Abstract
The red color of the pear peel in red-skinned European pear is due to the accumulation of anthocyanins. Numerous transcription factors play pivotal roles in anthocyanin biosynthesis, with zinc finger proteins frequently implicated in the regulation of this process via MYB10, as documented in earlier studies. In this article, we identified a zinc finger protein gene, named PbZAT12, that does not rely on PbMYB10, to regulate anthocyanin biosynthesis. The PbZAT12 protein was localized within the nucleus and exhibited a positive influence on the accumulation of anthocyanins in the peel of ‘Red Zaosu’ pears. Moreover, overexpression of PbZAT12 resulted in a significant up-regulation of PbDFR, PbANS, and PbUFGT expression levels in pear fruitlets. Y1H assays demonstrated a direct binding ability of PbZAT12 to proPbDFR, proPbANS, and proPbUFGT, which was supported by a dual luciferase assay, indicating its potential to activate the transcriptional activity of these promoters. However, in contrast to its effect on the aforementioned promoters, PbZAT12 did not exhibit an activation of PbMYB10. In summary, our findings suggest that a zinc finger transcription factor, PbZAT12, exerts a positive influence on anthocyanin biosynthesis in pear fruit through direct upregulation of the expression levels of PbDFR, PbANS, and PbUFGT.
1. Introduction
Anthocyanin, a vital secondary metabolite, imparts vibrant red, blue, and purple hues to diverse tissues [1]. These pigments give plants special colors that attract pollinators and animals to help with seed dispersal [2]. Additionally, they function as natural antioxidants, contributing significantly to human health and well-being [3,4]. Anthocyanin accumulation is a complex process regulated by various factors, including light exposure [5,6], temperature variations [3,7,8], sugar availability, and plant hormone signaling [9,10]. The biosynthesis process of anthocyanin in plants is closely related to the light signal transduction pathway, and sunlight up-regulates anthocyanin accumulation [11,12,13,14]. Light exerts a pivotal regulatory influence on the anthocyanin biosynthesis pathway by modulating the expression of both structural and regulatory genes, thereby facilitating the enhanced accumulation of anthocyanins [15]. Overexpression of PAP1 in Arabidopsis thaliana seedlings promotes anthocyanin accumulation and significantly increases DFR transcription levels [16]. Light enhances the anthocyanins in apple through the promotion of expression of key anthocyanin transcription factors, specifically MdMYB1 (MdMYBA) and MdMYB10 [11,17], in pear, by PbMYB10 and PbMYB114 [18,19].
A combination of enzymatic processes and transcriptional regulators regulates the biosynthesis of anthocyanin [20,21,22,23]. Enzymes involved in this process include flavonol synthase (FLS), dihydroflavonol reductase (DFR), leucoanthocyanidin dioxygenase/anthocyanidin synthase (LDOX/ANS), anthocyanidin reductase (ANR), and UDP-glucose: flavonoid 3-glucosyltransferase (UFGT) and other related enzymes [24,25]. Meanwhile, the accumulation of anthocyanin is primarily mediated by MYBs, basic Helix-Loop-Helix (bHLHs), and WD40, which can form a MYB-bHLH-WD40 (MBW) protein complex in plants [25,26].
Zinc finger proteins, as a group of transcription factors, are involved in diverse physiological and biochemical processes spanning across a wide array of organisms [27]. Based on their structural characteristics, these proteins can be categorized into various types, including C8, C6, C4, C2H2, C3HC4, C4HC3, C2HC, and LIM (C2HC5) [28]. Within the diverse range of zinc finger proteins, C2H2-type (cysteine2/histidine2-type) zinc finger proteins are most closely associated with stress-related transcription factors [27]. As a subfamily of zinc finger proteins, BBX family members have been extensively implicated in the regulation of anthocyanin biosynthesis [29,30]. The zinc finger protein ZAT12, which is the same as a member of the zinc finger proteins family, was initially identified as a responsive gene to HL (RHL41, also named ZAT12) [31]. RHL41 plays a role in modulating the expression of multiple light- and oxidative-stress-responsive genes in Arabidopsis [32], and it has been shown to increase anthocyanin levels [31]. However, the specific molecular mechanism by which it regulates anthocyanin biosynthesis has not been studied yet.
