Abstract
Studying the biostimulation effect of amino acids indicated their possible role in salt stress mitigation. In this investigation, six exogenous amino acids (alanine (Ala), arginine (Arg), glutamine (Glu), glycine (Gly), methionine (Met), and proline (Pro)) at 0.5 g/L were sprayed to evaluate their impact on lettuce plants cultivated under simulated salt stress conditions. Photosynthetic pigments, ion absorption, endogenous amino acids contents, catalase (CAT), and peroxidase (POD) enzyme activities were determined. A significant alleviation of salt stress was noticed when EAAs were used in the stress-induced plants, and applying Gly, Met, and Pro improved the plant status under salt stress conditions. The highest electric conductivity (568 μS/g) was testified from the control treatment (50 mM NaCl), while applying exogenous amino acids reduced electrical conductivity (EC), and the result was located between 469 and 558 μS/g. AAs alleviated Cl- anions in the lettuce leaves by 25% in comparison to control plants. Na+ cations were alleviated when the stress-induced plants were sprayed with amino acids. In contrast, applying amino acids promoted K+ uptake, and Arg presented the highest contents (3226 μg/g). AAs promoted chlorophyll (chl a and chl b) concentrations compared to the control treatment, and Met produced the maximum chl a content, while the carotene (car) contents significantly augmented when Gly, Met, and Pro were applied. AAs were highly generated in non-stressed treatment (Std) compared to the control. Under simulated salinity stress, Met and Pro application enhanced proteinogenic amino acids expression. Compared to Ctl treatment, peroxidase enzyme activities significantly diminished in the other treatments, which fell by over 40% when Gly, Met, and Pro were sprayed.
1. Introduction
The main scientific concern revolves around environmental stresses and their effect on crop productivity. The FAO estimates that salinity affects more than 6% of the world’s land, making it a significant constraint on crop productivity. Therefore, salinity stress is a major challenge that needs to be addressed to ensure sustainable farming practices and crop productivity [1]. Among the different abiotic stresses, salt stress is a genuine threat to vegetable crops’ productivity, mainly in arid zones such as Egypt, where more than 25% of the plants suffer from salt stress [2] due to drainage water from the agricultural activities returning to the Nile or its canals. Lettuce (Lactuca sativa L.) is a main leafy vegetable that grows better during the cold season in temperatures ranging between 7 and 24 °C [3,4]. Lettuce provides the human body with a nutritious blend of minerals, fibers, vitamins, and antioxidants that promote good health. In addition, it also helps to lower blood cholesterol levels and alleviates anxiety [5,6,7].
Lettuce is a pro-health plant characterized by its low fat, carbohydrate, and water content. Additionally, it is abundant in essential nutrients, with a particular emphasis on potassium, which is crucial for regulating proper hydration levels. Moreover, it contains selenium, another vital nutrient element, an antioxidant that prevents cancer, colon, prostate, and lung diseases. It also has many essential amino acids for protein formation and for enhancing the immunological system [8]. Lettuce’s productivity is negatively impacted by salt stress, as it is moderately sensitive to its productivity [9]. Salt stress creates an osmotic pressure that hinders water uptake, limiting growth. This stress also leads to physiological and nutritional imbalances due to the absorption of toxic ions [10]. It also triggers oxidative consequences by producing excessively reactive oxygen species (ROS), which damage lipids and proteins [11]. These effects primarily disrupt physiological processes, particularly photosynthesis and protein synthesis [12].
Biostimulants have gained substantial attention as a sustainable method for detoxifying heavy metals, stimulating natural toxins, controlling biotic stress, and stimulating water and nutrient uptake. Amino acids are beneficial for stress defense, photosynthesis, an increased nutrient uptake, as precursors to hormones, and for growth parameters [13]. Applying biostimulants to mitigate salt stress as an alternative to synthetic chemicals is key to a sustainable farming system [14]. Amino acids are precursors and constituents of proteins, which are well-known for promoting cell growth. Since amino acids are the basic building blocks of proteins, they are vital in plant growth, development, and metabolite synthesis. Amino acids have various beneficial roles in plant development due to their structure as protein units, which play a crucial function in glutamine biosynthesis [15] and as plant growth regulators [16]. Amino acids have a dual function as the providers of organic nitrogen and as building blocks for proteins.
Moreover, amino acids (AAs) act as essential molecules that implement diverse physiological functions within the internal plant tissues [17]. In addition, AAs play a crucial nutritional role in seed germination [18]. Under salinity conditions, amino acids work as osmolytes that promote ion transport regulation, stomatal opening control, heavy metals detoxification, enzyme activation, gene expression, and redox homeostasis maintenance [19,20].
There are various roles of alanine in plants via its accumulation as a general stress response molecule implicated in plant protection against temperature extremes, hypoxia, drought, heavy metal contamination, and some biotic stresses. It participates in lignin biosynthesis and ethylene synthesis and is also converted to the osmoprotective compound and antioxidant homo-glutathione [21]. Arginine is a biostimulant, one of the most multi-purpose amino acids with the highest N/C ratio. It is a precursor in polyamines synthesis and molecule signaling [22,23]. Glutamine induces several transcription factors responsible for plant defense against abiotic stress [24]. In addition to its role as a metabolic fuel, glutamine regulates plant gene expression, growth, and stress responses. Thus, glutamine is a functional amino acid that plays essential functions in plant nutrition [25]. Glycine is broadly applied in plant nutrition. It produces a wide range of amino-chelate fertilizers [26]. Methionine is an essential sulfur-containing amino acid, and it plays a crucial role in several diverse purposes in living organisms [27]. Methionine effectively regulates plant growth and development under environmental cues [28]. Proline accumulation occurs under drought [29], salt [30], low temperature [31], and heavy metal contamination [32]. Under stress conditions, proline stabilizes subcellular structures, scavenges free radicals, and buffers cellular redox potential [33].
