Mineral and Metabolome Analyses Provide Insights into the Cork Spot Disorder on ‘Akizuki’ Pear Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Mineral Content Analysis
2.3. Enzyme Activity Analysis of POD, CAT, and SOD and Content Detection of MDA
2.4. Metabolite Extraction and Analysis
2.5. Data Pre-Processing and Metabolite Identification
2.6. Multivariate Statistical Analysis
2.7. Differential Metabolite Analysis
2.8. Statistical Analysis
3. Results
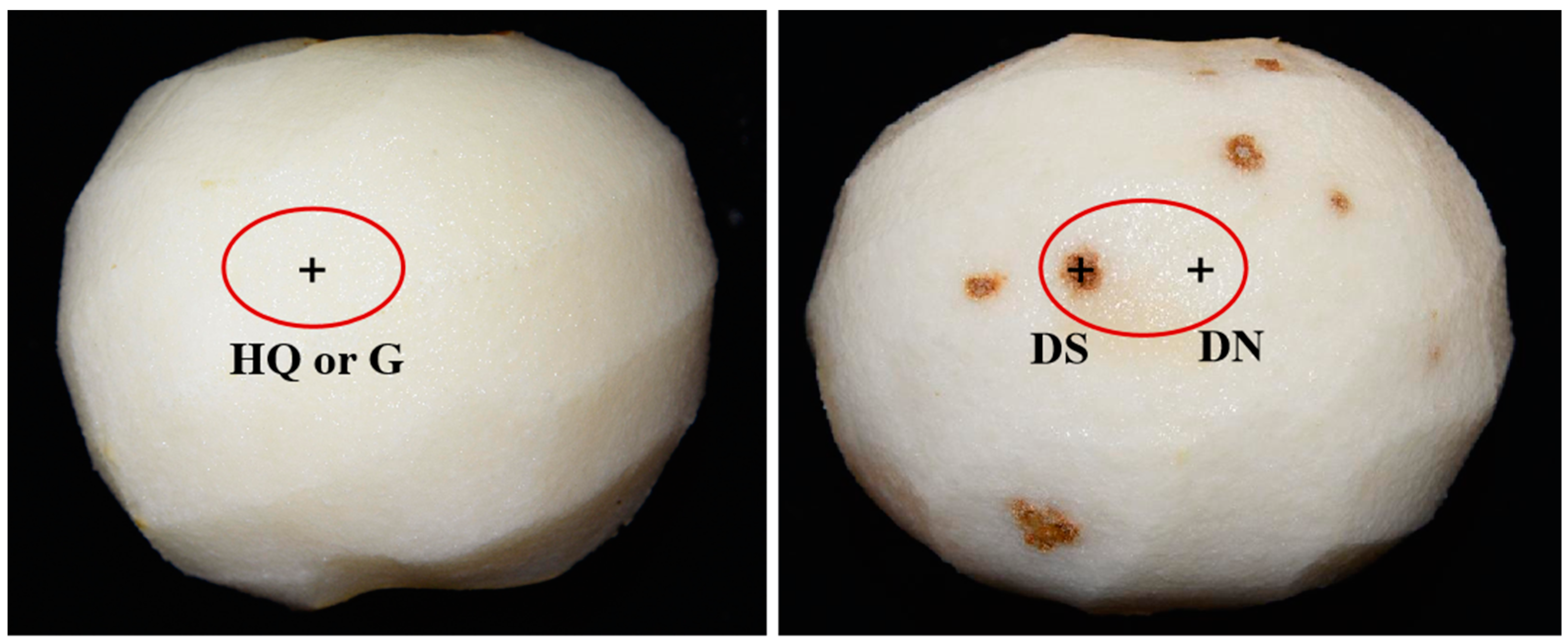
3.1. Observation of the Cork Spot Symptoms at Harvest Time
3.2. Analysis of Mineral Element Contents among Different Fruit Fleshes
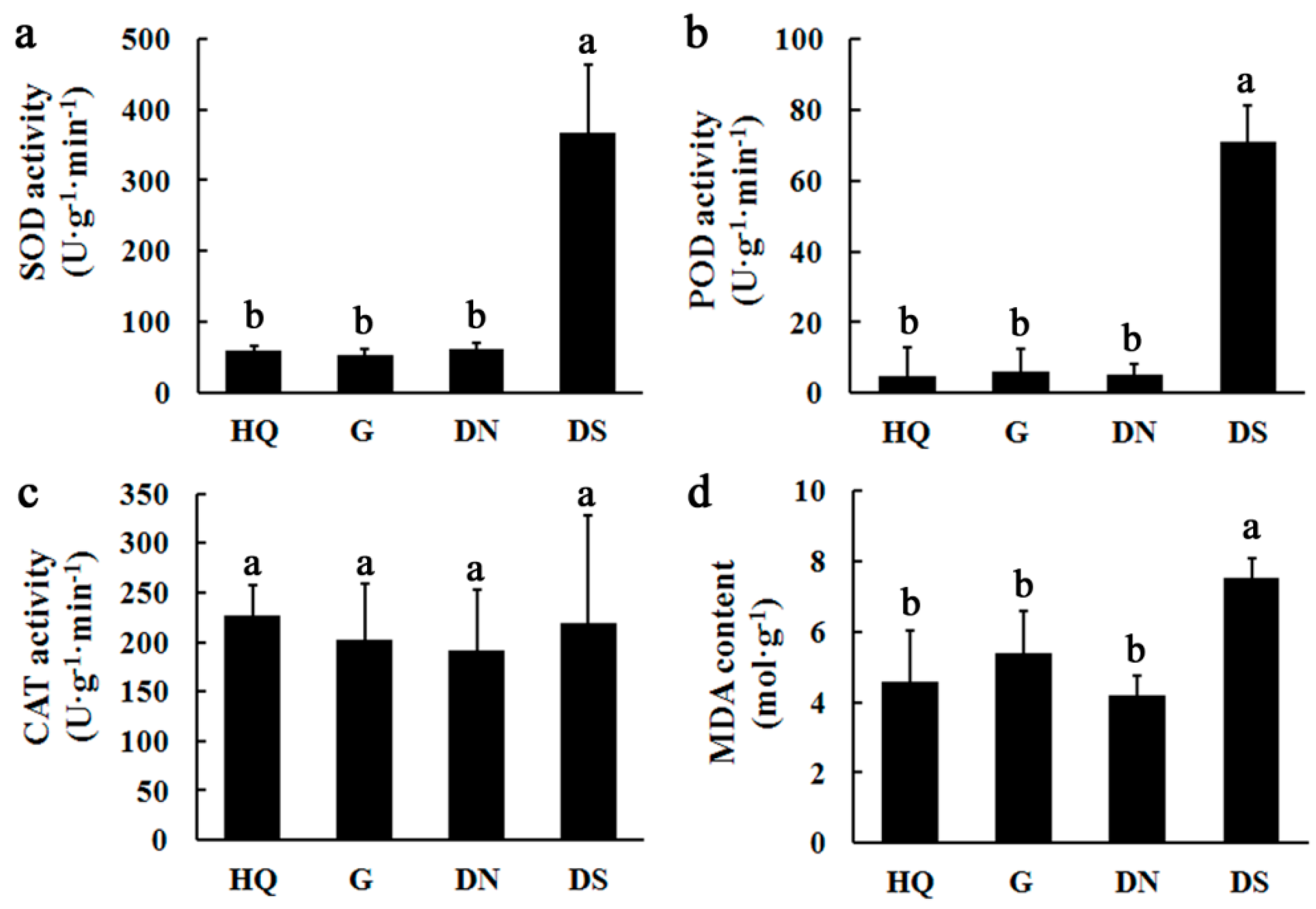
3.3. Detection of Antioxidant Enzyme Activities and MDA Contents
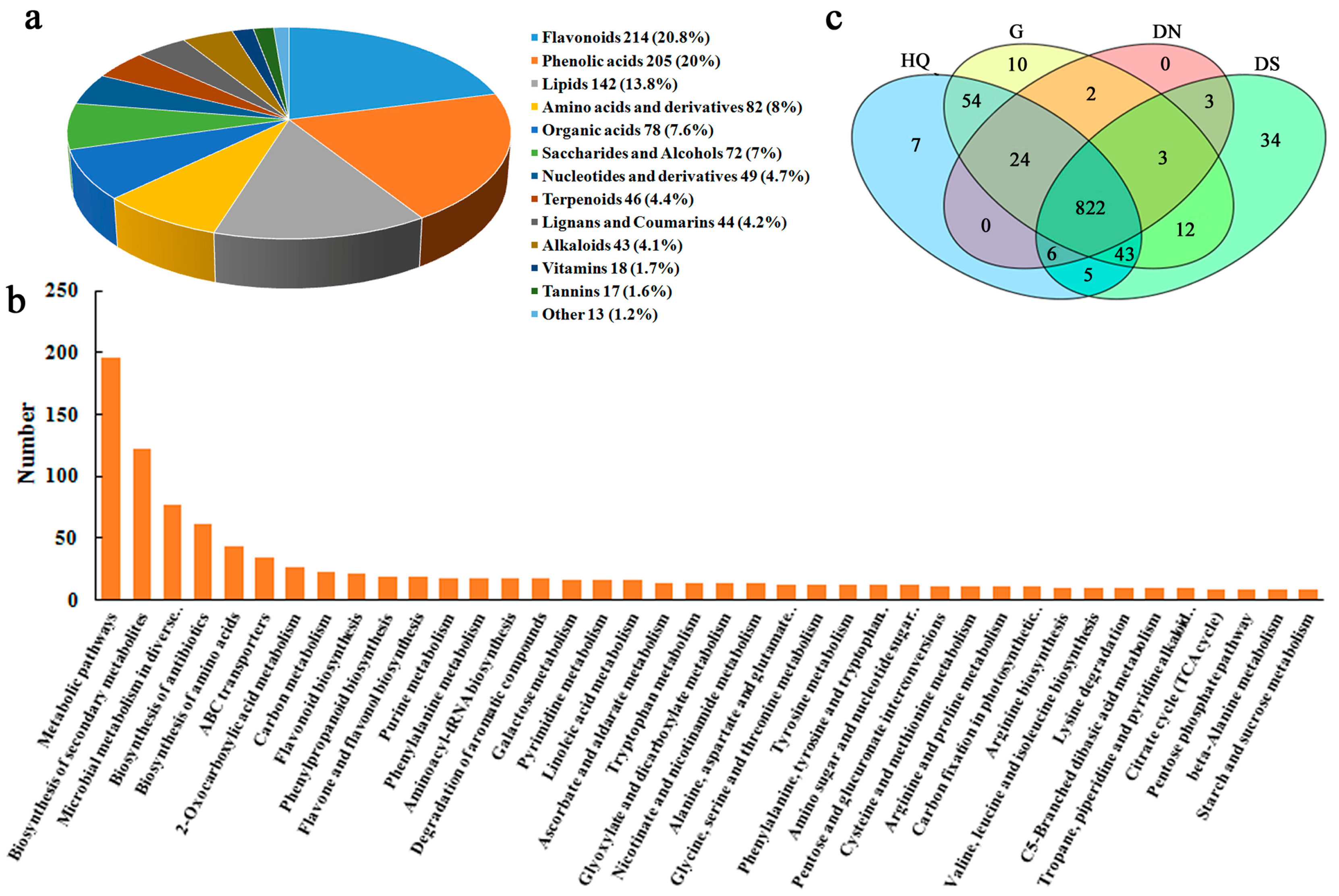
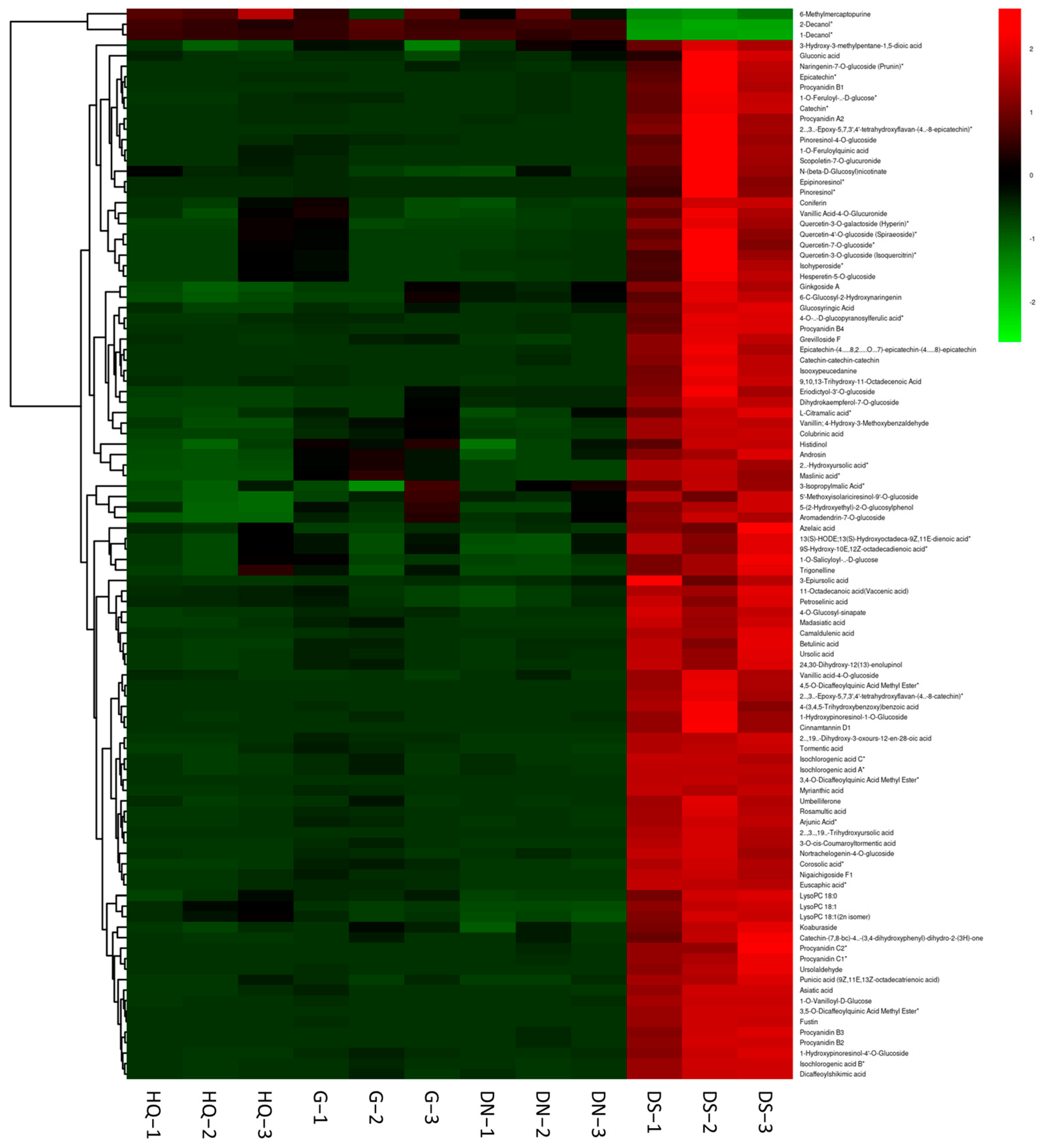
3.4. Identification of Metabolites
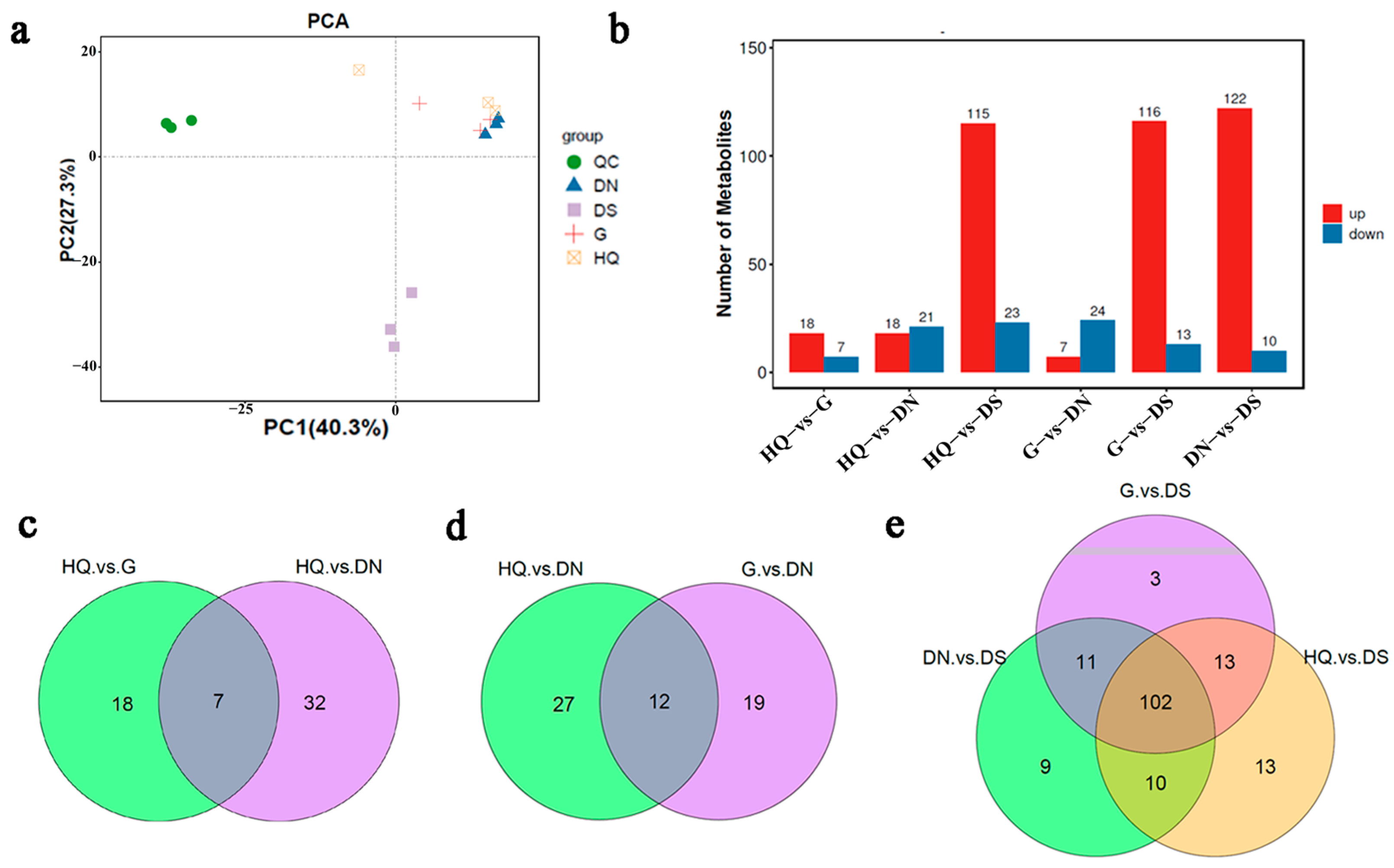
3.5. Metabolite Profiling Analysis and Differential Metabolite Identification
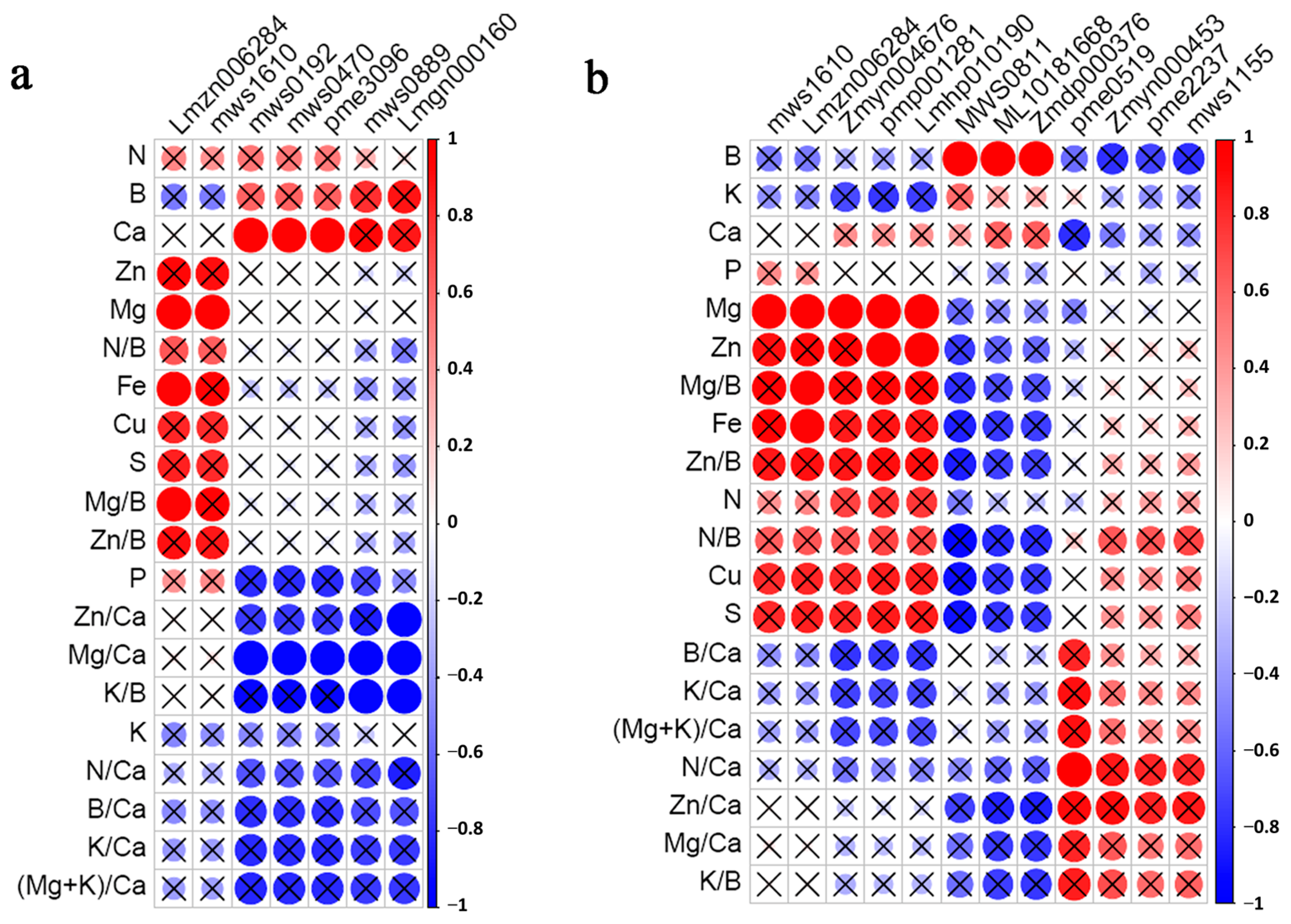
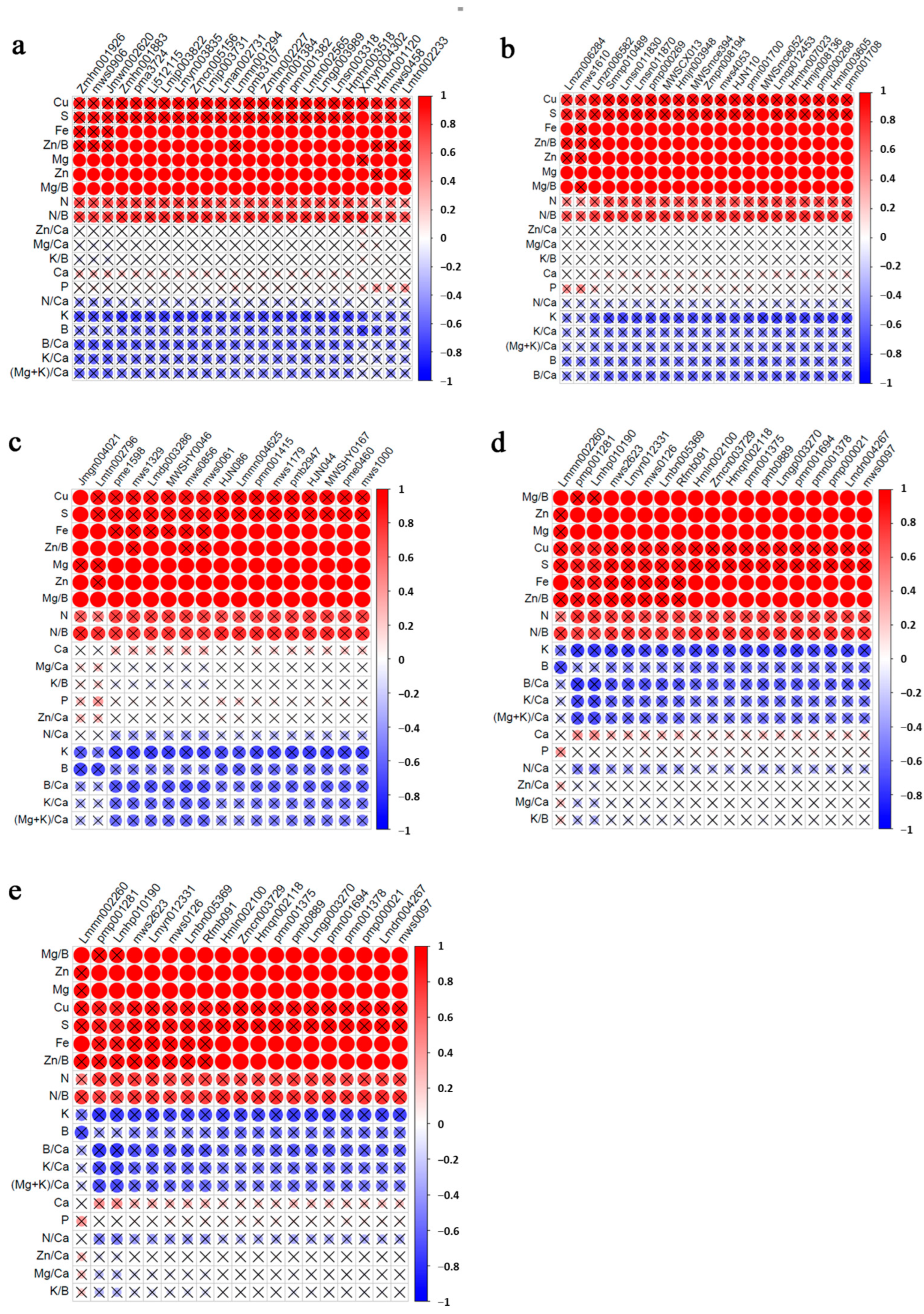
3.6. Correlation Analysis of Mineral Elements and Differential Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lu, G.; Li, Z.; Zhang, X.; Wang, R.; Yang, S. Expression Analysis of Lignin-Associated Genes in Hard End Pear (P. pyrifolia Whangkeumbae) and Its Response to Calcium Chloride Treatment Conditions. J. Plant Growth Regul. 2015, 34, 251–262. [Google Scholar] [CrossRef]
- Liu, X.; Fan, H.; Liu, D.; Liu, J.; Shen, Y.; Zhang, J.; Wei, J.; Wang, C. Transcriptome and Metabolome Analyses Provide Insights into the Watercore Disorder on ‘Akibae’ Pear Fruit. Int. J. Mol. Sci. 2021, 22, 4911. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xu, Y.; Wang, R.; Ma, C. Investigation and Prevention of Cork Spot Disorder in ‘Akizuki’ Pear (P. pyrifolia Nakai). HortScience 2019, 54, 480–486. [Google Scholar] [CrossRef]
- Cui, Z.; Jiao, Q.; Wang, R.; Ma, C. Investigation and analysis of relationship between mineral elements alteration and cork spot physiological disorder of Chinese pear ‘Chili’ (P. bretschneideri Rehd.). Sci. Hortic. 2020, 260, 108883. [Google Scholar] [CrossRef]
