Abstract
Soilless cultivation represents a promising method for the future of the horticulture industry as it offers advantages such as improved quality control over the growth environment and mitigation of uncertainties related to soil, water, and nutrient availability. In this study, we aimed to investigate the effects of different environments, specifically greenhouse (GH) and open-field (OF), on the growth, phenotypic characteristics, physio-biochemical properties, qualitative parameters, and antioxidant capacity of strawberries cultivated using a soilless system. The aforementioned parameters were measured in both the GH and OF settings. Our findings revealed that the growth, yield, and morphological parameters were significantly higher in the GH environment compared to the OF. However, when considering fruit quality indices such as fruit texture firmness, fruit dry matter percentage, taste index (TSS/TA ratio), and post-harvest shelf-life, the OF cultivation method exhibited significantly superior results. Moreover, various aspects, related to plant physiology and biochemistry, antioxidant enzyme activity, total antioxidant capacity (DPPH), vitamin C content, and secondary metabolites, were found to be significantly higher in the OF environment compared to the GH. Overall, the results of our study suggest that OF soilless cultivation outperforms GH cultivation in terms of fruit quality, antioxidant capacity, and post-harvest shelf-life. Despite the observed decrease in fruit growth and yield, soilless strawberries grown in OF are likely to yield a final product of higher quality and nutritional value compared to those cultivated in a GH environment. These findings highlight the potential of OF soilless cultivation as a viable approach for strawberry production, emphasizing the importance of considering not only yield but also qualitative aspects and the nutritional value. Further exploration and optimization of soilless cultivation techniques in OF settings could contribute to the advancement of sustainable horticultural practices.
1. Introduction
Fruits are known to be healthy foods because of their high nutritional content, and improving the quality of products, along with higher production, is a major emphasis in modern agriculture. Apart from the genetic potential of a variety, the other components that can affect the quality of fruits are the growth environment and agro-techniques [1]. One of the most delicious and nutritious soft fruits in the world is the strawberry (Fragaria × ananassa). The extract of this fruit has a high antioxidant capacity and can trap free-radical species [2].
Strawberries cultivation is one of the most profitable horticultural enterprises in many countries. Over the past 20 years, the level of strawberry cultivation has doubled worldwide. The amount of strawberry cultivated in Iran is also expanding [3]. Strawberries are generally produced in open-field systems on raised beds. This plant has a shallow root system, and thus, has high water requirements [4]. However, soil-borne strawberry diseases are major limitations that can influence plant agronomic performance and cause economic losses in conventional production fields [5]. In addition, soil culture problems (such as salinity and unsuitable soil characteristics) and water resource limitations in many countries, especially Iran, have contributed to the expansion of soilless culture [6]. However, ensuring a consistent and reliable food supply requires dedicated focus on the implementation of innovative and optimized methodologies [7]. Soilless culture has grown in response to increasing regulatory constraints on fumigants [8]. A suitable combination of soilless cultivation beds can be the best growing environment in terms of water and plant storage capacity, reducing toxicity, increasing ventilation, and balance in nutrition compared to soil cultivation [9]. In soilless growing systems, the density of plant roots is confined to a small volume of the growth medium. This system exhibits high rates of metabolic activity, respiration, and growth [10]. Compared to the traditional open-field production system, the soilless system offers more accurate control over the supply of water, the root zone temperature, nutrients, etc. Therefore, the soilless system has many advantages, including cleaner fruit production, no need for soil sterilization, higher productivity owing to easier and more accurate control of production factors, enhanced off-season production, reduction of labor requirements for harvest, and more crops per year [11]. In addition, better vegetative growth parameters in soilless systems than in traditional open fields have led to an increase in the number of fruits and the yield of good-quality strawberry fruits [12].
Focusing on the effects of the environment and cultivation practices on the nutrient and phytochemical contents of strawberries grown in hydroponics is an interesting topic in recent studies [13]. Greenhouses, as protected culture systems, are becoming more popular than open-field cultures. This culture system has beneficial properties, such as frost protection, increased yields, extended harvesting periods, and control over several major plant diseases [8]. However, there are disadvantages to greenhouse soilless systems compared to open-field soilless systems, such as a higher initial capital investment and a higher disease risk [11]. Although the genotype and environmental variation were extensively studied for the phytochemicals and nutrients in strawberries, the variation caused by the cultivation practices has drawn less attention. Therefore, one of the main concerns in determining the soilless cultivation system type is understanding its benefits. Soilless cultivation practices are flexible growing methods that allow growers to have full control over the growing environment, including the active root zone, and help to increase the efficiency of water usage while maintaining its quality in a dry area.
The importance of changing soil cultivation and moving towards soilless cultivation, due to the reduction of healthy water resources and soil fertility, the increasing demand for food due to population growth, as well as the improvement of food hygiene and health [11], and the reduction of water consumption [14], is obvious and undeniable. Soilless cultivation, in addition to controlling the root environment and managing nutrition to improve the quality and increase the yield [15], can be a solution for overcoming the unfavorable characteristics of water and soil, and making use of non-cultivable lands. This should be considered in both open-field and greenhouse cultivation.
Today, the value and quality of food products, especially in horticulture, have a more health-oriented and fresh definition than in the past [16]. Aroma, taste, dry matter content, antioxidants, and fruit color are important factors that determine fruit quality. Research has shown that environmental factors such as light, temperature, and relative humidity have a direct effect on fruit quality. Consistent light intensity throughout the season has been found to significantly increase the product quality [17]. A study demonstrated that strawberries grown in an environment with sufficient light (direct sunlight) produce more carbohydrates and dry matter than those grown under low-light conditions [18]. Sufficient light intensity has also been positively correlated with vitamin C content in vegetables, including strawberries [19]. On the other hand, color, particularly in fruits and vegetables, plays a crucial role the choices made by consumers. It has been proven that a low light intensity or indirect light directly affects pigment synthesis, thereby reducing the attractiveness of fruits and vegetables, such as radishes. Insufficient light not only reduces the product quality but also increases the levels of anti-health and nutritional substances, such as oxalate [16]. Another pivotal aspect to consider is the impact of plastic covers on the photosynthetically active radiation (PAR) and ultraviolet (UV) radiation, both of which play a crucial role in preserving the bioactive compounds of fruits. Research indicates that the antioxidant capacity of blueberry fruits cultivated under plastic covers is significantly diminished in comparison to those grown in open-fields. This underscores the detrimental effect of plastic covers on the preservation of bioactive compounds in fruits [20].