Moreover, the application of amino acids promotes the photosynthesis process and co-enzyme formation [34] as well as supports plant organisms facing various environmental stresses [19,35], where building proteins in plants have been impacted with an exogenous amino acids supplementation [36]. The exogenous application of amino acids can increase nitrogen status and concentration of mineral elements in plant tissues [37]. Recently, the safe production of crops, particularly fresh vegetables, has been vital regarding human health [38,39]. Applying amino acids such as glycine is more sustainable than synthetic fertilizers [40]. While the present research sheds light on the physiological and biochemical alterations under salinity stress conditions, the primary objective of this study was to test the hypothesis that the application of amino acids (alanine, arginine, glutamine, glycine, methionine, and proline) through foliar supplementation can alleviate the adverse impact of salinity stress on lettuce plants.
2. Materials and Methods
2.1. Experimental Conditions
Dachny cultivar lettuce seeds were sowed in pots filled with peat and bio-humus substrate. The substrate contains 350 mg/kg nitrogen, 270 mg/kg phosphorus, and 200 mg/kg potassium. After three weeks, once two true leaves had emerged, saline water containing 50 mM NaCl was used to water the pots to the field capacity, with the salinity treatment beginning at this point. Seedlings were sprayed (500 mL for each treatment) with amino acid solutions (0.5 g/L), while control plants were sprayed and rinsed with distilled water. The seedlings were grown in a greenhouse maintained at 25 °C during the day and 18–20 °C at night, with a relative humidity of around 70% and 10 h of artificial blue light (400–500 nm) daily. The greenhouse was ventilated to prevent the occurrence of plant diseases [41]. Amino acids were applied twice a week until 35 days. Six individual amino acids (Sigma-Aldrich, St. Louis, MO, USA) were sprayed (alanine (Ala), arginine (Arg), glutamine (Glu), glycine (Gly), methionine (Met), and proline (Pro)) in addition to salt treatment (Ctl) and standard treatment (Std). Finally, the samples were collected for morphological, physiological, and biochemical measurements in the laboratory.
2.2. Determination of the Morphological and Physio-Biochemical Attributes
The following parameters were measured: leaf number per plant, leaf area, fresh weight (FW), and dry matter. The dry matter content was determined by drying the leaves at 50 °C till a constant mass was achieved [42]. Moisture contents (MCs) were calculated using the leaves’ fresh weight and dry matter. Photosynthetic pigments (chlorophyll and carotene) were measured using spectrophotometric methods as follows: 200 mg of leaves were ground in 90% acetone, and the absorptions at 663 nm and 644 nm for chlorophylls and 452.5 nm for carotenoids were registered [43,44] and expressed in mg g–1 FW. Titration methods were applied to measure the titratable acidity (TTA) and expressed in mg/100 g [45]. Ascorbic acid was measured according to the 2,6-dichlorophenolindophenol method [46].
2.3. Determination of Ion Contents
Ion (Cl−, Na+, K+) contents were determined as follows: 200 mg of fresh leaves were extracted using an ultrasonic device (TTS, Sapfir, Moscow, Russia) with 25 mL of deionized water for 30 min at 40 °C, at a 35 kHz ultra-sound power level. The resulting samples were cooled and passed through a 0.45 µm porous filter. The extracted samples were then subjected to ion-selective electrodes to determine the potassium, sodium, and chloride ion concentrations. K+ electrode (ELIT-031), Na+ electrode (ELIS-112), and Cl- electrode (ELIT-261) were used, along with an ITAN ionometer (Itan-1, TomskAnalit, Tomsk city, Russia). The potassium/sodium ratio was determined after measuring the ions’ concentration in the samples. The electrolyte contents in the samples were measured by the solution’s electrical conductivity using an Expert-002 conductometer (Expert-002, Ekoniks, Moscow, Russia). The change in the electrical conductivity of the samples in µS/g is proportional to the concentration of electrolytes and reflects the degree of ion accumulation in plant tissues [47,48,49].
2.4. Analyses of Amino Acids Concentration
Determination of amino acids was conducted using an Amino Acid Analyzer Hitachi L-8800 system [50] as follows. Preliminary acid hydrolysis was carried out to measure amino acid composition in the samples. A concentrated hydrochloric and trifluoroacetic acid mixture was applied. For total acid hydrolysis, 300 μL of a freshly prepared hydrolyzing mixture was added to the sample in a molybdenum glass ampoule, then frozen in liquid nitrogen, evacuated, and melted. Hydrolysis was carried out at 155 °C for 1 h. Amino acid analysis was conducted on an Amino Acid Analyzer Hitachi L-8800 system (Tokyo, Japan). For system calibration, standard amino acids were applied. Colored products were detected by absorption at 570 nm for all amino acids but proline (440 nm). Data were processed by MultiChrom software v. 1.71a (Ampersand Ltd., Moscow, Russia) [51].
2.5. Catalase and Peroxidase Measurement
A reaction mixture consisting of 50 mM Na–phosphate buffer (pH 7.8), 50 mM guaiacol, 2% H2O2, and 0.1 mL of enzyme extraction were prepared to measure peroxidase activity. The absorbance was taken at 470 nm using a spectrophotometer, and POD was quantified as one unit, causing a 0.01 increase in absorbance at 470 nm in one minute. The results were expressed as U/g FW/min. [52]. Catalase (CAT) activity was determined by adding 0.1 mL of plant homogenate to 2 mL of 0.03% hydrogen peroxide solution to initiate the reaction. A control sample was also prepared using 0.1 mL. After 10 min, the reaction was measured by adding 4% ammonium molybdate, and the color intensity was measured at a wavelength of 410 nm against the control sample, where 2 mL of water was used instead of peroxide [53].
2.6. Statistical Analysis
A complete randomized design (CRD) with five replicates was employed for the experiment. R programming was used to conduct a one-way ANOVA analysis. The data were presented as mean ± SD and compared using the Tukey test (p ˂ 0.05). Correlation matrix and agglomerative hierarchical clustering were performed using XLSTAT v. 2023.