- Hayama, H.; Mitani, N.; Yamane, T.; Inoue, H.; Kusaba, S. Characteristics of Cork Spot Like Disorder in Japanese Pear ‘Akizuki’ and ‘Oushuu’. Hortic. Res. 2017, 16, 79–87. [Google Scholar] [CrossRef]
- Tomala, K.; Trzak, M. Occurrence of cork spot (pit) in ‘alexander lucas’ pears depends on fruit mineral element content. Acta Hortic. 1994, 368, 570–577. [Google Scholar] [CrossRef]
- Raese, T. Disorders of d’Anjou pears-including alfalfa greening, cork, and black end. In Proceedings of the 76th Annual Meeting Washington State Horticultural Association, Atlanta, GA, USA, 19–24 June 1980; pp. 172–176. [Google Scholar]
- Faust, M.; Shear, C.B. Corking disorders of apples: A physiological and biochemical review. Bot. Rev. 1968, 34, 441–469. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, X.; Wang, R.; Ma, C. X-ray μCT analysis to characterize cork spot disorder in Chinese pear ‘Chili’ (P. bretschneideri Rehd.). Postharvest Biol. Technol. 2020, 170, 111321. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, N.; Li, D.; Wang, R.; Ma, C. Nitrendipine-Treatment Increases Cork Spot Disorder Incidence in Pear ‘Akituki’ (Pyrus pyrifolia Nakai.) by Altering Calcium Distribution Inside the Fruit. Plants 2021, 10, 994. [Google Scholar] [CrossRef]
- Tamura, F. Occurrence of Physiological Disorders in Japanese Pear Fruit and Advances in Research on these Disorders. Hortic. Res. 2017, 16, 373–381. [Google Scholar] [CrossRef]
- Lombard, P.B.; Westwood, M.N. Performance of six pear cultivars on clonal Old Home, double rooted, and seedling rootstocks. J. Am. Soc. Hortic. Sci. 1976, 101, 214–216. [Google Scholar]
- Mitani, N.; Hayama, H.; Yamane, T.; Kusaba, S. Effects of Flower Position, Flowering Time, and Ethephon Treatment, which Affect the Fruit Maturation Time, on the Cork Spot-Like Disorder of Japanese Pear ‘Akizuki’ and ‘Oushuu’. Hortic. Res. 2017, 16, 471–477. [Google Scholar] [CrossRef]
- Stahly, E.A.; Benson, N.R. Calcium levels of ‘Golden Delicious’ apples as influenced by calcium sprays, 2,3,5-triiodobenzoic acid, and other plant growth regulator sprays [Pitting, physiological diseases]. J. Am. Soc. Hortic. Sci. 1976, 101, 120–122. [Google Scholar] [CrossRef]
