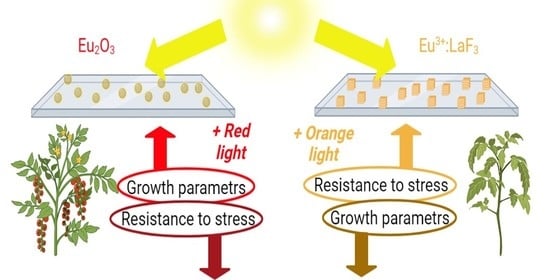
Two Types of Europium-Based Photoconversion Covers for Greenhouse Farming with Different Effects on Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoparticles and Study of Their Properties
2.2. The Glass Surface Application of NF
2.3. Fluorescence Spectroscopy
2.4. Planting and Growing Conditions
2.5. Measurement of Leaf Chlorophyll Content
2.6. Measurement of Plant Morphological Parameters
2.7. Measurement of the Kinetics of Photoinduced Changes in Chlorophyll a Fluorescence (FChl) and the Intensity of Carbon Dioxide Assimilation and Transpiration
2.8. Accounting for the Development of Late Blight on Tomato Leaves during Natural and Artificial Infection
2.9. Statistical Analysis
3. Results
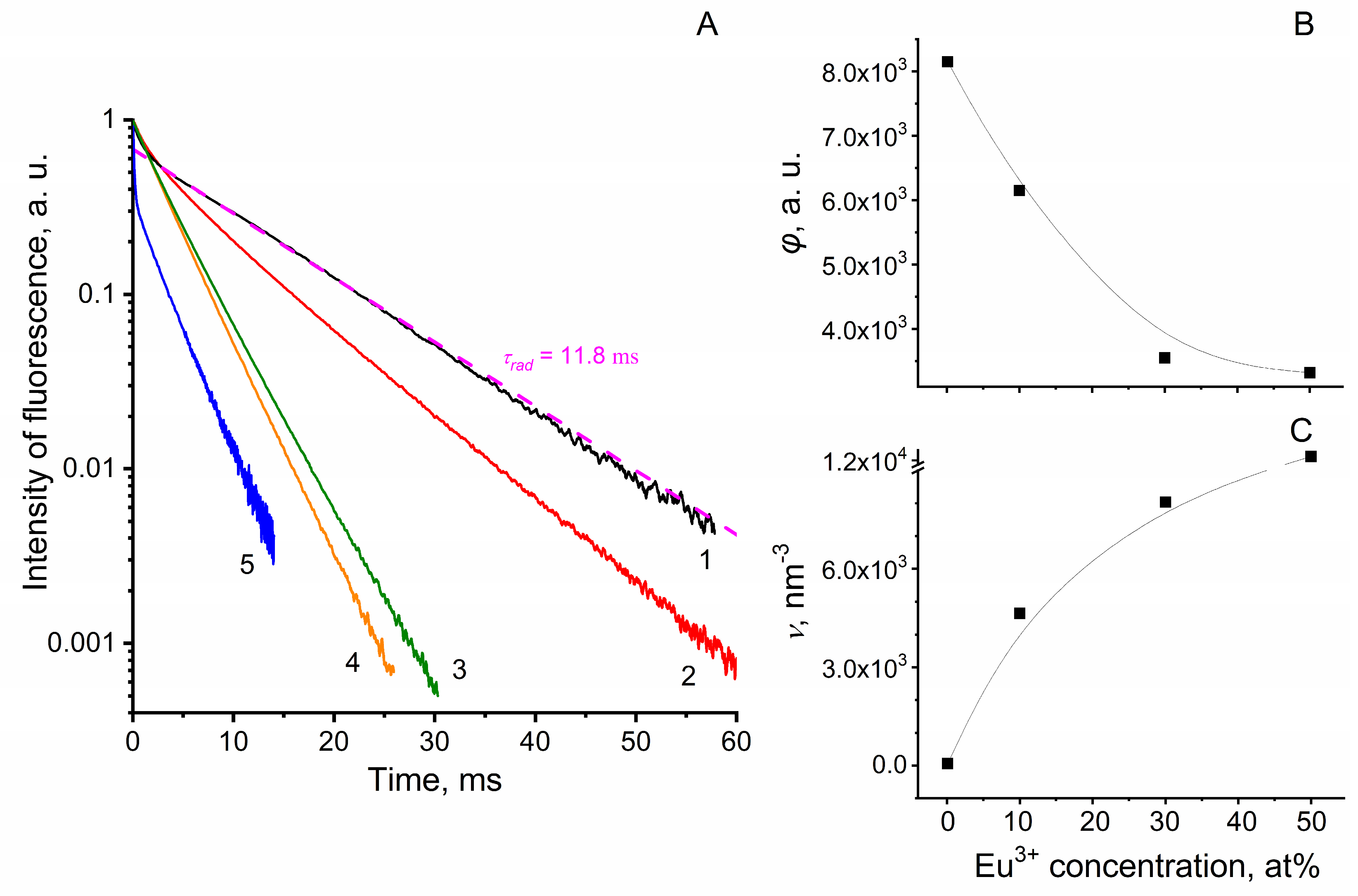
3.1. Selection of the Optimal Concentration of Europium Ions in Eu3+:LaF3 Nanoparticles for Use in Photoconversion Covers
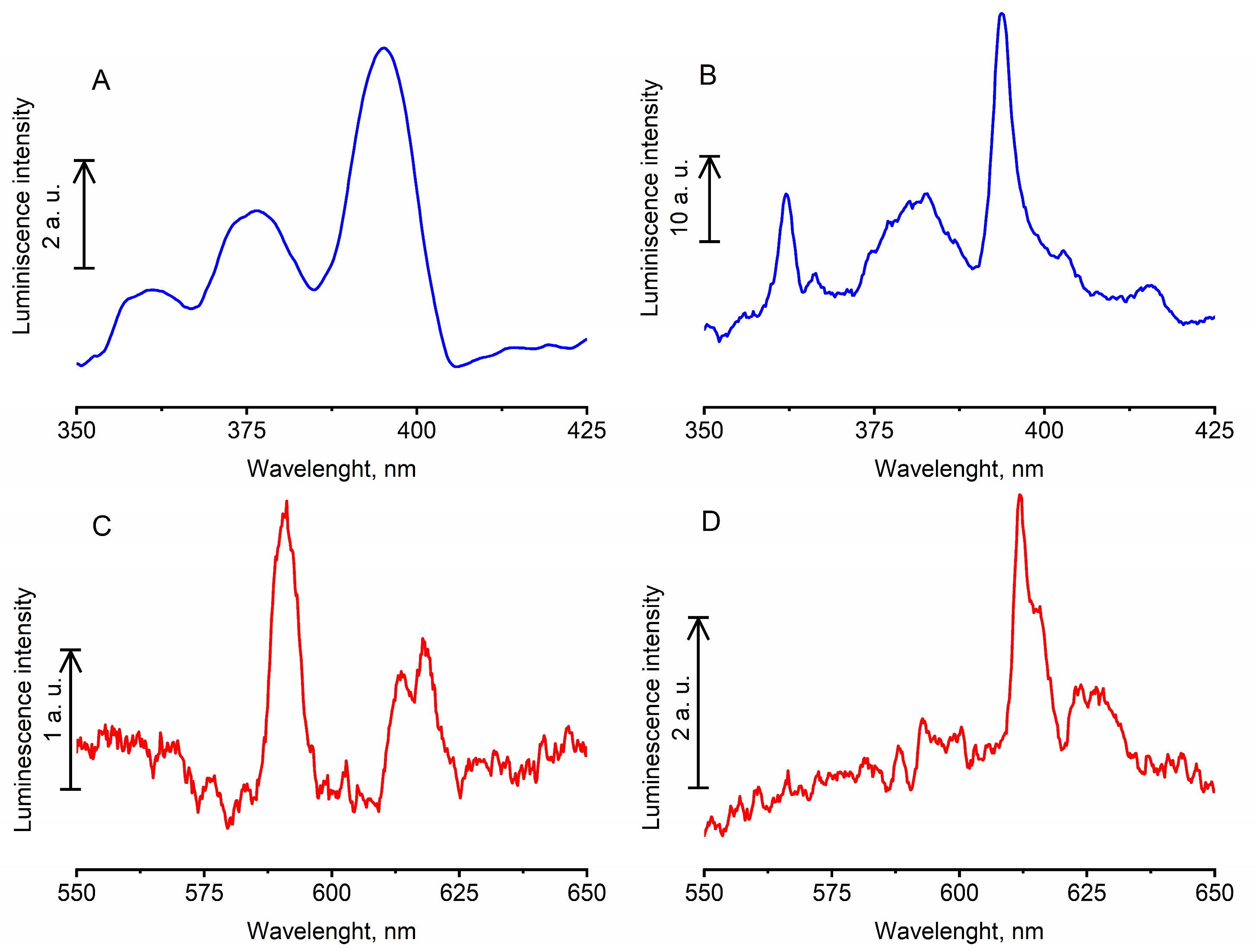
3.2. Properties of NF
3.3. Optical Properties of PCC
3.4. Plant Growth under PCC
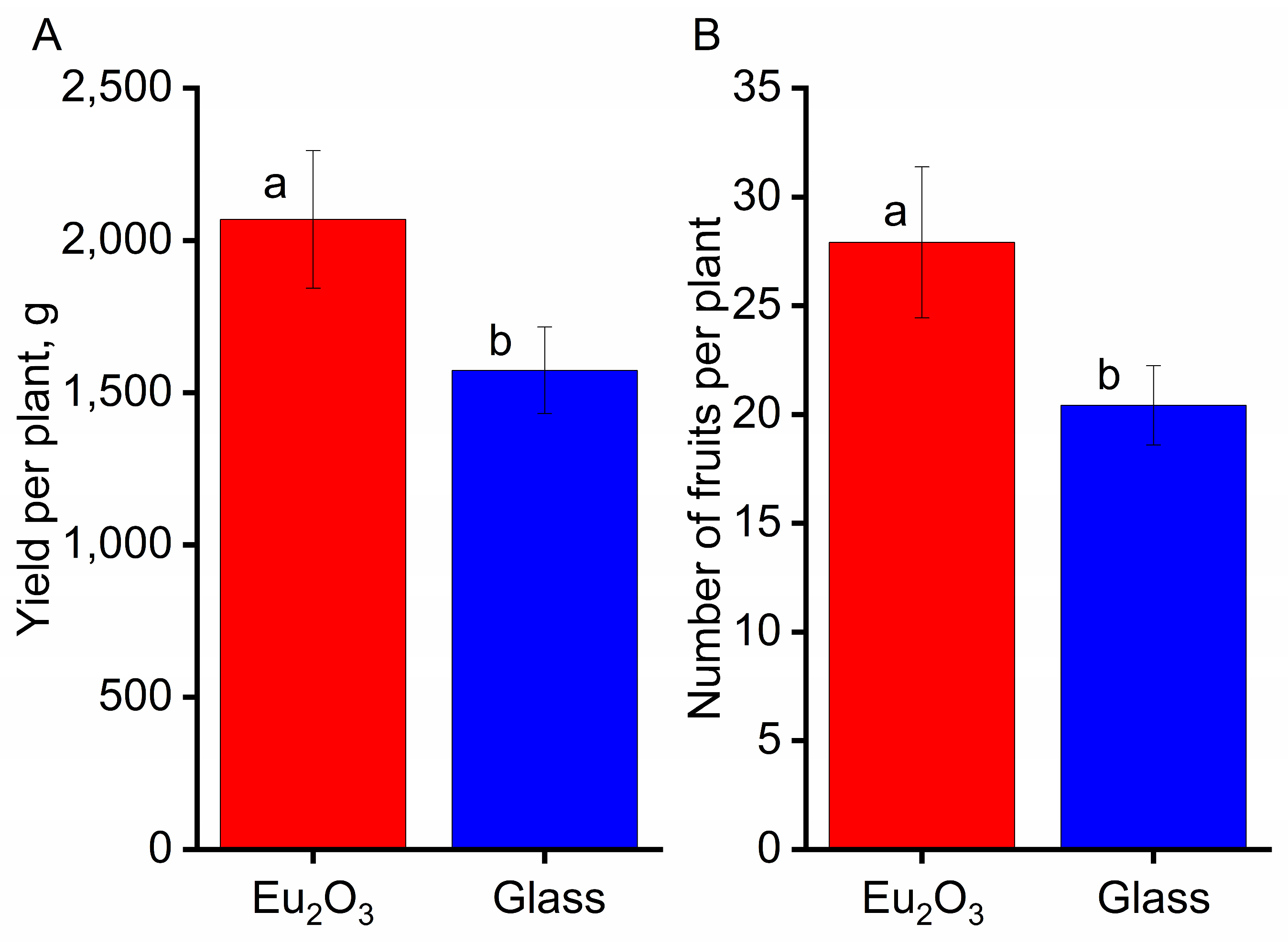
3.4.1. The Effect of PCC on Plant Growth and Development
3.4.2. Effect of PCC on Gas Exchange in Leaves
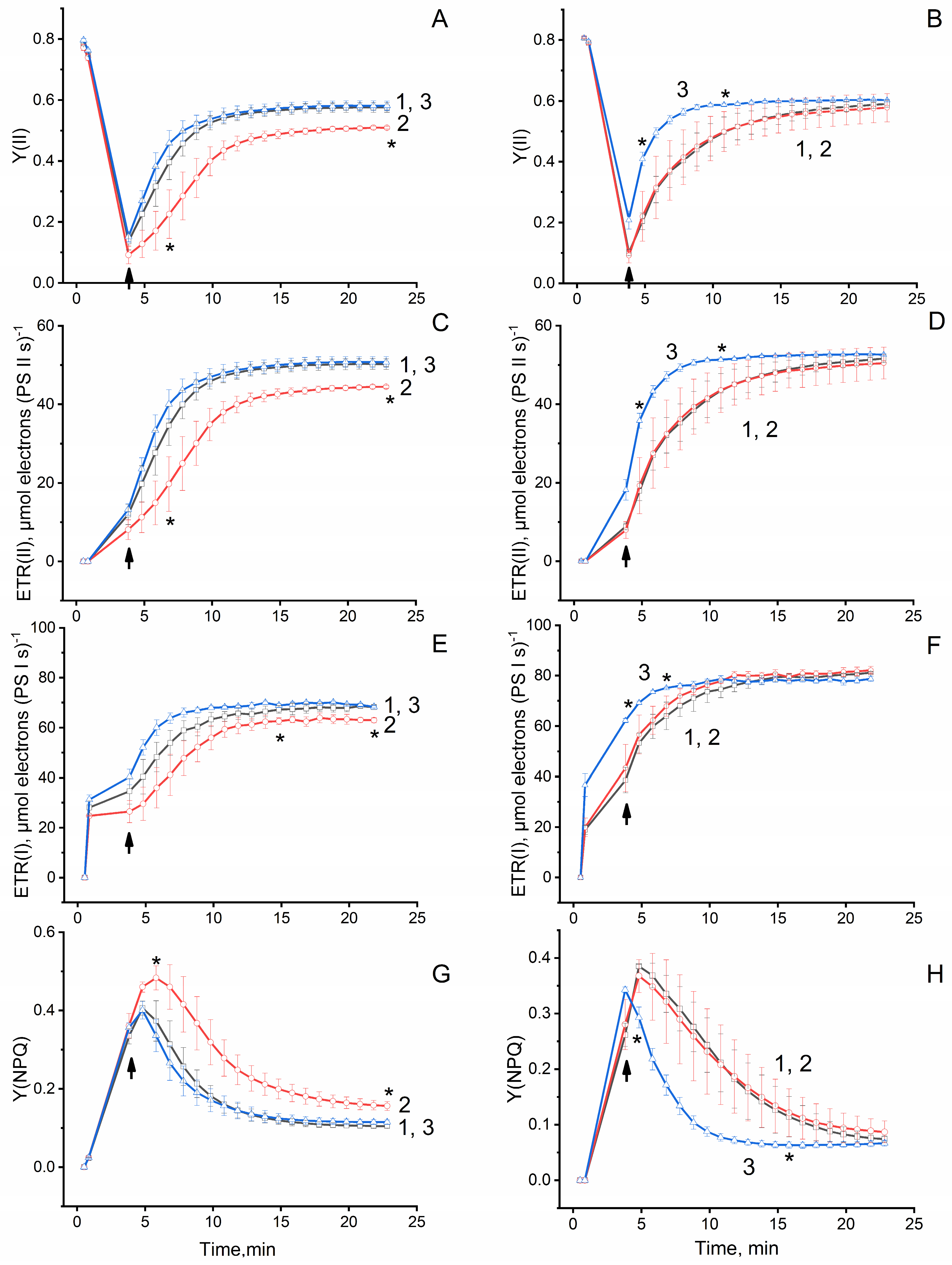
3.4.3. Effect of PCC on Photochemical Activity
3.5. Effect of PCC-Eu3+:LaF3 on Plant Resistance to Abiotic and Biotic Stress Factors
3.5.1. Plant Resistance to Abiotic Factors
3.5.2. Resistance of Tomato Plants to Late Blight Pathogen P. infestans
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Lin, W. Metal–Organic Frameworks for Artificial Photosynthesis and Photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Shevela, D.; Björn, L.O.; Govindjee, L. Photosynthesis: Solar Energy for Life; World Scientific: Singapore, 2019; p. 10522. [Google Scholar] [CrossRef]
- Kruse, O.; Rupprecht, J.; Mussgnug, J.H.; Dismukes, G.C.; Hankamer, B. Photosynthesis: A Blueprint for Solar Energy Capture and Biohydrogen Production Technologies. Photochem. Photobiol. Sci. 2005, 4, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Rye, R.; Holland, H. Paleosols and the Evolution of Atmospheric Oxygen: A Critical Review. Am. J. Sci. 1998, 298, 621–672. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, G.C.; Klimov, V.V.; Baranov, S.V.; Kozlov, Y.N.; DasGupta, J.; Tyryshkin, A. The Origin of Atmospheric Oxygen on Earth: The Innovation of Oxygenic Photosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 2170–2175. [Google Scholar] [CrossRef]
- Bolton, J.R.; Hall, D.O. The maximum efficiency of photosynthesis*. Photochem. Photobiol. 1991, 53, 545–548. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. What Is the Maximum Efficiency with Which Photosynthesis Can Convert Solar Energy into Biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving Photosynthetic Efficiency for Greater Yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Grinberg, M.A.; Vodeneev, V.A.; Il’in, N.V.; Mareev, E.A. Laboratory Simulation of Photosynthesis in a Wide Range of Electromagnetic and Radiation Environment Parameters. Astron. Rep. 2023, 67, 71–77. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving Photosynthesis and Crop Productivity by Accelerating Recovery from Photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Leone, G.; De la Cruz Valbuena, G.; Cicco, S.R.; Vona, D.; Altamura, E.; Ragni, R.; Molotokaite, E.; Cecchin, M.; Cazzaniga, S.; Ballottari, M.; et al. Incorporating a Molecular Antenna in Diatom Microalgae Cells Enhances Photosynthesis. Sci. Rep. 2021, 11, 5209. [Google Scholar] [CrossRef]
