Abstract
This study utilized the Fourier-based method to analyze the morphology of over 4000 leaves from more than 190 accessions selected from the Givaudan Citrus Variety Collection at the University of California Riverside, one of the world’s most diverse collections of citrus and closely related genera. Our analysis revealed significant variations in leaf morphology among the major citrus species groups, and hybrid varieties produced through breeding exhibited intermediate leaf morphology compared to their parental citrus species. We found a positive correlation between leaf area in native citrus species and temperature in lower tropical latitudes, while negative/positive associations between aspect ratio and temperature/rainfall were also observed, respectively. These results suggest that citrus leaves may have evolved into larger but thinner leaves to increase their photosynthetic capacity per unit area while maintaining water balance by reducing water loss through transpiration. Our analysis also indicates that the existing biodiversity observed in citrus species can be attributed to their migrations across the foothills of the Himalayas, southward to the islands of Indonesia, and northward to the islands of Japan. Our study supports the hypothesis that citrus species have adapted to warm areas lacking extreme daily and nightly temperatures, where a large number of species of origin are found. Overall, this study presents a promising approach to investigate the morphological variation in citrus leaves, which could potentially aid in the selection and breeding of citrus cultivars with superior physiological traits and deepen our understanding of citrus diversity.
1. Introduction
Citrus fruits are highly valued for their nutritional content, including vitamin C, folate, potassium, and antioxidant compounds. They are cultivated in more than 100 countries in tropical and subtropical regions and are a significant contributor to the economy of California, generating over USD 7 billion in revenue as of 2018 []. Despite their worldwide value and importance, breeding efforts for citrus face several challenges. The Rutaceae family, to which citrus belongs, is diverse, and the phylogeny of citrus after domestication by humans is complex []. Citrus also has an extended juvenile period, and many varieties have partial sterility or incompatibility. Due to these challenges, creating populations and performing crosses is costly; therefore, alternative analyses have played a vital role in the improvement of citrus cultivar, and populations and new varieties are consequently created with intention and low error.
Beyond the use of fresh citrus fruit in prepared food and beverages, citrus fruit, flowers, and leaves are highly valued for their production of oils and other secondary metabolites, which are utilized in various industries such as food and beverage, fragrances, and pharmaceuticals. Despite extensive research on citrus flowers and fruits, relatively less attention has been given to the inheritance of foliar traits in this plant family, which presents a missed opportunity. Leaves are a primary source of glucose production through the photosystems and the Calvin cycle. These sugars are converted into mobile sucrose, which is translocated through the phloem to the developing fruit. The sucrose is then transformed into different forms of sugar such as glucose, galactose, fructose, or complex carbohydrates as needed by the tissues []. Additionally, the precursors for key metabolites are believed to be translocated through the same process.
Citrus and its closely related species have their origins in Southeast Asia, specifically during the late Miocene period. This era was marked by significant climatic changes that triggered a series of speciation events, giving rise to various citrus cultivars such as kumquats (Fortunella japonica), citrons (Citrus medica), early mandarins (Citrus reticulata), pummelos (Citrus maxima), and papedas (Citrus cavaleriei). In the Pliocene, citrus species traveled south into Australasia, creating the diverse species of microcitrus, which is currently believed to be derived from some ancestor of Fortunella. There is still some debate on whether certain citrus relatives like Poncirus trifoliata are within the citrus genus, despite being compatible in breeding and root stocks.
Citrus leaves have variable leaf morphologies, including many leaves from the same node (multifoliate) and a rare trait known as phyllodes (petiolar wings) [,]. Trees usually initiate the growth of vegetative leaves twice a year, primarily after fruiting and in the fall, following flowering and fertilization. In addition to these distinct growth phases, trees also produce new leaves continuously throughout the year, albeit in smaller numbers. The exact timing and environmental factors that trigger this growth have yet to be fully evaluated. The development of citrus leaves encompasses a juvenile phase preceding maturity, during which the leaf tissue exhibits a soft and pliable nature. Following a period of approximately three to five months, the leaves undergo maturation, resulting in their firming and darkening. In this study, we aim to explore the phylogenetic relationship among citrus accessions through leaf shape morphology, which can shed light on the history of origination, domestication, and adaptation in citrus.
The overall morphology of the juvenile leaves resembles that of the mature leaves, including the petiole, blade, and apex from the beginning of the unfolding of the leaf. The variation within the canopy of a single tree can be large, and the position within the tree and conditions during the juvenile phase of the leaves seem to affect the overall size and some aspects of the morphology []. Although there can be a significant variation within the canopy of a single tree, much of this variation is correlated with the overall size of the leaf, as demonstrated by previous research [].
Advancements in mathematical and computational tools have made shape analysis more accessible and efficient. For example, elliptic Fourier descriptors (EFDs) have been used to quantify leaf shape in various species, including grape [], tomato [], and Passiflora []. EFDs are a set of descriptors that capture the shape of a curve. They are derived from the Fourier series, a mathematical technique used to represent any periodic function as a combination of sine and cosine waves. By applying the Fourier series to the curve, EFDs provide a concise representation of its shape characteristics. In addition to elliptic Fourier descriptors (EFDs), there are several other methods that can be used for shape analysis, such as landmarks, pseudo-landmarks [,], persistent homology [], and various geometric morphometrics and deep learning techniques. Each of these methods has its own unique strengths and limitations, offering researchers a diverse toolkit for analyzing shapes in a variety of applications. Despite these advances, many important crops, such as citrus, still rely on older publications like the citrus leaf descriptors published in 1999 []. This gap in knowledge leaves us with uncertainty about the biological and heritable variation captured by these methods. To gain a complete understanding of the genetic basis of leaf shape in citrus, it is crucial to include both linear and morphometric measures of shape.