- Al-Ani, A.M. Postharvest Physiology of ‘Anjou’ Pear Fruit: Relations between Mineral Nutrition and Cork Spot, Respiration, and Ethylene Evolution. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 28 February 1978. [Google Scholar]
- McAlpine, D. Bitter pit on apple and pears. The latest results in preservation measures. Phytopath 1921, 11, 370. [Google Scholar]
- Facteau, T.J.; Cahn, H.; Mielke, E.A. Mineral Concentrations in Individual ‘d’Anjou’ Pear Fruit with and without Corkspot 1. J. Food Qual. 2010, 23, 513–519. [Google Scholar] [CrossRef]
- Kawamata, S. Occurrence of ‘Yuzuhada’, a physiological disorder of Japanese pear (Rehd. cv. ‘Nijisseiki’), in relation to its nutrient content and respiration rates. Sci. Hortic. 1978, 8, 143–153. [Google Scholar] [CrossRef]
- Mason, J.L.; Welsh, M.F. Cork spot (pit) of Anjou pear related to calcium concentration in fruit. Hortic. Sci. 1970, 5, 447. [Google Scholar]
- Woodbridge, C.G. Calcium level of pear tissues affected with cork and black end. Hortic. Sci. 1971, 6, 451–453. [Google Scholar] [CrossRef]
- Richardson, D.G.; Lombard, P.B. Cork spot of anjou pear: Control by calcium sprays. Commun. Soil Sci. Plant Anal. 1979, 10, 383–389. [Google Scholar] [CrossRef]
- Raese, J.T.; Drake, S.R.; Staiff, D.C. Influence of different calcium materials and spray timing on mineral composition, yield, fruit quality, and control of fruit disorders of ‘anjou’ pears. J. Plant Nutr. 1995, 18, 823–838. [Google Scholar] [CrossRef]
- Raese, J.T.; Drake, S.R. Calcium Foliar Sprays for Control of Alfalfa Greening, Cork Spot, and Hard End in ‘Anjou’ Pears. J. Plant Nutr. 2006, 29, 543–552. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kobayashi, T.; Kougo, T.; Fujita, T.; Sato, S.; Moriguchi, T. Prevention of New Cork Spot-like Physiological Disorder in ‘Kurenainoyume’ Apples by Pre-harvest Fruit Bagging. Hortic. J. 2018, 87, 174–183. [Google Scholar] [CrossRef]
- Richardson, D.G.; Al-Ani, A.M. Cork spot of ‘d’Anjou’ pear fruit relative to criticl calcium concentration and other minerals. Acta Hortic. 1982, 28, 895–905. [Google Scholar]
- Dan, C.; Righetti, T.L.; Mielke, E.; Facteau, T. Mineral Analysis from Corkspotted and Normal ‘Anjou’ Pear Fruit. J. Am. Soc. Hortic. Sci. 1990, 115, 969–974. [Google Scholar]