3. Results
The results presented in Figure 1 demonstrate a detrimental impact of salt stress on the morphological characteristics of lettuce plants compared to the standard treatment (non-treated). However, applying exogenous amino acids through spraying in combination with salt treatment significantly (p < 0.05) mitigated the salt stress. The maximum number of leaves (Figure 1A) was observed when Gly was used. Irrigating lettuce with only salt water treatment (Ctl) reduced the leaf number per plant compared to the Std treatment (distilled water) by 30%. Under salt treatment, EAAs promoted water absorption and nutrient uptake. The maximum fresh weight (6.52 g) and dry matter (391 mg) were observed in Pro treatment, while the minor weights (2.0 g and 135 mg, respectively) were obtained in the stress-induced plants (Ctl). Based on the presented data, lettuce leaves naturally responded to salinity conditions, as evidenced by their shrinkage (Figure 1D). When exposed to salt stress, the average leaf area (LA) decreased by 42%, with the largest LA (39.3 cm2) observed in the control group (Std). Conversely, the smallest LA was observed in the Ala treatment group, producing less than 25 cm2 LA. The statistical analysis indicated that the moisture content (MC) of lettuce leaves (Figure 1E), which ranged from 92.3 to 95.3%, was not significantly affected by exogenous amino acids (AAs). Photosynthesis is the primary metabolic process for enhancing growth and development [10,53]. Our findings generally show that photosynthetic pigments were reduced under stress conditions, while AAs promoted them. Chlorophyll content was highly expressed in non-treated plants (9.1 mg/L) and vice versa in salt-treated treatments, where the Chl a was reduced by around 25% (Figure 2). The result of Arg statistically did not differ from the Ctl treatment. Pro and Met presented the highest chlorophyll results under salt stress. In the higher plants, chlorophyll b content represents 50% of chlorophyll a. The results of this study align with the overall findings, indicating that subjecting lettuce plants to salt stress reduced chlorophyll b levels in their leaves. The application of Met treatment led to the determination of the minimum amount of chlorophyll b content (2.8 mg/L). Conversely, Arg treatment increased chlorophyll b levels by over 20% more than the salt treatment (Ctl).
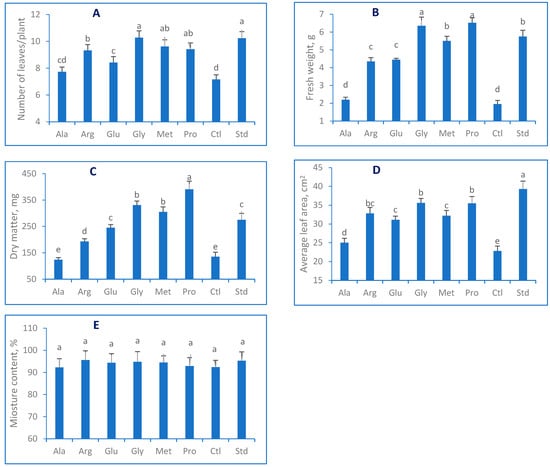
Figure 1.
(A) Number of leaves/plants; (B) fresh weight; (C) dry matter; (D) moisture contents; (E) average leaf area of Lactuca sativa sprayed with exogenous amino acids (Ala, Arg, Glu, Gly, Met, Pro) under salt stress (50 mM NaCl) conditions. Columns that share the same letter(s) statistically do not differ at p ≤ 0.05.
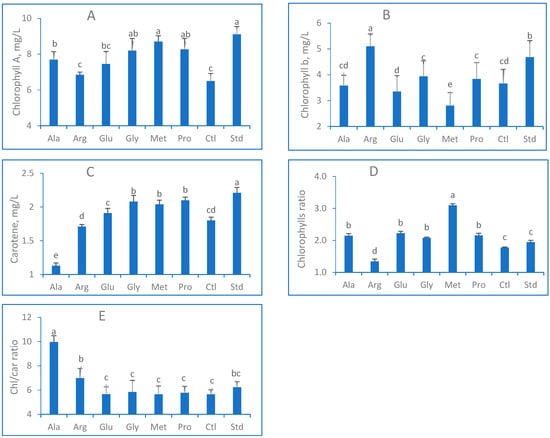
Figure 2.
(A) Chlorophyll a contents; (B) chlorophyll b contents; (C) carotene contents; (D) chlorophyll ratio; (E) chl- car ratio of Lactuca sativa sprayed with exogenous amino acids (Ala, Arg, Glu, Gly, Met, Pro) under salt stress (50 mM NaCl) conditions. Columns that share the same letter(s) statistically do not differ at p ≤ 0.05.
Carotene demonstrates a crucial function in photosynthesis, especially under stress conditions, namely protecting leaves’ tissues from excess light [54]. Non-stressed plants (Std) shared the maximum contents of carotene (2.21 mg/L), while the least contents (1.7 mg/L) were detected in the salt treatments (Figure 2C). When Gly, Met, and Pro were applied, the carotene contents significantly increased (>2.0 mg/L). Salt treatments promoted chlorophyll ratio (Chl ratio) except for Arg treatment, which registered the minor ratio (1.34), whereas Met treatment presented the maximum ratio (3.1). The investigation showed no significant differences regarding the Chl/Car ratio among the examined treatments. The ratio was located between 5.6 and 5.8, except for Ala (10.0) and Arg (7.0), whereas the non-stressed treatment exhibited a ratio of 6.2.
A considerable variation was observed when ascorbic acid and acidity were measured (Figure 3). The highest ascorbic acid contents were registered from Arg and Std treatments. Salt treatment decreased ascorbic acid compared to non-stressed (Std), which presented the most elevated ascorbic acid (23.2 mg/100 g). Glu and Ctl treatments presented the highest leaf acidity at more than 9.25%, while the Arg, Gly, Met, and Pro treatments decreased acidity to under 5.0%. Std and Ala treatments registered a balanced acidity (around 5.5%). As expected, more electrical conductivity was promoted in salt-stressed treatment than in non-stressed treatment (Figure 3C). Control treatment exhibited the highest electrical conductivity (568 μS/g), while applying EAAs decreased electrical conductivity, and the results ranged between 469 and 558 μS/g. The least EC (460 μS/g) was observed in standard treatment.
