Morpho-Physiological Responses of Three Italian Olive Tree (Olea europaea L.) Cultivars to Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Drought Stress Treatment
2.2. Soil Water Content
2.3. Relative Water Content of Leaves and Stems
2.4. Stomatal Density
2.5. Leaf Gas Exchange and Chlorophyll Fluorescence
2.6. Electrolyte Leakage
2.7. Malondialdehyde Content
2.8. Pigments Quantification
2.9. Statistical Analysis
3. Results
3.1. Drought Effects on Plant Water Status and Biochemical Responses
3.2. Impact of Water Deficit on the Photosynthetic Process
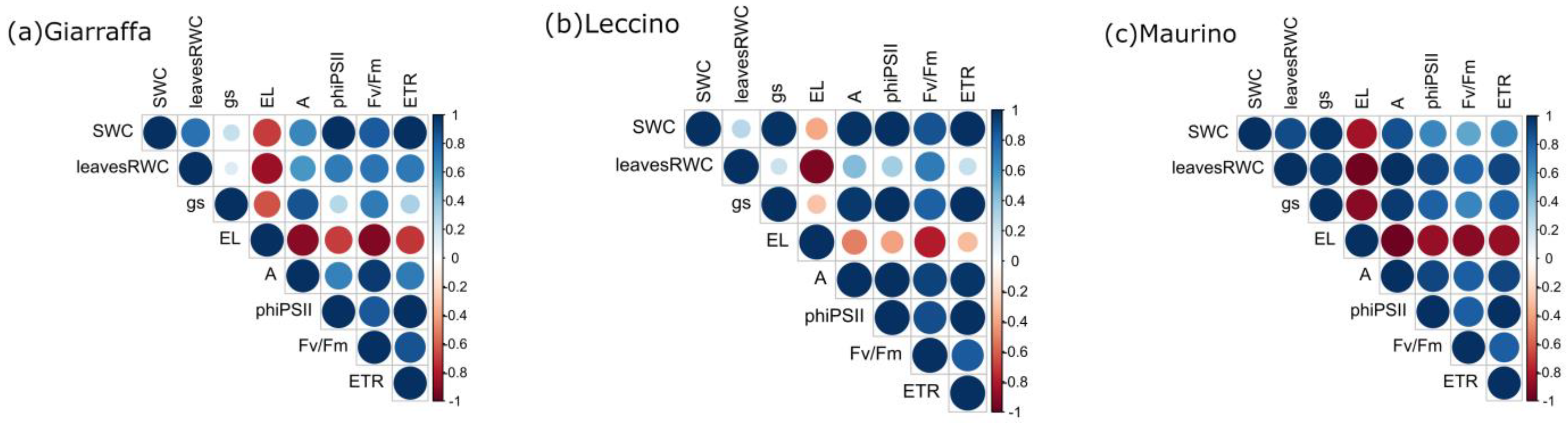
3.3. Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Vergni, L.; Todisco, F. Spatio-Temporal Variability of Precipitation, Temperature and Agricultural Drought Indices in Central Italy. Agric. For. Meteorol. 2011, 151, 301–313. [Google Scholar] [CrossRef]
- Douville, H.; Raghavan, K.; Renwick, J. Water Cycle Changes. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the IPCC; IPCC: Geneva, Switzerland, 2021; ISBN 978-1-00-915789-6. [Google Scholar]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Conesa, M.A.; Ochogavía, J.M.; Perdomo, J.A.; Francis, D.M.; Ribas-Carbó, M.; Savé, R.; Flexas, J.; Medrano, H.; Cifre, J. Physiological and Morphological Adaptations in Relation to Water Use Efficiency in Mediterranean Accessions of Solanum Lycopersicum. Plant Cell Environ. 2011, 34, 245–260. [Google Scholar] [CrossRef] [PubMed]
- El Yamani, M.; Sakar, E.H.; Boussakouran, A.; Rharrabti, Y. Physiological and Biochemical Responses of Young Olive Trees (Olea europaea L.) to Water Stress during Flowering. Arch. Biol. Sci. 2019, 71, 123–132. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of Water Stress on Gas Exchange, Water Relations, Chlorophyll Content and Leaf Structure in the Two Main Tunisian Olive (Olea europaea L.) Cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Lawlor, D.W. Limitation to Photosynthesis in Water-Stressed Leaves: Stomata vs. Metabolism and the Role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-Inhibition of Photosynthesis in C3 Plants: Stomatal and Non-Stomatal Limitations Revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Centritto, M.; Loreto, F.; Chartzoulakis, K. The Use of Low [CO2] to Estimate Diffusional and Non-Diffusional Limitations of Photosynthetic Capacity of Salt-Stressed Olive Saplings. Plant Cell Environ. 2003, 26, 585–594. [Google Scholar] [CrossRef]
- Hoshika, Y.; Paoletti, E.; Centritto, M.; Gomes, M.T.G.; Puértolas, J.; Haworth, M. Species-Specific Variation of Photosynthesis and Mesophyll Conductance to Ozone and Drought in Three Mediterranean Oaks. Physiol. Plant. 2022, 174, e13639. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of Water Deficit on Leaf Phenolic Composition, Gas Exchange, Oxidative Damage and Antioxidant Activity of Four Greek Olive (Olea europaea L.) Cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kitao, M.; Lei, T.T.; Koike, T.; Tobita, H.; Maruyama, Y. Susceptibility to Photoinhibition of Three Deciduous Broadleaf Tree Species with Different Successional Traits Raised under Various Light Regimes. Plant Cell Environ. 2000, 23, 81–89. [Google Scholar] [CrossRef]
- The World of Olive Oil. Available online: https://www.internationaloliveoil.org/the-world-of-olive-oil/ (accessed on 3 March 2023).