- Shen, L.; Yin, X. Solar Spectral Management for Natural Photosynthesis: From Photonics Designs to Potential Applications. Nano Converg. 2022, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shen, R.; Mo, L.; Yang, X.; Chen, X.; Wang, H.; Li, Y.; Hu, C.; Lei, B.; Zhang, X.; et al. Improving Plant Photosynthesis through Light-Harvesting Upconversion Nanoparticles. ACS Nano 2022, 16, 18027–18037. [Google Scholar] [CrossRef]
- Paskhin, M.O.; Yanykin, D.V.; Gudkov, S.V. Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review. Horticulturae 2022, 8, 885. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Vonshak, A. Scaling up Microalgal Cultures to Commercial Scale. Eur. J. Phycol. 2017, 52, 407–418. [Google Scholar] [CrossRef]
- Szyjka, S.J.; Mandal, S.; Schoepp, N.G.; Tyler, B.M.; Yohn, C.B.; Poon, Y.S.; Villareal, S.; Burkart, M.D.; Shurin, J.B.; Mayfield, S.P. Evaluation of Phenotype Stability and Ecological Risk of a Genetically Engineered Alga in Open Pond Production. Algal Res. 2017, 24, 378–386. [Google Scholar] [CrossRef]
- Bella, F.; Sibí, M. Review of Luminescence-Based Light Spectrum Modifications Methods and Materials for Photovoltaics Applications. Materials 2023, 16, 3112. [Google Scholar] [CrossRef]
- Fang, M.J.; Tsao, C.W.; Hsu, Y.J. Semiconductor Nanoheterostructures for Photoconversion Applications. J. Phys. D Appl. Phys. 2020, 53, 143001. [Google Scholar] [CrossRef]
- LLEAF. Available online: https://lleaf.com/ (accessed on 28 April 2023).
- UbiGro Greenhouse Film|Shop Greenhouse Covering Materials. Available online: https://ubigro.com/ (accessed on 28 April 2023).
- De Salvador, F.R.; Scarascia Mugnozza, G.; Vox, G.; Schettini, E.; Mastrorilli, M.; Bou Jaoudé, M. Innovative Photoselective and Photoluminescent Plastic Films for Protected Cultivation. Acta Hortic. 2008, 801, 115–121. [Google Scholar] [CrossRef]
- González, A.; Rodríguez, R.; Bañón, S.; Franco, J.A.; Fernández, J.A.; Salmerón, A.; Espí, E. Strawberry and Cucumber Cultivation under Fluorescent Photoselective Plastic Films Cover. Acta Hortic. 2003, 614, 407–413. [Google Scholar] [CrossRef]
- Edser, C. Light Manipulating Additives Extend Opportunities for Agricultural Plastic Films. Plast. Addit. Compd. 2002, 4, 20–24. [Google Scholar] [CrossRef]
- Hamada, K.; Shimasaki, K.; Nishimura, Y.; Oyama-Egawa, H.; Yoshida, K. Effects of Red, Blue and Yellow Fluorescent Films on Proliferation and Organogenesis in Cymbidium and Phalaenopsis PLB in Vitro. Acta Hortic. 2011, 907, 381–384. [Google Scholar] [CrossRef]
- Hamada, K.; Shimasaki, K.; Ogata, T.; Nishimura, Y.; Nakamura, K.; Oyama-Egawa, H.; Yoshida, K. Effects of Spectral Composition Conversion Film and Plant Growth Regulators on Proliferation of Cymbidium Protocorm Like Body (PLB) Cultured In Vitro. Environ. Control Biol. 2010, 48, 127–132. [Google Scholar] [CrossRef][Green Version]
- Hemming, S.; van Os, E.A.; Hemming, J.; Dieleman, J.A. The Effect of New Developed Fluorescent Greenhouse Films on the Growth of Fragaria x Ananassa “Elsanta”. Eur. J. Hortic. Sci. 2006, 71, 145–154. [Google Scholar]
- Hidaka, K.; Yoshida, K.; Shimasaki, K.; Murakami, K.; Yasutake, D.; Kitano, M. Spectrum Conversion Film for Regulation of Plant Growth. J. Fac. Agric. Kyushu Univ. 2008, 53, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Ke-li, Z.; Liang-jie, Y.; Mei-yun, X.; You-zu, Y.; Ju-tang, S. The Application of Lights-Conversed Polyethylene Film for Agriculture. Wuhan Univ. J. Nat. Sci. 2002, 7, 365–367. [Google Scholar] [CrossRef]
- Nishimura, Y.; Wada, E.; Fukumoto, Y.; Aruga, H.; Shimoi, Y. The Effect of Spectrum Conversion Covering Film on Cucumber in Soilless Culture. Acta Hortic. 2012, 956, 481–487. [Google Scholar] [CrossRef]
- Novoplansky, A.; Sachs, T.; Cohen, D.; Bar, R.; Bodenheimer, J.; Reisfeld, R. Increasing Plant Productivity by Changing the Solar Spectrum. Sol. Energy Mater. 1990, 21, 17–23. [Google Scholar] [CrossRef]
