Yield and Antioxidant Quality of Habanero Chili Pepper by Supplementing Potassium with Organic Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Vegetal Material
2.3. Establishment of the Experiment
2.4. Assessed Variables
2.4.1. Agronomic Variables
2.4.2. Variables for Fruit Quality
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Yield Components
3.2. Components of the Biochemical-Antioxidant Quality
3.3. Interaction of Antioxidant Means
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguirre-Mancilla, C.L.; Iturriaga de la Fuente, G.; Ramírez-Pimentel, J.G.; Covarrubias-Prieto, J.G.; Chablé-Moreno, F.; Raya-Pérez, J.C. El chile (C. annuum L.), cultivo y producción de semilla. Cienc. Tecnol. Agropec. Méx. 2017, 5, 19–27. [Google Scholar]
- Herrera, A.A.; Cervantes, O.F.; Antuna, G.O.; García, R.J.; Rodríguez, M.D.; Rodríguez, H.S.; Andrio, E.E.; Mendoza, E.M. Deterioro de la calidad de la semilla de chile piquín de cuatro colectas en Querétaro y Guanajuato. Rev. Mex. Cienc. Agríc. 2018, 9, 1627–1638. [Google Scholar] [CrossRef]
- Kumar, A.; Elad, Y.; Tsechansky, L.; Abrol, V.; Lew, B.; Offenbach, R.; Graber, E.R. Biochar potential in intensive cultivation of Capsicum annuum L. (sweet pepper): Crop yield and plant protection. J. Sci. Food Agric. 2018, 98, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Duval, M.E.; Galantini, J.A.; Martínez, J.M.; Iglesias, J.O. Comparación de índices de calidad de suelos agrícolas y naturales basados en el carbono orgánico. Cienc. Suelo 2016, 34, 197–209. [Google Scholar]
- Yang, H.; Liu, H.; Zheng, J.; Huang, Q. Effects of regulated deficit irrigation on yield and water productivity of chili pepper (Capsicum annuum L.) in the arid environment of Northwest China. Irrig. Sci. 2018, 36, 61–74. [Google Scholar] [CrossRef]
- Moreno-Reséndez, A.; Briceño-Contreras, E.; Valenzuela-Núñez, L.; Hernández-Herrera, J. Capítulo VI: Abonos orgánicos: Una alternativa sustentable en la agricultura. In Tópicos Selectos de Sustentabilidad, un Reto Permanente Para el Nuevo Milenio; Clave Editorial: Tijuana, Mexico, 2020; Chapter VI; pp. 125–164. [Google Scholar]
- Yuan, J.; Sha, Z.M.; Hassani, D.; Zhao, Z.; Cao, L.K. Assessing environmental impacts of organic and inorganic fertilizer on daily and seasonal greenhouse gases effluxes in rice field. Atmos. Environ. 2017, 155, 119–128. [Google Scholar] [CrossRef]
- Verma, B.C.; Pramanik, P.; Bhaduri, D. Organic fertilizers for sustainable soil and environmental management. In Nutrient Dynamics for Sustainable Crop Production; Meena, R.S., Ed.; Springer: Singapore, 2020; pp. 289–313. [Google Scholar] [CrossRef]
- Instituto Interamericano de Cooperación para la Agricultura (IICA). Aumenta 137% el Valor de las Importaciones de Fertilizantes Químicos de América Latina y el Caribe. 2022. Available online: https://blog.iica.int/blog (accessed on 20 December 2022).