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 3 March 2023).
- Alba, V.; Montemurro, C.; Sabetta, W.; Pasqualone, A.; Blanco, A. SSR-Based Identification Key of Cultivars of Olea europaea L. Diffused in Southern-Italy. Sci. Hortic. 2009, 123, 11–16. [Google Scholar] [CrossRef]
- Albertini, E.; Torricelli, R.; Bitocchi, E.; Raggi, L.; Marconi, G.; Pollastri, L.; Di Minco, G.; Battistini, A.; Papa, R.; Veronesi, F. Structure of Genetic Diversity in Olea europaea L. Cultivars from Central Italy. Mol. Breed. 2011, 27, 533–547. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Vendramin, G.G.; Chiappetta, A. Genetic Biodiversity of Italian Olives (Olea europaea) Germplasm Analyzed by SSR Markers. Sci. World J. 2014, 2014, e296590. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Espejo, A.; Fernández, J.E.; Torres-Ruiz, J.M.; Rodriguez-Dominguez, C.M.; Perez-Martin, A.; Hernandez-Santana, V. The Olive Tree under Water Stress. In Water Scarcity and Sustainable Agriculture in Semiarid Environment: Tools, Strategies, and Challenges for Woody Crops; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-813164-0. [Google Scholar]
- Piccini, C.; Cai, G.; Dias, M.C.; Araújo, M.; Parri, S.; Romi, M.; Faleri, C.; Cantini, C. Olive Varieties under Uv-b Stress Show Distinct Responses in Terms of Antioxidant Machinery and Isoform/Activity of Rubisco. Int. J. Mol. Sci. 2021, 22, 11214. [Google Scholar] [CrossRef]
- Rossi, L.; Francini, A.; Minnocci, A.; Sebastiani, L. Salt Stress Modifies Apoplastic Barriers in Olive (Olea europaea L.): A Comparison between a Salt-Tolerant and a Salt-Sensitive Cultivar. Sci. Hortic. 2015, 192, 38–46. [Google Scholar] [CrossRef]
- Fernández, J.E. Understanding Olive Adaptation to Abiotic Stresses as a Tool to Increase Crop Performance. Environ. Exp. Bot. 2014, 103, 158–179. [Google Scholar] [CrossRef]
- Carr, M.K.V. The Water Relations and Irrigation Requirements of Olive (Olea europaea L.): A Review. Exp. Agric. 2013, 49, 597–639. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, G. Comparative Effects of Drought Stress on Leaf Anatomy of Two Olive Cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Sofo, A.; Dichio, B.; Xiloyannis, C.; Masia, A. Lipoxygenase Activity and Proline Accumulation in Leaves and Roots of Olive Trees in Response to Drought Stress. Physiol. Plant. 2004, 121, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Correia, S.; Serôdio, J.; Silva, A.M.S.; Freitas, H.; Santos, C. Chlorophyll Fluorescence and Oxidative Stress Endpoints to Discriminate Olive Cultivars Tolerance to Drought and Heat Episodes. Sci. Hortic. 2018, 231, 31–35. [Google Scholar] [CrossRef]
- Faraloni, C.; Cutino, I.; Petruccelli, R.; Leva, A.R.; Lazzeri, S.; Torzillo, G. Chlorophyll Fluorescence Technique as a Rapid Tool for in Vitro Screening of Olive Cultivars (Olea europaea L.) Tolerant to Drought Stress. Environ. Exp. Bot. 2011, 73, 49–56. [Google Scholar] [CrossRef]
- Pierantozzi, P.; Torres, M.; Bodoira, R.; Maestri, D. Water Relations, Biochemical—Physiological and Yield Responses of Olive Trees (Olea europaea L. Cvs. Arbequina and Manzanilla) under Drought Stress during the Pre-Flowering and Flowering Period. Agric. Water Manag. 2013, 125, 13–25. [Google Scholar] [CrossRef]
- de Pascali, M.; Vergine, M.; Sabella, E.; Aprile, A.; Nutricati, E.; Nicolì, F.; Buja, I.; Negro, C.; Miceli, A.; Rampino, P.; et al. Molecular Effects of Xylella Fastidiosa and Drought Combined Stress in Olive Trees. Plants 2019, 8, 437. [Google Scholar] [CrossRef]
- D’Agostino, N.; Taranto, F.; Camposeo, S.; Mangini, G.; Fanelli, V.; Gadaleta, S.; Miazzi, M.M.; Pavan, S.; di Rienzo, V.; Sabetta, W.; et al. GBS-Derived SNP Catalogue Unveiled Wide Genetic Variability and Geographical Relationships of Italian Olive Cultivars. Sci. Rep. 2018, 8, 15877. [Google Scholar] [CrossRef]
- Tosca, A.; Valagussa, M.; Martinetti, L.; Frangi, P. Biochar and Green Compost as Peat Alternatives in the Cultivation of Photinia and Olive Tree. Acta Hortic. 2021, 1305, 257–262. [Google Scholar] [CrossRef]
- Bilskie, J. Soil Water Status: Content and Potential; Campbell Scientific, Inc.: Logan, UT, USA, 2001; Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjj_97LrZiAAxV5yAIHHVo3CJIQFnoECA4QAQ&url=https%3A%2F%2Fs.campbellsci.com%2Fdocuments%2Ffr%2Ftechnical-papers%2Fsoilh20c.pdf&usg=AOvVaw3jbEEwVoaHKEhJVP65Jm9a&opi=89978449 (accessed on 19 July 2023).