- Sánchez-Lanuza, M.B.; Menéndez-Velázquez, A.; Peñas-Sanjuan, A.; Navas-Martos, F.J.; Lillo-Bravo, I.; Delgado-Sánchez, J.M. Advanced Photonic Thin Films for Solar Irradiation Tuneability Oriented to Greenhouse Applications. Materials 2021, 14, 2357. [Google Scholar] [CrossRef]
- Schettini, E.; de Salvador, F.R.; Scarascia-Mugnozza, G.; Vox, G. Radiometric Properties of Photoselective and Photoluminescent Greenhouse Plastic Films and Their Effects on Peach and Cherry Tree Growth. J. Hortic. Sci. Biotechnol. 2015, 86, 79–83. [Google Scholar] [CrossRef]
- Simakin, A.V.; Ivanyuk, V.V.; Dorokhov, A.S.; Gudkov, S.V. Photoconversion Fluoropolymer Films for the Cultivation of Agricultural Plants Under Conditions of Insufficient Insolation. Appl. Sci. 2020, 10, 8025. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Z.; Dong, R.; Xie, G.; Zhou, J.; Wu, K.; Zhang, H.; Cai, Q.; Lei, B. Characterization and Properties of a Sr2Si5N8:Eu2+-Based Light-Conversion Agricultural Film. J. Rare Earths 2020, 38, 539–545. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kim, J.H.; Park, K.S.; Namgoong, J.W.; Hwang, T.G.; Kim, J.P.; Son, J.E. Quantitative Methods for Evaluating the Conversion Performance of Spectrum Conversion Films and Testing Plant Responses under Simulated Solar Conditions. Hortic. Environ. Biotechnol. 2020, 61, 999–1009. [Google Scholar] [CrossRef]
- In Yoon, H.; Hyeun Kang, J.; Kim, D.; Eek Son, J. Seedling Quality and Photosynthetic Characteristic of Vegetables Grown Under a Spectrum Conversion Film. J. Bio-Environ. Control. 2021, 30, 110–117. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Parrish, C.H.; Hebert, D.; Jackson, A.; Ramasamy, K.; McDaniel, H.; Giacomelli, G.A.; Bergren, M.R. Optimizing Spectral Quality with Quantum Dots to Enhance Crop Yield in Controlled Environments. Commun. Biol. 2021, 4, 124. [Google Scholar] [CrossRef] [PubMed]
- Ivanyuk, V.V.; Shkirin, A.V.; Belosludtsev, K.N.; Dubinin, M.V.; Kozlov, V.A.; Bunkin, N.F.; Dorokhov, A.S.; Gudkov, S.V. Influence of Fluoropolymer Film Modified with Nanoscale Photoluminophor on Growth and Development of Plants. Front. Phys. 2020, 8, 616040. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Yanykin, D.V.; Simakin, A.V.; Paskhin, M.O.; Ivanyuk, V.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; Gudkov, S.V. Cultivation of Solanum lycopersicum under Glass Coated with Nanosized Upconversion Luminophore. Appl. Sci. 2021, 11, 10726. [Google Scholar] [CrossRef]
- Minich, A.S.; Minich, I.B.; Shaitarova, O.V.; Permyakova, N.L.; Zelenchukova, N.S.; Ivanitskiy, A.E.; Filatov, D.A.; Ivlev, G.A. Vital Activity of Lactuca Sativa and Soil Microorganisms under Fluorescent Films. TPSU Bull. 2011, 8, 78–84. [Google Scholar]
- Golovatskaya, I.F.; Minich, A.S.; Bolshakova, M.A. Regulation and Development of Brassica oleracea L. Plants Growth with the Help of Sunlight Correction. Tomsk. State Univ. J. Biol. 2012, 2, 151–165. [Google Scholar]
- Zelenchukova, N.S.; Ivanitsky, A.E.; Agaeva, S.A.; Tishkina, V.N. Productivity, Ascorbic Acid Synthesis and Catalase Activity in Lactuca sativa L. Leaves under Plastic Films. TSPU Bull. 2013, 8, 55–59. [Google Scholar]
- Yanykin, D.V.; Burmistrov, D.E.; Simakin, A.V.; Ermakova, J.A.; Gudkov, S.V. Effect of Up-Converting Luminescent Nanoparticles with Increased Quantum Yield Incorporated into the Fluoropolymer Matrix on Solanum lycopersicum Growth. Agronomy 2022, 12, 108. [Google Scholar] [CrossRef]
- Yanykin, D.V.; Paskhin, M.O.; Simakin, A.V.; Burmistrov, D.E.; Pobedonostsev, R.V.; Vyatchinov, A.A.; Vedunova, M.V.; Kuznetsov, S.V.; Ermakova, J.A.; Alexandrov, A.A.; et al. Plant Photochemistry under Glass Coated with Upconversion Luminescent Film. Appl. Sci. 2022, 12, 7480. [Google Scholar] [CrossRef]