- Gómez, L.B.; González, E.T.; Soria, F.M. Prediction of potasium requirements for Capsicum chínense Jacq. production in southeast México. Trop. Subtrop. Agroecosyst. 2008, 8, 69–80. [Google Scholar]
- Borges, G.L.; Cervantes, C.L.; Ruiz, N.J.; Soria, F.M.; Reyes, O.V.; Villanueva, C.E. Capsaicinoides en chile habanero (Capsicum chinense Jacq.) bajo diferentes condiciones de humedad y nutrición. Terra Latinoam. 2010, 28, 35–41. [Google Scholar]
- INTAGRI. Guía de Fertilizantes Potásicos para Cultivos; Serie Nutrición Vegetal Núm. 142. Artículos Técnicos de INTAGRI; INTAGRI: Celaya, Mexico, 2020; 6p. [Google Scholar]
- INTAGRI. Cómo Enfrentar la Crisis de los Fertilizantes; Serie Nutrición Vegetal, Núm. 153. Artículos Técnicos de INTAGRI; INTAGRI: Celaya, Mexico, 2022; 5p. [Google Scholar]
- Morales-Casco, L.A.; Zúniga-González, C.A. Impactos del cambio climático en la agricultura y seguridad alimentaria. Rev. Iberoam. Bioecon. Cambio Clim. 2016, 2, 269–291. [Google Scholar] [CrossRef]
- Nichols, M. Good agricultural practises (gap) and greenhouse crops. Acta Hort. (ISHS) 2007, 742, 135–138. [Google Scholar] [CrossRef]
- SAGARPA: Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación; Organización de las Naciones Unidas para la Alimentación y la Agricultura. Compendio de Indicadores de Gestión y Resultados 2017. Programa de Concurrencia con las Entidades Federativas; SAGARPA: Mexico City, Mexico, 2018; 110p. [Google Scholar]
- Muñoz-Ramírez, L.S. Selección de Variedades de Chile Habanero (Capsicum chinense J.) con Fines Industriales. Ph.D. Thesis, Centro de Investigación Científica de Yucatán A.C (CICY), Mérida, Mexico, 2020; 146p. [Google Scholar]
- Menichini, F.; Tundis, R.; Bonesi, M.; Monica, R.; Loizzo, C.F.; Giancarlo, S.; Cindio, D.B.; Peter, J.; Houghton, C.F.M. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq.cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Guzman, I.; Paul, W.B.; Connell, A.O.M. Heat, color, and flavor compounds in capsicum fruit. Biol. Act. Phytochem. 2011, 41, 109–126. Available online: https://www.springerlink.com/content/v46t237483544px8/ (accessed on 20 February 2023).
- Wang, S.Y. Maximizing antioxidants in fruits. Acta Hort. (ISHS) 2010, 877, 81–93. Available online: http://www.actahort.org/books/877/877_5.htm (accessed on 3 February 2023). [CrossRef]
- Bennett, D.J.; Kirby, G.W. Constitution and biosynthesis of capsaicin. J. Chem. Soc. C 1968, 442–446. [Google Scholar] [CrossRef]
- Padayatt, S.J.; Daruwala, R.; Wang, Y.; Eck, P.K.; Song, J.; Koh, W.S.; Levine, M. Vitamin C: From molecular actions to optimum intake. In Handbook of Antioxidants, 2nd ed.; Cadenzas, E., Packer, L., Eds.; CRC Press: Washington, DC, USA, 2003; pp. 117–145. [Google Scholar]
- Silverstein, R.M.; Webster, F.X. Spectotrometric Identification of Organic, 6th ed.; John Wiley and Sons: Hoboken, NJ, USA, 1988. [Google Scholar]
- Fisher, R.A. The Design of Experiments, 2nd ed.; Oliver and Boyd. Edinburg: London, UK, 1937; 260p. [Google Scholar]
- Statistical Analysis System Institute. User’s Guide of SAS; SAS Institute Inc.: Cary, NC, USA, 2002; 550p. [Google Scholar]
- Salas, P.L.; Pablo, P.R.; Esparza, R.J.R.; Álvarez, R.V.P.; Palomo, G.A.; Rodríguez, D.N.; Márquez, H.C. Rendimiento y calidad de forraje hidropónico producido bajo fertilización orgánica. Terra Latinoam. 2010, 28, 355–360. [Google Scholar]
- Eghball, B. Nitrogen mineralization from field-applied beef cattle manure or compost. Soil Sci. Soc. Am. J. 2000, 64, 2024–2030. [Google Scholar] [CrossRef]
- Aram, K.; Rangarajan, A. Compost for nitrogen fertility management of bell pepper in a drip-irrigate plasticulture system. Hort. Sci. 2005, 40, 577–581. [Google Scholar]
- Joshi, R.; Singh, J.; Vig, A.P. Vermicompost as an effective organic fertilizer andbiocontrol agent: Effect on growth, yield and quality of plants. Rev. Environ. Sci. Biotech. 2015, 14, 137–159. [Google Scholar] [CrossRef]
- Ramírez, E.L.; Castillo, C.C.A.; Navarro, E.A.; Carrillo, E.A. Efecto de productos con reguladores de crecimiento sobre la floración y amarre de fruto en chile habanero. Rev. Chapingo Ser. Hortic. 2005, 11, 93–98. [Google Scholar] [CrossRef]
- SIAP (Servicio de Inspección Agroalimentaria y Pesquera). Anuario Estadístico de la Producción de Chile Habanero en Condiciones de Invernadero 2012. Available online: http://www.siap.gob.mx/index.php?option=com_wrapper&view=wrapper&Itemid=350 (accessed on 7 December 2022).