In this study, a large and diverse germplasm collection of citrus, comprising over 4000 leaves from more than 190 unique accessions, was analyzed from all the major branches of the genus and closely related genera to provide a comprehensive analysis of leaf shape in citrus, with a focus on the primary leaf blade. Our objective was to identify the variations in leaf blade shape between the native citrus species and explore the environmental and physiological factors responsible for these differences as the different species evolved.
2. Materials and Methods
2.1. Selecting Citrus Accessions
We utilized the Affymetrix Axiom® Citrus HD Genotyping Array, which contains 58,000 SNPs, to genotype the entire GCVC, consisting of more than 1000 accessions []. Phylogeny and local ancestry analyses have been performed with these high-density SNP data. The analysis of over 50,000 SNPs showed that 382 of the 920 citrus accessions in the GCVC have genomes that differ due to recent recombination, while the others are members of clonal lineages in which divergence has occurred only by mutation. We further selected 250 of the 382 genetically distinct accessions for a USDA-funded research project focused on investigating the genomic factors underlying leaf metabolite variability. As part of this study, we conducted leaf morphology analysis on a subset of 190 accessions selected from these 250 genetically distinct accessions.
2.2. Collecting Citrus Leaves
Leaves were collected from the Givaudan Citrus Variety Collection (GCVC) at the University of California Riverside (33.967522, −117.331686), minimizing the influence of confounding environmental factors and allowing for a more accurate analysis of leaf shape morphology. All trees are planted in the same location and climate as a common garden. We collected ten fully grown leaves from two clonally identical trees, for each of the 190 unique citrus varieties included in this study during the fall of 2021 and 2022. The modified collection method utilized in this study is consistent with the approach employed by Iwata in 2003. The method entails collecting mature and undamaged leaves one or two nodes below the branch apex, exclusively from leaves that receive full sunlight in the outer canopy. For consistency, leaves are collected from all four sides of the trees, as recommended by the International Plant Genetic Resources institute et al. (1999).
2.3. Retrieving Historical Climate Data
Climate data were obtained from the WorldClim database for the southeastern Eurasian continent, where native citrus species were initially found. This database comprises a comprehensive collection of climate data from 1970 to 2000. We utilized the “rgdal” and “raster” packages to import this information into R. We collaborated with experts associated with the Citrus Variety Collection and the USDA germplasm station for citrus and dates to select suitable locations for the climate data.
2.4. Leaf Scanning and Allometric Measurements with MuLES
Central leaflets of citrus leaves were scanned using an Epson Perfection V600 Photo Document scanner at a resolution of 300 dots per inch (dpi). After scanning, images were batch-cropped and analyzed using a custom macro-program named MuLES (multiple leaf sample extraction system) that was developed by our team []. MuLES automatically extracts binary masks and measures primary allometric features of each leaf. We performed the Wald test for normal distribution on each measurement within varieties, and all the measurement datasets exhibited a normal distribution. For this dataset, our focus was specifically on the central leaflets of the specimen.
2.5. Elliptical Fourier Analysis
Fourier analysis was selected due to its ease of use and relevance to the specific research question. Elliptical Fourier analysis (EFA) is a mathematical method used to analyze the shape of planar curves []. This method employs the use of elliptical Fourier descriptors (EFDs) to represent a curve. EFDs are derived from the Fourier series, a mathematical technique that allows the representation of any periodic function as a combination of sine and cosine waves with varying frequencies. By utilizing EFDs, the curve is transformed into a series of descriptors, providing a concise and effective representation of its shape characteristics. These coefficients can be used to reconstruct the curve, and the resulting set of coefficients is referred to as the EFA signature. The major steps in EFA involve the digitization of the curve, normalization, calculation of the Fourier descriptors, and selection of the appropriate number of harmonics. EFA has been widely used in many applications to capture complex shape variations []. In this study, we applied EFA to analyze the morphometrics of citrus leaves in a two-dimensional plane for closed contours. We used the Fourier tools in the Momocs package in R to implement this method [].
2.6. Principal Component Analysis
Principal component analysis (PCA) is a multivariate statistical technique used to identify patterns and structure in high-dimensional data by reducing its dimensionality []. The method involves transforming the original variables into a new set of uncorrelated variables called principal components (PCs), which represent linear combinations of the original variables. The first PC captures the largest amount of variation in the data, and each subsequent PC captures the remaining variation in descending order of importance. PCA is commonly used in data exploration and visualization, as well as feature extraction and data compression. In this study, PCs were collected from coordinate data from leaf masks generated in ImageJ using elliptical Fourier descriptors [] in the R package Momocs.
2.7. Thin Plate Spline
Thin plate splines (TPSs) are a spline-based technique used for data interpolation and smoothing. The concept of a TPS was initially introduced in geometric design by Duchon [], and the name ‘thin plate spline’ refers to a physical analogy involving the bending of a thin sheet of metal. Like the rigidity of the metal, the TPS fit also resists bending, resulting in a penalty that considers the smoothness of the fitted surface. Initially applied to fish morphology, the ‘momocs’ function (in Momocs package) displays a heat map or morphing grid that highlights the areas of variability on the shape itself.