- Xu, Z.; Zhou, G. Responses of Leaf Stomatal Density to Water Status and Its Relationship with Photosynthesis in a Grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef]
- Diaz-Espejo, A.; Nicolás, E.; Fernández, J.E. Seasonal Evolution of Diffusional Limitations and Photosynthetic Capacity in Olive under Drought. Plant Cell Environ. 2007, 30, 922–933. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Buckley, T.N.; Egea, G.; de Cires, A.; Hernandez-Santana, V.; Martorell, S.; Diaz-Espejo, A. Most Stomatal Closure in Woody Species under Moderate Drought Can Be Explained by Stomatal Responses to Leaf Turgor. Plant Cell Environ. 2016, 39, 2014–2026. [Google Scholar] [CrossRef] [PubMed]
- Ethier, G.J.; Livingston, N.J. On the Need to Incorporate Sensitivity to CO2 Transfer Conductance into the Farquhar-von Caemmerer-Berry Leaf Photosynthesis Model. Plant Cell Environ. 2004, 27, 137–153. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R.; Long, S.P. Improved Temperature Response Functions for Models of Rubisco-Limited Photosynthesis. Glob. Change Biol. 2001, 21, 253–259. [Google Scholar] [CrossRef]
- Pons, T.L.; Flexas, J.; Von Caemmerer, S.; Evans, J.R.; Genty, B.; Ribas-Carbo, M.; Brugnoli, E. Estimating Mesophyll Conductance to CO2: Methodology, Potential Errors, and Recommendations. J. Exp. Bot. 2009, 60, 2217–2234. [Google Scholar] [CrossRef]
- Loreto, F.; Harley, P.C.; Marco, G.D.; Sharkey, T.D. Estimation of Mesophyll Conductance to CO2 Flux by Three Different Methods. Plant Physiol. 1992, 98, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Guidotti, D.; Sebastiani, L.; Tognetti, R. Changes in Assimilation Capacity during Leaf Development in Broad- Leaved Prunus Persica and Sclerophyllous Olea europaea. J. Hortic. Sci. Biotechnol. 2007, 82, 69–78. [Google Scholar] [CrossRef]