Temperature plays a fundamental role in plant phenology and growth, with optimal levels varying among plant species [21]. It indirectly affects leaf stomatal conductance through alterations in the plant water status and vapor pressure, subsequently directly influencing transpiration and photosynthesis [22,23]. Elevated temperatures can hinder plant physiological and developmental processes by reducing the leaf water content and stomatal conductance, increasing the leaf temperature (due to diminished evaporative cooling [24]), augmenting transpiration rates, diminishing photosynthesis, and ultimately, impeding shoot growth, metabolite production, and overall plant growth [23,25].
Stomatal conductance serves as a measure of gas and water exchange between plant leaves and the surrounding environment. Environmental factors, particularly relative humidity, play a crucial role in controlling stomatal function [26]. Similar to temperature, relative humidity is a vital determinant for plant growth and survival [27]. Stomata, by regulating water and carbon dioxide exchange, significantly influence the plant water status, photosynthesis, primary metabolism, drought tolerance, and resource competition [28].
The variables of light intensity, temperature, and relative humidity play a crucial role in the growth and survival of plants and are significantly influenced by environmental factors. When these variables are present at extremely low or high levels, they can induce stress in plants. However, maintaining them within acceptable ranges can enhance both the quantity and quality of plant products [29]. Consequently, it appears that soilless cultivation in the open field, with its distinct ability to effectively manage nutrients (ensuring balanced mineral nutrients and optimal ion availability during plant growth), can potentially improve the quality and antioxidant capacity of crops. This improvement can be attributed to factors such as a higher and more uniform light intensity, as well as a greater temperature differential (DIF). Additionally, soilless cultivation systems in open fields may offer plants better tolerance to temperature and humidity stresses when compared to greenhouses and enclosed spaces. Such advancements have the potential to increase horticultural crops’ productivity and enhance the nutritional value of food. In light of this, the primary objective of this study was to investigate and compare the growth characteristics, physio-biochemical attributes, and quality parameters of strawberries cultivated using a soilless cultivation system in both open-field and greenhouse environments.
2. Materials and Methods
2.1. Experimental Site and Cropping System
The experiment was conducted on the campus of Islamic Azad University, Mahabad (Iran). The cultivation of Albion strawberry (Fragaria × ananassa ‘Albion’) was conducted in two cultivation systems, a soilless greenhouse (SG) and a soilless open-field (SOF) system, based on a complete randomized block design with four replicates between April and September 2021 (Table 1).

Table 1.
Environmental conditions of soilless cultured strawberry (Fragaria × ananassa ‘Albion’).
In the open-field and greenhouse cultural systems, strawberry plants were cultivated in a soilless growing system in 20 L grow bags measuring 75 cm in length, 15 cm in width, and 25 cm in depth, filled with coconut coir and perlite substrates (1:1, V/V) under natural light and temperature conditions (four replicates, and each replicate had eight plants). The plants were watered with a drip irrigation system (one drip per plant with a flow rate of 2.3 L/hThe pots were placed on benches with a 1% slope to ensure free drainage. The preparation of the nutrient solution was according to the Caruso method [30] (Table 2). The conductivity and pH were kept at approximately 1.3 dS/m and around 5.8, respectively. Optimum fertigation was attained by measuring the drainage amount obtained from the drainage channels placed underneath the growing bags on day one. Fertigation was also calculated depending on the drainage. In the open field, when the light intensity exceeded 925 (µmol m−2 s−1), a green shading net was used. Throughout each experiment, harvesting was performed when the fruit was fully ripe (completely red).

Table 2.
Chemical composition of the hydroponic nutrient solutions.
2.2. Growth and Morphological Parameters
In each replication at the final harvest, the fresh weight of the crown, leaf, root, fruit, and plant (root + crown + leaf), and the mean of a single fruit from the randomly selected plants were taken. During the period of flowering and fruiting, the rate of fruit set was calculated as a percentage by counting the total number of flowers and fruits formed. The numbers of fruits per plant during the season and crowns per plant after harvesting and removing the plants from the bed were separated and counted. Small, deformed, immature, and physiologically disturbed fruits were separated and calculated as the percentage of non-marketable fruits. Fruits of the same size and approximately the same ripeness were selected and stored at two different temperatures (2 °C and 22 °C), and their weight loss changes at the same time (10 days, the minimum duration of treatment) were recorded. Moreover, their maximum durability was assessed and determined based on the market indicators. The leaves, roots, and crowns were oven-dried (70 °C for 24 h) to measure the dry weight. Dry matter and fruit juice were calculated. The fruit firmness was measured using a Lutron FG-5020 device (Lutron FG-5020-TAIWAN-probe: 5 mm) [31]. The measurements of the fruit diameter and length throughout the growing season, as well as the crown diameter and root length, were documented post-harvest using a digital caliper.
2.3. Biochemical and Physiological Parameters
2.3.1. Photosynthetic Pigments
The measurement of chlorophyll pigment (a, b, total, and carotenoids) content was conducted following the protocol developed by Smith and Benitez [32]. A spectrophotometer (Lambda 25 UV/VIS, Perkin-Elmer, Waltham, MA, USA) was utilized for the measurements, with specific wavelengths assigned for each pigment: 663 nm for chlorophyll a, 645 nm for chlorophyll b, and 470 nm for carotenoids. This established protocol ensured standardized and accurate quantification of the chlorophyll pigment content. The content of carotene pigments in the leaves was measured using the method described by Berger [33].
2.3.2. Physiological Parameters
The membrane stability index was assessed by measuring the amount of electrolyte leakage (EL) in the leaves according to the method of Lutts et al. and expressed as a percentage [34]. A leaf porometer (SN: LP2402, Decagon. Hopkins Ct, Pullman, WA, USA) was used to measure the stomatal conductivity (SC, in mol H2O m−2 s−1) of the leaves.