- Brown, J.W. Chemical studies in the physiology of apples. V. Methods of ash analysis and the effect of environment on the mineral constitution of the apple. Ann. Bot. 1926, 40, 129–147. [Google Scholar] [CrossRef]
- Taylor, M.D.; Locascio, S.J. Blossom-End Rot: A Calcium Deficiency. J. Plant Nutr. 2004, 27, 123–139. [Google Scholar] [CrossRef]
- Ho, L.C.; White, P.J. A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann. Bot. 2005, 95, 571–581. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- Zhu, Z.-J.; Schultz, A.W.; Wang, J.; Johnson, C.H.; Yannone, S.M.; Patti, G.J.; Siuzdak, G. Liquid chromatography quadrupole time-of-flight mass spectrometry characterization of metabolites guided by the METLIN database. Nat. Protoc. 2013, 8, 451–460. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Wang, H.W.; Prabakaran, N.; Jeyalakshmi, K.; Kwon, M.; Manoharan, K.; Kim, W. 2,4-dichlorophenoxyacetic acid-induced leaf senescence in mung bean (Vigna radiata L. Wilczek) and senescence inhibition by co-treatment with silver nanoparticles. Plant Physiol. Biochem. 2011, 49, 168–177. [Google Scholar] [CrossRef]
- Vellosillo, T.; Vicente, J.; Kulasekaran, S.; Hamberg, M.; Castresana, C. Emerging Complexity in Reactive Oxygen Species Production and Signaling during the Response of Plants to Pathogens. Plant Physiol. 2010, 154, 444–448. [Google Scholar] [CrossRef]
- Jabs, T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 1999, 57, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.; Dixon, R.A. The Oxidative Burst in Plant Disease Resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Fluhr, R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000, 5, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Jones, R.L. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J. 2001, 25, 19–29. [Google Scholar]
- Sibgha, N.; Muhammad, A. Alleviation of adverse effects of salt stress on sunflower (Helianthus annuus L.) by exogenous application of salicylic acid: Growth and photosynthesis. Pak. J. Bot. 2008, 40, 1657–1663. [Google Scholar]
- Cui, Z.; Wang, N.; Duan, Y.; Xu, X.; Wang, R.; Zhang, S.; Ma, C. High-Resolution Microstructure Analysis of Cork Spot Disordered Pear Fruit “Akizuki” (Pyrus pyrifolia Nakai) Using X-Ray CT. Front. Plant Sci. 2021, 12, 715124. [Google Scholar] [CrossRef]