- Galmés, J.; Flexas, J.; Keys, A.J.; Cifre, J.; Mitchell, R.A.C.; Madgwick, P.J.; Haslam, R.P.; Medrano, H.; Parry, M.A.J. Rubisco Specificity Factor Tends to Be Larger in Plant Species from Drier Habitats and in Species with Persistent Leaves. Plant Cell Environ. 2005, 28, 571–579. [Google Scholar] [CrossRef]
- Loriaux, S.D.; Avenson, T.J.; Welles, J.M.; Mcdermitt, D.K.; Eckles, R.D.; Riensche, B.; Genty, B. Closing in on Maximum Yield of Chlorophyll Fluorescence Using a Single Multiphase Flash of Sub-Saturating Intensity. Plant Cell Environ. 2013, 36, 1755–1770. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Pou, A.; Zwieniecki, M.A.; Holbrook, N.M. On Measuring the Response of Mesophyll Conductance to Carbon Dioxide with the Variable J Method. J. Exp. Bot. 2012, 63, 413–425. [Google Scholar] [CrossRef]
- Grassi, G.; Magnani, F. Stomatal, Mesophyll Conductance and Biochemical Limitations to Photosynthesis as Affected by Drought and Leaf Ontogeny in Ash and Oak Trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Trupiano, D.; Polzella, A.; De Zio, E.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Ben Youssef, N.; Scippa, G.S. Unraveling Physiological, Biochemical and Molecular Mechanisms Involved in Olive (Olea europaea L. Cv. Chétoui) Tolerance to Drought and Salt Stresses. J. Plant Physiol. 2018, 220, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative Impacts of Water Stress on the Leaf Anatomy of a Drought-Resistant and a Drought-Sensitive Olive Cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Oddo, E.; Virgilio, F.; Grisafi, F. Effects of Water Deficit on the Leaf Water Relations of Pot-Grown Olive Cultivars. Plant Stress 2008, 2, 56–63. [Google Scholar]
- Melaouhi, A.; Baraza, E.; Escalona, J.M.; El-AouOuad, H.; Mahjoub, I.; Bchir, A.; Braham, M.; Bota, J. Physiological and Biochemical Responses to Water Deficit and Recovery of Two Olive Cultivars (Olea europaea L., Arbequina and Empeltre Cvs.) under Mediterranean Conditions. Theor. Exp. Plant Physiol. 2021, 33, 369–383. [Google Scholar] [CrossRef]