Relative water content (RWC) was measured using the fresh weight (FW), turgid weight (TW), and constant dry weight (DW) of leaves, according to the method described by Ritchie [35]. Total soluble solids (TSS) (°Brix) of the fruits were recorded using a portable refractometer (Atago Co., Ltd., Tokyo, Japan) at 20 °C. Titratable acidity (TA) was evaluated by titrating the juice with 0.1 N NaOH. The fruit flavor or maturity index was determined using the TSS/TA ratio [36].
2.3.3. Biochemical Parameters
Superoxide dismutase (SOD) activity was assayed based on the reduction of NBT (nitroblue tetrazolium) [37]. The peroxidase (POD) activity was determined using the potassium phosphate buffer method and measuring the absorbance changes at 470 nm and 25 °C [38]. Catalase (CAT) activity was measured by monitoring the decrease in H2O2 at 240 nm for 1 min at 25 °C, and the amount of enzyme that caused a reduction in absorbance at 240 nm of 0.01 per minute was considered as CAT activity [39].
Colorimetric determination of leaf proline was performed using the acid-ninhydrin method based on Bates [40].
Measurement of total antioxidant capacity in the fruits was carried out based on percent quenching of the 2-diphenyl-1-picrylhydrazyl radical (DPPH) decrease in absorbance of the radical at 517 nm using a spectrophotometer (Lambda 25 UV/VIS, Perkin-Elmer) [41].
The total flavonoid content of fruits was measured using the aluminum chloride method and expressed in mg quercetin/g of strawberry fruit based on the calibration curve [42]. The total phenolic content (TPC) and total anthocyanins content (TAC) of fruit were measured according to the Folin–Ciocalteu method in terms of mg gallic acid per gram of fresh weight and the pH difference method, respectively [42,43]. The vitamin C (ascorbic acid) content was measured by iodine titration with potassium iodide in the presence of a 1% starch reagent [44].
In harvested strawberries, phenylalanine ammonia-lyase (PAL) activity was determined according to the Zucker (1965) method, based on the production of cinnamate for 1 h at 30 °C and the absorbance change at 290 nm [45].
2.4. Statistical Analysis
The data for each harvest were analyzed using SPSS software (version 24). The data were analyzed using a t-test at a significance level of p < 0.05. Results are presented as the mean ± standard deviation. Pearson’s correlation analysis was applied to evaluate the relationships between pairs of variables.
3. Results and Discussion
3.1. Growth and Morphological Parameters
Most plant growth parameters in the greenhouse environment were significantly higher than those in the open field (p < 0.05; Table 3). Growth parameters, including the fresh weight of leaves, dry and fresh weight of the crown, fresh weight of roots, total plant weight, mean of single fruit weight, root and crown diameter, fruit length, number of roots, root volume, number of fruits per plant, and fruit loss, were significantly higher in the greenhouse cultivation than in the open-field culture (Table 3, Figure 1) (p < 0.05). However, parameters such as fruit firmness, fruit dry matter, fruit juice, fruit set, and the shelf-life of fruits at 2 °C and 22 °C were significantly higher in the open-field than in the greenhouse conditions (Table 3) (p < 0.05). There was no significant difference between the dry weight of leaves, dry weight of roots, number of crowns, and percentage of unmarketable fruits under the greenhouse and open-field cultivation conditions (p > 0.05, Table 3).

Table 3.
Effect of different growing environments on the morphological and growing characteristics of soilless cultured strawberry (Fragaria × ananassa ‘Albion’) (mean ± SD, n = 8).
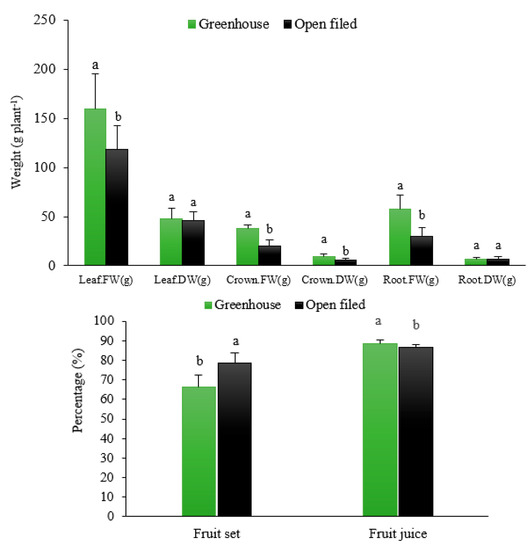
Figure 1.
Effect of different growing environments on the fresh (FW) and dry weight (DW) of aerial and underground organs (top) and fruit set and fruit juice (bottom) of soilless cultured strawberry (Fragaria × ananassa ‘Albion’). Different letters show significant differences between treatments (p < 0.05) (mean ± SD).
The coefficients of the significantly studied traits are shown in Figure 2. This analysis reflects the degree of interaction between growth and morphological parameters in strawberry plants (fruits + crowns + roots), which further facilitates a better understanding of the interactions between plant growth influenced by different cultural conditions (greenhouse and open field). To provide a deeper insight into these correlations, the analysis was separately performed for greenhouse and open-field planting. The correlation matrix for fruit properties significantly varied between greenhouse and open-field planting (Figure 2). According to the correlation coefficient values, statistically significant negative correlations were observed among fruit juice and fruit set, fruit loss at 2 °C and fruit shelf-life at 2 °C, fruit loss at 2 °C and fruit shelf-life at 22 °C, and fruit dry matter and fruit juice. This significant negative correlation indicated a higher fruit quality in soilless greenhouse strawberries. In contrast, significant positive correlations were observed for the fruit dry matter and crown dry weight. The vegetative components of strawberries in greenhouse conditions were higher than those in open-field conditions and had a higher ability to produce flowers and fruits, which has been confirmed by other studies [46,47].