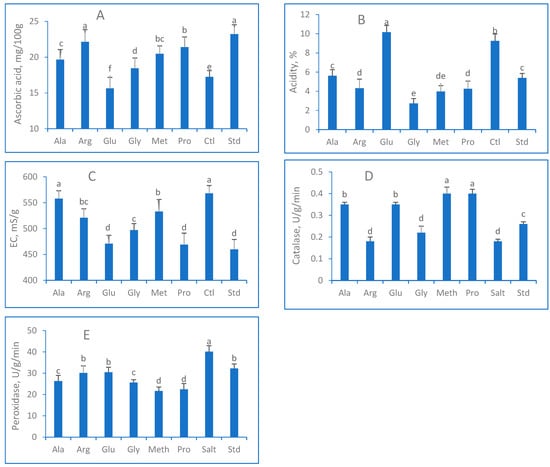
Figure 3.
(A) Number of leaves/plants; (B) fresh weight; (C) dry matter; (D) moisture contents; (E) average leaf area of Lactuca sativa sprayed with exogenous amino acids (Ala, Arg, Glu, Gly, Met, Pro) under salt stress (50mM NaCl) conditions. Columns that share the same letter(s) statistically do not differ at p ≤ 0.05.
Under salinity stress, plant defense-related activity (Peroxidase) was significantly augmented (Figure 3D) in comparison to the standard plants and, conversely, when the catalase activity was evaluated (Figure 3E). Peroxidases are involved in several physiological processes in plants, particularly in response to biotic and abiotic stresses. Additionally, they aid in the elimination of reactive oxygen species (ROS) produced under salt stress conditions and can prevent oxidative damage to plant cells [11,55]. Peroxidase activity significantly reduced all exogenous amino acid treatments, whereas the maximum deduction was observed in Met (21.66 U/g/min). The control treatment (salt) exhibited the maximum peroxidase concentration (40.12 U/g/min). Catalase activity was reduced by 42% at salinity treatment compared to Std, whereas spraying EAAs promoted catalase activity under salinity conditions. Met and Pro presented the highest results (0.40 U/g/min), while the most negligible concentration (0.18) was determined when Arg and Ctl were applied.
Figure 4 shows the expression of Cl, Na, and K contents in lettuce leaves in μg/g FW. As expected, salinity treatments promoted chloride contents (1438 μg/g) in plant tissues, while AAs reduced Cl concentration in the leaves by 25% compared to the stressed plants. In contrast, the least significant chloride amounts (970 and 980 μg/g) were observed in Std and Pro treatments. Similar to the results for other parameters, a similar trend was observed in the case of sodium concentration, with Na ions being reduced when salt-stressed plants were treated with AAs applied. Gly treatment exhibited the highest mitigation results (−30%) compared to the stressed plants, and its impact is close to Std treatment (1231 μg/g). In contrast, the application of EAAs promoted potassium absorption compared to the control treatment (Figure 4C). Gly presented the highest K+ concentration (3118 μg/g) in comparison to the other AAs and yet a reduced concentration (2810 μg/g) in the Pro treatment, whereas the highest potassium content resulted from Std with a 25% increase compared to salt-treated plants (2547 μg/g).
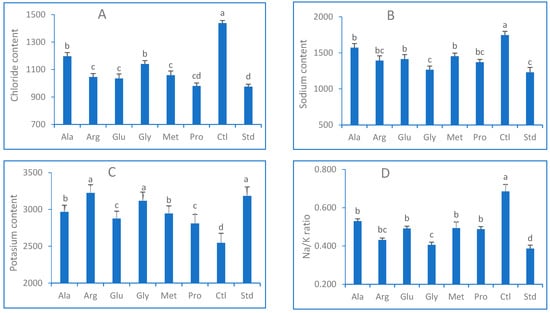
Figure 4.
(A) Chloride content; (B) sodium content; (C) potassium content; (D) sodium/potassium ratio of Lactuca sativa sprayed with exogenous amino acids (Ala, Arg, Glu, Gly, Met, Pro) under salt stress (50mM NaCl) conditions. Columns that share the same letter(s) statistically do not differ at p ≤ 0.05.
The sodium/potassium ratio (Figure 4D) varied from 0.39 in non-stressed plants (Std) to 0.69 in salt-stressed plants susceptible to salt stress. However, the application of AAs reduced the Na/K ratio by increasing potassium uptake and decreasing sodium absorption. The lowest ratio (0.41) was observed when Gly was sprayed compared to the other AAs, while Ala treatment resulted in the highest ratio (0.53).
Proteinogenic metabolism: Seventeen endogenous amino acids were determined to examine the impact of exogenous amino acids on proteinogenic metabolism (Table 1). In general, endogenous amino acids are expressed at higher levels in the standard treatment (non-stressed) than in the control treatment (stressed). The standard treatment exhibited the highest asparagine content (2.55 g/100 g) compared to the stressed plants, which ranged from 2.04 in Ala treatment to 2.35 in Gly treatment. Conversely, Pro treatment increased endogenous threonine content by over 40% compared to the control treatment, while the lowest content was received from the Ala treatment. Similar results were obtained when measuring serine and glycine, where Met treatment increased the levels of serine (1.02 g/100 g) and glycine (1.18 g/100 g) in the stressed leaves compared to other stressed treatments. Its impact was not statistically different from that of the standard and Gly treatments (Table 1).

Table 1.
Effect of foliar application of exogenous amino acids on concentration of endogenous amino acids and protein concentration on lettuce leaves grown under salinity stress conditions.
Determining proline levels revealed an increase in concentration in salt-treated plants in comparison to the standard, excluding Ala, which had the lowest proline content of less than 1.07 g/100 g. Under salt stress conditions, Met treatment increased proline content (1.43 g/100 g). Glutamine was located between 1.35 and 3.56 g/100 g, and Arg and Pro treatments increased glutamine concentration in salt-treated plants by over 65% compared to the control. Spraying Met on salt-treated lettuce plants increased the concentration of endogenous amino acids alanine (1.31 g/100 g), valine (1.44 g/100 g), and methionine (245 g/100 g), while Gly treatment improved isoleucine (1.061 g/100 g) and leucine (2.004 g/100 g) than other investigated AAs. The findings were comparable to those observed in Std treatment. In contrast, the Ala treatment resulted in a reduction in the expression of several endogenous amino acids, such as alanine (1.09 g/100 g), valine (1.21 g/100 g), isoleucine (0.877 g/100 g), tyrosine (0.568 g/100 g), and lysine (1.200 g/100 g). Furthermore, salt stress decreased the phenylalanine content in the leaves compared to non-stressed leaves. However, applying the Gly led to an increase in the expression of phenylalanine in lettuce leaves (1.263 g/100 g).