- Pardossi, A.; Incrocci, L.; Incrocci, G.; Malorgio, F.; Battista, P.; Bacci, L.; Rapi, B.; Marzialetti, P.; Hemming, J.; Balendonck, J. Root Zone Sensors for Irrigation Management in Intensive Agriculture. Sensors 2009, 9, 2809–2835. [Google Scholar] [CrossRef]
- Pardossi, A.; Marzialetti, P.; Spe, C.V.; Bibbiani, C. I substrati e la coltivazione delle piante in contenitore. Fertilitas Agrorum 2009, 3, 22–31. [Google Scholar]
- Flower, D.J.; Ludlow, M.M. Contribution of Osmotic Adjustment to the Dehydration Tolerance of Water-Stressed Pigeon Pea (Cajanus cajan (L.) Millsp.) Leaves. Plant Cell Environ. 1986, 9, 33–40. [Google Scholar] [CrossRef]
- Jones, H.G.; Tardieu, F. Modelling Water Relations of Horticultural Crops: A Review. Sci. Hortic. 1998, 74, 21–46. [Google Scholar] [CrossRef]
- Boussadia, O.; Mariem, F.B.; Mechri, B.; Boussetta, W.; Braham, M.; Hadj, S.B.E. Response to Drought of Two Olive Tree Cultivars (Cv Koroneki and Meski). Sci. Hortic. 2008, 116, 388–393. [Google Scholar] [CrossRef]
- Traversari, S.; Francini, A.; Traversi, M.L.; Emiliani, G.; Sorce, C.; Sebastiani, L.; Giovannelli, A. Can Sugar Metabolism in the Cambial Region Explain the Water Deficit Tolerance in Poplar? J. Exp. Bot. 2018, 69, 4083–4097. [Google Scholar] [CrossRef] [PubMed]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Sofo, A.; Dichio, B.; Montanaro, G.; Xiloyannis, C. Photosynthetic Performance and Light Response of Two Olive Cultivars under Different Water and Light Regimes. Photosynthetica 2009, 47, 602–608. [Google Scholar] [CrossRef]