- Villa, C.M.M.; Catalán, V.E.A.; Inzunza, I.M.A.; Román, L.A. Población de plantas y manejo de la solución nutrimental de capsicum chínense Jacq. en invernadero. In Proceedings of the Memoria. VI Reunión Nacional de Innovación Agrícola León, Guanajuato, Mexico, 12–14 October 2011. [Google Scholar]
- Swart, E.A.M.; Marcelis, I.F.M.; Voorrips, R.E. Variation in relative growth rate and growth traits in wild and cultivated Capsicum accessions grown under different temperatures. J. Hortic. Sci. Biotechnol. 2006, 81, 1029–1037. Available online: http://www.pubhort.org/jhsb/Vol81/81_6/6.htm (accessed on 27 December 2022). [CrossRef]
- Gómez, A.R.; Lázaro, J.G.; León, N.A.A. Producción de frijol (Phaseolus vulgaris L.) y Rábano (Rhabanus sativus L.) en huertos biointensivos en el trópico húmedo de Tabasco. Univ. Cient. 2008, 24, 11–20. [Google Scholar]
- Nieto-Garibay, A.; Murillo, A.B.; Troyo, D.E.; Larrinaga, M.J.A.; García, H.J.L. El uso de composta como alternativa ecológica para la producción sostenible del chile (Capsicum annuum L.) en zonas áridas. Interciencia 2002, 27, 417–421. [Google Scholar]
- Rodríguez, D.N.; Cano, R.P.; Figueroa, V.U.; Favela, C.E.; Moreno, R.A.; Márquez, H.C.; Ochoa, M.E.; Preciado, R.P. Uso de abonos orgánicos en la producción de tomate en invernadero. Terra Latinoam. 2009, 27, 319–327. [Google Scholar]
- Jayanthi, L.; Sekara, J.; Basha, S.A.; Andparthasarathi, K. Influence of Vermifertilizer on Soil Quality, Yield and Quality of Chilli, Capsicum annuum. Online Int. Interdiscip. Res. J. 2014, 4, 206–218. [Google Scholar]
- Saraswathy, N.; Prabhakaran, J. Efficacy of Vermicompost from Vegetable Market Wastes on Yield Responses of Tomato (Lycopersicon esculentum Mill.). Int. J. Curr. Biotechnol. 2014, 2, 12–15. [Google Scholar]
- Najar, I.A.; Khan, A.B. Effect ofvermicompos on growth and productivity of tomato (Lycopersicon esculentum) under field conditions. Acta Biol. Malays. 2013, 2, 12–21. [Google Scholar]
- Terada, N.; Dissanayake, K.; Okada, C.; Sanada, A.; Koshio, K. Micro-Tom tomato response to fertilization rates and the effect of cultivation systems on fruit yield and quality. Horticulturae 2023, 9, 367. [Google Scholar] [CrossRef]
- Wu, Y.; Si, W.; Yan, S.; Wu, L.; Zhao, W.; Zhang, J.; Zhang, F.; Fan, J. Water consumption, soil nitrate-nitrogen residue and fruit yield of drip-irrigated greenhouse tomato under various irrigation levels and fertilization practices. Agric. Water Manag. 2023, 277, 108092. [Google Scholar] [CrossRef]
- Aza, G.C.; Núñez, P.G.H.; Ochoa, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep. 2011, 30, 695–706. Available online: http://www.springerlink.com/content/c5208660653078m7/ (accessed on 26 January 2023).