On the contrary, the minimum phenylalanine (1.048 g/100 g) was obtained from salt-treated plants when sprayed with Arg (Table 1). The concentration of ornithine in salt-treated plants ranged from 0.188 in Pro to 0.242 g/100 g in Gly. There are no statistically significant differences between the obtained results of Std and Arg treatments (0.229 g/100 g). Lysine contents were increased by more than 1.200 g/100 g in all studied treatments except for the control (0.892) treatment. The histidine concentration represents half of the lysine. Similarly, when arginine was determined, the same trend was registered. Excluding Ctl treatment, which registered the least contents (0.828 g/100 g), the other treatments increased arginine contents, and the data were located between 1.073 and 1.33 g/100 g. Lastly, the protein content of the salt-stressed leaves varied from 14.62 g/100 g (Ctl) to 21.53 g/100 g (Met), with the Std treatment exhibiting a protein content of 20.68 g/100 g, which was not significantly different from the Met treatment.
Correlation matrix and agglomerative hierarchical clustering:
A correlation test was performed using XLSTAT v. 2023 (Figure 5A). Regarding the obtained coefficients, a robust significant correlation (≥0.70) was observed among the phonological attributes (NLP, FW, and DM) of lettuce plants. Also, a moderate positive correlation was registered between morphological characteristics and endogenous amino acid concentration, except for Orn, which negatively correlated with NLP, FW, and DM. Electrical conductivity has a perfect positive correlation (≥0.80) with chloride and sodium contents, photosynthetic pigments, peroxidase antioxidant enzyme, and acidity; moreover, a moderate negative correlation (− 0.70 ≥ − 0.40) with endo-proteinogenic amino acids concentration, ascorbic acid, and catalase was observed. Agglomerative clustering is the primary type of hierarchical clustering performed on group traits in clusters regarding their similarity, and the output is a tree-based representation of the traits, called dendrogram or AGNES (agglomerative nesting). The illustrated dendrogram (Figure 5B) was calculated according to Ward’s linkage method. The dendrogram divided data into two main groups: the first cluster (C1) includes catalase enzymes, chl a, morphological traits, and endo-amino acids, while the second cluster (C2) includes peroxidase enzymes, Na, Cl, K, EC, and chl b.
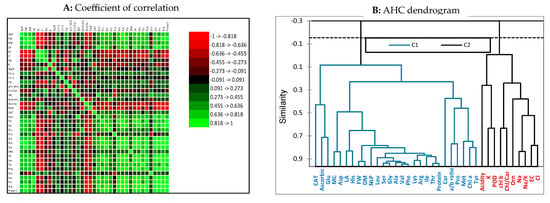
Figure 5.
(A) Coefficients of correlation for the different pairs of studied parameters of lettuce plant cultivated under salinity stress and sprayed with amino acids; (B) agglomerative hierarchical clustering dendrogram (AHC) of the studied attributes.
4. Discussion
Crops in arid regions are typically subjected to numerous environmental stresses, including salt, drought, temperature fluctuations, and toxic metal contamination. These factors can reduce crop growth and productivity, depending on the severity of the stress [10,56]. Salt stress destructively impacts plant growth, development, and quality by making alterations to the physiological and biochemical processes, reducing productivity, especially in arid regions such as the Mediterranean region [57], where the salinity of water exceeds the threshold tolerated by crops [58,59]. The problem is also worsened at high temperatures and drought [60]. The present study examined the influence of exogenous amino acids on lettuce under simulated salinity stress conditions. The findings revealed that EAAs can mitigate the harmful effects of salinity on lettuce plants. In the following discussion, we explore the biological mechanisms that plants use to enhance their tolerance to salt stress and the application of exogenous amino acids has been proposed as a sustainable approach for mitigating salt stress [61,62]. EAAs promoted seed germination and plant growth [63], and the plant-derived protein hydrolyzate improved the fresh weight, dry biomass, and plant growth of lettuce [64], soybean [65], and lentil [66] cultivated under salt stress. Salt stress significantly impacted the morphology of lettuce in the current study, primarily due to a reduced water uptake and difficulties in nutrient uptake. [67]. Our study demonstrated that Met and Pro were more effective in mitigating salinity stress than Ala and Arg. The application of EAAs helped to alleviate the negative impacts of salinity stress on the mass, number, and average leaf area of lettuce, with Met and Pro treatments exhibiting more significant results than the control treatment. The beneficial effects of EAAs on growth and development have been widely documented in other crops [61,68]. Salinity is the primary environmental stressor that disrupts the physiological processes in plants, including photosynthesis and pigment production [69,70,71]. In this investigation, we detected that the chlorophyll content was reduced by 5% in control (50 mM NaCl) than the standard treatment (distilled water). While amino acids alleviate the deterioration of photosynthetic pigments when sprayed on salt-treated plants, they were found to actively protect proteins and photosystems against the negative effects of salinity stress [72] and work as osmolytes to an equilibrium of the cellular osmotic potential and manage nutrient uptake [73].
Salt conditions lead to accumulating high levels of sodium ions in plant tissues, disrupting the homeostasis of other nutrient elements such as potassium, and causing physiological problems and ion imbalances [74,75]. The results of this study revealed that despite the high accumulation of sodium ions in plant tissues exposed to 50 mM sodium chloride, the application of AAs led to a sodium and chloride reduction. Alternatively, the treatment with EAAs promoted potassium content, particularly in conditions where salt causes cell desiccation and ionic and osmotic imbalances [65]. Incorporating EAAs into lettuce leaves may be linked to the storage of precursors for protein synthesis, which could facilitate the rapid recovery of metabolic processes following salinity stress [76]. Various investigations have also highlighted the vital function of amino acids in ionic homeostasis regulation [77,78]. The lettuce leaves generated proline under salinity stress, which is consistent with the observed responses in multiple studies investigating salt stress. [79,80,81,82,83].