- Brunetti, C.; Gori, A.; Marino, G.; Latini, P.; Sobolev, A.P.; Nardini, A.; Haworth, M.; Giovannelli, A.; Capitani, D.; Loreto, F.; et al. Dynamic Changes in ABA Content in Water-Stressed Populus Nigra: Effects on Carbon Fixation and Soluble Carbohydrates. Ann. Bot. 2019, 124, 627–644. [Google Scholar] [CrossRef]
- Clemente-Moreno, M.J.; Gago, J.; Díaz-Vivancos, P.; Bernal, A.; Miedes, E.; Bresta, P.; Liakopoulos, G.; Fernie, A.R.; Hernández, J.A.; Flexas, J. The Apoplastic Antioxidant System and Altered Cell Wall Dynamics Influence Mesophyll Conductance and the Rate of Photosynthesis. Plant J. Cell Mol. Biol. 2019, 99, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Hoshika, Y.; Fares, S.; Pellegrini, E.; Conte, A.; Paoletti, E. Water Use Strategy Affects Avoidance of Ozone Stress by Stomatal Closure in Mediterranean Trees—A Modelling Analysis. Plant Cell Environ. 2020, 43, 611–623. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence-a Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Llorente, B.E. Drought Stress in Plants: A Review on Water Relations. Biosci. Res. 2009, 6, 20–27. [Google Scholar]
- Marino, G.; Pallozzi, E.; Cocozza, C.; Tognetti, R.; Giovannelli, A.; Cantini, C.; Centritto, M. Assessing Gas Exchange, Sap Flow and Water Relations Using Tree Canopy Spectral Reflectance Indices in Irrigated and Rainfed Olea europaea L. Environ. Exp. Bot. 2014, 99, 43–52. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-Induced Electrolyte Leakage: The Role of K+-Permeable Channels and Involvement in Programmed Cell Death and Metabolic Adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]

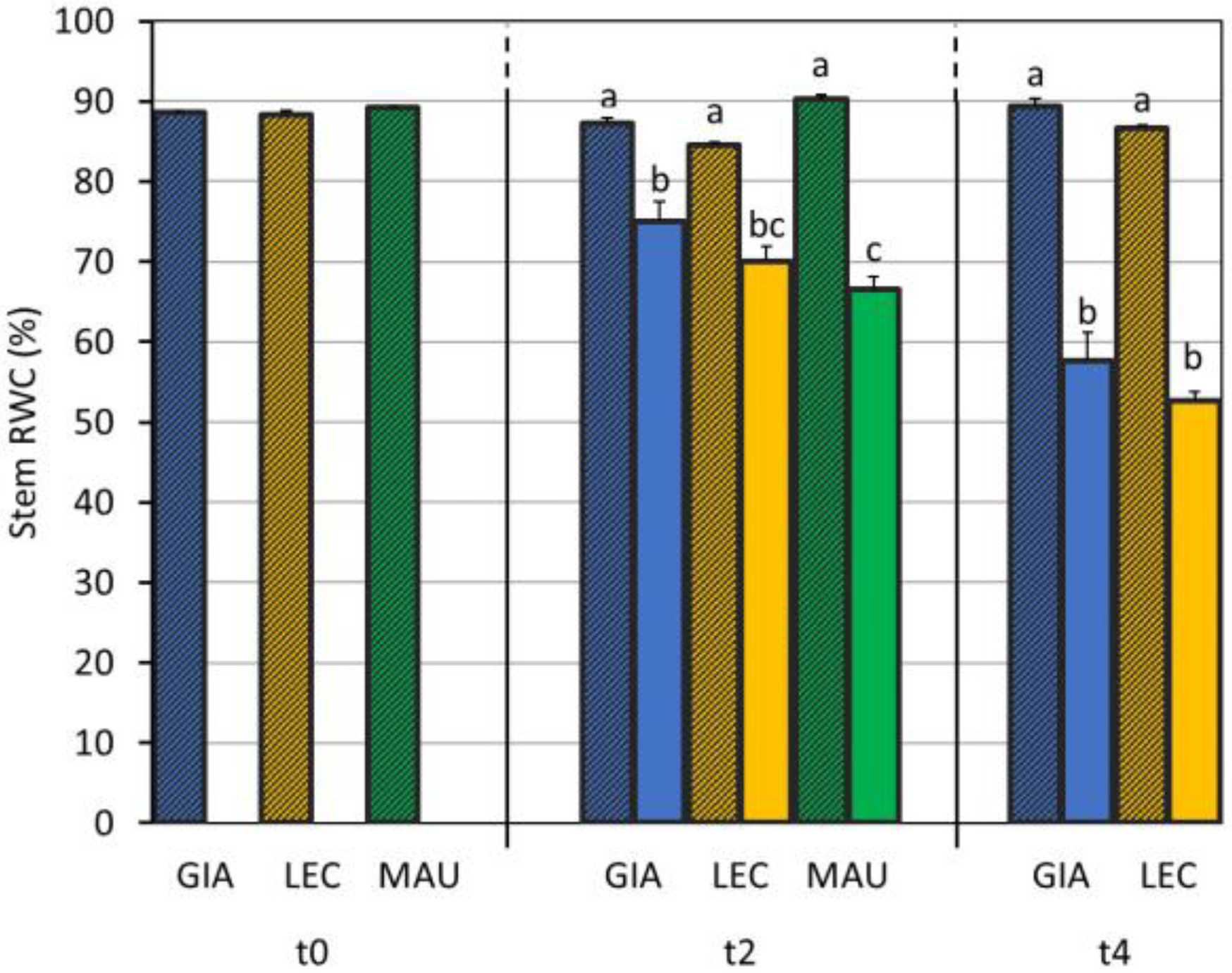

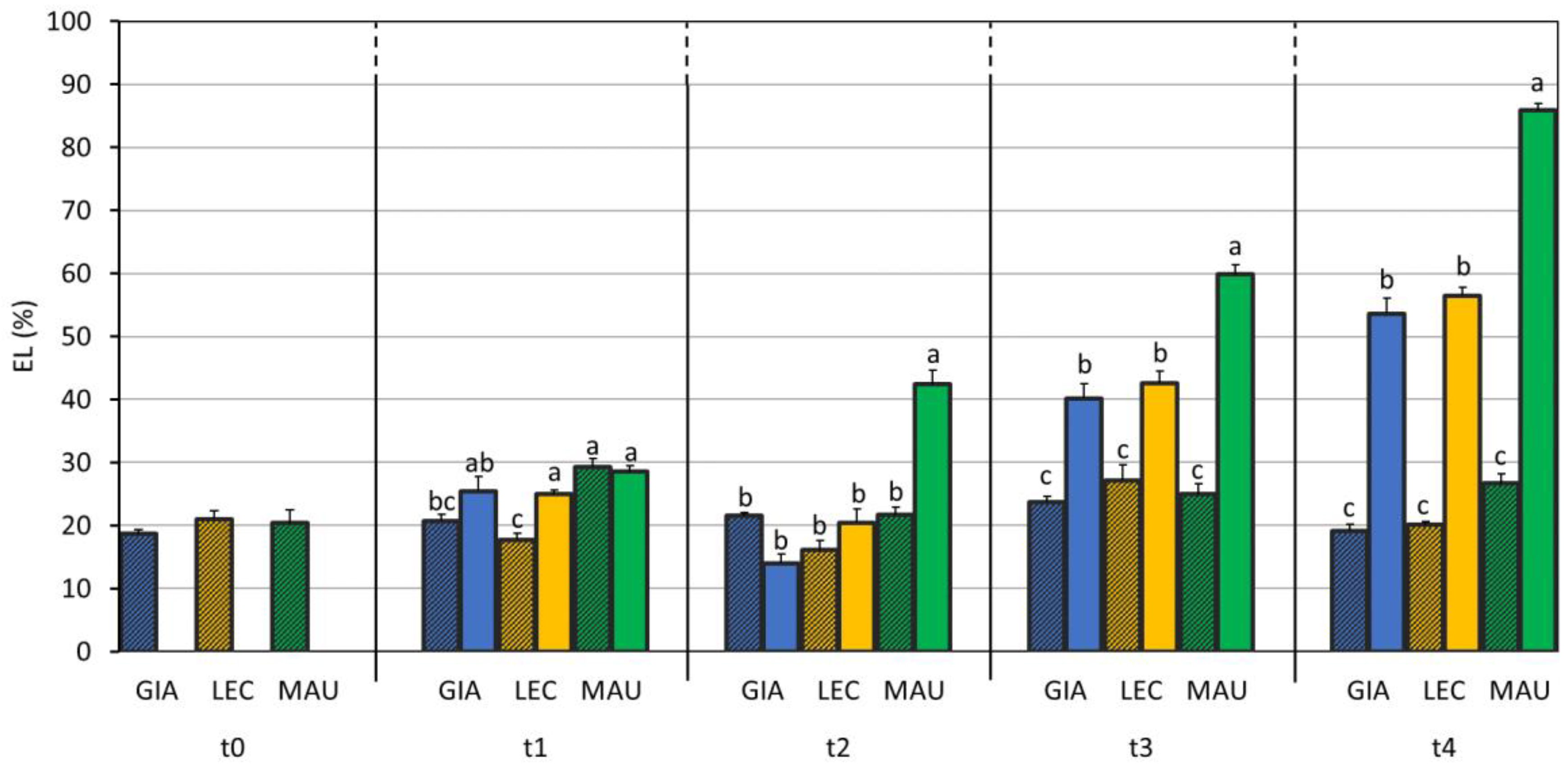
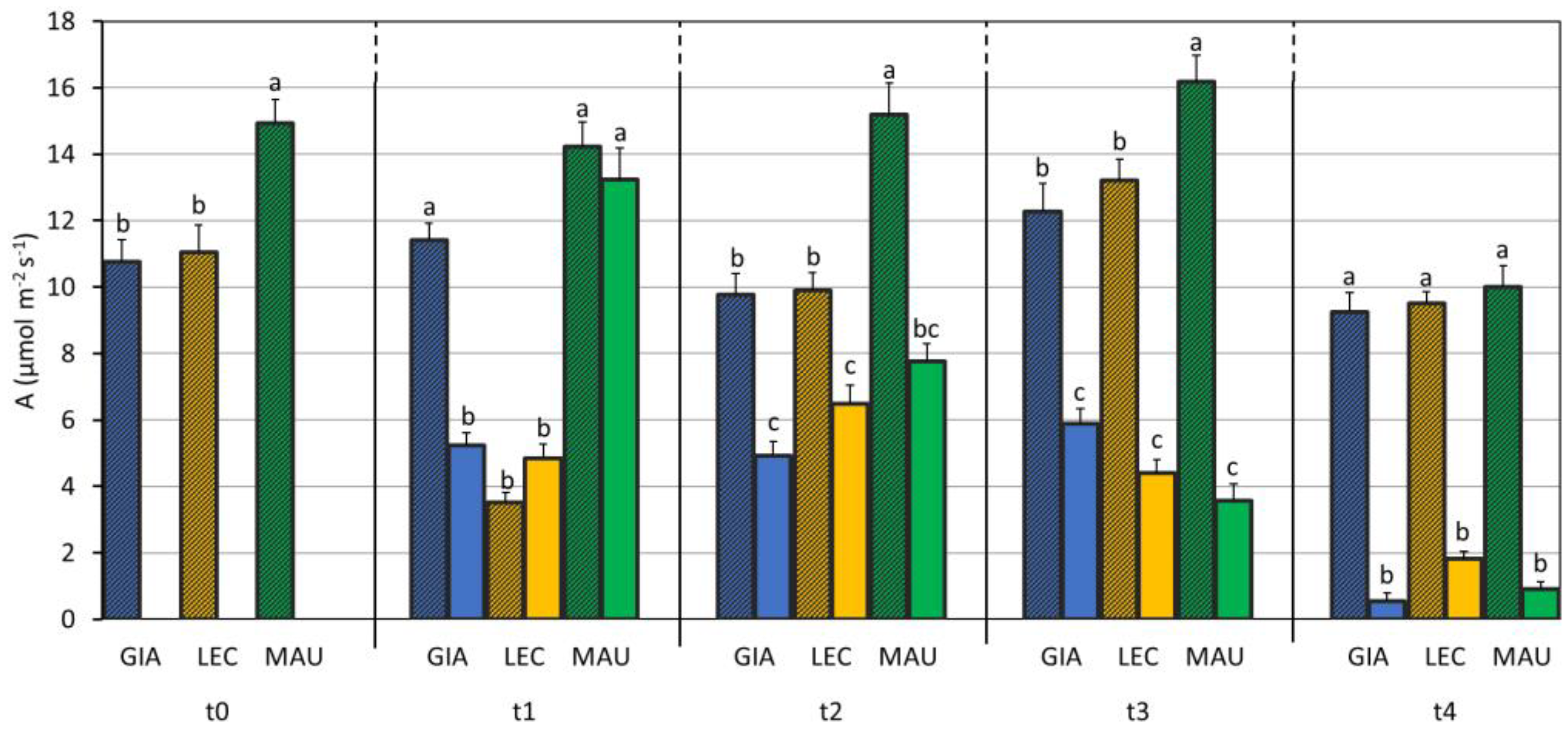

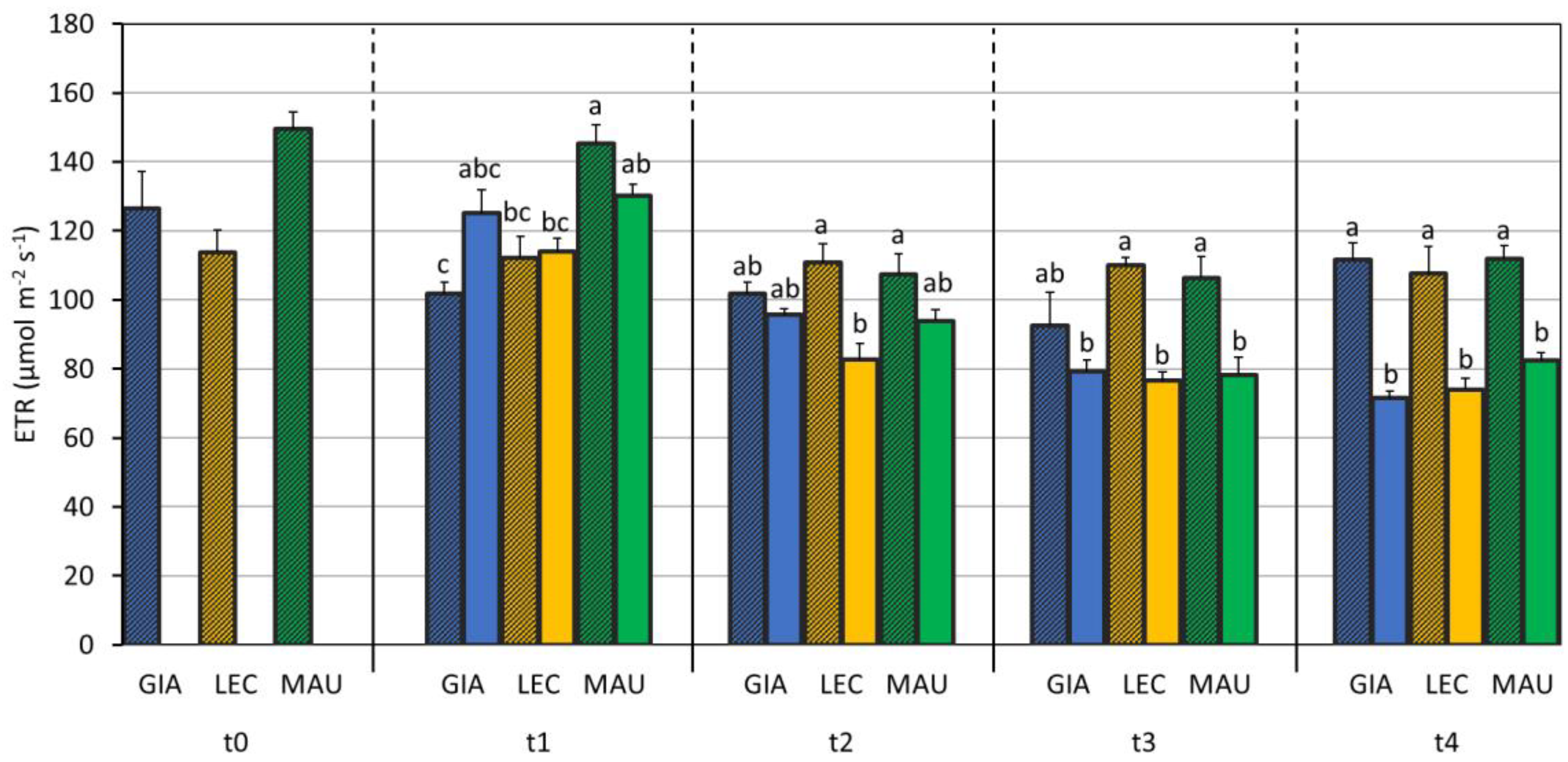

| SWC (%) | Leaf RWC (%) | Stem RWC (%) | SD (n./mm2) | gs (mol m−2 s−1) | EL (%) | MDA (mmol/kg DW) | Chl a + b (µg/mg DW) | Car (µg/mg DW) | |
|---|---|---|---|---|---|---|---|---|---|