3. Results and Discussion
3.1. Comparison between Native Citrus Species
Figure 1a illustrates the geographical distribution of the original citrus species and major groups, including pummelos, mandarins, kumquats, citrons, papedas, microcitrus, and the citrus relative trifoliates found in the southeastern Eurasian continent. The phylogenetic relationship among the original citrus species was analyzed using principal component analysis (PCA) based on five leaf shape traits, including length, width, area, aspect ratio, and circularity. Significant variations were observed among these major citrus species groups in terms of leaf length (Feret’s diameter), leaf breadth (also called leaf width), leaf area, and leaf aspect ratio (width/length), as depicted in Supplementary Figure S5a–d. The pummelos have the widest and longest leaves among the Citrus species, second only to the citrons, and both species are typically found in more tropical zones. As species dispersed and established and evolved in more arid or temperate zones, such as Australasica and the variety of mandarins in northern regions, there is a tendency for leaf size to decrease, accompanied by a decrease in aspect ratio, indicating the development of narrower leaves. This trend can also be observed in kumquats native to Japan and coastal China (Figure 1a,b).
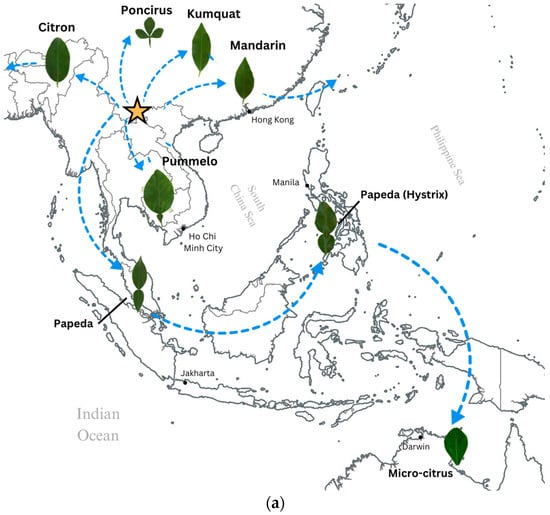
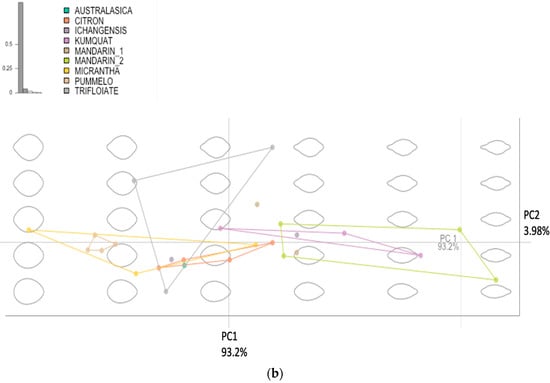
Figure 1.
(a) A map of the southeastern Eurasian continent showing the native ecological points of botanical discovery of these Citrus species. Each leaf image is a representative of a particular variety within the citrus genus. Each image is representative of the citrus species found within that native range. The arrows do not imply direct phylogenetic relationship. Each arrow indicates migration and spread of citrus. (b) Principal component analysis of the original citrus species. Each shape connected to each point represents the mean shape for that category of citrus compiled from the leaf dataset. The shapes are pulled from the range of possible shapes within the dataset and put into a morphospace to better visualize the data.
3.2. Paleoclimatic Effect on Leaf Morphology
Environmental factors, such as annual mean temperature, elevation, and latitude, of the locations where citrus species evolved likely played a critical role in the natural selection and dispersion of different citrus species as the genus evolved from its ancestral origin in southeastern China. Thus, we investigated the potential associations between the primary leaf shape traits, including their width, aspect ratio, area, and length. The primary climatic factors included in the analysis were rainfall and the mean temperature of the geographical regions belonging to the citrus species (Figure 2a,b). The analysis revealed a positive correlation between leaf area and temperature, whereas aspect ratio (length divided by width) showed a negative correlation with temperature and a positive correlation with rainfall. This is because the increasing temperature would increase leaf area, particularly in the width. An increasing width and little or no change in leaf length would decrease the ratio value. This also explains why aspect ratio increases with rainfall, because leaves do not necessarily increase in size or width with merely an increase in temperature as the arid species of microcitrus remain small despite a higher mean annual temperature. Inversely, citrus species on colder climates tend to have narrower leaves. Due to the colder temperatures, the plant body may not support larger plant organs despite the more regular annual precipitation. There are several studies that support our observations regarding the relationships between leaf size, aspect ratio, temperature, and rainfall. Most notably, the data observed follow similar trends seen in previously collected paleoclimatic evidence [,]. More modern evidence also follows these trends such as Wright et. al, who examined the leaf traits of 214 plant species from tropical rainforests and found that leaf size was positively correlated with both temperature and rainfall, whereas leaf aspect ratio was negatively correlated with temperature and positively correlated with rainfall []. Evaluating the linear leaf parameters in relation to leaf area using log-transformed scales is a well-established practice in leaf morphology research. This approach allows for valuable insights into the linear relationship of leaf dimensions in a way that eliminates exponential changes from outlier and extreme values, facilitating a better understanding of the underlying patterns and dynamics of leaf morphology. By employing this methodology, researchers can uncover important trends and characteristics that contribute to our knowledge of leaf morphology [,]. Supplementary Figures S1 and S2 illustrate the strong linear correlation observed between leaf length and leaf area, as well as between leaf width and leaf area, when the data are represented on log-transformed scales. These additional figures provide further evidence supporting the consistent relationship between these leaf parameters and leaf areas across different groups of citrus species.
