B.; Ciura, J.; Szymańska, R.; Kruk, J. Improving photosynthesis, plant productivity and abiotic stress tolerance—Current trends and future perspectives. J. Plant Physiol. 2018, 231, 415–433. [Google Scholar] [CrossRef]
- Dinis, L.T.; Malheiro, A.; Luzio, A.; Fraga, H.; Ferreira, H.; Gonçalves, I.; Pinto, G.; Correia, C.; Moutinho-Pereira, J. Improvement of grapevine physiology and yield under summer stress by kaolin-foliar application: Water relations, photosynthesis and oxidative damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
- Silva, J.R.; Rodrigues, W.P.; Ferreira, L.S.; de Paula Bernado, W.; Paixão, J.S.; Patterson, A.E.; Poni, S. Deficit irrigation and transparent plastic covers can save water and improve grapevine cultivation in the tropics. Agri. Water Manag. 2018, 202, 66–80. [Google Scholar] [CrossRef]
- Tombesi, S.; Cincera, I.; Frioni, T.; Ughini, V.; Gatti, M.; Palliotti, A.; Poni, S. Relationship among night temperature, carbohydrate translocation and inhibition of grapevine leaf photosynthesis. Environ. Exp. Bot. 2019, 157, 293–298. [Google Scholar] [CrossRef]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef]
- Jimenes, I.M.; Mayer, N.A.; Dias, C.T.D.S.; Filho, J.A.S.; da Silva, S.R. Influence of clonal rootstocks on leaf nutrient content, vigor and productivity of young ‘Sunraycer’ nectarine trees. Sci. Hortic. 2018, 235, 279–285. [Google Scholar] [CrossRef]
- Ferreira, T.D.O.; da Costa, R.R.; Félix, D.T.; Neto, E.R.D.A.; Cruz, M.D.M.; de Lima, M.A.C. Quality and antioxidant potential of ‘BRS Magna’ grapes harvested in the first half of the year under different training systems and rootstocks in a tropical region. Cien. Agrot. 2019, 43, e029518. [Google Scholar] [CrossRef]
- Leão, P.C.D.S.; Chaves, A.R.D.M. Training systems and rootstocks on yield and agronomic performance of ‘Syrah’ grapevine in the Brazilian semiarid. Ciência Agrotecnol. 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Gladstone, E.A.; Dokoozlian, N.K. Influence of leaf area density and 487 trellis/training system on the light micro-climate within grapevine canopies. Vitis 2003, 42, 123–132. [Google Scholar]
- Sanchez-Rodriguez, L.A.; Spósito, M.B. Influence of the trellis/training system on the physiology and production of Vitis labrusca cv. Niagara Rosada in Brazil. Sci. Hortic. 2020, 261, 109043. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta (BBA) Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Not. 2008, 1, 27–35. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolotis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Teisseire, H.; Guy, V. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Peixoto, P.H.P.; Cambraia, J.; Sant’anna, R.; Mosquim, P.R.; Moreira, M.A. Aluminium effects on lipid peroxidation and the activities of enzymes of oxidative metabolism in sorghum. Rev. Brasil Fis. Veg. 1999, 11, 37–143. [Google Scholar]