- Kozukue, N.; Jae, S.H.; Etsuko, K.; Sin, J.L.; Joung, A.K.; Kap, R.L.; Carol, E.L.; Mendel, F. Analysis of eight capsaicinoids in peppers and pepper-containing foods by high-performance liquid chromatography and liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2005, 53, 9172–9181. [Google Scholar] [CrossRef]
- Broderick, C.E.; Cooke, P.H. Fruit composition, tissues, and localization of antioxidants and capsaicinoids in capsicum peppers by fluorescence microscopy. Acta Hort. (ISHS) 2009, 841, 85–90. Available online: http://www.actahort.org/books/841/841_7.htm (accessed on 20 January 2023). [CrossRef]
- Manju, P.R.; Sreelathakumary, I. Quality parameters in hot chilli (Capsicum chinense Jacq.). J. Trop. Agric. 2002, 40, 7–10. [Google Scholar]
- Eyal, B.; Mazourek, M.; Connell, O.M.; Curry, J.; Thorup, T.; Kede, L.; Jahn, M.; Llan, P. Molecular mapping of capsaicinoid biosynthesis genes and quantitative trait loci analysis for capsaicinoid content in Capsicum. Theor. Appl. Genet. 2003, 108, 79–86. [Google Scholar]
- Sanju, S.M.; Sailen, G.P.; Devi, T.K.T.; Ramya, R.A.; Fiyaz, D.S.P. Effect of sowing time of crop geometry on the capsaicinoid content in Bhootjolokia (Capsicum chinenese Jacq.). J. Food Sci. Technol. 2012, 51, 1974–1981. [Google Scholar]
- Ramírez, A.; Pacheco, E. Composición química y compuestos bioactivos presentes en pulpas de piña, guayaba y guanábana. Interciencia 2011, 36, 71–75. [Google Scholar]
- Nuez, F.; Gil, O.R.; Costa, G.J. El Cultivo de Pimiento Chiles y Ajíes; Ediciones Mundi-Presa: Barcelona, España, 2003; ISBN 9788471146090. [Google Scholar]
- Kopsell, D.A.; Lefsrud, M.G.; Kopsell, D.E. Pre-harvest cultural growing conditions can influence carotenoid phytochemical concentrations in vegetable crops. Acta Hort. (ISHS) 2009, 841, 283–294. Available online: http://www.actahort.org/books/841/841_34.htm (accessed on 23 January 2023). [CrossRef]
- Moreno, S.A.L.; Cantú, J.A.G.; Cárdenas, M.L.A.B.; González, M.A.N.C.; González, H.G.A.; Garza, J.A.V. Determinación de carotenoides y clorofila en frutos de cuatro variedades de chile (Capsicum sp.). In Proceedings of the XII Congreso Nacional de Ciencia y Tecnología de Alimentos, Guanajuato, Mexico, 27–28 May 2010. [Google Scholar]