In the majority of the studies, endogenous amino acids displayed a consistent pattern, with amino acid concentration increasing as the level of salt stress decreased. Amino acid content was the highest in standard plants, while the lowest levels were observed in the treatments with the highest salinity stress, except for proline, which constituted a significant proportion of the total amino acid content [84]. In response to salinity stress, plants accumulate higher levels of osmolytes such as proline [69,85], which has a low molecular weight and is widely recognized as the primary osmoprotectant that helps in regulating salt tolerance, protecting membrane integrity, and stabilizing enzymes and proteins [86]. Moreover, amino acids can be vital to maintain cellular osmotic potential and regulate ion transport [85]. As per this study, the application of EAAs has led to increased endogenous amino acid contents in lettuce leaf tissues under salinity stress, resulting in improved plant adaptation and osmotic adjustment. This phenomenon has also been detected in other crops such as lentil, soybean, and cucumber, where amino acids have been shown to alleviate salt stress [71,87,88] because they are critical to plant metabolites in protein synthesis and other vital cellular functions.
5. Conclusions
To sum up, the application of AAs has a beneficial impact on the growth, physiological, and biochemical traits of lettuce plants. Our findings prove that amino acid applications as biostimulants can be a crucial tool for mitigating the negative effect of environmental stresses, including salt stress caused by climate change. However, this investigation only provided a brief insight into the potential damage to crops caused by salinity stress in arid regions due to climate change. Therefore, in the future, applying biostimulants based on exogenous amino acids may play a critical role in mitigating and alleviating abiotic stress and promoting sustainable farming practices. To reduce the negative impact of salt stress, our finding recommends spraying methionine or proline at 0.5 g/L on lettuce farms.
Author Contributions
Conceptualization, M.A. and M.P.; methodology, M.A., L.V., O.S., L.B.; software, M.A. and M.P; validation, M.A., B.A. (Bekzad Amantayev), and B.A. (Bauyrzhan Arinov); formal analysis, M.A.; investigation, M.A., M.P.; resources, E.L.; data curation, M.A. and A.K.; writing—original draft preparation, M.A. and L.B.; writing—review and editing, M.Z.; supervision, L.V.; project administration, M.A. and L.V.;funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Mostafa Abdelkader is funded by a full scholarship (2958AC) from the Ministry of Higher Education, Egypt. This work was supported by the RUDN University Strategic Academic Leadership Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar]
- ICARDA. ICARDA Annual Report 2009; International Center for Agricultural Research in the Dry Areas: Aleppo, Syria, 2010; p. iv + 68. [Google Scholar]
- Jenni, S. Rib discoloration: A physiological disorder induced by heat stress in crisphead lettuce. HortScience 2005, 40, 2031–2035. [Google Scholar] [CrossRef]
- Wei, S.; Yang, X.; Huo, G.; Ge, G.; Liu, H.; Luo, L.; Hu, J.; Huang, D.; Long, P. Distinct metabolome changes during seed germination of lettuce (Lactuca sativa L.) in response to thermal stress as revealed by untargeted metabolomics analysis. Int. J. Mol. Sci. 2020, 21, 1481. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Camejo, D.; Frutos, A.; Mestre, T.C.; del Carmen Piñero, M.; Rivero, R.M.; Martínez, V. Artificial light impacts the physical and nutritional quality of lettuce plants. Hortic. Environ. Biotechnol. 2020, 61, 69–82. [Google Scholar] [CrossRef]
- Baslam, M.; Pascual, I.; Sánchez-Díaz, M.; Erro, J.; García-Mina, J.M.; Goicoechea, N. Improvement of nutritional quality of greenhouse-grown lettuce by arbuscular mycorrhizal fungi is conditioned by the source of phosphorus nutrition. J. Agric. Food Chem. 2011, 59, 11129–11140. [Google Scholar] [CrossRef] [PubMed]
- Anilakumar, K.R.; Harsha, S.N.; Mallesha, S.; Sharma, R.K. Lettuce: A promising leafy vegetable with functional properties. Def. Life Sci. J. 2017, 2, 178. [Google Scholar] [CrossRef]
- Uenluekara, A.; Cemek, B.; Karaman, S.; Erşahin, S. Response of lettuce (Lactuca sativa var. crispa) to salinity of irrigation water. N. Z. J. Crop Hortic. Sci. 2008, 36, 265–273. [Google Scholar] [CrossRef]
- Abdelkader, M.; Geioushy, R.A.; Fouad, O.A.; Khaled, A.G. Investigation the activities of photosynthetic pigments, antioxidant enzymes and inducing genotoxicity of cucumber seedling exposed to copper oxides nanoparticles stress. Sci. Hortic. 2022, 305, 111364. [Google Scholar]
- Hossain, M.S.; Dietz, K.-J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.A.; Matos, R.; Mereiter, S.; Pinto, M.T.; Polónia, A.; Gartner, F.; et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine 2019, 40, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Cheng, Q.; Sun, W. The Effects of Amino Acids, Phenols and Protein Hydrolysates asBiostimulants on Sustainable Crop Production and AlleviatedStress. Recent Pat. Biotechnol. 2022, 16, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.M.; Gaplaev, M.S.; Terekbaev, A.A.; Puchkov, M.Y. The influence of biostimulants on tomato plants cultivated under hydroponic systems. J. Hortic. Res. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef]
- Sierras, N.; Botta, A.; Staasing, L.; Martinez, M.J.; Bru, R. Understanding the effect of amino acids based biostimulant by an enantiomeric analysis of their active principles and a proteomic profiling approach. Acta Hortic. 2016, 1148, 93–100. [Google Scholar] [CrossRef]