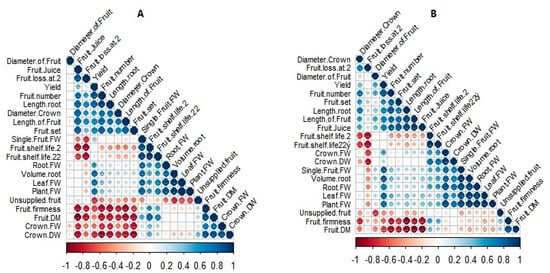
Figure 2.
Pearson correlations between morphological and growing characters of soilless cultured strawberry (Fragaria × ananassa ‘Albion’) in the greenhouse (A) and open-field (B) conditions.
3.2. Biochemical and Physiological Parameters
The results showed that the photosynthetic pigments, including chlorophyll a, chlorophyll b, and carotenoids, in strawberries grown in open-field soilless culture were significantly lower than those in strawberries grown in greenhouse soilless culture (p < 0.05; Table 4).

Table 4.
Effect of different growing environments on the photosynthetic pigments of soilless-grown strawberry (Fragaria × ananassa ‘Albion’) (mean ± SD).
The analysis of the physiological and biochemical parameters in leaves showed that the proline content, EL, and the activity of antioxidant enzymes (SOD, CAT, and POD) in strawberries grown in the open-field soilless culture were significantly higher than those grown in the soilless greenhouse environment (p < 0.05, Table 5, Figure 3). These results can be explained by the higher environmental pressure on plants grown under open-field compared to greenhouse conditions. In contrast, the relative water content and stomatal conductivity (SC) of leaves were significantly lower in the soilless open-field growing environment than in the soilless greenhouse environment (p < 0.05). The total soluble solids (TSS), TSS/TA ratio, PAL activity, vitamin C, flavonoid and phenol content, and DPPH in strawberries grown in the open-field soilless culture were significantly higher than those in strawberries grown in the greenhouse environment (Table 5, Figure 3 and Figure 4, p < 0.05).

Table 5.
Effect of different growing environments on the physiological and biochemical parameters of soilless cultured Albion strawberry (Fragaria × ananassa Albion) (mean ± SD).
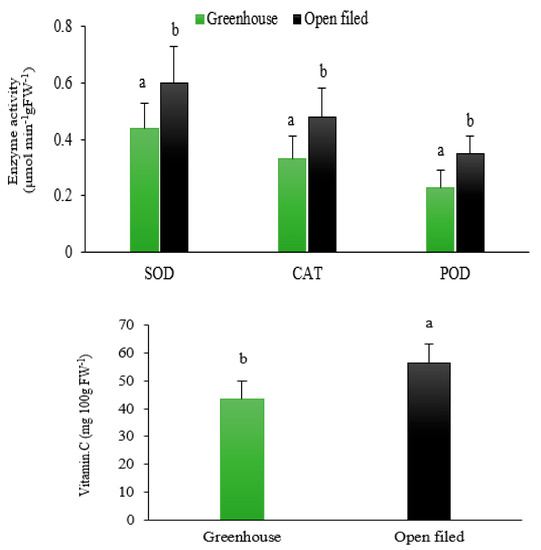
Figure 3.
Effect of different growing environments on superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) activities, and vitamin C content of soilless cultured strawberry (Fragaria × ananassa ‘Albion’). Different letters show significant difference between treatments (p < 0.05) (mean ± SD).
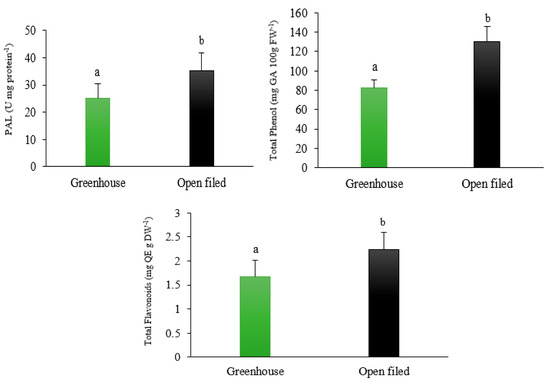
Figure 4.
Effect of different growing environments on phenylalanine ammonia-lyase (PAL), total phenol, and flavonoids of soilless cultured strawberry (Fragaria × ananassa ‘Albion’). Different letters show significant difference between treatments (p < 0.05) (mean ± SD).
The amounts of photosynthetic pigments markedly increase under greenhouse conditions compared to that under open-field conditions. Ibrahim et al. reported that three varieties of Labisia pumila Benth, grown in a greenhouse, showed a higher content of photosynthetic pigments than open-field-grown plants [48]. It has been suggested that the photosynthetic response of leaves to increased photosynthetic photon flux density (PPFD) during plant growth in open fields can be related to intricate interactions between acclimation of the photosynthetic machinery, photoinhibition damage, and repair [49]. Therefore, strawberry plants grown in the open field and exposed to higher light conditions might exhibit photoinhibition under high PPFD conditions [50]. The significant and strong positive correlation between photosynthetic pigments in strawberries grown in the greenhouse supports this assumption (Figure 5).
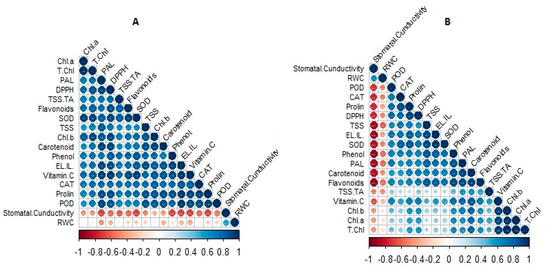
Figure 5.
Pearson’s correlations between photosynthetic pigments and biochemical parameters of soilless cultured strawberry (Fragaria × ananassa ‘Albion’) in greenhouse (A) and open-field (B) conditions.