- Mínguez, M.M.I.; Dámaso, H.M.B. Separation and quantification of the carotenoid pigments in red peppers (Capsicum annuum L.), paprika, and oleoresin by reversed-phase HPLC. J. Agric. Food Chem. 1993, 41, 1616–1620. [Google Scholar] [CrossRef]
- Rusu, O.-R.; Mangalagiu, I.; Amăriucăi-Mantu, D.; Teliban, G.-C.; Cojocaru, A.; Burducea, M.; Mihalache, G.; Roșca, M.; Caruso, G.; Sekara, A.; et al. Interaction Effects of Cultivars and Nutrition on Quality and Yield of Tomato. Horticulturae 2023, 9, 541. [Google Scholar] [CrossRef]
- Duarte, A.; Caixeirinho, D.; Miguel, M.G.; Sustelo, V.; Nunes, C.; Mendes, M.; Marreiros, A. Vitamin C content of citrus from conventional versus organic farming systems. Acta Hort. (ISHS) 2010, 868, 389–394. Available online: http://www.actahort.org/books/868/868_52.htm (accessed on 12 January 2023). [CrossRef]
- Urban, L.; Berti, L.; Bourgaud, F.; Gautier, H.; Léchaudel, M.; Joas, J.; Sallanon, H. The effect of environmental factors on biosynthesis of carotenoids and polyphenolics in fruits and vegetables: A review and prospects. Acta Hort. (ISHS) 2009, 841, 339–344. Available online: http://www.actahort.org/books/841/841_42.htm (accessed on 19 January 2023). [CrossRef]
- Pérez, L.A.; José, J.M.; López, N.E.; Núñez, D.F.; Carbonell, B.A.A. Effects of agricultural practices on color, carotenoids composition, and minerals contents of sweet peppers, cv. almuden. J. Agric. Food Chem. 2007, 55, 8158–8164. [Google Scholar] [CrossRef] [PubMed]
- Ayuso-Yuste, M.C.; González-Cebrino, F.; Lozano-Ruiz, M.; Fernández-León, A.M.; Bernalte-García, M.J. Influence of ripening stage on quality parameters of five traditional tomato varieties grown under organic conditions. Horticulturae 2022, 8, 313. [Google Scholar] [CrossRef]
| N | P | K | Ca | Mg | Na | B | Fe | Cu | Mn | Zn | PH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | ||||||||||||
| Recommended doses | 240 * | 200 * | 240 * | 168.0 | 48.7 | 23.0 | 0.4 | 3.2 | 0.0 | 1.9 | 0.2 | |
| Liquid earthworm humus | 0.06 ++ | 0.08 ++ | 14.4 ++ | 21.0 | 22.0 | 136.0 | 27.3 | 17.0 | 1.8 | 3.2 | 2.5 | 8.2 |
| Vermicompost | 0.16 + | 0.108 + | 24.3 + | 11.0 | 23.0 | 15.7 | 15.0 | 1.8 | 0.3 | 7.2 | ||
| Variation Source | GL | Measured Squares | |||||||
|---|---|---|---|---|---|---|---|---|---|
| REND (t·ha−1) | PFP (g) | PPF (g) | NFP | DPF (cm) | DEF (cm) | AFF (m2) | AFP (cm) | ||