- Atilio, J.B.; Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar] [CrossRef]
- Rai, V.K. Role of Amino Acids in Plant Responses to Stresses. Biol. Plant. 2002, 45, 481–487. [Google Scholar] [CrossRef]
- Cheng, Y.; Tian, Q.; Zhang, W.-H. Glutamate receptors are involved in mitigating effects of amino acids on seed germination of Arabidopsis thaliana under salt stress. Environ. Exp. Bot. 2016, 130, 68–78. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The synthesis and role of β-alanine in plants. Front. Plant Sci. 2019, 10, 921. [Google Scholar] [CrossRef]
- Patel, P.; Kadur Narayanaswamy, G.; Kataria, S.; Baghel, L. Involvement of nitric oxide in enhanced germination and seedling growth of magnetoprimed maize seeds. Plant Signal. Behav. 2017, 12, e1293217. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Miao, F.; Wang, Y.; Liu, H.; Wang, X.; Wang, H.; Guo, J.; Shao, R.; Yang, Q. L-Arginine alleviates the reduction in photosynthesis and antioxidant activity induced by drought stress in maize seedlings. Antioxidants 2023, 12, 482. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.D.S.; Alvarez-Pizarro, J.C.; Costa, J.H.; Paula, S.d.O.; Prisco, J.T.; Gomes-Filho, E. Putative role of glutamine in the activation of CBL/CIPK signalling pathways during salt stress in sorghum. Plant Signal. Behav. 2017, 12, 522–536. [Google Scholar] [CrossRef]
- Kan, C.-C.; Chung, T.-Y.; Juo, Y.-A.; Hsieh, M.-H. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genom. 2015, 16, 731. [Google Scholar] [CrossRef] [PubMed]
- Zargar Shooshtari, F.; Souri, M.K.; Hasandokht, M.R.; Jari, S.K. Glycine mitigates fertilizer requirements of agricultural crops: Case study with cucumber as a high fertilizer demanding crop. Chem. Biol. Technol. Agric. 2020, 7, 19. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M.; Ashraf, M.; Sadiq, M. Exogenous application of L-methionine mitigates the drought-induced oddities in biochemical and anatomical responses of bitter gourd (Momordica charantia L.). Sci. Hortic. 2020, 267, 109333. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P.J. Salinity, osmolytes and compatible solutes. In Salinity: Environment—Plants—Molecules; Springer: Dordrecht, The Netherlands, 2002; pp. 181–204. [Google Scholar] [CrossRef]
- Naidu, B.P.; Paleg, L.G.; Aspinall, D.; Jennings, A.C.; Jones, G.P. Amino acid and glycine betaine accumulation in cold-stressed wheat seedlings. Phytochemistry 1991, 30, 407–409. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Amin, A.A.; Gharib, F.A.; El-Awadi, M.; Rashad, E.-S.M. Physiological response of onion plants to foliar application of putrescine and glutamine. Sci. Hortic. 2011, 129, 353–360. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef] [PubMed]
- Das, C.; Sengupta, T.; Chattopadhyay, S.; Setua, M.; Das, N.K.; Saratchandra, B. Involvement of kinetin and spermidine in controlling salinity stress in mulberry (Morus alba L. cv. S 1). Acta Physiol. Plant. 2002, 24, 53–57. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Souri, M.K.; Sooraki, F.Y.; Moghadamyar, M. Growth and quality of cucumber, tomato, and green bean under foliar and soil applications of an aminochelate fertilizer. Hortic. Environ. Biotechnol. 2017, 58, 530–536. [Google Scholar] [CrossRef]
- Tejada, M.; Rodríguez-Morgado, B.; Paneque, P.; Parrado, J. Effects of foliar fertilization of a biostimulant obtained from chicken feathers on maize yield. Eur. J. Agron. 2018, 96, 54–59. [Google Scholar] [CrossRef]
- Souri, M.K.; Hatamian, M. Aminochelates in plant nutrition: A review. J. Plant Nutr. 2019, 42, 67–78. [Google Scholar] [CrossRef]
- Alsadon, A.; Al-Helal, I.; Ibrahim, A.; Abdel-Ghany, A.; Al-Zaharani, S.; Ashour, T. The effects of plastic greenhouse covering on cucumber (Cucumis sativus L.) growth. Ecol. Eng. 2016, 87, 305–312. [Google Scholar] [CrossRef]
- Voronov, S.; Pleskachiov, Y.; Shitikova, A.; Zargar, M.; Abdelkader, M. Diversity of the Biological and Proteinogenic Characteristics of Quinoa Genotypes as a Multi-Purpose Crop. Agronomy 2023, 13, 279. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In Advances in Photosynthesis Research; Springer: Berlin/Heidelberg, Germany, 1984; pp. 9–12. [Google Scholar]
- Abdelkader, M.M.; Elsayed, H.M.A. Biodiversity of Photosynthetic Pigments, Macronutrients Uptake and Fruit Quality of Tomato Genotypes. Russ. J. Plant Physiol. 2022, 69, 50. [Google Scholar] [CrossRef]
- Schvambach, M.I.; Andriolli, B.V.; de Souza, P.F.; Oliveira, J.L.B.; Pescador, R. Conservation of crisp lettuce in different post-harvest storage conditions. Rev. Ceres 2020, 67, 256–262. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Eremeeva, N.B.; Makarova, N.V.; Zhidkova, E.M.; Maximova, V.P.; Lesova, E.A. Ultrasonic and microwave activation of raspberry extract: Antioxidant and anti-carcinogenic properties. Foods Raw Mater. 2019, 7, 264–273. [Google Scholar]
- Wardak, C.; Pietrzak, K.; Morawska, K.; Grabarczyk, M. Ion-Selective Electrodes with Solid Contact Based on Composite Materials: A Review. Sensors 2023, 23, 5839. [Google Scholar] [CrossRef]
- Pukhalskaya, N.V.; Kudrin, A.A.; Bolshakova, L.S.; Pukhovsky, A.V.; Sychev, V.G. Rapid Method for the Ionometric Determination of Potassium Content in Leaves and Its Distribution over Physiological Pools. State Scientific Institution All-Russian Scientific Research Institute of Agrochemistry D.N. Ryanishnikova RU 2465575 C2, 27 October 2012. Available online: https://elibrary.ru/item.asp?id=37770861 (accessed on 10 June 2023).