- Zhang, L.; Kamitakahara, H.; Sasaki, R.; Oikawa, A.; Itai, A. Effect of exogenous GA4 + 7 and BA + CPPU treatments on fruit lignin and primary metabolites in Japanese pear ‘Gold Nijisseiki’. Sci. Hortic. 2020, 272, 109593. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, C.; Zhang, Y.; Li, C.; Li, X.; Yu, Q.; Wang, S.; Wang, X.; Chen, X.; Feng, S. Transcriptomic and metabolomic analysis provides insights into anthocyanin and procyanidin accumulation in pear. BMC Plant Biol. 2020, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.J.; Wu, X.; Gao, X.; Li, L.F.; Xie, Z.H.; Gu, C.; Zhang, S.L. Metabolome and transcriptome analyses unravel the inhibition of embryo germination by abscisic acid in pear. Sci. Hortic. 2022, 292, 110652. [Google Scholar]
- Li, Z.; Hu, J.; Wu, Y.; Wang, J.; Song, H.; Chai, M.; Cong, L.; Miao, F.; Ma, L.; Tang, W. Integrative analysis of the metabolome and transcriptome reveal the phosphate deficiency response pathways of alfalfa. Plant Physiol. Biochem. 2022, 170, 49–63. [Google Scholar] [CrossRef]
- Rudell, D.R.; Mattheis, J.P.; Hertog, M.L.A.T.M. Metabolomic Change Precedes Apple Superficial Scald Symptoms. J. Agric. Food Chem. 2009, 57, 8459–8466. [Google Scholar] [CrossRef] [PubMed]
- Falchi, R.; D’Agostin, E.; Mattiello, A.; Coronica, L.; Spinelli, F.; Costa, G.; Vizzotto, G. ABA regulation of calcium-related genes and bitter pit in apple. Postharvest Biol. Technol. 2017, 132, 1–6. [Google Scholar] [CrossRef]
- Chen, L.; Xu, M.; Zheng, Y.; Men, Y.; Sheng, J.; Shen, L. Growth promotion and induction of antioxidant system of tomato seedlings (S. lycopersicum L.) by endophyte TPs-04 under low night temperature. Sci. Hortic. 2014, 176, 143–150. [Google Scholar] [CrossRef]
- Vincken, J.P.; Heng, L.; Groot, A.D.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Sawai, S.; Saito, K. Triterpenoid biosynthesis and engineering in plants. Front. Plant Sci. 2011, 2, 25. [Google Scholar] [CrossRef]