| Blocks | 2 | 13.7 ns | 9100 ns | 0.212 ns | 2313 * | 0.010 ns | 0.034 ns | 8.08 * | 42.2 * |
| Nutrition (A) | 4 | 118 * | 77,810 * | 3.42 ** | 423 ns | 0.190 ** | 0.071 * | 33.1 ** | 70.4 ** |
| Error A | 8 | 41.1 | 26835 | 0.433 | 819 | 0.045 | 0.005 | 6.58 | 25.8 |
| Variety (B) | 2 | 15.9 ns | 10,607 ns | 13.0 ** | 4633 ** | 0.260 ** | 0.297 ** | 7.36 * | 25.4 |
| A×B Interaction | 6 | 58.0 ns | 38,189 ns | 1.34 ** | 2903 ** | 0.025 ns | 0.070 * | 8.36 * | 53.2 ** |
| Error B | 20 | 27.4 | 18,031 | 0.212 | 648 | 0.040 | 0.023 | 2.37 | 12.8 |
| CV (%) | 15.4 | 15.4 | 8.29 | 15.3 | 5.85 | 6.54 | 18.8 | 5.74 | |
| PFP (g) | REND (t∙ha−1) | PPF (g) | NFP | AFF (m2) | AFP (cm) | DPF (cm) | DEF (cm) | |
|---|---|---|---|---|---|---|---|---|
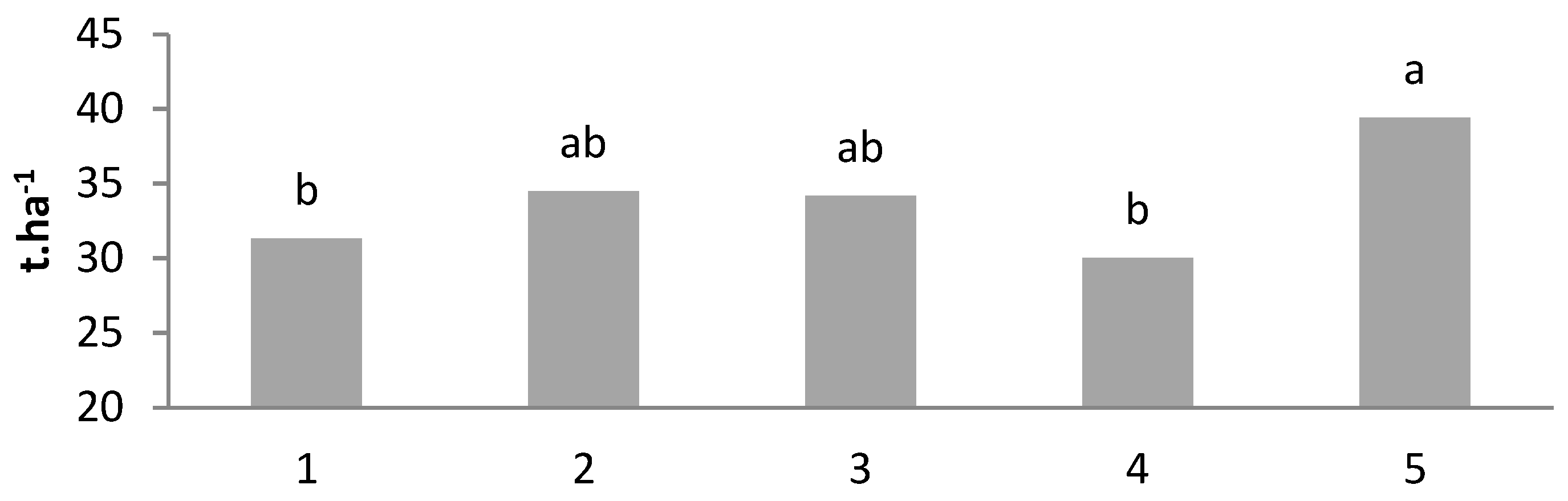
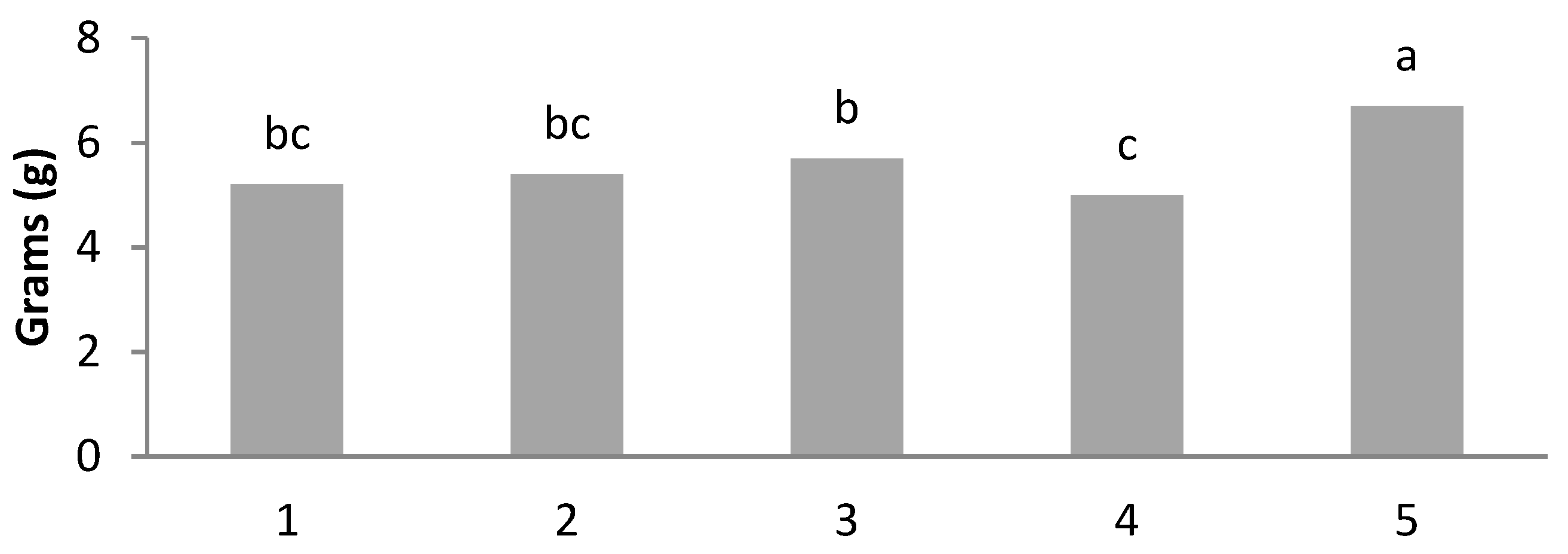
| T1 | 803 ± 19.5 b | 31.3 ± 0.83 b | 5.21 ± 0.16 bc | 159 ± 9.20 a | 1.13 ± 0.12 a | 63.8 ± 0.99 a | 3.43 ± 0.15 ab | 2.48 ± 0.09 a |
| T2 | 884 ± 29.5 ab | 34.5 ± 0.57 ab | 5.36 ± 0.71 bc | 173 ± 8.66 a | 0.84 ± 0.09 b | 60.8 ± 1.17 ab | 3.43 ± 0.14 ab | 2.35 ± 0.06 ab |
| T3 | 876 ± 30.9 ab | 34.2 ± 0.91 ab | 5.68 ± 1.31 b | 167 ± 7.15 a | 0.67 ± 0.05 b | 64.2 ± 1.33 a | 3.70 ± 0.08 a | 2.37 ± 0.06 ab |
| T4 | 769 ± 28.7 b | 30.0 ± 0.71 b | 4.97 ± 1.01 c | 172 ± 9.56 a | 0.66 ± 0.02 b | 58.2 ± 1.12 b | 3.31 ± 0.12 b | 2.24 ± 0.07 b |
| T5 | 1010 ± 19.3 a | 39.4 ± 0.81 a | 6.56 ± 0.90 a | 159 ± 10.6 a | 0.78 ± 0.04 b | 64.8 ± 1.45 a | 3.40 ± 0.11 b | 2.32 ± 0.01 ab |
| Varieties | ||||||||
| (1) Campeche | 840 ± 36.7 a | 32.8 ± 0.95 a | 4.76 ± 0.73 c | 183 ± 9.12 a | 0.86 ± 0.11 a | 62.2 ± 1.42 ab | 3.57 ± 0.09 a | 2.50 ± 0.02 a |
| (2) Palenque | 873 ± 40.3 a | 34.1 ± 0.77 a | 5.34 ± 0.53 b | 167 ± 9.29 ab | 0.85 ± 0.09 a | 64.5 ± 1.65 a | 3.48 ± 0.17 ab | 2.34 ± 0.02 b |
| (3) Jaguar | 893 ± 39.7 a | 34.8 ± 0.83 a | 6.58 ± 0.89 a | 148 ± 11.4 b | 0.73 ± 0.05 a | 55.7 ± 2.31 b | 3.31 ± 0.15 b | 2.22 ± 0.09 b |
| PPF (g) | NFP | AFF (m2) | AFP (cm) | |
|---|---|---|---|---|
| T1V1 | 5.00 ± 0.30 efg | 169 ± 9.6 bcd | 1.24 ± 0.39 ab | 62.4 ± 2.50 bcd |
| T1V2 | 5.23 ± 0.15 def | 120 ± 8.6 f | 1.37 ± 0.20 a | 64.7 ± 1.95 bc |
| T1V3 | 5.40 ± 0.36 cde | 189 ± 10.9 abc | 0.79 ± 0.18 de | 64.1 ± 2.62 bc |
| T2V1 | 5.26 ± 0.45 de | 165 ± 9.8 cd | 1.06 ± 0.30 bc | 62.1 ± 1.23 bcd |