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of Amino Acids on Sulfonated Polystyrene Resins. An Improved System. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar] [CrossRef]
- Trofimova, L.; Ksenofontov, A.; Mkrtchyan, G.; Graf, A.; Baratova, L.; Bunik, V. Quantification of Rat Brain Amino Acids: Analysis of the Data Consistency. Curr. Anal. Chem. 2016, 12, 349–356. [Google Scholar] [CrossRef]
- Sun, D.; Liang, G.; Xie, J.; Lei, X.; Mo, Y. Improved preservation effects of litchi fruit by combining chitosan coating with ascorbic acid treatment during postharvest storage. Afr. J. Biotechnol. 2010, 9, 3272–3279. [Google Scholar]
- Kolesnichenko, V.V.; Kolesnichenko, A.V. The influence of high Cd2+ concentrations on lipid peroxidation and antioxidant system function of wheat (Triticum aestivum) and rye (Secale cereale) etiolated shoots. J. Stress Physiol. Biochem. 2012, 8, 5–15. [Google Scholar]
- Thayer, S.S.; Björkman, O. Carotenoid distribution and deepoxidation in thylakoid pigment-protein complexes from cotton leaves and bundle-sheath cells of maize. Photosynth. Res. 1992, 33, 213–225. [Google Scholar] [CrossRef]
- Vicuna, D. The Role of Peroxidases in the Development of Plants and Their Responses to Abiotic Stresses. Ph.D. Thesis, Technological University Dublin, Dublin, Ireland, 2005. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Garabello, C.; Contartese, V.; Ferrante, A. Effect of exogenous application of salt stress and glutamic acid on lettuce (Lactuca sativa L.). Sci. Hortic. 2022, 299, 111027. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; D’anna, F. Effect of salt stress in lettuce cultivation. Acta Hortic. 2003, 609, 371–375. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional value. J. Am. Soc. Hortic. Sci. 2016, 141, 12–21. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Zavala-Gonzalez, E.A.; Camara-Zapata, J.M.; Martínez-Nicolás, J.J.; Simón, I.; Simón-Grao, S.; García-Sánchez, F. Effect of foliar application of amino acids on the salinity tolerance of tomato plants cultivated under hydroponic system. Sci. Hortic. 2020, 272, 109509. [Google Scholar] [CrossRef]
- Sh Sadak, M.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar]
- Abdelkader, M.; Voronina, L.; Puchkov, M.; Shcherbakova, N.; Pakina, E.; Zargar, M.; Lyashko, M. Seed Priming with Exogenous Amino Acids Improves Germination Rates and Enhances Photosynthetic Pigments of Onion Seedlings (Allium cepa L.). Horticulturae 2023, 9, 80. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Peña Calzada, K.; Olivera Viciedo, D.; Habermann, E.; Calero Hurtado, A.; Lupino Gratão, P.; De Mello Prado, R.; Lata-Tenesaca, L.F.; Martinez, C.A.; Ajila Celi, G.E.; Rodríguez, J.C. Exogenous application of amino acids mitigates the deleterious effects of salt stress on soybean plants. Agronomy 2022, 12, 2014. [Google Scholar] [CrossRef]
- Fardus, J.; Hossain, M.S.; Fujita, M. Modulation of the antioxidant defense system by exogenous L-glutamic acid application enhances salt tolerance in lentil (Lens culinaris Medik.). Biomolecules 2021, 11, 587. [Google Scholar] [CrossRef]
- Al-Maskri, A.; Al-Kharusi, L.; Al-Miqbali, H.; Khan, M.M. Effects of salinity stress on growth of lettuce (Lactuca sativa) under closed-recycle nutrient film technique. Int. J. Agric. Biol. 2010, 12, 377–380. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Rohman, M.M.; Anee, T.I.; Huang, Y.; Fujita, M. Exogenous silicon protects Brassica napus plants from salinity-induced oxidative stress through the modulation of AsA-GSH pathway, thiol-dependent antioxidant enzymes and glyoxalase systems. Gesunde Pflanz. 2018, 70, 185–194. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Zhang, B. Transcriptome analysis and functional identification of GmMYB46 in soybean seedlings under salt stress. PeerJ 2021, 9, e12492. [Google Scholar] [CrossRef]
- Abdelkader, M.; Voronina, L.; Shelepova, O.; Puchkov, M.; Loktionova, E.; Zhanbyrshina, N.; Yelnazarkyzy, R.; Tleppayeva, A.; Ksenofontov, A. Monitoring Role of Exogenous Amino Acids on the Proteinogenic and Ionic Responses of Lettuce Plants under Salinity Stress Conditions. Horticulturae 2023, 9, 626. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M.; Fukuzawa, K. Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem. Med. 1980, 23, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.C.; Chiconato, D.A.; de Mello Prado, R.; Junior, G.D.S.S.; Gratao, P.L.; Felisberto, G.; Viciedo, D.O.; Dos Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef]
- Ahmad, P.; Abass Ahanger, M.; Nasser Alyemeni, M.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251, 3. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, M.A.S.; Babar, M.A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Karabal, E.; Yücel, M.; Öktem, H.A. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 2003, 164, 925–933. [Google Scholar] [CrossRef]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Jiménez-Bremont, J.F.; Becerra-Flora, A.; Hernández-Lucero, E.; Rodríguez-Kessler, M.; Acosta-Gallegos, J.A.; Ramírez-Pimentel, J.G. Proline accumulation in two bean cultivars under salt stress and the effect of polyamines and ornithine. Biol. Plant. 2006, 50, 763–766. [Google Scholar] [CrossRef]
- Eraslan, F.; Inal, A.; Savasturk, O.; Gunes, A. Changes in antioxidative system and membrane damage of lettuce in response to salinity and boron toxicity. Sci. Hortic. 2007, 114, 5–10. [Google Scholar] [CrossRef]
- Santander, C.; Ruiz, A.; García, S.; Aroca, R.; Cumming, J.; Cornejo, P. Efficiency of two arbuscular mycorrhizal fungal inocula to improve saline stress tolerance in lettuce plants by changes of antioxidant defense mechanisms. J. Sci. Food Agric. 2020, 100, 1577–1587. [Google Scholar] [CrossRef]
- Xie, E.; Wei, X.; Ding, A.; Zheng, L.; Wu, X.; Anderson, B. Short-term effects of salt stress on the amino acids of Phragmites australis root exudates in constructed wetlands. Water 2020, 12, 569. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing salt tolerance of plants: From metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Peng, X.; Han, L.; Hou, L.; Li, B. Effects of exogenous spermidine on root metabolism of cucumber seedlings under salt stress by GC-MS. Agronomy 2020, 10, 459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).