We identified a light-responsive gene, PbZAT12, through transcriptome analysis of ‘Red Zaosu’ pear fruit peel reddening under high temperature and high light. PbZAT12 contains the conserved C2H2 domain, and we propose that it enhances anthocyanin biosynthesis in red skin pear. In this study, we identified PbZAT12 through differential gene screening in the transcriptome of ‘Red Zaosu’ pear, which showed a correlation with anthocyanin content in the pear peel. We validated the promotion of anthocyanin accumulation by PbZAT12 through transient injection in pear peel. In PbZAT12-OE, we screened for some structural genes that exhibited similar expression levels to PbZAT12 by qRT-PCR. Furthermore, we confirmed the direct binding of PbZAT12 to the promoter regions of these genes and its enhancement of promoter activity through Y1H experiments and the dual-luciferase reporter assay system. Therefore, we demonstrated that PbZAT12 positively regulates anthocyanin biosynthesis in pear peel by directly modulating the expression of structural genes involved in anthocyanin biosynthesis, and we tentatively inferred that this regulation occurs independently of PbMYB10. These findings contribute to enriching the regulatory network of anthocyanin biosynthesis in pear peel and are of significant importance in uncovering the regulatory differences of different transcription factors in anthocyanin biosynthesis.
2. Materials and Methods
2.1. Plant Materials
In 2018, for our study, we carefully chose the fruit of ‘Red Zaosu’ (Pyrus bretschneideri Rehd.) as our primary experimental material. These fruits were obtained from a well-established commercial orchard situated in Mei County, Shaanxi Province, China. The peels of fruitlets overexpressing PbZAT12 were harvested 5 days post injection in 35 DAFB (Days after full bloom), and the harvested fruit samples were rapidly frozen in liquid nitrogen to preserve their biochemical composition and enzymatic activity. Subsequently, they were stored at −80 °C to maintain their integrity during long-term storage. This freezing and storage process allowed for accurate measurements of anthocyanin concentrations and facilitated the extraction of RNA for further analysis.
The dual luciferase assay was conducted using Nicotiana benthamiana seedlings, which were cultivated in a controlled environment. The seedlings were grown in a light incubator set at a temperature of 22 °C, with a photoperiod consisting of 16 h of light followed by 8 h of darkness.
2.2. Transcriptome Sequencing
Three types of samples were collected from the fruit of ‘Red Zaosu’: red-striped peel (GS), green-striped peel (RS), and all-red peel (AR). Three replicates were collected for each sample type. RNA was extracted from the peel samples and transcriptome libraries were constructed. Illumina HiSeq 2000 (Illumina, San Diego, CA, USA) sequencing was performed on the libraries. For specific details regarding transcriptome construction and data analysis, please refer to Zhai et al. [24].
2.3. Anthocyanin Concentration Measurements
In this study, we implemented a slightly modified version of a previously established method [33] to extract and determine total anthocyanin from our samples. The absorbance values were measured simultaneously at 520 nm and 700 nm using a Microporous plate spectrophotometer (Multiskan GO; Thermo Scientific, Waltham, MA, USA). The buffer liquid was used as a negative control.
2.4. Sequence Alignment and Phylogenetic Analysis of Proteins
To align the ZAT proteins from different species, we used the DNAMAN program from LynnonBiosoft, CA, USA. For the phylogenetic analysis, we used the MEGA 5.0 program (Mega Limited, Auckland, New Zealand) and applied the NJ method with the JTT model.
2.5. RNA Extraction and Expression Analysis
The qRT-PCR reactions were conducted using SYBR Premix Ex Taq II (TaKaRa, Dalian, China) following the manufacturer’s instructions and performed on an Icycler iQ5 instrument (Bio-Rad, Berkeley, CA, USA). Each sample was subjected to three biological replicates, and each biological replicate was run with three technical replicates. The specific method of RNA extraction and setting up the QRT-PCR system is based on the method in Yao et al.’s article [19]. The resulting qRT-PCR data were analyzed using the 2−ΔΔCT method, with each biological replicate undergoing three separate analyses.
Please refer to Supplemental Table S1 for the primer sequences used for actin, anthocyanin biosynthetic genes, and PbZAT12.
2.6. Subcellular Localization Experiments of Gene
The complete coding sequence of PbZAT12 was amplified using PCR from cDNA extracted from the peel of ‘Red Zaosu’. Subsequently, it was inserted into the plant binary expression vector pCambia 2300 along with a GFP tag, driven by the CaMV35S promoter, resulting in the creation of the construct p35S::GFP-PbZAT12. These constructs were then transformed into Agrobacterium tumefaciens strain EHA105 (ToloBio, Shanghai, China) for the purpose of introducing them into the cells of Nicotiana benthamiana leaves.