- Moses, T.; Pollier, J.; Thevelein, J.M.; Goossens, A. Bioengineering of plant (tri)terpenoids: From metabolic engineering of plants to synthetic biology invivo and invitro. New Phytol. 2013, 200, 27–43. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Z.; Qi, Y.; Liu, Y.; Ding, Y.; Tian, X.; Ren, X. MdCAX affects the development of the ‘Honeycrisp’ bitter pit by influencing abnormal Ca distribution. Postharvest Biol. Technol. 2021, 171, 111341. [Google Scholar] [CrossRef]
- Witney, G.W.; Kushad, M.M.; Barden, J.A. Induction of bitter pit in apple. Sci. Hortic. 1991, 47, 173–176. [Google Scholar] [CrossRef]
- Lurie, S.; Watkins, C.B. Superficial scald, its etiology and control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]
- Shear, C.B. Incidence of cork spot as related to calcium in the leaves and fruit of York Imperial apples. J. Am. Soc. Hortic. Sci. 1972, 97, 61–64. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M. Boron Toxicity and Deficiency in Agricultural Plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Askew, H.O.; Chittenden, E. Part III. Effect of Borax Sprays on the Boron Status of Fruit and Incidence of “Internal Cork” in Apples. J. Hortic. Sci. Biotechnol. 1937, 14, 242–245. [Google Scholar] [CrossRef]
- Feng, S. Study on the Characteristics of Disease and Control of Bitter Pit and Watercore in Pear Trees. Master’s Thesis, Yangzhou University, Yangzhou, China, 16 December 2017. [Google Scholar]
- Bush, D.S. Calcium Regulation in Plant Cells and its Role in Signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 95–122. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Quiles, A.; Hernando, I.; Pérez-Munuera, I. Effect of calcium propionate on the microstructure and pectin methy-lesterase activity in the parenchyma of fresh-cut Fuji apples. J. Sci. Food Agric. 2010, 87, 511–519. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Guo, L.; Liu, Y.; Jin, L.; Syed, B.H.; Du, W.; Deng, Z.; Peng, S. Transcriptome Changes Associated with Boron Deficiency in Leaves of Two Citrus Scion-Rootstock Combinations. Front. Plant Sci. 2017, 8, 317. [Google Scholar] [CrossRef]
- Yermiyahu, U.; Nir, S.; Ben-Hayyim, G.; Kafkafi, U. Quantitative competition of calcium with sodium or magnesium for sorption sites on plasma membrane vesicles of melon (Cucumis melo L.) root cells. J. Membr. Biol. 1994, 138, 55–63. [Google Scholar] [CrossRef] [PubMed]