- De Sousa Filho, P.C.; Lima, J.F.; Serra, O.A. From Lighting to Photoprotection: Fundamentals and Applications of Rare Earth Materials. J. Braz. Chem. Soc. 2015, 26, 2471–2495. [Google Scholar] [CrossRef]
- Chen, Q.H.; Shi, S.Y.; Zhang, W. gong Study on the Structure and Luminescent Properties of the Coordinated Eu2O3 Ethanol Colloids. Mater. Chem. Phys. 2009, 114, 58–62. [Google Scholar] [CrossRef]
- Feng, J.; Shan, G.; Maquieira, A.; Koivunen, M.E.; Guo, B.; Hammock, B.D.; Kennedy, I.M. Functionalized Europium Oxide Nanoparticles Used as a Fluorescent Label in an Immunoassay for Atrazine. Anal. Chem. 2003, 75, 5282–5286. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, X.; Gao, Z. Determination of Trace Amounts of Mercury Using Hierarchically Nanostructured Europium Oxide. Talanta 2010, 82, 1924–1928. [Google Scholar] [CrossRef]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Sudarsan, V.; Van Veggel, F.C.J.M.; Herring, R.A.; Raudsepp, M. Surface Eu3+ Ions Are Different than “Bulk” Eu3+ Ions in Crystalline Doped LaF3 Nanoparticles. J. Mater. Chem. 2005, 15, 1332–1342. [Google Scholar] [CrossRef]
- Janssens, S.; Williams, G.V.M.; Clarke, D. Systematic Study of Sensitized LaF3: Eu3+ Nanoparticles. J. Appl. Phys. 2011, 109, 23506. [Google Scholar] [CrossRef]
- Vistovskyy, V.; Malyi, T.; Vas’kiv, A.; Chylii, M.; Mitina, N.; Zaichenko, A.; Gektin, A.; Voloshinovskii, A. Luminescent Properties of LuPO4-Pr and LuPO4-Eu Nanoparticles. J. Lumin. 2016, 179, 527–532. [Google Scholar] [CrossRef]
- Huignard, A.; Buissette, V.; Franville, A.C.; Gacoin, T.; Boilot, J.P. Emission Processes in YVO4:Eu Nanoparticles. J. Phys. Chem. B 2003, 107, 6754–6759. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced Photocatalytic Degradation of Methylene Blue and Methyl Orange by ZnO:Eu Nanoparticles. Appl. Catal. B 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Chen, W.; Malm, J.-O.; Zwiller, V.; Huang, Y.; Liu, S.; Wallenberg, R.; Bovin, J.-O.; Samuelson, L. Energy Structure and Fluorescence of Eu2+ in ZnS:Eu Nanoparticles. Phys. Rev. B 2000, 61, 11021. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Pietrasik, E.; Goryczka, T.; Pisarski, W.A. Structural and Luminescence Properties of Silica Powders and Transparent Glass-Ceramics Containing LaF3:Eu3+ Nanocrystals. J. Am. Ceram. Soc. 2018, 101, 4654–4668. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, J.; Cao, X. Facile Synthesis and Photoluminescence of Europium Ion Doped LaF3 Nanodisks. Eur. J. Inorg. Chem. 2007, 2007, 3863–3867. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Zhang, Y.; Zheng, Y.; Xu, Z.; Liu, R. Structure and Luminescent Properties of Electrodeposited Eu3+-Doped CaF2 Thin Films. Thin Solid Films 2014, 562, 478–484. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Dąbrowski, P.; Cetner, M.D.; Samborska, I.A.; Łukasik, I.; Brestic, M.; Zivcak, M.; Tomasz, H.; Mojski, J.; Kociel, H.; et al. A Comparison between Different Chlorophyll Content Meters under Nutrient Deficiency Conditions. J. Plant Nutr. 2016, 40, 1024–1034. [Google Scholar] [CrossRef]
- Walz, H. DUAL-PAM-100 DUAL-PAM/F MANUAL. Available online: https://www.walz.com/files/downloads/manuals/dual-pam-100/DualPamEd05.pdf (accessed on 17 July 2023).
- Yamori, W.; Makino, A.; Shikanai, T. A Physiological Role of Cyclic Electron Transport around Photosystem I in Sustaining Photosynthesis under Fluctuating Light in Rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef]
- Available online: https://www.walz.com/files/downloads/manuals/gfs-3000/GFS-3000_Manual_9.pdf (accessed on 17 July 2023).