| T2V2 | 4.56 ± 0.37 fg | 207 ± 7.5 a | 0.71 ± 0.18 e | 64.5 ± 2.01 bc |
| T2V3 | 6.30 ± 0.53 b | 148 ± 10.7 def | 0.76 ± 0.14 e | 55.6 ± 3.32 e |
| T3V1 | 4.26 ± 0.51 g | 202 ± 8.9 ab | 0.59 ± 0.15 e | 61.4 ± 2.84 bcd |
| T3V2 | 5.36 ± 0.62 cde | 181 ± 9.4 abcd | 0.68 ± 0.16 e | 65.1 ± 2.12 bc |
| T3V3 | 7.43 ± 0.48 a | 118 ± 8.2 f | 0.73 ± 0.35 e | 66.1 ± 3.01 b |
| T4V1 | 3.60 ± 0.36 h | 188 ± 9.2 abc | 0.69 ± 0.05 e | 55.7 ± 3.63 e |
| T4V2 | 5.33 ± 0.32 de | 167 ± 5.8 bcd | 0.65 ± 0.05 e | 58.3 ± 2.81 e |
| T4V3 | 6.00 ± 0.43 bc | 160 ± 7.1 cde | 0.64 ± 0.07 e | 60.5 ± 3.87 cde |
| T5V1 | 5.66 ± 0.15 bcd | 192 ± 8.7 abc | 0.74 ± 0.11 e | 63.7 ± 3.16 bcd |
| T5V2 | 6.23 ± 0.20 b | 159 ± 4.2 cde | 0.84 ± 0.14 | 58.7 ± 3.05 e |
| T5V3 | 7.80 ± 0.26 a | 125 ± 6.2 ef | 0.76 ± 0.09 e | 72.0 ± 2.64 a |
| FV | GL | Capsaicin (CAPs: mg/Kg) | Vitamin C (VC: mg/100 g) | Carotenoids (CT: mg/100 g) | β-Carotenes (Βc: mg/mL) | Xanthophylls (Xa: mg/Kg) |
|---|---|---|---|---|---|---|
| Blocks | 2 | 25,455 ns | 102 ns | 241 ns | 1.6 ns | 191 ns |
| Nutrition (A) | 4 | 27,838 ns | 5225 ** | 2097 ** | 19.3 ** | 242 ns |
| Error A | 8 | 31,275 ns | 80 ns | 530 ns | 1.5 ns | 71.2 ns |
| Variety (B) | 2 | 7174 ns | 1453 ** | 3882 ** | 24.4 ** | 5639 ** |
| Interaction (A×B) | 6 | 14,796 ns | 3147 ** | 25522 ** | 2.2 ** | 275 ** |
| Error B | 20 | 17,605 | 69 | 278 | 0.7 | 97.6 |
| CV (%) | 7.5 | 4.5 | 33.2 | 17.6 | 28.7 |
| CAPs. mg/Kg | VC mg/100 g | CT mg/100 g | βC mg/mL | Xa mg/Kg | |
|---|---|---|---|---|---|
| T1 | 1735 ± 46.7 | 198 ± 3.27 a | 1.70 ± 0.03 ab | 6.95 ± 0.57 a | 36.4 ± 3.48 |
| T2 | 1737 ± 35.6 | 149 ± 2.26 c | 1.55 ± 0.04 b | 5.36 ± 0.46 b | 38.6 ± 2.61 |
| T3 | 1730 ± 37.9 | 200 ± 3.67 a | 1.29 ± 0.05 b | 4.25 ± 0.38 bc | 39.2 ± 3.54 |
| T4 | 1799 ± 41.4 | 164 ± 4.07 b | 2.33 ± 0.03 a | 3.73 ± 0.56 c | 30.7 ± 2.57 |
| T5 | 1857± 45.9 | 199 ± 5.65 a | 1.07 ± 0.02 b | 3.31 ± 0.42 c | 27.4 ± 2.08 |
| Varieties | |||||
| (1) Campeche | 1749 ± 30.4 | 171 ± 7.9 b | 2.17 ± 38.1 a | 6.14 ± 0.66 a | 56.8 ± 3.37 a |
| (2) Palenque | 1773 ± 35.8 | 186 ± 5.8 a | 1.40 ± 42.4 b | 4.36 ± 0.57 b | 24.8 ± 2.67 b |
| (3) Jaguar | 1793 ± 29.8 | 189 ± 4.1 a | 1.21 ± 48.8 b | 3.66 ± 0.71 b | 21.8 ± 3.02 b |
| VC (mg/100 g) | CT (mg/100 g) | βC (mg/mL) | Xa mg/Kg | |
| T1V1 | 212 ± 2.78 bc | 1.74 ± 0.05 bcd | 8.83 ± 0.98 a | 50.1 ± 3.58 b |