| Samples | N (mg/kg) | Zn (mg/kg) | B (mg/kg) | Fe (mg/kg) | Cu (mg/kg) | Ca (mg/kg) | K (mg/kg) | Mg (mg/kg) | P (mg/kg) | S (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| HQ | 3.19 ± 0.57 ab | 5.34 ± 0.87 b | 60.23 ± 9.41 a | 10.75 ± 1.36 d | 1.45 ± 0.25 c | 745.43 ± 78.03 a | 9401.12 ± 1323.53 bc | 580.75 ± 43.76 c | 761.51 ± 20.12 d | 496.95 ± 4.85 c |
| G | 2.02 ± 0.14 c | 4.80 ± 0.32 b | 49.80 ± 0.97 b | 21.45 ± 1.01 c | 1.19 ± 0.01 c | 194.91 ± 5.33 c | 12,995.33 ± 170.44 a | 678.39 ± 13.65 b | 1086.65 ± 23.38 a | 472.42 ± 31.23 cd |
| DN | 2.88 ± 0.06 b | 5.90 ± 0.93 b | 32.03 ± 0.56 c | 24.24 ± 1.14 b | 3.30 ± 0.05 b | 107.94 ± 8.97 d | 9932.20 ± 26.67 b | 478.84 ± 9.55 d | 885.68 ± 8.91 c | 712.39 ± 9.35 b |
| DS | 3.75 ± 0.29 a | 14.68 ± 1.12 a | 31.97 ± 0.60 c | 57.90 ± 8.91 a | 6.65 ± 0.05 a | 483.5 ± 11.26 b | 7381.43 ± 105.21 d | 1301.83 ± 19.90 a | 941.08 ± 7.39 b | 1141 ± 94.86 a |
| Name | Compound | Class | Log2 (G/HQ) | p-Value | VIP | log2 (DN/HQ) | p-Value | VIP |
|---|---|---|---|---|---|---|---|---|
| mws0470 | Methylmalonic acid | Organic acids | −1.13 | 0.000682108 | 14.90 | −1.05 | 0.00474035 | 12.73 |
| mws0192 | Succinic acid | Organic acids | −1.16 | 0.000609659 | 15.13 | −1.09 | 0.004809102 | 13.01 |
| pme3096 | Aminomalonic acid | Organic acids | −1.21 | 0.000230108 | 2.95 | −1.12 | 0.002645329 | 2.52 |
| Lmgn000160 | 3-Ureidopropionic acid | Organic acids | −0.48 | 0.014183107 | 1.94 | −1.01 | 0.005539138 | 2.39 |
| Lmzn006284 | 2α-Hydroxyursolic acid | Triterpenes | 4.21 | 0.008486822 | 3.21 | 2.09 | 0.002040357 | 1.27 |
| mws1610 | Maslinic acid | Triterpenes | 4.15 | 0.011787435 | 3.18 | 1.97 | 0.005822207 | 1.22 |
| mws0889 | D-Threonic acid | Saccharides and alcohols | −0.76 | 0.004782529 | 1.66 | −0.96 | 0.000892064 | 1.64 |
| Name | Compound | Class | Log2 (DN/HQ) | p-Value | VIP | Log2 (DN/G) | p-Value | VIP |
|---|---|---|---|---|---|---|---|---|
| pme2237 | Dulcitol | Saccharides and alcohols | 0.47 | 0.004784837 | 5.77 | 0.64 | 0.001583311 | 9.10 |
| pme0519 | D-Sucrose | Saccharides and alcohols | 0.81 | 0.013778942 | 2.37 | 0.55 | 0.045412344 | 2.66 |
| mws1155 | D-Mannitol | Saccharides and alcohols | 0.67 | 0.015519951 | 3.89 | 0.84 | 0.011389627 | 6.04 |