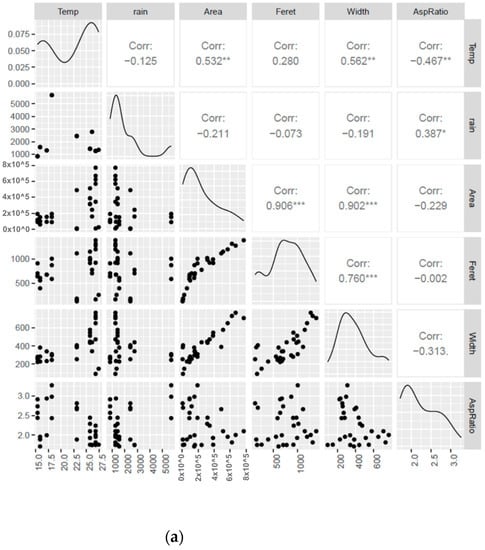
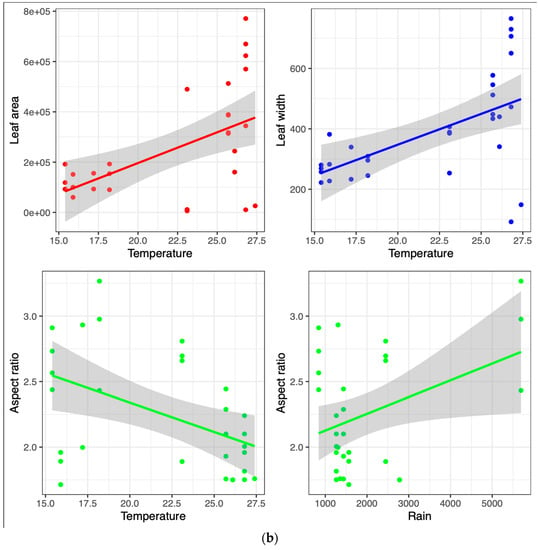
Figure 2.
Statistical analysis of climate data and morphology data. (a) Pairwise correlation of climate data and allometric variables. Variables include mean annual rainfall, mean annual temperature, aspect ratio, length, width, area. Significance levels: * denotes a p-value less than 0.05; ** represents a p-value less than 0.01; *** indicates a p-value less than 0.001. (b) Detected stronger correlations between weather metrics of mean annual temperature and mean annual precipitation with allometric variables of leaf width (blue dots), leaf area (red dots), and aspect ratio (green dots).
One crucial aspect of plants adapting to different climates is the increase in effective leaf area, which can be achieved by developing additional leaves and leaf blades in various forms. In the citrus genus and the Rutaceae family, two unique structures are observed: the multifoliate form, typically consisting of three leaves (trifoliate), and the presence of petiolar wings, also known as phyllodes []. Phyllodes are often found in more tropical or temperate climates, while trifoliates are known to be more cold tolerant and can be found in both temperate and more northern areas. A prime example of this can be seen in papedas, which are frequently encountered in Southeast Asia. Different subgroups of papedas can be found in the tropical islands of Indonesia and the Philippines, all of which retain the distinctive petiole wings. While older citrus relatives exhibit both structures in repetitive patterns, the unifoliate form has gradually become dominant in modern production varieties, possibly due to indirect selection by humans.
The positive correlation between leaf area and temperature may be explained by the fact that larger leaves can more effectively dissipate excess heat through transpiration [,]. In warm environments, larger leaves may also confer an advantage by capturing more light, thereby increasing the rate of photosynthesis, as long as water is not limiting. The negative correlation between leaf aspect ratio and temperature, as well as the positive correlation with rainfall, can be attributed to plants’ need to balance water loss through transpiration with their ability to fix carbon through photosynthesis. In hot and dry environments, plants may have evolved to have thicker leaves with smaller aspect ratios to reduce water loss through transpiration []. However, in warm and humid environments, plants may have evolved to have thinner leaves with larger aspect ratios to increase their photosynthetic capacity per unit area, while still maintaining water balance. The specific direction of these observed associations with rainfall may depend on the distinct water requirements and adaptations of each plant species.
Several studies have provided evidence for a negative correlation between leaf area and temperature due to water loss and gas exchange []. For example, Ocheltree et al., 2020 [], found that larger leaves have higher rates of transpiration and are more susceptible to water stress under high temperature conditions. In addition, Shi et al., 2020 [], demonstrated that leaf size decreases with increasing temperature, as smaller leaves may help to reduce water loss and maintain gas exchange under high temperature stress. However, there is also evidence for a positive correlation between leaf area and temperature in certain conditions. Wright et al., 2017 [], showed that mean daily and nightly temperatures can drastically influence the upper limit of leaf size. Li et al., 2006 [], in their collection of over 9000 flora from herbarium collections, found that leaf size decreases with increasing latitude, suggesting that the right temperatures and resources allow for larger leaves in lower latitudes. These contradictory findings indicate that the relationship between leaf size and temperature can be intricate and influenced by multiple factors, including species, latitude, and resource availability.