- James, W.C. Illustrated Series of Assessment Keys for Plant Diseases, Their Preparation and Usage. Can. Plant Dis. Surv. 1971, 51, 39–65. [Google Scholar]
- Filippov, A.V. Late Blight of Potatoes. Plant Prot. Quar. 2012, 5, 61–88. [Google Scholar]
- Orlovskii, Y.V.; Popov, A.V.; Orlovskaya, E.O.; Vanetsev, A.S.; Vagapova, E.A.; Rähn, M.; Sammelselg, V.; Sildos, I.; Baranchikov, A.E.; Grachev, P.V.; et al. Comparison of Concentration Dependence of Relative Fluorescence Quantum Yield and Brightness in First Biological Window of Wavelengths for Aqueous Colloidal Solutions of Nd3+: LaF3 and Nd3+: KY3F10 Nanocrystals Synthesized by Microwave-Hydrothermal Treatment. J. Alloys Compd. 2018, 756, 182–192. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Flier, W.G. The Biology of Phytophthora infestans at Its Center of Origin*. Annu. Rev. Phytopathol. 2005, 43, 171–190. [Google Scholar] [CrossRef]
- Yu, L.; Song, H.; Lu, S.; Liu, Z.; Yang, L.; Kong, X. Luminescent Properties of LaPO4:Eu Nanoparticles and Nanowires. J. Phys. Chem. B 2004, 108, 16697–16702. [Google Scholar] [CrossRef]
- Syamchand, S.S.; Sony, G. Europium Enabled Luminescent Nanoparticles for Biomedical Applications. J. Lumin. 2015, 165, 190–215. [Google Scholar] [CrossRef]
- Yan, D.; Lei, B.; Chen, B.; Wu, X.J.; Liu, Z.; Li, N.; Ge, J.; Xue, Y.; Du, Y.; Zheng, Z.; et al. Synthesis of High-Quality Lanthanide Oxybromides Nanocrystals with Single-Source Precursor for Promising Applications in Cancer Cells Imaging. Appl. Mater. Today 2015, 1, 20–26. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sharma, P.; Kumar, S.; Alex, S.A.; Kumar, R.; Mehta, S.K.; Mukherjee, A.; Umar, A. A comparative multi-assay approach to study the toxicity behaviour of Eu2O3 nanoparticles. J. Mol. Liq. 2018, 269, 783–795. [Google Scholar] [CrossRef]
- Kattel, K.; Park, J.Y.; Xu, W.; Kim, H.G.; Lee, E.J.; Bony, B.A.; Heo, W.C.; Chang, Y.; Kim, T.J.; Do, J.Y.; et al. Water-soluble ultrasmall Eu2O3 nanoparticles as a fluorescent imaging agent: In vitro and in vivo studies. Colloids Surf. A Physicochem. Eng. Asp. 2012, 394, 85–91. [Google Scholar] [CrossRef]
- Olifirenko, V.; Abduraimova, A.; Kang, M.S.; Raja, I.S.; Duisenbayeva, B.; Molkenova, A.; Khamkhash, L.; Hwang, Y.-H.; Han, D.-W.; Atabaev, T.S. Potential applicability of polyethyleneimine PEI-coated Eu2O3 and Dy2O3 nanoparticles for contrast enhancement in computed tomography. Nano Express 2021, 2, 010022. [Google Scholar] [CrossRef]
- Zhang, Q.; Pratt, E.C.; Tamura, R.; Ogirala, A.; Hsu, H.T.; Farahmand, N.; O’Brien, S.; Grimm, J. Ultrasmall downconverting nanoparticle for enhanced Cerenkov imaging. Nano Lett. 2021, 21, 4217–4224. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Andreev, S.N.; Barmina, E.V.; Bunkin, N.F.; Kartabaeva, B.B.; Nesvat, A.P.; Stepanov, E.V.; Taranda, N.I.; Khramov, R.N.; Glinushkin, A.P. Effect of Visible Light on Biological Objects: Physiological and Pathophysiological Aspects. Phys. Wave Phenom. 2017, 25, 207–213. [Google Scholar] [CrossRef]
- Pearcy, R.W. Sunflecks and Photosynthesis in Plant Canopies. Annu. Rev. Plant Biol. 2003, 41, 421–453. [Google Scholar] [CrossRef]
- Elizabete Carmo-Silva, A.; Salvucci, M.E. The Regulatory Properties of Rubisco Activase Differ among Species and Affect Photosynthetic Induction during Light Transitions. Plant Physiol. 2013, 161, 1645–1655. [Google Scholar] [CrossRef]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco Activase Is a Key Regulator of Non-Steady-State Photosynthesis at Any Leaf Temperature and, to a Lesser Extent, of Steady-State Photosynthesis at High Temperature. Plant J. 2012, 71, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Dau, H. New Trends in Photobiology: Short-Term Adaptation of Plants to Changing Light Intensities and Its Relation to Photosystem II Photochemistry and Fluorescence Emission. J. Photochem. Photobiol. B 1994, 26, 3–27. [Google Scholar] [CrossRef]
- Hansen, U.P.; Moldaenke, C.; Tabrizi, H.; Ramm, D. The Effect of Transthylakoid Proton Uptake on Cytosolic PH and the Imbalance of ATP and NAPDH/H+ Production as Measured by CO2- and Light-Induced Depolarisation of the Plasmalemma. Plant Cell Physiol. 1993, 34, 681–695. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Dark Recovery of the Chl a Fluorescence Transient (OJIP) after Light Adaptation: The QT-Component of Non-Photochemical Quenching Is Related to an Activated Photosystem I Acceptor Side. Biochim. Biophys. Acta (BBA)—Bioenerg. 2006, 1757, 787–797. [Google Scholar] [CrossRef]
- Allen, J.F.; Gantt, E.; Golbeck, J.H.; Osmond, B.; Schansker, G.; Yuan, Y.; Strasser, R.J. Chl a Fluorescence and 820 Nm Transmission Changes Occurring During a Dark-to-Light Transition in Pine Needles and Pea Leaves: A Comparison. In Photosynthesis. Energy from the Sun; Springer: Dordrecht, The Netherlands, 2008; pp. 945–949. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Rehman, M.; Fahad, S.; Saleem, M.H.; Hafeez, M.; Rahman, M.; Liu, F.; Deng, G. Red Light Optimized Physiological Traits and Enhanced the Growth of Ramie (Boehmeria nivea L.). Photosynthetica 2020, 58, 922–931. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Seif, M.; Arab, M.; Zare Mehrjerdi, M.; Li, T.; Lastochkina, O. Growth and Photosynthetic Performance of Calendula Officinalis under Monochromatic Red Light. Int. J. Hortic. Sci. Technol. 2018, 5, 123–132. [Google Scholar] [CrossRef]
- XiaoYing, L.; ShiRong, G.; ZhiGang, X.; XueLei, J.; Tezuka, T. Regulation of Chloroplast Ultrastructure, Cross-Section Anatomy of Leaves, and Morphology of Stomata of Cherry Tomato by Different Light Irradiations of Light-Emitting Diodes. HortScience 2011, 46, 217–221. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The Impact of the Phytochromes on Photosynthetic Processes. Biochim. Biophys. Acta (BBA)—Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to Lower Red to Far-Red Light Ratios Improve Tomato Tolerance to Salt Stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Duchovskis, P.; Urbonavičiūtė, A.; Samuolienė, G.; Jankauskienė, J.; Sakalauskaitė, J.; Šabajevienė, G.; Sirtautas, R.; Novičkovas, A. The Effect of Light-Emitting Diodes Lighting on the Growth of Tomato Transplants. Zemdirb.-Agric. 2010, 97, 98. [Google Scholar]