| pmp001281 | LysoPC 18:1 | Lipids | −0.98 | 0.015533774 | 1.69 | −0.41 | 0.008074391 | 1.34 |
| Lmhp010190 | LysoPC 18:1(2n isomer) | Lipids | −0.97 | 0.034051831 | 1.64 | −0.48 | 0.016473192 | 1.45 |
| Zmyn004676 | 17-Hydroxylinolenic acid | Lipids | −1.25 | 0.01554908 | 1.02 | −0.90 | 0.034279247 | 1.11 |
| Zmyn000453 | Isocitric acid | Organic acids | 0.46 | 0.002719251 | 3.27 | 0.50 | 0.035685538 | 4.03 |
| MWS0811 | L-Pipecolic acid | Organic acids | −1.21 | 0.031421359 | 2.30 | −0.97 | 0.014435477 | 2.87 |
| mws1610 | Maslinic acid | Triterpenes | 1.97 | 0.005822207 | 1.22 | −2.19 | 0.022651996 | 3.22 |
| Lmzn006284 | 2α-Hydroxyursolic acid | Triterpenes | 2.09 | 0.002040357 | 1.27 | −2.12 | 0.017226549 | 3.24 |
| ML10181668 | Cycloleucine | Amino acids and derivatives | −1.15 | 0.008737324 | 2.88 | −0.70 | 0.003361136 | 2.90 |
| Zmdp000376 | 4-Guanidinobutanal | Others | −1.21 | 0.017358602 | 2.81 | −0.74 | 0.001701593 | 2.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhu, Y.; Ji, P.; Li, A.; Qiu, Z.; Cheng, Y.; Wang, R.; Ma, C.; Song, J.; Cui, Z.; et al. Mineral and Metabolome Analyses Provide Insights into the Cork Spot Disorder on ‘Akizuki’ Pear Fruit. Horticulturae 2023, 9, 818. https://doi.org/10.3390/horticulturae9070818
Yang Y, Zhu Y, Ji P, Li A, Qiu Z, Cheng Y, Wang R, Ma C, Song J, Cui Z, et al. Mineral and Metabolome Analyses Provide Insights into the Cork Spot Disorder on ‘Akizuki’ Pear Fruit. Horticulturae. 2023; 9(7):818. https://doi.org/10.3390/horticulturae9070818
Chicago/Turabian StyleYang, Yingjie, Yanlin Zhu, Piyu Ji, Anqi Li, Zhiyun Qiu, Yuanyuan Cheng, Ran Wang, Chunhui Ma, Jiankun Song, Zhenhua Cui, and et al. 2023. "Mineral and Metabolome Analyses Provide Insights into the Cork Spot Disorder on ‘Akizuki’ Pear Fruit" Horticulturae 9, no. 7: 818. https://doi.org/10.3390/horticulturae9070818
APA StyleYang, Y., Zhu, Y., Ji, P., Li, A., Qiu, Z., Cheng, Y., Wang, R., Ma, C., Song, J., Cui, Z., Liu, J., Nie, Y., Zhou, X., & Li, D. (2023). Mineral and Metabolome Analyses Provide Insights into the Cork Spot Disorder on ‘Akizuki’ Pear Fruit. Horticulturae, 9(7), 818. https://doi.org/10.3390/horticulturae9070818