3.3. Comparison of Hybrid Varieties
The diversity of leaf shape among the original citrus species is remarkable, as shown in Supplementary Figure S5a–d, even when only focusing on the primary leaf blade, but how does the morphology of these original species influence the hybrids and more modern varieties we use today? The principal component analysis comparing hybrid and original species all in one plane shows a portion of these morphological relationships (Figure 3). The clustering of these different leaf morphologies in the principal component analysis among the native citrus groups follows similar groupings (Figure 3) to the genotype principal component analysis found in previous studies. Several hybrid varieties were included, such as sour orange, grapefruit, and lemons and sweet oranges, to investigate the phylogenetic relationships among the original citrus species and increase the resolution and power of our analysis. Continuing the pattern observed in Figure 1b, mandarins, kumquats, and sour oranges also cluster together toward the right-hand side. This finding is consistent with the existing literature which indicates that type 1 mandarins and kumquats have similar native ranges, suggesting similar environmental pressures [,]. In the PCA plot, papeda and papeda hybrids cluster closely together, indicating a high level of similarity. While the variability of the upper leaf blade in papeda and papeda hybrids is relatively low, including other leaf features—such as the petiolar wing —would likely help to distinguish these two groups. Citrons, pummelos, and their hybrid varieties are predominantly located on the left side of the PCA plot, all of which display wider aspect ratios. The citron is located at the lower end of the coordinate plane, while lemon is situated much higher. Citron hybrids, which may or may not involve lemon as a parent, are located closer to the center of the plot. Despite inheriting at least 50% of genetic material from citron, the morphology of citron hybrids has significantly deviated, likely due to the influence of the other parent. This divergence is particularly evident in the case of lemons, where the traits and characteristics have deviated even more from those of the citron parent. Citrons bear more spherical features, thus placing them lower in the chart, but their hybrid offspring, citron-hybrid and lemon, are higher up, showing influence from their other parent and strong divergence from the original citron along the plane of PC2. Mandarins and their hybrid forms, sour and sweet orange, occupy the right-hand side of the graph due to their narrower shape. These mandarins are closely related and thus morphologically close to each other, presenting some indications on the kind of intentional selection made on these varieties to produce specific results. Grapefruits are a hybrid cross between two morphological extremes, making it an excellent example of the changes in leaf morphology. Grapefruits are positioned in the middle of the graph, bridging the gap between their parent categories of pummelos on the left and sweet oranges on the right. This intermediate positioning showcases a phenotype that exhibits characteristics falling between the two extremes on this coordinate plane. Figure 4 further illustrates this observation. Overall, the principal component analysis shows the strong influence which the original citrus species can have on their hybrid offspring. Adaptations in the environments in which these species first evolved can still be observed in future lines even if changed or diminished through generations.
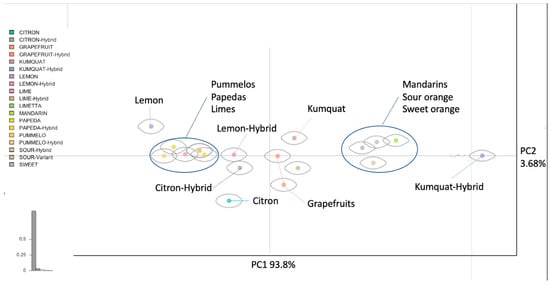
Figure 3.
Principal component analysis of the original citrus species and their hybrids. Each shape connected to each point represents the mean shape for that category of citrus compiled from the leaf dataset. The shapes are pulled from the range of possible shapes within the dataset and put into a morphospace to better visualize the data.
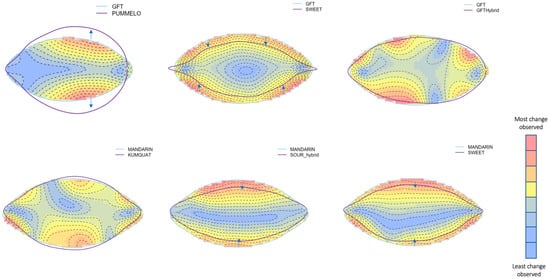
Figure 4.
Thin plate spline morphology comparisons of pure and hybridized versions of several common citrus variety categories. Blue areas represent the areas of little or no change between the two shapes provided. The distance between the outlines of the two shapes also shows some level of deviation. Regions in the red and yellow spectra show the large areas of change between the two shapes. Each shape outlined in purple and blue represents the mean shape of a specific citrus category extracted from the larger dataset. Each comparison focuses on two shapes only.
3.4. Limitations of Current Works
Although increasing the diversity of individuals from specific varieties and phyletic groups can improve the resolution of the analysis, the increased complexity of the study population may also make it more challenging to definitively determine species groups due to the long history of crosses between these groups, which is why these groups are compared in groups and categories that are relevant according to the existing literature. While having a more extensive variety collection would be ideal, with over 1400 unique varieties, there are still limitations. The GCVC is as diverse as it is because the accessions from around the world amassed over a span of 100 years. Some of these varieties are kept for their relevance to desirable consumer traits and others are kept for conserving biodiversity. As a result, there is some unintentional bias in the quantities of samples available. For example, while the GCVC has many hybrid descendants of the original Japanese wild mandarin (C. ryukyuensis) known as the Satsuma lines, it does not contain a copy of the actual progenitor, which is held in collections in Japan. To address these limitations, including species and accessions from multiple collections around the world, increasing the number of leaves sampled per tree, and using more advanced algorithms may help enhance the accuracy and reliability of morphological studies on citrus leaves.