- Devi, S.R.; Prasad, M.N.V. Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1986, 138, 157–165. [Google Scholar] [CrossRef]
- Nelson, N.A. A photometric adaptation of Somogy method for the determination of glucose. J. Biol. Chem. 1944, 135, 136–175. [Google Scholar]
- SAS Institute. SAS Version 9 (Vol. 2); SAS Institute Incorporated: Cary, NC, USA, 2000. [Google Scholar]
- Bertamini, M.; Grando, M.S.; Zocca, P.; Pedrotti, M.; Lorenzi, S.; Cappellin, L. Linking monoterpenes and abiotic stress resistance in grapevines. BIO Web Conf. 2019, 13, 01003. [Google Scholar] [CrossRef]
- Sucu, S.; Yağcı, A.; Yıldırım, K. Changes in Morphological, Physiological Traits and Enzyme Activity of Grafted and Ungrafted Grapevine Rootstocks under Drought Stress. Erwerbs-Obstbau 2018, 60, 127–136. [Google Scholar] [CrossRef]
- Miller, G.; Coutu, J.; Shulaev, V.; Mittler, R. Reactive Oxygen Signaling in Plants. In Annual Plant Reviews Online; Wiley: Hoboken, NJ, USA, 2018; pp. 189–201. [Google Scholar] [CrossRef]
- Ollat, N.; Bordenave, L.; Tandonnet, J.P.; Boursiquot, J.M.; Marguerit, E. Grapevine rootstocks: Origins and perspectives. Acta Hortic. 2016, 1136, 11–22. [Google Scholar] [CrossRef]
- Ollat, N.; Cookson, S.J.; Lauvergeat, V.; Marguerit, E.; Barrieu, F.; Gambetta, G.A.; Goutouly, J.P.; Tandonnet, J.P.; Vivin, P.; Delrot, S. Grapevine roots: The dark side. Acta Hortic. 2017, 1188, 213–226. [Google Scholar] [CrossRef]
- Ollat, N.; Cookson, S.; Destrac-Irvine, A.; Lauvergeat, V.; Ouaked-Lecourieux, F.; Marguerit, E.; Barrieu, F.; Dai, Z.; Duchêne, E.; Gambetta, G.; et al. Grapevine adaptation to abiotic stress: An overview. Acta Hortic. 2018, 1248, 497–512. [Google Scholar] [CrossRef]
- Kraus, C.; Pennington, T.; Herzog, K.; Hecht, A.; Fischer, M.; Voegele, R.T.; Kicherer, A. Effects of canopy architecture and microclimate on grapevine health in two training systems. Vitis 2018, 57. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A Guide for its Bootstrap procedures in multiple comparisons. Ciência Agrotecnol. 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Heuvel, J.E.V. Influence of Grapevine Training Systems on Vine Growth and Fruit Composition: A Review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar] [CrossRef]
| Trellis Height | Rootstock | ||
|---|---|---|---|
| ‘IAC 766’ | ‘106-8 Mgt’ | ||
| Flowering | |||
| qP | 1.6 m | 0.57 ± 0.02 aA | 0.49 ± 0.01 bB |
| 2.0 m | 0.54 ± 0.02 bA | 0.56 ± 0.01 aA | |
| NPQ | 1.6 m | 2.03 ± 0.07 bB | 2.38 ± 0.01 aA |
| 2.0 m | 2.64 ± 0.14 aA | 2.08 ± 0.04 bB | |