| Cultivar (C) | |||||||||
| Giarraffa | 89 ± 52 a | 64.0 ± 16.1 | 69.2 ± 17.9 | 34.4 ± 7.6 c | 0.086 ± 0.039 b | 26.3 ± 12.5 b | 5.28 ± 0.98 b | 3.93 ± 0.82 | 0.70 ± 0.15 |
| Leccino | 76 ± 50 b | 60.5 ± 15.1 | 66.7 ± 17.0 | 38.4 ± 5.0 b | 0.070 ± 0.040 c | 27.4 ± 13.3 b | 3.78 ± 0.50 c | 3.66 ± 0.71 | 0.65 ± 0.13 |
| Maurino | 76 ± 49 b | 61.3 ± 17.6 | 69.4 ± 20.0 | 48.4 ± 5.4 a | 0.112 ± 0.051 a | 37.7 ± 21.1 a | 7.26 ± 1.33 a | 3.49 ± 1.93 | 0.63 ± 0.32 |
| p-value | 0.014 | 0.080 | 0.051 | <0.001 | <0.001 | <0.001 | <0.001 | 0.837 | 0.649 |
| Treatment (S) | |||||||||
| CTRL | 117 ± 26 a | 82.0 ± 8.6 a | 88.0 ± 2.1 a | 40.1 ± 9.3 | 0.119 ± 0.037 a | 21.9 ± 4.8 b | 4.93 ± 1.67 b | 3.43 ± 1.17 | 0.61 ± 0.19 |
| DS | 26 ± 21 b | 50.9 ± 10.8 b | 64.4 ± 9.8 b | 40.7 ± 7.6 | 0.052 ± 0.029 b | 41.2 ± 20.1 a | 5.94 ± 1.72 a | 4.10 ± 1.31 | 0.73 ± 0.23 |
| p-value | <0.001 | <0.001 | <0.001 | 0.203 | <0.001 | <0.001 | 0.004 | 0.116 | 0.143 |
| C × S | |||||||||
| p-value | 0.715 | 0.041 | 0.001 | <0.001 | <0.001 | <0.001 | 0.422 | 0.636 | 0.824 |
| A (µmol m−2 s−1) | Φ PSII | Fv/Fm | ETR (µmol m−2 s−1) | gm (mol m−2 s−1) | Ls (%) | Lm (%) | Lb (%) | Vcmax (µmol m−2 s−1) | Jmax (µmol m−2 s−1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar (C) | ||||||||||
| Giarraffa | 7.79 ± 3.87 b | 0.149 ± 0.032 b | 0.797 ± 0.048 | 100.6 ± 21.5 b | 0.110 ± 0.043 | 44.5 ± 10.7 a | 27.5 ± 10.4 | 28.0 ± 17.9 | 57.1 ± 13.4 | 74.9 ± 16.2 ab |
| Leccino | 7.14 ± 3.78 b | 0.154 ± 0.034 ab | 0.797 ± 0.055 | 100.1 ± 19.3 b | 0.139 ± 0.077 | 43.9 ± 5.2 ab | 21.6 ± 8.0 | 34.5 ± 12.7 | 55.8 ± 12.1 | 87.4 ± 13.4 a |
| Maurino | 10.66 ± 5.48 a | 0.166 ± 0.039 a | 0.800 ± 0.047 | 111.7 ± 26.1 a | 0.112 ± 0.053 | 38.9 ± 11.6 b | 27.5 ± 11.1 | 33.6 ± 14.7 | 49.1 ± 23.0 | 68.8 ± 19.4 b |
| p-value | <0.001 | <0.001 | 0.860 | <0.001 | 0.061 | 0.026 | 0.295 | 0.196 | 0.520 | 0.054 |