The stomatal conductance and relative water content of leaves were the two physiological parameters that were significantly higher in strawberries grown in greenhouses than in those grown in open fields. Stomata play a critical role in regulating the fluxes of water and carbon dioxide between plants and the atmosphere. Therefore, stomatal responses to changes in the environment can be integrated with changes in stomatal conductance [51]. Previous studies have shown that the stomatal conductance and relative water content of leaves are sensitive to changing environmental factors, such as vapor pressure deficit, light, and the CO2 concentration [52]. In addition, it has been confirmed that stomatal conductance in plants under UV-blocking covers is higher [53]. Therefore, the differences observed in stomatal conductance in the greenhouse and open field may be attributed to variations in the UVB impact and signify the complexity of the effects of UV on stomata [54]. Furthermore, scientific evidence has established that maintaining an optimal level of relative humidity (40–60%) can effectively reduce the leaf temperature and enhance the stomatal conductance [55]. Consequently, it is plausible to suggest that the greenhouse environment, with its suitable average relative humidity (49%), promoted increased stomatal conductance compared to the open field (22%). Consequently, this led to an improvement in the relative water content of leaves and better thermoregulation, with a positive effect on the photosynthesis activity. The validity of this argument was supported by a significant correlation observed between these two parameters, as depicted in Figure 5. The low relative humidity induces high transpiration that can induce a high water demand, with possibly stressful conditions for crops.
The enzymatic antioxidant activity, including SOD, POD, and CAT, as well as the levels of proline and electrolyte leakage in the leaves of open-field-grown strawberries, exhibited significantly higher values compared to those of greenhouse-grown strawberries. This disparity suggests that the open-field cultivation environment imposed greater environmental stresses, such as fluctuating changes in light intensity and temperature [56]. It is noteworthy that in the open field, the daily temperature reached higher levels (24 °C) during the day and lower levels (15.5 °C) during the night. Additionally, the light intensity was considerably higher (957 µmol m−2 s−1) compared to the greenhouse condition. These conditions of temperature and light in the open field can be considered as stress factors for strawberries, resulting in an upregulation of enzymatic antioxidant activity and the accumulation of proline, an amino acid known for its stress-responsive role. SOD, a critical component of the active oxygen scavenging system of plant chloroplasts, can quickly respond to environmental changes within a few hours [57]. SOD is an enzyme that facilitates the conversion of superoxide radicals (O2−) into hydrogen peroxide (H2O2) and molecular oxygen (O2) [58]. Consequently, it can be inferred that the cellular levels of H2O2 are directly influenced by the production of O2− [59]. Additionally, CAT and POD use an organic substrate as an electron donor to reduce H2O2 to water and protect plants during oxidative stress [60]. Hydrogen peroxide is recognized as one of the most significant reactive oxygen species (ROS), and at lower concentrations, it is a signaling molecule in biological systems. It arises from the incomplete regeneration of atmospheric oxygen during vital cellular processes, including photorespiration [61]. H2O2 functions as a pivotal signaling molecule involved in plants’ response to various abiotic stresses, such as extreme temperatures, drought, and excessive radiation [62]. The homeostasis of H2O2 is maintained through the action of antioxidant enzyme systems, including catalase and peroxidase, which rapidly eliminate H2O2 [63]. Due to its small size and relatively long half-life (1 ms), H2O2 can readily traverse cell membranes, thereby facilitating its role as a signaling molecule [64]. H2O2 possesses diverse functions and regulates numerous physiological processes within plants, such as cell wall reinforcement, aging, photosynthesis, stomatal opening, and the cell cycle. However, it is important to note that excessive concentrations of H2O2 can induce cellular and plant damage [65]. In other words, at low concentrations, H2O2 acts as a signaling molecule, while at higher concentrations, it triggers cell death [66].
Numerous studies have consistently demonstrated that enhanced proline accumulation is concomitant with increased activities of SOD, POD, and CAT, thereby signifying the activation of an antioxidative defense mechanism through elevated proline production [67]. Consequently, the effectiveness and synchronization of enzymatic and non-enzymatic antioxidant compounds within the plant are imperative for regulating the concentration of H2O2, as corroborated by the findings of the present study (Figure 5). Moreover, under prevailing environmental conditions, including light intensity, temperature, and relative humidity, the stress induced by high temperatures [68], particularly during July and August (28/30 °C), appears to have heightened the leaf electrolyte leakage in the open field. This perturbation in the stability of the leaf cell membrane in strawberries cultivated in open fields, prompted by environmental stresses such as high temperatures, potentially contributes to an increase in electrolyte leakage. Consequently, this leads to a subsequent reduction in the maximum photochemical performance of photosystem II (PSII), ultimately diminishing the content of photosynthetic pigments [69]. The results of the correlation analysis and mean comparisons lend support to this assertion (Table 4 and Table 5, Figure 5).
Based on the observations recorded during the present investigation, the total soluble solids, TSS/TA ratio, PAL activity, vitamin C content, total antioxidant capacity, flavonoid, and phenol content were increased in the fruits of open-field-grown strawberries. Therefore, the soilless culture of strawberries in open-field conditions could have positive effects on the fruit quality, increasing TSS, nutrition, and secondary metabolites such as flavonoid content, and phenol content. As PAL is a key regulatory enzyme for flavonoid biosynthesis during fruit ripening, an increase in the activity of this enzyme can increase the levels of flavonoids in the fruit [70]. Previous studies have reported an increase in vitamin C concentrations under open-field culture conditions due to a response to environmental abiotic stress [71]. It has been suggested that increasing the vitamin C concentration is a response to abiotic stress through de novo synthesis or due to its regeneration from dihydrolipoic acid [71]. Phenolics and flavonoids play important roles in the detoxification of free radicals during an increase in environmental stress [72]. Contradictory reports on the effects of environmental stress on phenolic and flavonoid compounds in strawberries can be found in the literature. However, most of them described a differential accumulation of phenolic and flavonoid compounds that was dependent on the type of abiotic stress, concluding that these compounds favored oxidative damage protection under abiotic stress [73]. The increase in the total antioxidant capacity (DPPH) in fruits of open-field-grown strawberries in this study can further confirm this claim.