| ETR (μmol m−2 s−1 electrons) | 1.6 m | 148.58 ± 7.07 aA | 133.86 ± 5.51 bB |
| 2.0 m | 123.03 ± 3.16 bB | 158.15 ± 9.93 aA | |
| gs (mol m−2 s−1) | 1.6 m | 0.23 ± 0.01 aB | 0.25 ± 0.01 aA |
| 2.0 m | 0.17 ± 0.001 bB | 0.22 ± 0.01 bA | |
| E (mmol m−2 s−1 water vapor) | 1.6 m | 7.06 ± 0.42 aB | 7.59 ± 0.30 aA |
| 2.0 m | 6.82 ± 0.24 aA | 6.47 ± 0.17 bB | |
| WUE | 1.6 m | 4.53 ± 0.25 aA | 4.42 ± 0.11 bA |
| 2.0 m | 4.56 ± 0.18 aB | 5.66 ± 0.15 aA | |
| A (μmol m−2 s−1 CO2) | 1.6 m | 34.75 ± 1.38 aA | 36.17 ± 1.25 aA |
| 2.0 m | 29.51 ± 1.51 bB | 38.82 ± 2.48 aA | |
| Ci (μmol mol−1 CO2) | 1.6 m | 179.93 ± 3.74 aA | 148.67 ± 5.70 aB |
| 2.0 m | 175.31 ± 5.76 aA | 103.21 ± 6.11 bB | |
| A/Ci | 1.6 m | 0.19 ± 0.001 aB | 0.26 ± 0.02 bA |
| 2.0 m | 0.17 ± 0.01 bB | 0.32 ± 0.01 aA | |
| Cl b (mg 100 g−1 leaves) | 1.6 m | 16.40 ± 2.01 aA | 12.22 ± 0.43 aB |
| 2.0 m | 13.02 ± 0.66 bA | 12.69 ± 2.14 aA | |
| POD (µmol mg−1 min−1 protein) | 1.6 m | 36.52 ± 1.37 aA | 34.83 ± 1.52 aA |
| 2.0 m | 39.15 ± 0.83 aA | 32.97 ± 2.26 aB | |
| CAT (µg mKat−1 protein) | 1.6 m | 5.68 ± 1.26 aA | 6.11 ± 3.20 aA |
| 2.0 m | 2.12 ± 0.77 bB | 4.70 ± 1.69 aA | |
| Berry ripening | |||
| Cl a (mg 100 g−1 leaves) | 1.6 m | 35.18 ± 0.93 bA | 36.03 ± 3.25 aA |
| 2.0 m | 55.28 ± 2.60 aA | 31.31 ± 3.35 bB | |
| Cl b (mg 100 g−1 leaves) | 1.6 m | 16.03 ± 0.23 bA | 17.67 ± 1.71 aA |
| 2.0 m | 26.57 ± 0.67 aA | 14.35 ± 1.98 bB | |
| Cl total (mg 100 g−1 leaves) | 1.6 m | 51.21 ± 0.84 bA | 53.71 ± 4.55 aA |
| 2.0 m | 81.85 ± 3.20 aA | 45.66 ± 5.32 bB | |
| SOD (mg U−1 protein) | 1.6 m | 4665.88 ± 19.91 bA | 3689.94 ± 34.25 aB |
| 2.0 m | 5027.41 ± 17.90 aA | 3463.00 ± 32.14 aB | |
| CAT (µg mKat−1 protein) | 1.6 m | 14.61 ± 2.61 bA | 14.12 ± 0.41 bA |
| 2.0 m | 54.62 ± 1.58 aB | 86.19 ± 3.98 aA | |
| Trellis Height | Rootstock | |||
|---|---|---|---|---|
| 1.6 m | 2.0 m | ‘IAC 766’ | ‘106-8 Mgt’ | |
| Flowering | ||||
| Fv/Fm | 0.86 ± 0.02 b | 0.90 ± 0.02 a | 0.86 ± 0.01 b | 0.90 ± 0.02 a |
| SPAD index | 33.47 ± 2.79 a | 32.58 ± 2.38 a | 35.08 ± 1.27 a | 30.97 ± 1.63 b |
| Cl a (mg 100 g−1 leaves) | 33.27 ± 5.69 a | 30.82 ± 4.34 a | 35.55 ± 3.41 a | 28.55 ± 3.88 a |
| Cl total (mg 100 g−1 leaves) | 47.59 ± 8.10 a | 43.68 ± 5.61 a | 50.26 ± 5.54 a | 41.01 ± 5.12 b |
| SOD (mg U−1 protein) | 4141.42 ± 38.62 a | 4331.28 ± 51.27 a | 4561.96 ± 39.92 a | 3910.74 ± 24.95 b |
| Lipid peroxidation (μmol g−1 leaves) | 11.02 ± 1.04 a | 9.59 ± 0.09 a | 12.79 ± 1.06 a | 7.82 ± 0.08 b |
| Berry ripening | ||||
| SPAD index | 35.86 ± 2.93 a | 37.09 ± 1.88 a | 37.97 ± 2.25 a | 34.98 ± 1.66 b |
| POD (µmol mg−1 min−1 protein) | 23.45 ± 3.64 a | 22.64 ± 3.18 a | 25.37 ± 2.66 a | 20.72 ± 2.07 b |
| Yield | ||||
| Yield (kg−1 plant) | 4.16 ± 0.08 b | 5.76 ± 1.04 a | 5.07 ± 0.09 a | 4.85 ± 0.08 a |