- Brazaityte, A.; Duchovskis, P.; Urbonaviciute, A.; Samuoliene, G.; Jankauskiene, J.; Kasiuleviciute-Bonakere, A.; Bliznikas, Z.; Novickovas, A.; Breive, K.; Zukauskas, A. The Effect of Light-Emitting Diodes Lighting on Cucumber Transplants and after-Effect on Yield. Zemdirb.-Agric. 2009, 96, 102–118. [Google Scholar]
- Saleem, M.H.; Gohar, F.; Muhammaf, I.F.; Rehman, O.; Naseem, N.; Iqbal, M.; Hassan, A. Effect of Different Colors of Lights on Growth and Antioxidants Capacity in Rapeseed (Brassica napus L.) Seedlings. Ann. Agric. Crop Sci. 2019, 4, 1045. [Google Scholar]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The Effect of Light Quality on Seed Germination, Seedling Growth and Selected Biochemical Properties of Stevia Rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on Net Photosynthetic Rate, Growth and Leaf Stomata of Chrysanthemum Plantlets in Vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Castillo, J.A. Influence of Light on Plant–Phyllosphere Interaction. Front. Plant Sci. 2018, 9, 1482. [Google Scholar] [CrossRef]
- Carvalho, S.; Folta, K. Expanding Genetic Potential with Light Environmentally Modified Organisms. Crit. Rev. Plant Sci. 2015, 2689, 486–508. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Karlsson, M.; Rosberg, A.K.; Dorais, M.; Naznin, M.T.; Khalil, S.; Bergstrand, K.J. Light and Microbial Lifestyle: The Impact of Light Quality on Plant–Microbe Interactions in Horticultural Production Systems—A Review. Horticulturae 2019, 5, 41. [Google Scholar] [CrossRef]
- Losi, A.; Gärtner, W. A Light Life Together: Photosensing in the Plant Microbiota. Photochem. Photobiol. Sci. 2021, 20, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roldán, M.C.; Garre, V.; Guarro, J.; Mariné, M.; Roncero, M.I.G. Role of the White Collar 1 Photoreceptor in Carotenogenesis, UV Resistance, Hydrophobicity, and Virulence of Fusarium Oxysporum. Eukaryot. Cell 2008, 7, 1227–1230. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of Plant Growth and Drought Tolerance by Native Microorganisms (AM Fungi and Bacteria) from Dry Environments: Mechanisms Related to Bacterial Effectiveness. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Bergstrand, K.J.; Hartmann, R.; Gharaie, S.; Wohanka, W.; Dorais, M.; Rosberg, A.K. Ornamental Flowers in New Light: Artificial Lighting Shapes the Microbial Phyllosphere Community Structure of Greenhouse Grown Sunflowers (Helianthus annuus L.). Sci. Hortic. 2017, 216, 234–247. [Google Scholar] [CrossRef]
- Canessa, P.; Schumacher, J.; Hevia, M.A.; Tudzynski, P.; Larrondo, L.F. Assessing the Effects of Light on Differentiation and Virulence of the Plant Pathogen Botrytis Cinerea: Characterization of the White Collar Complex. PLoS ONE 2013, 8, e84223. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Gharaie, S.; Vaas, L.A.I.; Rosberg, A.K.; Windstam, S.T.; Karlsson, M.E.; Bergstrand, K.J.; Khalil, S.; Wohanka, W.; Alsanius, B.W. Light Spectrum Modifies the Utilization Pattern of Energy Sources in Pseudomonas sp. DR 5-09. PLoS ONE 2017, 12, e0189862. [Google Scholar] [CrossRef]
- Kim, H.; Ridenour, J.B.; Dunkle, L.D.; Bluhm, B.H. Regulation of Stomatal Tropism and Infection by Light in Cercospora Zeae-Maydis: Evidence for Coordinated Host/Pathogen Responses to Photoperiod? PLoS Pathog. 2011, 7, e1002113. [Google Scholar] [CrossRef]
- Ballario, P.; Vittorioso, P.; Magrelli, A.; Talora, C.; Cabibbo, A.; Macino, G.; Sapienza, L.; di Biopatologia Umana, D.; Umberto, P.I.; Regina Margherita, V. White Collar-1, a Central Regulator of Blue Light Responses in Neurospora, Is a Zinc Finger Protein. EMBO J. 1996, 15, 1650–1657. [Google Scholar] [CrossRef]
- Choudhury, R.A.; McRoberts, N. Temperature and Light Effects on in Vitro Germination of Peronospora Effusa Sporangia. Trop. Plant Pathol. 2018, 43, 572–576. [Google Scholar] [CrossRef]
- Cetz-Chel, J.E.; Balcázar-López, E.; Esquivel-Naranjo, E.U.; Herrera-Estrella, A. The Trichoderma Atroviride Putative Transcription Factor Blu7 Controls Light Responsiveness and Tolerance. BMC Genom. 2016, 17, 327. [Google Scholar] [CrossRef]
- Ansari, K.I.; Doyle, S.M.; Kacprzyk, J.; Khan, M.R.; Walter, S.; Brennan, J.M.; Arunachalam, C.S.; McCabe, P.F.; Doohan, F.M. Light Influences How the Fungal Toxin Deoxynivalenol Affects Plant Cell Death and Defense Responses. Toxins 2014, 6, 679–692. [Google Scholar] [CrossRef]
- Balint-Kurti, P.; Simmons, S.J.; Blum, J.E.; Ballaré, C.L.; Stapleton, A.E. Maize Leaf Epiphytic Bacteria Diversity Patterns Are Genetically Correlated with Resistance to Fungal Pathogen Infection. Mol. Plant-Microbe Interact. 2010, 23, 473–484. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, S.A.; Yun, H.K. Inhibition of Botrytis Cinerea and Accumulation of Stilbene Compounds by Light-Emitting Diodes of Grapevine Leaves and Differential Expression of Defense-Related Genes. Eur. J. Plant Pathol. 2015, 143, 753–765. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhao, F.F.; Zhang, M.; Lin, H.H.; Xi, D.H. Effects of Light Quality on the Interaction between Cucumber Mosaic Virus and Nicotiana Tabacum. J. Phytopathol. 2015, 163, 1002–1013. [Google Scholar] [CrossRef]