Previous studies have reported that a higher and positive DIF can enhance the soluble sugar concentration and improve the fruit quality [74]. In line with these findings, the results of the present experiment confirm the positive impact of high DIF in the open field (8.5 °C) compared to the greenhouse (3 °C). Wu et al. also demonstrated that DIF levels above 8 and below 14 optimally stimulate the activity of the sucrose biosynthesis enzyme, sucrose phosphate synthase, leading to an increase in the fruit sugar content [75]. Consequently, the higher taste index (TSS/TA) observed in the open field, as compared to the greenhouse, could be attributed to the elevated sugar content. Additionally, it has been established that plants respond to thermal stress by biosynthesizing secondary metabolites (SMs), with phenols and flavonoids being highly sensitive to temperature variations [76]. In the present study, the observed increase in temperature likely induced localized thermal stress, resulting in elevated levels of secondary metabolites, such as phenols and flavonoids, in the open field. Moreover, considering the well-established role of SMs in post-harvest fruit quality and shelf-life [77], it is reasonable to infer that the enhanced shelf-life of fruits in the open-field, as observed in this study, can be attributed to the increased accumulation of SMs compared to the greenhouse. Moreover, many studies have demonstrated that the main difference between fruits produced under UV exclusion conditions (greenhouses) is that they have a significantly lower content of secondary compounds and antioxidants than fruits produced under open-field conditions [78,79]. Considering the more regular, positive, and significant correlation between secondary compounds and antioxidants in strawberries grown in the greenhouse, as well as the strong and significant negative correlation between stomatal conductance and secondary compounds and antioxidants in strawberries grown in the open environment (Figure 5), it can be argued that sensitivity to environmental changes, including UV, caused these observations.
4. Conclusions
Overall, the results of this study showed that strawberries cultivated in the greenhouse and in the open-field soilless condition showed different phenotypic, physiological, and biochemical reactions. While strawberries grown under greenhouse conditions performed better in terms of vegetative growth, photosynthesis, and yield, open-field cultivation showed superior results in terms of the fruit quality, flavor index, enzymatic antioxidant activity, antioxidant compounds, and fruit shelf-life. These findings suggest that open-field soilless cultivation may yield distinct responses compared to greenhouse cultivation and should be tested on a wider range of cultivars to draw more accurate conclusions. The limited availability of detailed studies on stress gradients in open-field strawberry cultivation, compared to greenhouse cultivation, indicates a lack of understanding regarding how different environments in soilless cultivation respond to stress and impact the final product yield.
Author Contributions
Conceptualization, M.J.N. and A.F.; data curation, J.R.D.; formal analysis, M.A.; methodology, J.R.D. and M.A.; project administration, M.J.N.; software, J.R.D.; supervision, M.J.N.; writing—original draft, J.R.D.; writing—review and editing, M.J.N., A.F. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Research data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pandey, S.; Sinh, J.; Singh, S.K.; Mourya, I.B. Influence of growing environment on growth, yield and chemical composition of strawberry (Fragaria × ananassa) fruits under open vs. naturally ventilated polyhouse conditions. Indian J. Agric. Sci. 2015, 85, 1540–1545. [Google Scholar]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, B.; Naeimi, A.; Badsar, M. Designing an integrated model for strawberry growers’ behavior toward implementation of good agricultural practices in Iran. Environ. Dev. Sustain. 2021, 24, 10924–10944. [Google Scholar] [CrossRef]
- Cormier, J.; Depardieu, C.; Letourneau, G.; Boily, C.; Gallichand, J.; Caron, J. Tensiometer-based irrigation scheduling and water use efficiency of field-grown strawberries. Agron. J. 2020, 112, 2581–2597. [Google Scholar] [CrossRef]
- Maas, J.L. Strawberry disease management. In Diseases of Fruits and Vegetables; Springer: Berlin/Heidelberg, Germany, 2004; Volume II, pp. 441–483. [Google Scholar]
- Jabbar, A.; Tehranifar, A.; Shoor, M.; Nemati, S.H. Effect of different media on some growth, flowering and biochemical parameters of two cultivars of gladiolus (Gladiolus grandiflorus L.) under soilless conditions. J. Ornam. Plants 2018, 8, 205–215. [Google Scholar]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. Sci. Total. Environ. 2020, 698, 134154. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Baruzzi, G.; Massetani, F.; Faedi, W. Strawberry production in forced and protected culture in Europe as a response to climate change. Can. J. Plant Sci. 2012, 92, 1021–1036. [Google Scholar] [CrossRef]
- Thakur, N.; Nigam, M.; Awasthi, G.; Shukla, A.; Shah, A.A.; Negi, N.; Khan, S.A.; Casini, R.; Elansary, H.O. Synergistic soil-less medium for enhanced yield of crops: A step towards incorporating genomic tools for attaining net zero hunger. Funct. Integr. Genom. 2023, 23, 86. [Google Scholar] [CrossRef]
- Palencia, P.; Martínez, F.; Vázquez, M.A. Oxyfertigation and Transplanting Conditions of Strawberries. Agronomy 2021, 11, 2513. [Google Scholar] [CrossRef]
- Cecatto, A.P.; Calvete, E.O.; Nienow, A.A.; da Costa, R.C.; Mendonça, H.F.C.; Pazzinato, A.C. Culture systems in the production and quality of strawberry cultivars. Acta Sci. Agron. 2013, 35, 471–478. [Google Scholar] [CrossRef]
- Maher, M.; Shylla, B.; Sharma, D.; Sharma, U.; Kuchay, M. Yield and quality of polyhouse grown strawberries as affected by soilless media and jeevamrit. Int. J. Chem. Stud. 2020, 8, 585–589. [Google Scholar] [CrossRef]