| pH | 3.39 ± 0.09 a | 3.31 ± 0.08 b | 3.35 ± 0.08 a | 3.36 ± 0.11 a |
| Soluble solids (°Brix) | 14.19 ± 1.36 b | 15.25 ± 1.55 a | 15.13 ± 1.63 a | 14.31 ± 1.36 a |
| Titratable acidity (% tartaric acid) | 0.98 ± 0.16 a | 0.94 ± 0.16 a | 0.90 ± 0.18 b | 1.02 ± 0.11 a |
| SS/TA ratio | 14.91 ± 3.15 a | 16.78 ± 4.31 a | 17.52 ± 4.40 a | 14.17 ± 2.25 b |
| Reducing sugar (%) | 10.56 ± 1.89 a | 10.98 ± 1.70 a | 10.58 ± 1.53 a | 10.96 ± 2.04 a |
| Trellis Height | Rootstock | ||
|---|---|---|---|
| ‘IAC 766’ | ‘106-8 Mgt’ | ||
| Flowering | |||
| qP | 1.6 m | 0.55 ± 0.02 aA | 0.44 ± 0.02 aA |
| 2.0 m | 0.45 ± 0.01 bA | 0.41 ± 0.01 bB | |
| NPQ | 1.6 m | 2.53 ± 0.14 bB | 3.29 ± 0.09 aA |
| 2.0 m | 3.03 ± 0.08 aB | 3.24 ± 0.10 aA | |
| ETR (μmol m−2 s−1 electrons) | 1.6 m | 124.38 ± 3.50 aA | 114.14 ± 5.64 aB |
| 2.0 m | 100.70 ± 5.96 bA | 100.11 ± 4.87 bA | |
| gs (mol m−2 s−1) | 1.6 m | 0.28 ± 0.01 aA | 0.14 ± 0.001 aB |
| 2.0 m | 0.26 ± 0.001 bA | 0.13 ± 0.001 aB | |
| A (μmol m−2 s−1 CO2) | 1.6 m | 36.13 ± 1.27 aA | 31.40 ± 1.06 aB |
| 2.0 m | 31.00 ± 0.38 bA | 29.74 ± 1.30 aA | |
| Ci (μmol mol−1 CO2) | 1.6 m | 129.29 ± 8.21 bA | 132.43 ± 3.20 bA |
| 2.0 m | 162.48 ± 3.37 aA | 151.59 ± 7.21 aA | |
| SPAD index | 1.6 m | 29.11 ± 2.72 aA | 21.57 ± 0.57 bB |
| 2.0 m | 30.34 ± 0.83 aA | 26.75 ± 1.35 aB | |
| Cl a (mg 100 g−1 leaves) | 1.6 m | 47.19 ± 7.00 aA | 39.12 ± 3.54 bB |
| 2.0 m | 44.65 ± 1.53 aA | 48.28 ± 4.67 aA | |
| CAT (µg mKat−1 protein) | 1.6 m | 35.55 ± 3.33 aA | 32.65 ± 0.98 aB |
| 2.0 m | 19.86 ± 0.86 bA | 9.41 ± 0.95 bB | |
| Berry ripening | |||
| Cl a (mg 100 g−1 leaves) | 1.6 m | 40.93 ± 3.44 aA | 23.44 ± 2.00 bB |
| 2.0 m | 32.98 ± 2.00 bA | 29.75 ± 2.16 aA | |
| Cl b (mg 100 g−1 leaves) | 1.6 m | 17.48 ± 2.58 aA | 10.30 ± 0.79 aB |
| 2.0 m | 13.84 ± 1.58 bA | 12.68 ± 2.02 aA | |
| Cl total (mg 100 g−1 leaves) | 1.6 m | 58.42 ± 5.86 aA | 33.75 ± 2.78 bB |
| 2.0 m | 46.82 ± 1.60 bA | 42.43 ± 3.27 aA | |
| POD (µmol mg−1 min−1 protein) | 1.6 m | 15.72 ± 3.36 bB | 28.36 ± 2.67 aA |
| 2.0 m | 24.68 ± 1.57 aB | 30.27 ± 1.92 aA | |
| CAT (µg mKat−1 protein) | 1.6 m | 24.04 ± 4.03 aB | 49.40 ± 2.93 bA |
| 2.0 m | 29.90 ± 1.89 aB | 82.95 ± 8.44 aA | |
| Variable | Trellis Height | Rootstock | ||
|---|---|---|---|---|
| 1.6 m | 2.0 m | ‘IAC 766’ | ‘106-8 Mgt’ | |
| Flowering | ||||
| Fv/Fm | 0.84 ± 0.03 a | 0.86 ± 0.03 a | 0.87 ± 0.01 a | 0.82 ± 0.01 b |
| E (mmol m−2 s−1 water vapor) | 7.76 ± 2.00 a | 8.37 ± 1.66 a | 9.75 ± 0.38 a | 6.38 ± 0.56 b |
| WUE | 4.42 ± 0.62 a | 3.83 ± 0.44 a | 3.66 ± 0.29 b | 4.60 ± 0.44 a |
| A/Ci | 0.19 ± 0.01 a | 0.21 ± 0.01 a | 0.21 ± 0.03 a | 0.22 ± 0.02 a |
| Cl b (mg 100 g−1 leaves) | 18.35 ± 2.43 a | 20.03 ± 1.38 a | 20.05 ± 2.23 a | 18.33 ± 1.67 a |