The p35S::GFP-PbZAT12 plasmid-carrying Agrobacterium tumefaciens strain EHA105 was cultured on LB solid medium at 28 °C for 48 h. Subsequently, the Agrobacterium cells were resuspended in an infiltration buffer containing 10 mM MgCl2, 10 mM MES, and 200 μM acetosyringone and incubated at room temperature until reaching an optical density (OD600) of 0.8. The resulting suspension was then used to infiltrate the cells of Nicotiana benthamiana leaves using the methods described by Jiang et al. [34]. Following infiltration, the Nicotiana benthamiana leaf samples were kept in shaded conditions at room temperature for 48 h, and the resulting GFP fluorescence was observed using an Olympus BX63 microscope. The primer sequences used for subcellular localization can be found in Supplemental Table S1.
2.7. Transient Expression Assay in Skin of Pear Fruitlets
The complete coding DNA sequence (CDS) of PbZAT12 was fully cloned into the pBI121 binary vector, resulting in the generation of PbZAT12-OE plasmids. The plasmids were then introduced into Agrobacterium tumefaciens strain EHA105 for a transient expression assay in the skin of pear fruitlets. The same protocol for Agrobacterium incubation as the subcellular localization experiments was followed. For the injection of the skin of pear fruitlets, the method outlined in Spolaore et al. [35] was utilized, while the injection volume was carried out according to Zhai et al. [24]. To visualize GUS staining, the plant materials were stained with 5-bromo-4-chloro-3-indolyl glucuronide (X-Gluc) as described in Spolaore et al. [35].
2.8. Yeast One-Hybrid (Y1H) Assay
The Y1H assays were conducted following the instructions provided by the Matchmaker Gold Yeast One-Hybrid System Kit (Clontech, Mountain View, CA, USA). First, 800 bp fragments of the promoters of the structural genes were inserted into pAbAi to create the pAbAi-baits. Subsequently, the complete CDS of PbZAT12 was introduced into the pGADT7 vector, resulting in the generation of the AD-PbZAT12 vector. The pAbAi-bait vectors were then linearized and individually transformed separately into the Y1H Gold cell. Transformants were selected on SD/-Ura agar plates that lacked uracil, and successful integration into the Y1HGold genome was confirmed by Colony PCR analysis using 630495 (Clontech). After determining the minimum inhibitory concentration of Aureobasidin A (AbA) for the bait reporter yeast strains, the AD-PbZAT12 vector was introduced into the bait yeast strains. Subsequently, the transformed yeast strains were screened on an SD/-Leu/AbA plate. The experiments were conducted in triplicate, with each experiment repeated three times.
2.9. Dual Luciferase Assay
To obtain the promoter sequences of PbANS, PbDFR, and PbUFGT, genomic DNA isolated from ‘Red Zaosu’ was used as the template for PCR amplification. The primers used for amplification were designed based on the R045Q (TaKaRa, Dalian, China) and gene-specific sequences (Supplementary Table S1). The amplified promoter sequences were ligated into the HindIII and BamHI restriction sites of the pGreenII 0800-LUC vector, which were named proPbANS, proPbDFR, and proPbUFGT, respectively [36]. The full-length coding sequences of PbZAT12 were inserted into the pGreenII 0029 62-SK vector [36].
Subsequently, the recombinant plasmids containing the PbZAT12 gene and the pSoup helper plasmid were separately introduced into the Agrobacterium tumefaciens strain EHA105 [36]. Agrobacterium tumefaciens cells carrying the PbZAT12-SK construct were mixed individually with the proPbANS, proPbDFR, or proPbUFGT constructs in a 1:1 ratio. The bacterial mixture was then injected into leaves of 4-week-old Nicotiana benthamiana plants. After injection, the plants were grown in a growth chamber for 3 days. The specific method is based on the method in Yao et al.’s article [19].
3. Results
3.1. Identification and Sequence Analysis of PbZAT12 TF in Pear
We discovered a ZAT transcription factor (TF) (GenBank accession number: XP_009337587.1) through a transcriptome study, which exhibited the greatest difference in RS vs. GS expression, and FPKM (Fragments Per Kilobase Million) > 0 (Figure 1a). To further identify zinc finger transcription factors in pear, we examined the conserved domains of the Arabidopsis thaliana AtZAT12 protein (NP_200790.1) in the GenBank database. This analysis revealed 73 ZAT genes from white pear genomic genes in the NCBI database. Phylogenetic analysis indicated that the two candidate ZAT proteins clustered together with AtZAT12 proteins (Figure 1b). Consequently, we designated the ZAT TF (XP_009337587.1) as PbZAT12. Sequence alignment and phylogenetic tree analysis revealed that PbZAT12 had the highest protein sequence identity with AtZAT12 (Figure 1c).