- De Wit, M.; Spoel, S.H.; Sanchez-Perez, G.F.; Gommers, C.M.M.; Pieterse, C.M.J.; Voesenek, L.A.C.J.; Pierik, R. Perception of Low Red: Far-Red Ratio Compromises Both Salicylic Acid- and Jasmonic Acid-Dependent Pathogen Defences in Arabidopsis. Plant J. 2013, 75, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Demkura, P.V.; Ballaré, C.L. UVR8 Mediates UV-B-Induced Arabidopsis Defense Responses against Botrytis Cinerea by Controlling Sinapate Accumulation. Mol. Plant 2012, 5, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Yu, N.; Li, W.; Vanhaelewyn, L.; Hamshou, M.; Van Der Straeten, D.; Smagghe, G. An Ultraviolet B Condition That Affects Growth and Defense in Arabidopsis. Plant Sci. 2018, 268, 54–63. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B Irradiation Changes Specifically the Secondary Metabolite Profile in Broccoli Sprouts: Induced Signaling Overlaps with Defense Response to Biotic Stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef]
- Gouinguené, S.P.; Turlings, T.C.J. The Effects of Abiotic Factors on Induced Volatile Emissions in Corn Plants. Plant Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef]
- Kook, H.S.; Park, S.H.; Jang, Y.J.; Lee, G.W.; Kim, J.S.; Kim, H.M.; Oh, B.T.; Chae, J.C.; Lee, K.J.; Moo Kim, H. Blue LED (Light-Emitting Diodes)-Mediated Growth Promotion and Control of Botrytis Disease in Lettuce. Acta Agric. Scand. Sect. B—Soil. Plant Sci. 2013, 63, 271–277. [Google Scholar] [CrossRef]
- Young, H.M.; George, S.; Narváez, D.F.; Srivastava, P.; Schuerger, A.C.; Wright, D.L.; Marois, J.J. Effect of Solar Radiation on Severity of Soybean Rust. Phytopathology 2012, 102, 794–803. [Google Scholar] [CrossRef][Green Version]
- Nagendran, R.; Lee, Y.H. Green and Red Light Reduces the Disease Severity by Pseudomonas Cichorii JBC1 in Tomato Plants via Upregulation of Defense-Related Gene Expression. Phytopathology 2015, 105, 412–418. [Google Scholar] [CrossRef]
- Khanam, N.N.; Ueno, M.; Kihara, J.; Honda, Y.; Arase, S. Suppression of Red Light-Induced Resistance in Broad Beans to Botrytis Cinerea by Salicylic Acid. Physiol. Mol. Plant Pathol. 2005, 66, 20–29. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.P.; Yu, H.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Light Quality Affects Incidence of Powdery Mildew, Expression of Defence-Related Genes and Associated Metabolism in Cucumber Plants. Eur. J. Plant Pathol. 2010, 127, 125–135. [Google Scholar] [CrossRef]
- Suthaparan, A.; Torre, S.; Stensvand, A.; Herrero, M.L.; Pettersen, R.I.; Gadoury, D.M.; Gislerød, H.R. Specific Light-Emitting Diodes Can Suppress Sporulation of Podosphaera Pannosa on Greenhouse Roses. Plant Dis. 2010, 94, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Vaknin, M.; Ben-Naim, Y.; Rubin, A.E. Light Suppresses Sporulation and Epidemics of Peronospora Belbahrii. PLoS ONE 2013, 8, e81282. [Google Scholar] [CrossRef] [PubMed]
- Zahirul, S.; Islam Slam, I.; Mohammad, M.; Abadoost, B.; Sadia, S.; Bekal Ekal, B.; Kris, K.; Lambert Ambert, L. Red Light-Induced Systemic Disease Resistance against Root-Knot Nematode Meloidogyne javanica and Pseudomonas syringae Pv. Tomato DC 3000. J. Phytopathol. 2008, 156, 708–714. [Google Scholar] [CrossRef]
- Xu, H.; Fu, Y.N.; Li, T.L.; Wang, R. Effects of Different LED Light Wavelengths on the Resistance of Tomato against Botrytis Cinerea and the Corresponding Physiological Mechanisms. J. Integr. Agric. 2017, 16, 106–114. [Google Scholar] [CrossRef]
- Zahirul Islam, S.; Honda, Y.; Sawa, Y.; Babadoost, M. Characterization of Antifungal Glycoprotein in Red-Light-Irradiated Broadbean Leaflets. Mycoscience 2002, 43, 471–473. [Google Scholar] [CrossRef]
- Shirasawa, H.; Ueno, M.; Kihara, J.; Arase, S. Protective Effect of Red Light against Blast Disease Caused by Magnaporthe Oryzae in Rice. Crop Prot. 2012, 39, 41–44. [Google Scholar] [CrossRef]
- Islam, S.Z.; Honda, Y.; Sonhaji, M. Phototropism of Conidial Germ Tubes of Botrytis Cinerea and Its Implication in Plant Infection Processes. Plant Dis. 2007, 82, 850–856. [Google Scholar] [CrossRef]
- Shibuya, T.; Itagaki, K.; Tojo, M.; Endo, R.; Kitaya, Y. Fluorescent Illumination with High Red-to-Far-Red Ratio Improves Resistance of Cucumber Seedlings to Powdery Mildew. HortScience 2011, 46, 429–431. [Google Scholar] [CrossRef]
- Cerrudo, I.; Keller, M.M.; Cargnel, M.D.; Demkura, P.V.; de Wit, M.; Patitucci, M.S.; Pierik, R.; Pieterse, C.M.J.; Ballaré, C.L. Low Red/Far-Red Ratios Reduce Arabidopsis Resistance to Botrytis Cinerea and Jasmonate Responses via a COI1-JAZ10-Dependent, Salicylic Acid-Independent Mechanism. Plant Physiol. 2012, 158, 2042–2052. [Google Scholar] [CrossRef]
- Legard, D.E.; Xiao, C.L.; Mertely, J.C.; Chandler, C.K. Effects of Plant Spacing and Cultivar on Incidence of Botrytis Fruit Rot in Annual Strawberry. Plant Dis. 2007, 84, 531–538. [Google Scholar] [CrossRef]
- Elad, Y.; Israeli, L.; Fogel, M.; Rav David, D.; Kenigsbuch, D.; Chalupowicz, D.; Maurer, D.; Lichter, A.; Silverman, D.; Biton, S.; et al. Conditions Influencing the Development of Sweet Basil Grey Mould and Cultural Measures for Disease Management. Crop Prot. 2014, 64, 67–77. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, L.; Chi, Y. Spring phenology rather than climate dominates the trends in peak of growing season in the Northern Hemisphere. Glob. Chang. Biol. 2023, 29, 4543–4555. [Google Scholar] [CrossRef]





| C. sativus | S. lycopersicum | |||||
|---|---|---|---|---|---|---|
| Control | Eu3+:LaF3 | Eu2O3 | Control | Eu3+:LaF3 | Eu2O3 | |
| Stem lenght, cm | - | - | - | 165 ± 12 a’ | 159 ± 8 a’ | 205 ± 8 b’ |
| Leaves area, cm2 | 245 ± 23 a | 175 ± 16 b | 263 ± 29 a | 383 ± 26 a’ | 362 ± 24 a’ | 528 ± 40 b’ |
| Leaves number | - | - | - | 16.0 ± 0.7 a’ | 15.3 ± 0.5 a’ | 17.4 ± 1.6 a’ |
| Internodes length, cm | - | - | - | 7.7 ± 0.3 a’ | 8.0 ± 0.3 a’ | 7.8 ± 0.2 a’ |
| Dry weight/Fresh weight | 0.13 ± 0.01 a | 0.12 ± 0.01 a | 0.12 ± 0.01 a | 0.12 ± 0.02 a’ | 0.12 ± 0.01 a’ | 0.11 ± 0.01 a’ |
| Chlorophyll content, a.u. | 10.5 ± 0.3 a | 8.1 ± 0.5 a | 10.0 ± 0.2 a | 20.8 ± 1.5 a’ | 17.1 ± 1.3 ab’ | 15.3 ± 0.5 b’ |
| Chlorophyll determination formula | y = 0.636 + 0.036x | y = 0.63 + 0.0535x | ||||
| Chlorophyll content, mg Chl g−1 of fresh weight | 1.01 ± 0.03 a | 0.93 ± 0.06 a | 1.00 ± 0.02 a | 1.74 ± 0.13 a’ | 1.54 ± 0.12 ab’ | 1.44 ± 0.05 b’ |