| Cl total (mg 100 g−1 leaves) | 61.50 ± 8.96 a | 66.50 ± 4.21 a | 65.97 ± 6.70 a | 62.03 ± 4.21 a |
| POD (µmol mg−1 min−1 protein) | 31.63 ± 2.20 a | 32.30 ± 4.07 a | 34.41 ± 2.20 a | 29.52 ± 4.07 b |
| SOD (mg U−1 protein) | 6685.52 ± 11.71 a | 6681.96 ± 13.43 a | 7657.70 ± 26.93 a | 5709.78 ± 35.43 b |
| Lipid peroxidation (μmol g−1 leaves) | 9.76 ± 1.04 a | 9.63 ± 1.03 a | 9.17 ± 2.03 a | 10.22 ± 2.04 a |
| Berry ripening | ||||
| SPAD index | 44.14 ± 1.95 a | 45.12 ± 2.07 a | 46.14 ± 1.66 a | 43.12 ± 0.80 b |
| SOD (mg U−1 protein) | 3105.15 ± 29.44 b | 4110.78 ± 37.90 a | 3266.75 ± 46.78 b | 3949.17 ± 37.35 a |
| Lipid peroxidation (μmol g−1 leaves) | 8.55 ± 0.90 a | 9.44 ± 0.94 a | 8.76 ± 0.94 a | 9.22 ± 0.93 a |
| Yield | ||||
| Yield (kg−1 plant) | 1.52 ± 0.06 a | 1.55 ± 0.09 a | 1.73 ± 0.09 a | 1.35 ± 0.08 b |
| pH | 3.58 ± 0.16 a | 3.55 ± 0.12 a | 3.62 ± 0.16 a | 3.52 ± 0.10 a |
| Soluble solids (°Brix) | 15.99 ± 0.49 a | 16.32 ± 0.53 a | 16.32 ± 0.51 a | 15.99 ± 0.52 a |
| Titratable acidity (% tarctaric acid) | 0.68 ± 0.09 a | 0.75 ± 0.15 a | 0.73 ± 0.15 a | 0.70 ± 0.11 a |
| SS/TA | 23.28 ± 2.86 a | 24.50 ± 3.09 a | 23.88 ± 3.46 a | 23.90 ± 2.56 a |
| Reducing sugar (%) | 12.64 ± 1.38 a | 13.37 ± 1.42 a | 13.27 ± 1.73 a | 12.74 ± 1.04 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues Neto, F.J.; Pimentel Junior, A.; Modesto, L.R.; Moura, M.F.; Putti, F.F.; Boaro, C.S.F.; Ono, E.O.; Rodrigues, J.D.; Tecchio, M.A. Photosynthesis, Biochemical and Yield Performance of Grapevine Hybrids in Two Rootstock and Trellis Height. Horticulturae 2023, 9, 596. https://doi.org/10.3390/horticulturae9050596
Domingues Neto FJ, Pimentel Junior A, Modesto LR, Moura MF, Putti FF, Boaro CSF, Ono EO, Rodrigues JD, Tecchio MA. Photosynthesis, Biochemical and Yield Performance of Grapevine Hybrids in Two Rootstock and Trellis Height. Horticulturae. 2023; 9(5):596. https://doi.org/10.3390/horticulturae9050596
Chicago/Turabian StyleDomingues Neto, Francisco José, Adilson Pimentel Junior, Lenon Romano Modesto, Mara Fernandes Moura, Fernando Ferrari Putti, Carmen Silvia Fernandes Boaro, Elizabeth Orika Ono, João Domingos Rodrigues, and Marco Antonio Tecchio. 2023. "Photosynthesis, Biochemical and Yield Performance of Grapevine Hybrids in Two Rootstock and Trellis Height" Horticulturae 9, no. 5: 596. https://doi.org/10.3390/horticulturae9050596
APA StyleDomingues Neto, F. J., Pimentel Junior, A., Modesto, L. R., Moura, M. F., Putti, F. F., Boaro, C. S. F., Ono, E. O., Rodrigues, J. D., & Tecchio, M. A. (2023). Photosynthesis, Biochemical and Yield Performance of Grapevine Hybrids in Two Rootstock and Trellis Height. Horticulturae, 9(5), 596. https://doi.org/10.3390/horticulturae9050596