- Del Bubba, M.; Checchini, L.; Chiuminatto, U.; Doumett, S.; Fibbi, D.; Giordani, E. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of polyphenolic composition of four cultivars of Fragaria vesca L. berries and their comparative evaluation. J. Mass Spectrom. 2012, 47, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Gonnella, M.; Renna, M. The Evolution of soilless systems towards ecological sustainability in the perspective of a circular economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Nicola, S.; Savvas, D.; Voogt, W. Soilless cultivation through an intensive crop production scheme. Management strategies, challenges and future directions. Front. Plant Sci. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.M.; Leonardi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- Weston, L.; Barth, M. Preharvest factors affecting postharvest quality of vegetables. HortScience 1997, 32, 812–816. [Google Scholar] [CrossRef]
- Caruso, G.; Villari, A.; Villari, G. Quality characteristics of Fragaria vesca L. fruits influenced by NFT solution EC and shading. In Proceedings of the South Pacific Soilless Culture Conference (SPSCC 648), Palmerston North, New Zealand, 10–13 February 2003. [Google Scholar]
- Shinohara, Y. Growing Conditions and Quality of Vegetables: Effect of Light and Fertilizer Conditions on the Ascorbic Acid Content of Vegetables; Memoirs of Institute of Agriculture and Forestry-University of Tsukuba, Agricultural and Forestry Science: Ibaraki, Japan, 1987. [Google Scholar]
- Krishna, P.; Pandey, G.; Thomas, R.; Parks, S. Improving Blueberry Fruit Nutritional Quality through Physiological and Genetic Interventions: A Review of Current Research and Future Directions. Antioxidants 2023, 12, 810. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extremes 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Urban, J.J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef]
- Tuzet, A.; Perrier, A.; Leuning, R. A coupled model of stomatal conductance, photosynthesis and transpiration. Plant Cell Environ. 2003, 26, 1097–1116. [Google Scholar] [CrossRef]
- Crawford, A.J.; McLachlan, D.H.; Hetherington, A.M.; Franklin, K.A. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012, 22, R396–R397. [Google Scholar] [CrossRef] [PubMed]
- Feller, U.; Vaseva, I.I. Extreme climatic events: Impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Trishchenko, A.P.; Barr, A.G.; Black, T.A.; McCaughey, H. Modeling the Response of Canopy Stomatal Conductance to Humidity. J. Hydrometeorol. 2009, 10, 521–532. [Google Scholar] [CrossRef]
- Fanourakis, D.; Bouranis, D.; Giday, H.; Carvalho, D.R.; Nejad, A.R.; Ottosen, C.-O. Improving stomatal functioning at elevated growth air humidity: A review. J. Plant Physiol. 2016, 207, 51–60. [Google Scholar] [CrossRef]
- Gago, J.; de Menezes Daloso, D.; Figueroa, C.M.; Flexas, J.; Fernie, A.R.; Nikoloski, Z. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: A multispecies meta-analysis approach. Plant Physiol. 2016, 171, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Mariani, L. Agronomic management for enhancing plant tolerance to abiotic stresses: High and low values of temperature, light intensity, and relative humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef]
- Caruso, G.; Villari, G.; Melchionna, G.; Conti, S. Effects of cultural cycles and nutrient solutions on plant growth, yield and fruit quality of alpine strawberry (Fragaria vesca L.) grown in hydroponics. Sci. Hortic. 2011, 129, 479–485. [Google Scholar] [CrossRef]
- Døving, A.; Måge, F. Methods of Testing Strawberry Fruit Firmness. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2002, 52, 43–51. [Google Scholar] [CrossRef]
- Smith, J.H.; Benitez, A. Chlorophylls: Analysis in plant materials. In Modern Methods of Plant Analysis/Moderne Methoden der Pflanzenanalyse; Springer: Berlin/Heidelberg, Germany, 1955; pp. 142–196. [Google Scholar]
- Berger, S. Metoda ilościowego oznaczania karotenu (prowitamina A) i sumy karotenów w niektórych produktach roślinnych. Rocz. Państwowego Zakładu Hig. 1953, 4, 473–479. [Google Scholar]
- Lutts, S.; Kinet, J.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Nguyen, H.T.; Holaday, A.S. Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci. 1990, 30, 105–111. [Google Scholar] [CrossRef]
- Jungsakulrujirek, S.; Noomhorm, A. Effect of harvesting time and fruit size on titratable acidity, soluble solid and distribution of limonin in Thai tangerine juice. Int. J. Food Sci. Technol. 1998, 33, 367–374. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z.; Guan, L.; Gaughan, G.; Lin, G. Antioxidant Enzymes Regulate Reactive Oxygen Species during Pod Elongation in Pisum sativum and Brassica chinensis. PLoS ONE 2014, 9, e87588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, S.; Li, Z.; Liu, N.; Li, L.; Luo, L.; Peng, T.; Liu, W. Effects of the infestation by Actinote thalia pyrrha (Fabricius) on the physiological indexes of Mikania micrantha leaves. Acta Ecol. Sin. 2006, 26, 1330–1336. [Google Scholar] [CrossRef]
- Zeng, S.-X.; Wang, Y.-R.; Liu, H.-X. Some enzymatic reactions related to chlorophyll degradation in cucumber cotyledons under chilling in the light. Acta Phytophysiol Sin 1991, 17, 177–182. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Hosu, A.; Cristea, V.-M.; Cimpoiu, C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 152–178. [Google Scholar]
- Rahmatian, A.; Delshad, M.; Salehi, R. Effect of grafting on growth, yield and fruit quality of single and double stemmed tomato plants grown hydroponically. Hortic. Environ. Biotechnol. 2014, 55, 115–119. [Google Scholar] [CrossRef]
- Zucker, M. Sequential induction of phenylalanine ammonia-lyase and a lyase-inactivating system in potato tuber disks. Plant Physiol. 1968, 43, 365–374. [Google Scholar] [CrossRef]
- Cocco, C.; Andriolo, J.L.; Cardoso, F.L.; Erpen, L.; Schmitt, O.J. Crown size and transplant type on the strawberry yield. Sci. Agricola 2011, 68, 489–493. [Google Scholar] [CrossRef]