| C. sativus | S. lycopersicum | ||||||
|---|---|---|---|---|---|---|---|
| Control | Eu3+:LaF3 | Eu2O3 | Control | Eu3+:LaF3 | Eu2O3 | ||
| A, µmol CO2 m−2 s−1 | Dark | −0.5 ± 0.01 a | −0.54 ± 0.03 a | −0.7 ± 0.1 a | −0.19 ± 0.01 a’ | −0.20 ± 0.06 a’ | −0.19 ± 0.03 a’ |
| Light | 2.6 ± 0.2 a | 2.5 ± 0.1 a | 2.9 ± 0.1 a | 3.1 ± 0.3 a’ | 2.3 ± 0.4 b’ | 3.3 ± 0.1 a’ | |
| E, mol H2O m−2 s−1 | Dark | 1.6 ± 0.2 a | 1.6 ± 0.2 a | 1.6 ± 0.1 a | 0.12 ± 0.01 a’ | 0.11 ± 0.01 a’ | 0.12 ± 0.03 a’ |
| Light | 2.3 ± 0.1 a | 2 ± 0.3 a | 2.4 ± 0.1 a | 0.55 ± 0.1 a’ | 0.5 ± 0.1 a’ | 0.57 ± 0.05 a’ | |
| GCO2, mmol m−2 s−1 | Dark | 72 ± 16 a | 103 ± 20 ab | 112 ± 8 b | 12 ± 0.1 a’ | 10 ± 0.3 a’ | 37 ± 4 b’ |
| Light | 135 ± 33 a | 160 ± 53 a | 173 ± 12 a | 56 ± 2 a’ | 37 ± 13 a’ | 90 ± 9 b’ | |
| GH2O, mmol m−2 s−1 | Dark | 112 ± 25 a | 162 ± 31 ab | 175 ± 13 b | 19 ± 0.2 a’ | 16 ± 0.5 a’ | 57 ± 6 b’ |
| Light | 211 ± 51 a | 249 ± 83 a | 270 ± 18 a | 87 ± 3 a’ | 57 ± 21 a’ | 140 ± 14 b’ | |
| Ci, ppm | Dark | 400 ± 1 a | 399 ± 1 a | 400 ± 1 a | 414 ± 1 a’ | 416 ± 5 a’ | 403 ± 1 b’ |
| Light | 353 ± 1 a | 358 ± 11 a | 353 ± 3 a | 334 ± 2 a’ | 311 ± 11 a’ | 347 ± 5 b’ | |
| C. sativus | S. lycopersicum | |||||
|---|---|---|---|---|---|---|
| Control | Eu3+:LaF3 | Eu2O3 | Control | Eu3+:LaF3 | Eu2O3 | |
| Fv/Fm | 0.8 ± 0.01 a | 0.8 ± 0.01 a | 0.8 ± 0.01 a | 0.81 ± 0.01 a’ | 0.81 ± 0.01 a’ | 0.81 ± 0.01 a’ |
| Y(II) | 0.58 ± 0.02 a | 0.5 ± 0.01 b | 0.58 ± 0.02 a | 0.58 ± 0.02 a’ | 0.57 ± 0.05 a’ | 0.6 ± 0.01 a’ |
| ETR(II), µmol electrons (PII s)−1 | 50.2 ± 1.4 a | 44.2 ± 0.6 b | 50.8 ± 1.3 a | 50.8 ± 1.4 a’ | 49.8 ± 4.3 a’ | 52.6 ± 0.2 a’ |
| Y(NPQ) | 0.10 ± 0.01 a | 0.16 ± 0.02 b | 0.12 ± 0.01 a | 0.08 ± 0.02 a’ | 0.09 ± 0.03 a’ | 0.06 ± 0.01 b’ |
| ETR(I), µmol electrons (PI s)−1 | 67.8 ± 1.4 a | 63.4 ± 2 b | 69.2 ± 0.4 a | 80.3 ± 0.6 a’ | 81.5 ± 1.7 a’ | 77.7 ± 1.5 a’ |
| Y(I) | 0.92 ± 0.01 a | 0.93 ± 0.02 a | 0.89 ± 0.02 a | 0.78 ± 0.02 a’ | 0.73 ± 0.02 b’ | 0.8 ± 0.01 a’ |
| +4 °C | +40 °C | ||||||
|---|---|---|---|---|---|---|---|
| Control | Eu3+:LaF3 | Eu2O3 | Control | Eu3+:LaF3 | Eu2O3 | ||
| A, umol CO2 m−2 s−1 | Dark | −0.26 ± 0.01 a | −0.25 ± 0.03 a | −0.16 ± 0.01 a | −0.28 ± 0.01 a’ | −0.41 ± 0.05 b’ | −0.16 ± 0.04 c’ |
| Light | 0.28 ± 0.01 a | 1.00 ± 0.2 b | 0.55 ± 0.02 a | 2.60 ± 0.2 a’ | 2.90 ± 0.1 a’ | 1.00 ± 0.3 b’ | |
| E, mol CO2 m−2 s−1 | Dark | 0.14 ± 0.01 a | 0.14 ± 0.02 a | 0.14 ± 0.01 a | 0.17 ± 0.02 a’ | 0.17 ± 0.01 a’ | 0.18 ± 0.01 a’ |
| Light | 0.29 ± 0.01 a | 0.36 ± 0.05 a | 0.21 ± 0.01 b | 0.61 ± 0.03 a’ | 0.66 ± 0.03 a’ | 0.30 ± 0.04 b’ | |
| GCO2, mmol m−2 s−1 | Dark | 8.0 ± 0.8 a | 10.0 ± 1 a | 3.9 ± 0.6 b | 11.6 ± 1.5 a’ | 9.3 ± 0.1 a’ | 4.7 ± 0.1 b’ |
| Light | 6.8 ± 0.2 a | 11.7 ± 3.8 a | 3.6 ± 0.9 b | 32.2 ± 0.2 a’ | 28.7 ± 2 a’ | 7.3 ± 2.1 b’ | |
| GH2O, mmol m−2 s−1 | Dark | 12.6 ± 1 a | 15.7 ± 1.7 a | 6.1 ± 1 b | 18.1 ± 1.8 a’ | 14.5 ± 0.2 a’ | 7.4 ± 0.1 b’ |
| Light | 11.0 ± 0.3 a | 18.2 ± 6 a | 5.6 ± 1.4 b | 50.2 ± 2.4 a’ | 44.7 ± 3.1 a’ | 11.4 ± 3.3 b’ | |
| Ci, ppm | Dark | 425 ± 2 a | 420 ± 6 a | 441 ± 11 a | 416 ± 3 a’ | 431 ± 5 b’ | 432 ± 7 b’ |
| Light | 353 ± 1 a | 305 ± 9 b | 291 ± 22 b | 313 ± 2 a’ | 301 ± 5 b’ | 246 ± 1 c’ | |
| +4 °C | +40 °C | |||||
|---|---|---|---|---|---|---|
| Control | Eu3+:LaF3 | Eu2O3 | Control | Eu3+:LaF3 | Eu2O3 | |
| Fv/Fm | 0.76 ± 0.01 a | 0.72 ± 0.01 b | 0.73 ± 0.02 c | 0.76 ± 0.01 a’ | 0.75 ± 0.01 a’ | 0.73 ± 0.01 a’ |
| Y(II) | 0.2 ± 0.03 a | 0.37 ± 0.04 b | 0.25 ± 0.06 a | 0.59 ± 0.01 a’ | 0.62 ± 0.01 a’ | 0.44 ± 0.02 b’ |
| ETR(II), µmol electrons (PSII s)−1 | 17 ± 3 a | 32 ± 3 b | 22 ± 5 a | 51 ± 1 a’ | 54 ± 1 a’ | 38 ± 2 b’ |
| Y(NPQ) | 0.42 ± 0.03 a | 0.26 ± 0.03 b | 0.37 ± 0.05 a | 0.04 ± 0.01 a’ | 0.01 ± 0.01 b’ | 0.19 ± 0.02 c’ |
| ETR(I), µmol electrons (PSI s)−1 | 42 ± 7 a | 60 ± 0.1 b | 46 ± 5 a | 70 ± 3 a’ | 69 ± 3 a’ | 57 ± 4 b’ |
| Y(I) | 0.48 ± 0.06 a | 0.69 ± 0.01 b | 0.53 ± 0.06 a | 0.8 ± 0.03 a’ | 0.8 ± 0.03 a’ | 0.66 ± 0.05 b’ |
| Control | Eu3+:LaF3 | Eu2O3 | |
|---|---|---|---|
| B:G | 0.70 a | 0.66 b | 0.68 ab |
| R:B | 1.30 a | 1.40 b | 1.40 b |
| R:FR | 2.80 a | 2.82 a | 2.64 a |
| R:PPFD | 0.35 a | 0.36 b | 0.36 b |
| O:PPFD | 0.135 a | 0.138 b | 0.136 a |
| O:R | 0.380 a | 0.385 a | 0.370 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskhin, M.O.; Yanykin, D.V.; Popov, A.V.; Pobedonostsev, R.V.; Kazantseva, D.V.; Dorokhov, A.S.; Izmailov, A.Y.; Vyatchinov, A.A.; Orlovskaya, E.O.; Shaidulin, A.T.; et al. Two Types of Europium-Based Photoconversion Covers for Greenhouse Farming with Different Effects on Plants. Horticulturae 2023, 9, 846. https://doi.org/10.3390/horticulturae9070846
Paskhin MO, Yanykin DV, Popov AV, Pobedonostsev RV, Kazantseva DV, Dorokhov AS, Izmailov AY, Vyatchinov AA, Orlovskaya EO, Shaidulin AT, et al. Two Types of Europium-Based Photoconversion Covers for Greenhouse Farming with Different Effects on Plants. Horticulturae. 2023; 9(7):846. https://doi.org/10.3390/horticulturae9070846
Chicago/Turabian StylePaskhin, Mark O., Denis V. Yanykin, Alexander V. Popov, Roman V. Pobedonostsev, Dina V. Kazantseva, Alexey S. Dorokhov, Andrey Yu. Izmailov, Alexey A. Vyatchinov, Elena O. Orlovskaya, Artem T. Shaidulin, and et al. 2023. "Two Types of Europium-Based Photoconversion Covers for Greenhouse Farming with Different Effects on Plants" Horticulturae 9, no. 7: 846. https://doi.org/10.3390/horticulturae9070846
APA StylePaskhin, M. O., Yanykin, D. V., Popov, A. V., Pobedonostsev, R. V., Kazantseva, D. V., Dorokhov, A. S., Izmailov, A. Y., Vyatchinov, A. A., Orlovskaya, E. O., Shaidulin, A. T., Orlovskii, Y. V., Vodeneev, V. A., & Gudkov, S. V. (2023). Two Types of Europium-Based Photoconversion Covers for Greenhouse Farming with Different Effects on Plants. Horticulturae, 9(7), 846. https://doi.org/10.3390/horticulturae9070846