3.5. Dimensions of the Hybrid Varieties
The data points on the violin plots of the hybrid varieties generally exhibit deviations in mean, standard deviation, or range at different levels from the main clusters of data points in the original varieties, indicating a shift in leaf shape likely to have resulted from genetic contributions from other parental accessions (Figure S5a–d). For example, the leaf length of citron-hybrid is notably longer than that of citron (p-value = 0.001 by Welch’s two-tailed t-test, rejecting null hypothesis), while no significant difference was detected in leaf width between these two groups (p-value: 0.6538). Similarly, the leaf length of a kumquat-hybrid is longer than that of kumquat (p-value of 0.005 by Welch’s two-tailed t-test, rejecting null hypothesis), but there is no difference in leaf width between these two groups (p-value: 0.13). This evidence further confirms the presence of intermediate phenotypes between different varieties in citrus. Older and less domesticated species such as the trifoliates, found in colder and more northern regions of China, or papedas, both hystrix and ichangensis papeda, are often found in more tropical regions, have a wider distribution of points in these violin plots, demonstrating their diversity and larger range of phenotypes. The same diversity can be observed with the hybrid varieties, as clear shifts in data points between trifoliate and trifoliate hybrids contrast greatly from pummelo and pummelo hybrids. This evidence suggests that the magnitude of these intermediate phenotypes in the hybrids seems stronger with the more wild and less domesticated species in the collection. Such evidence suggests great value in preserving both wild and more domesticated forms of plant species. The log transformation of the leaf measurements reveals notable shifts in the linear relationship between leaf area and width, as well as between leaf area and length. Supplementary Figure S1 illustrates these shifts, particularly in the case of kumquat, where there is a significant increase in area with increasing length (steep slope) but a less pronounced increase in area from width (shallow slope). In contrast, mandarin 1 and mandarin 2 exhibit different slopes, with a steeper slope in width (Figure S1b) and a shallower slope in length (Figure S1a). Similar observations can be made in Figure S2c,d, comparing mandarins and kumquats. These findings provide evidence of morphological differences between kumquats and mandarins, despite their similar appearance and paleoclimatic growing ranges. This further supports the notion that these two groups represent distinct branching phyletic groups.
3.6. Intermediate Phenotypes
The leaf morphology of hybrids in citrus hybrids exhibits intermediate characteristics that can be observed even in younger trees before the formation of the first fruit. The use of the thin plate spline method reveals these intermediate forms, highlighting shared traits between the hybrids and their parent species (Figure 4). Despite the availability of these tools, not all major crops have undergone shape analysis, indicating the potential for novel discoveries in this area. In this method, the first object (represented by the blue line in each panel of Figure 4) is laid out on a grid or sheet. Then, the grid or sheet undergoes changes and morphs to fit the shape of the second object (shown in purple). Each change and difference incur a penalty to the surface, which is visualized in the heatmap presented in Figure 4. Both the distance between outlines and the difference in color indicated the magnitude of change between the two varieties. By employing this method, we can visualize both significant and subtle details among various varieties in our dataset. In this study, we focus on major citrus groups, including mandarin and its two hybrid offspring, sour and sweet orange, along with grapefruit, in comparison to its parent varieties, pummelos and sweet orange. The heat maps presented in Figure 4 depict the intermediate phenotype observed in grapefruit, exhibiting a profile that is round and wide, similar to its pummelo parent. However, the grapefruit’s profile is not as wide or rounded as the pummelo, and it also displays a smaller net area, likely influenced by its sweet orange parent. Mandarins, known for their narrow leaves, produce hybrid offspring with slightly thinner leaves. Sour oranges are the result of a cross between early mandarins and pummelos. These early mandarins can exhibit bitterness or sourness and lack the modern orange traits. The retention of the sour flavor suggests that the leaf shape may be influenced by the early mandarin parents. On the other hand, sweet oranges are derived from a cross between later mandarins and have an additional admixture of pummelo in their evolutionary history, resulting in a sweeter taste and other traits. Since this cross involves two mandarin oranges, the change in leaf morphology is minimal. Nevertheless, the minimal change observed underscores the limitations of relying solely on morphological analysis in the citrus biological system. Given the extensive hybridization of citrus varieties, this method of phenotyping has limited utility. However, integrating genetic evidence into leaf morphology studies will greatly enhance our understanding of specific variety parentages, as demonstrated in the studies by Wu et al. in 2018 and Hiraoka et al. in 2020.