| T1V2 | 179 ± 2.90 ef | 1.85 ± 0.02 bc | 6.60 ± 0.70 bc | 31.3 ± 3.85 cde |
| T1V3 | 203 ± 3.42 cd | 1.49 ± 0.01 bcde | 5.43 ± 1.00 de | 27.7 ± 4.27 def |
| T2V1 | 123 ± 3.45 bcd | 1.99 ± 0.05 b | 7.63 ± 0.97 b | 76.8 ± 2.56 a |
| T2V2 | 163 ± 2.76 gh | 1.80 ± 0.05 bcd | 4.43 ± 0.26 ef | 19.0 ± 1.53 f |
| T2V3 | 160 ± 2.30 h | 0.86 ± 0.02 e | 4.06 ± 0.56 gh | 20.1 ± 2.08 f |
| T3V1 | 213 ± 3.30 bc | 1.08 ± 0.02 e | 6.23 ± 0.47 cd | 66.1 ± 3.20 a |
| T3V2 | 193 ± 3.66 de | 1.33 ± 0.01 de | 3.50 ± 0.36 i | 32.7 ± 2.93 cd |
| T3V3 | 194 ± 3.15 de | 1.45 ± 0.04 cde | 3.03 ± 0.51 i | 18.8 ± 3.78 f |
| T4V1 | 110 ± 3.72 j | 4.69 ± 0.01 a | 3.76 ± 0.61 hi | 43.9 ± 2.06 bc |
| T4V2 | 221 ± 3.09 ab | 1.17 ± 0.01 e | 4.43 ± 0.37 ef | 24.1 ± 2.13 f |
| T4V3 | 160 ± 2.99 h | 1.14 ± 0.02 e | 3.00 ± 0.17 i | 23.9 ± 1.82 f |
| T5V1 | 195 ± 3.86 d | 1.32 ± 0.03 e | 4.23 ± 0.39 g | 46.9 ± 2.72 b |
| T5V2 | 176 ± 3.77 fg | 0.82 ± 0.01 e | 2.90 ± 0.27 i | 16.9 ± 1.55 f |
| T5V3 | 227 ± 2.90 a | 1.07 ± 0.01 e | 2.80 ± 0.40 i | 18.4 ± 1.47 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cortés, A.; Robledo-Torres, V.; Luna-García, L.R.; Mendoza-Villarreal, R.; Pérez-Rodríguez, M.Á. Yield and Antioxidant Quality of Habanero Chili Pepper by Supplementing Potassium with Organic Products. Horticulturae 2023, 9, 797. https://doi.org/10.3390/horticulturae9070797
González-Cortés A, Robledo-Torres V, Luna-García LR, Mendoza-Villarreal R, Pérez-Rodríguez MÁ. Yield and Antioxidant Quality of Habanero Chili Pepper by Supplementing Potassium with Organic Products. Horticulturae. 2023; 9(7):797. https://doi.org/10.3390/horticulturae9070797
Chicago/Turabian StyleGonzález-Cortés, Areli, Valentín Robledo-Torres, Laura Raquel Luna-García, Rosalinda Mendoza-Villarreal, and Miguel Ángel Pérez-Rodríguez. 2023. "Yield and Antioxidant Quality of Habanero Chili Pepper by Supplementing Potassium with Organic Products" Horticulturae 9, no. 7: 797. https://doi.org/10.3390/horticulturae9070797
APA StyleGonzález-Cortés, A., Robledo-Torres, V., Luna-García, L. R., Mendoza-Villarreal, R., & Pérez-Rodríguez, M. Á. (2023). Yield and Antioxidant Quality of Habanero Chili Pepper by Supplementing Potassium with Organic Products. Horticulturae, 9(7), 797. https://doi.org/10.3390/horticulturae9070797