| Treatment (S) | ||||||||||
| CTRL | 11.38 ± 3.40 a | 0.172 ± 0.031 a | 0.823 ± 0.010 a | 113.9 ± 19.6 a | 0.156 ± 0.058 a | 34.8 ± 6.1 b | 21.9 ± 6.9 b | 43.3 ± 6.1 a | 52.8 ± 12.4 | 76.2 ± 17.9 |
| DS | 4.96 ± 3.46 b | 0.137 ± 0.031 b | 0.768 ± 0.061 b | 91.9 ± 21.1 b | 0.087 ± 0.037 b | 50.7 ± 6.2 a | 30.7 ± 11.4 a | 18.6 ± 11.5 b | 54.9 ± 21.9 | 75.2 ± 18.6 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.015 | <0.001 | 0.706 | 0.945 |
| C × S | ||||||||||
| p-value | <0.001 | 0.001 | 0.091 | 0.034 | 0.732 | 0.067 | 0.140 | 0.271 | 0.419 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parri, S.; Romi, M.; Hoshika, Y.; Giovannelli, A.; Dias, M.C.; Piritore, F.C.; Cai, G.; Cantini, C. Morpho-Physiological Responses of Three Italian Olive Tree (Olea europaea L.) Cultivars to Drought Stress. Horticulturae 2023, 9, 830. https://doi.org/10.3390/horticulturae9070830
Parri S, Romi M, Hoshika Y, Giovannelli A, Dias MC, Piritore FC, Cai G, Cantini C. Morpho-Physiological Responses of Three Italian Olive Tree (Olea europaea L.) Cultivars to Drought Stress. Horticulturae. 2023; 9(7):830. https://doi.org/10.3390/horticulturae9070830
Chicago/Turabian StyleParri, Sara, Marco Romi, Yasutomo Hoshika, Alessio Giovannelli, Maria Celeste Dias, Francesca Cristiana Piritore, Giampiero Cai, and Claudio Cantini. 2023. "Morpho-Physiological Responses of Three Italian Olive Tree (Olea europaea L.) Cultivars to Drought Stress" Horticulturae 9, no. 7: 830. https://doi.org/10.3390/horticulturae9070830
APA StyleParri, S., Romi, M., Hoshika, Y., Giovannelli, A., Dias, M. C., Piritore, F. C., Cai, G., & Cantini, C. (2023). Morpho-Physiological Responses of Three Italian Olive Tree (Olea europaea L.) Cultivars to Drought Stress. Horticulturae, 9(7), 830. https://doi.org/10.3390/horticulturae9070830