- Grijalba, C.M.; Pérez-Trujillo, M.M.; Ruiz, D.; Ferrucho, A.M. Strawberry yields with high-tunnel and open-field cultivations and the relationship with vegetative and reproductive plant characteristics. Agron. Colomb. 2015, 33, 147–154. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Photosynthetic capacity, photochemical efficiency and chlorophyll content of three varieties of Labisia pumila Benth. Exposed to open field and greenhouse growing conditions. Acta Physiol. Plant 2011, 33, 2179–2185. [Google Scholar] [CrossRef]
- Adir, N.; Zer, H.; Shochat, S.; Ohad, I. Photoinhibition—A historical perspective. Photosynth. Res. 2003, 76, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Liu, X. Photosynthetic characteristics of linze jujube in conditions of high temperature and irradiation. Sci. Hortic. 2005, 104, 339–350. [Google Scholar] [CrossRef]
- Habermann, E.; De Oliveira, E.A.D.; Contin, D.R.; Martin, J.A.B.S.; Curtarelli, L.; Gonzalez-Meler, M.; Martinez, C.A. Stomatal Development and Conductance of a Tropical Forage Legume Are Regulated by Elevated [CO2] Under Moderate Warming. Front. Plant Sci. 2019, 10, 609. [Google Scholar] [CrossRef]
- Tanentzap, F.M.; Stempel, A.; Ryser, P. Reliability of leaf relative water content (RWC) measurements after storage: Consequences for in situ measurements. Botany 2015, 93, 535–541. [Google Scholar] [CrossRef]
- Katsoulas, N.; Bari, A.; Papaioannou, C. Plant Responses to UV Blocking Greenhouse Covering Materials: A Review. Agronomy 2020, 10, 1021. [Google Scholar] [CrossRef]
- Hideg, É.; Strid, Å. The effects of UV-B on the biochemistry and metabolism of plants. In UV-B Radiation and Plant Life; CAB International: Oxfordshire, UK, 2017; pp. 90–110. [Google Scholar]
- Han, W.; Yang, Z.; Huang, L.; Sun, C.; Yu, X.; Zhao, M. Fuzzy comprehensive evaluation of the effects of relative air humidity on the morpho-physiological traits of Pakchoi (Brassica chinensis L.) under high temperature. Sci. Hortic. 2019, 246, 971–978. [Google Scholar] [CrossRef]
- Haghshenas, M.; Nazarideljou, M.J.; Shokoohian, A. Phytochemical and Quality Attributes of Strawberry Fruit under Osmotic Stress of Nutrient Solution and Foliar Application of Putrescine and Salicylic Acid. J. Hortic. Sci. 2020, 7, 263–278. [Google Scholar]
- Scandalios, J.G. Response of Plant Antioxidant Defense Genes to Environmental Stress. Adv. Genet. 1990, 28, 1–41. [Google Scholar]
- Del Río, L.A.; Corpas, F.J.; Lopez-Huertas, E.; Palma, J.M. Plant superoxide dismutases: Function under abiotic stress conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–26. [Google Scholar]
- Ślesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kuk, Y.I.; Shin, J.S.; Burgos, N.R.; Hwang, T.E.; Han, O.; Cho, B.H.; Jung, S.; Guh, J.O. Antioxidative Enzymes Offer Protection from Chilling Damage in Rice Plants. Crop Sci. 2003, 43, 2109–2117. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, H.; Mittelstrass, K.; Kschieschan, S.; Bender, J.; Weigel, H.-J.; Overmyer, K.; Kangasjärvi, J.; Sandermann, H.; Langebartels, C. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ. 2002, 25, 717–726. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.D.; Van Breusegem, F. Hydrogen peroxide—A central hub for information flow in plant cells. AoB Plants 2012, 2012, pls014. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhris, M.; Abdallah, F.B. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Thiaw, S.; Hall, A.E. Comparison of selection for either leaf-electrolyte-leakage or pod set in enhancing heat tolerance and grain yield of cowpea. Field Crops Res. 2004, 86, 239–253. [Google Scholar] [CrossRef]
- Bhattarai, S.; Harvey, J.T.; Djidonou, D.; Leskovar, D.I. Exploring Morpho-Physiological Variation for Heat Stress Tolerance in Tomato. Plants 2021, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Halbwirth, H.; Puhi, I.; Hass, U.; Jezik, K.; Treutter, D.; Stich, K. Two-phase flavonoid formation in developing strawberry (Fragaria × ananassa) fruit. J. Agric. Food Chem. 2006, 54, 1479–1485. [Google Scholar] [CrossRef]
- Botella, M.; Hernández, V.; Mestre, T.; Hellín, P.; García-Legaz, M.F.; Rivero, R.M.; Martínez, V.; Fenoll, J.; Flores, P. Bioactive Compounds of Tomato Fruit in Response to Salinity, Heat and Their Combination. Agriculture 2021, 11, 534. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Veronneau, P.-Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Gao, Q.; Chen, J.-X. Effects of day and night temperature difference on growth, development, yield and fruit quality of tomatoes. Ying Yong Sheng Tai Xue Bao 2015, 26, 2700–2706. [Google Scholar]
- Wu, X.; Han, W.; Yang, Z.; Zhang, Y.; Zheng, Y. The difference in temperature between day and night affects the strawberry soluble sugar content by influencing the photosynthesis, respiration and sucrose phosphatase synthase. Hortic. Sci. 2021, 48, 174–182. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Galal, F.H.; Seufi, A.M. Effect of extreme temperature changes on phenolic, flavonoid contents and antioxidant activity of tomato seedlings (Solanum lycopersicum L.). PeerJ 2021, 9, e11193. [Google Scholar] [CrossRef]
- Mamat, S.F.; Azizan, K.A.; Baharum, S.N.; Noor, N.M.; Aizat, W.M. GC-MS and LC-MS analyses reveal the distribution of primary and secondary metabolites in mangosteen (Garcinia mangostana Linn.) fruit during ripening. Sci. Hortic. 2020, 262, 109004. [Google Scholar] [CrossRef]
- Ordidge, M.; García-Macías, P.; Battey, N.H.; Gordon, M.H.; John, P.; A Lovegrove, J.; Vysini, E.; Wagstaffe, A.; Hadley, P. Development of colour and firmness in strawberry crops is UV light sensitive, but colour is not a good predictor of several quality parameters. J. Sci. Food Agric. 2012, 92, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- García-Macías, P.; Ordidge, M.; Vysini, E.; Waroonphan, S.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Wagstaffe, A. Changes in the flavonoid and phenolic acid contents and antioxidant activity of red leaf lettuce (Lollo Rosso) due to cultivation under plastic films varying in ultraviolet transparency. J. Agric. Food Chem. 2007, 55, 10168–10172. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).