4. Conclusions
In this study, allometric variation in citrus leaf morphology was examined using EFDs, revealing that differences in leaf blade width contribute to this variation in both accessions and species. These findings are beneficial in two ways. First, it deepens our understanding of citrus with modern methods that can be shared worldwide. Second, the leaf morphology approach used can be beneficial in developing new breeding and research strategies for citrus crops. This approach can enable predictive modeling and a more informed genetic research, reducing both time and cost compared to traditional methods like genetic rescue and mutation breeding. The availability of sequenced genomes for various citrus species provides opportunities to explore diverse genetic associations between leaf shape, fruit, and flower traits with corresponding genomic regions. Future studies should integrate genetic and omics data to further examine the relationship between leaf morphology, fruit, and flower traits, and to advance our understanding of the biology and physiology of trait development. Overall, this study provides valuable insights into the heritability and environmental influences on citrus leaf morphology and highlights the potential of leaf morphology analysis as a tool for citrus breeding and research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9070793/s1, Figure S1: (a) Natural log comparison of area vs length for citrus species. (b) Natural log comparison of area vs width for citrus species. Any change in slope between each category indicates a difference in dimensions; Figure S2: (a,b,c,d) Natural log comparison of area vs length or width. Any change in slope between each category indicates a difference in dimensions; Figure S3: Principal component contribution graph with the y axis denoting the PC number, the x axis being the mean shape and standard deviations of the mean shapes across the spectrum of eigen values generated by the elliptical fourier analysis and translated into components. This is the same data used to generate the values in Figure 3a in the main text; Figure S4: World Clim map of coordinate points used for climate data. Coordinates listed as follows: C.maxima 16.015777, 103.448887, C.medica 18.616631, 81.47623–25.395202, 85.870762, C.micrantha hongensis 14.624152, 121.278345, C.micrantha hystrix 3.182375, 101.964380, C. trifoliata 25.840986, 110.765782, mandarin group north 31.267677, 115.70963, australasica −12.691577, 131.663257, 129.422046, Kumquat 25.388846, 118.098014; Figure S5: (a,b,c,d) violin plots of the distribution of leaf length, width area and aspect ratio in different species of Citrus. Each dot represents a single leaf; Figure S6: (a,b,c,d) Violin plots depicting the distribution of length, width, area, and aspect ratio for both hybrid and original varieties. In this publication, a hybrid is defined as a variety resulting from the intentional crossing of two distinctly different citrus types. This includes hybrids resulting from past generations or intentional crosses to achieve specific phenotypes (e.g., crossing a grapefruit with a sweet orange to create a sweet orange and grapefruit hybrid).
Author Contributions
Z.J., J.M.C. and M.L.R. conceived and designed the study, provided oversight throughout the research process, and contributed to writing the manuscript. R.C.T. collected and analyzed the data and wrote the initial draft of the manuscript. X.W., J.L., L.Y., Y.H. and Z.W. participated in data collection and analysis, and contributed to writing the manuscript. I.A.H., C.B., M.R., S.P.K., S.L., D.H.C., L.S., T.K. and D.S. participated in data analysis and interpretation, and contributed to writing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the U.S. Department of Agriculture National Institute of Food and Agriculture; USDA NIFA FACT grant 2019-67022-29930 to Z.J. and M.L.R.
Data Availability Statement
Leaf measurement data used for this study can be found here. Original masks of this dataset used for advanced morphometrics analysis are available here. Fourier descriptors are linked here. Samples of these species and varieties of citrus are available for purview in the University of California Riverside Herbarium, including distant relatives in the Rutaceae family. Samples of fruit, leaves and flowers are also available in the Gividuan Citrus Variety Collection upon approved request.
Acknowledgments
We would like to thank the curators of the Citrus Variety Collection for allowing access to their materials. David Karp for his intimate knowledge on the more ambiguous citrus species.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, S.; Wu, F.; Duan, Y.; Singerman, A.; Guan, Z. Citrus Greening: Management Strategies and Their Economic Impact. HortScience 2020, 55, 604–612. [Google Scholar] [CrossRef]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the Origin and Evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-Sink Transport of Sugar and Regulation by Environmental Factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R. Comparative Developmental Analysis of the Heteroblastic Leaf Series of Axillary Shoots of Acorus calamus L. (Araceae). Cellule 1973, 69, 251. [Google Scholar]
- Ballve, R.M.L.; Medina-Filho, H.P.; Bordignon, R. Identification of Reciprocal Hybrids in Citrus by the Broadness of the Leaf Petiole Wing. Braz. J. Genet. 1997, 20, 697–702. [Google Scholar] [CrossRef]
- Bruschi, P.; Grossoni, P.; Bussotti, F. Within-and among-Tree Variation in Leaf Morphology of Quercus petraea (Matt.) Liebl. Natural Populations. Trees-Struct. Funct. 2003, 17, 164–172. [Google Scholar] [CrossRef]
- Iwata, H.; Nesumi, H.; Ninomiya, S.; Takano, Y.; Ukai, Y. The Evaluation of Genotype× Environment Interactions of Citrus Leaf Morphology Using Image Analysis and Elliptic Fourier Descriptors. Breed. Sci. 2002, 52, 243–251. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Ranjan, A.; Martinez, C.C.; Headland, L.R.; Thiem, T.; Kumar, R.; Covington, M.F.; Hatcher, T.; Naylor, D.T.; Zimmerman, S.; et al. A Modern Ampelography: A Genetic Basis for Leaf Shape and Venation Patterning in Grape. Plant Physiol. 2014, 164, 259–272. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Kumar, R.; Headland, L.R.; Ranjan, A.; Covington, M.F.; Ichihashi, Y.; Fulop, D.; Jiménez-Gómez, J.M.; Peng, J.; Maloof, J.N.; et al. A Quantitative Genetic Basis for Leaf Morphology in a Set of Precisely Defined Tomato Introgression Lines. Plant Cell 2013, 25, 2465–2481. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Otoni, W.C. Morphometric Analysis of Passiflora Leaves: The Relationship between Landmarks of the Vasculature and Elliptical Fourier Descriptors of the Blade. Gigascience 2017, 6, giw008. [Google Scholar]
- Kent, J.T. Data Analysis for Shapes and Images. J. Stat. Plan. Inference 1997, 57, 181–193. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Sinha, N.R. Evolutionary and Environmental Forces Sculpting Leaf Development. Curr. Biol. 2016, 26, R297–R306. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; An, H.; Angelovici, R.; Bagaza, C.; Batushansky, A.; Clark, L.; Coneva, V.; Donoghue, M.J.; Edwards, E.; Fajardo, D.; et al. Topological Data Analysis as a Morphometric Method: Using Persistent Homology to Demarcate a Leaf Morphospace. Front. Plant Sci. 2018, 9, 553. [Google Scholar] [CrossRef]
- IPGRI. Descriptors for Citrus; International Plant Genetic Resources Institute: Rome, Italy, 1999. [Google Scholar]
- Hiraoka, Y. Application of High-Density SNP Genotyping Array in Citrus Germplasm Characterization and Genetic Dissection of Traits. 2020. Available online: Search.proquest.com (accessed on 8 January 2023).
- Bowman, C.S.; Traband, R.; Wang, X.; Knowles, S.P.; Lo, S.; Jia, Z.; Vorsa, N.; Herniter, I.A. Multiple Leaf Sample Extraction System (MuLES): A Tool to Improve Automated Morphometric Leaf Studies. Appl. Plant Sci. 2023, 11, e11513. [Google Scholar] [CrossRef] [PubMed]
- Lestrel, P.E. Fourier Descriptors and Their Applications in Biology; Cambridge University Press: Cambridge, UK, 1997; ISBN 9780521452014. [Google Scholar]
- Iwata, H.; Ebana, K.; Uga, Y.; Hayashi, T.; Jannink, J.-L. Genome-Wide Association Study of Grain Shape Variation among Oryza Sativa L. Germplasms Based on Elliptic Fourier Analysis. Mol. Breed. 2010, 25, 203–215. [Google Scholar] [CrossRef]
- Bonhomme, V.; Picq, S.; Gaucherel, C.; Claude, J. Momocs: Outline Analysis UsingR. J. Stat. Softw. 2014, 56, 24. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal Component Analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Duchon, J. Splines Minimizing Rotation-Invariant Semi-Norms in Sobolev Spaces. In Constructive Theory of Functions of Several Variables; Springer: Berlin/Heidelberg, Germany, 1977; pp. 85–100. [Google Scholar]
- Peppe, D.J.; Royer, D.L.; Cariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of Leaf Size and Shape to Climate: Global Patterns and Paleoclimatic Applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef]
- Bailey, I.W.; Sinnott, E.W. A botanical index of cretaceous and tertiary climates. Science 1915, 41, 831–834. [Google Scholar] [CrossRef]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S.; et al. Functional Traits and the Growth–mortality Trade-off in Tropical Trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- John, G.P.; Scoffoni, C.; Sack, L. Allometry of Cells and Tissues within Leaves. Am. J. Bot. 2013, 100, 1936–1948. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From Tropics to Tundra: Global Convergence in Plant Functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.R.A.; de Souza, E.H.; Souza, F.V.D.; Fadini, M.; Girardi, E.A.; Soares Filho, W.d.S. Genetic Variation of Citrus and Related Genera with Ornamental Potential. Euphytica 2015, 205, 503–520. [Google Scholar] [CrossRef]
- Raschke, K. Heat Transfer between the Plant and the Environment. Annu. Rev. Plant Physiol. 1960, 11, 111–126. [Google Scholar] [CrossRef]
- Knoerr, K.R.; Gay, L.W. Tree Leaf Energy Balance. Ecology 1965, 46, 17–24. [Google Scholar] [CrossRef]
- Gouveia, A.C.; Freitas, H. Modulation of Leaf Attributes and Water Use Efficiency in Quercus Suber along a Rainfall Gradient. Trees 2009, 23, 267–275. [Google Scholar] [CrossRef]
- Anyia, A.O.; Herzog, H. Water-Use Efficiency, Leaf Area and Leaf Gas Exchange of Cowpeas under Mid-Season Drought. Eur. J. Agron. 2004, 20, 327–339. [Google Scholar] [CrossRef]
- Ocheltree, T.W.; Nippert, J.B.; Prasad, P.V.V. A Safety vs Efficiency Trade-off Identified in the Hydraulic Pathway of Grass Leaves Is Decoupled from Photosynthesis, Stomatal Conductance and Precipitation. New Phytol. 2016, 210, 97–107. [Google Scholar] [CrossRef]
- Shi, P.; Yu, K.; Niinemets, Ü.; Gielis, J. Can Leaf Shape Be Represented by the Ratio of Leaf Width to Length? Evidence from Nine Species of Magnolia and Michelia (Magnoliaceae). Forests 2021, 12, 41. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global Climatic Drivers of Leaf Size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, D.; Shrestha, N.; Xu, X.; Wang, Q.; Jia, W.; Wang, Z. Spatiotemporal Variation in Leaf Size and Shape in Response to Climate. J. Plant Ecol. 2020, 13, 87–96. [Google Scholar] [CrossRef]
- Li, Y.Z.; Cheng, Y.J.; Yi, H.L.; Deng, X.X. Genetic Diversity in Mandarin Landraces and Wild Mandarins from China Based on Nuclear and Chloroplast Simple Sequence Repeat Markers. J. Hortic. Sci. Biotechnol. 2006, 81, 371–378. [Google Scholar] [CrossRef]
- Palma, A.; D’Aquino, S. Kumquat—Fortunella Japonica. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 271–278. ISBN 9780128031384. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).