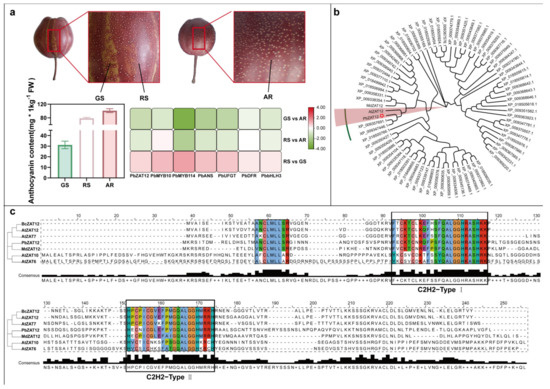
Figure 1.
Phylogenetic Analysis of Pear Genes from Different Plant Species. (a) RS, GS, and AR phenotypes of ‘Red Zaosu’ pear; anthocyanin content of PbZAT12; and log2.Fold_change values of anthocyanin-biosynthesis-related genes in the transcriptome (RS: Red stripes peel of ‘Red Zaosu’; GS: Green stripes peel of ‘Red Zaosu’; AR: Peel of all-red ‘Red Zaosu’). (b) Phylogenetic analysis of MdZAT12 and AtZAT12 in pear, apple, and Arabidopsis. The five-pointed stars represent PbZAT12, the red shaded areas represent the closest homologous genes to PbZAT12, and the green outlined areas represent multiple homologous genes clustered together with PbZAT12 in the phylogenetic tree. (c) Phylogenetic analysis of ZAT proteins in different plants and multiple sequence alignment of different ZAT proteins. The accession numbers of the proteins obtained from NCBI are listed in Supplementary Table S2. The C2H2 conserved domain is highlighted with a box in (c). Note: We named XP_009337587.1 as PbZAT12.
3.2. PbZAT12 Plays an Active Role in Anthocyanin Accumulation in Pear Fruitlets
To investigate whether the expression of PbZAT12 affects anthocyanin accumulation, we performed transient expression of PbZAT12-OE constructs on pear fruitlet peels using agrobacterium infiltration. Our results showed that compared to fruitlets injected with an empty vector, fruitlets with PbZAT12-OE constructs had significantly higher levels of anthocyanin accumulation on their peels (Figure 2a). To confirm successful infection on the fruitlet peels, we monitored the GUS signals (Figure 2b). Moreover, the expression level of PbZAT12 was significantly increased in the peel of PbZAT12-OE ‘Zaosu’ fruitlets (Figure 2c). These findings suggest that PbZAT12 plays a role in promoting anthocyanin accumulation in the skin of pear fruitlets.
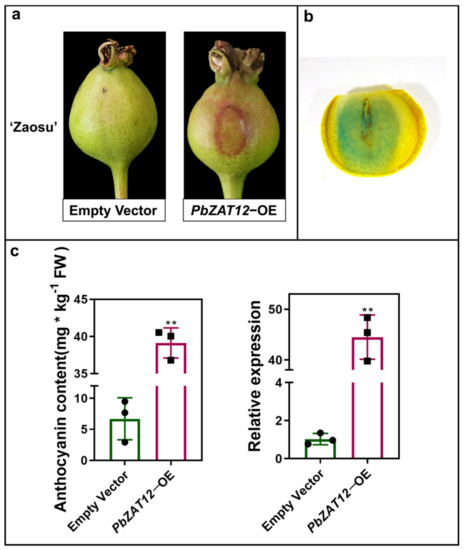
Figure 2.
Anthocyanin content and gene expression level around transient expression in ‘Zaosu’ pear fruit peel. (a) Pear fruit peel coloration around overexpressed PbZAT12 fruitlets. (b) The validity of infection of the skin of GUS-stained ‘Zaosu’ fruitlets. (c) The expression levels of PbZAT12 and anthocyanin concentration of overexpressed PbZAT12 fruitlets. Significant differences were analyzed with Student’s t-test: ** p < 0.01.
3.3. PbZAT12 Accumulates Anthocyanin by up-Regulating Anthocyanin-Related Genes in Skin of Pear Fruitlets
To identify potential downstream genes regulated by PbZAT12, we performed real-time PCR analysis of structural genes and transcription factors involved in anthocyanin biosynthesis in the peel of PbZAT12-OE pear fruitlets. The findings revealed a substantial increase in the expression levels of PbDFR, PbANS, and PbUFGT in the peel of PbZAT12-OE fruitlets compared to those injected with an empty vector (Figure 3). These findings suggest that PbZAT12 promotes anthocyanin accumulation in the peel of pear fruitlets by regulating the expression of genes involved in anthocyanin biosynthesis.
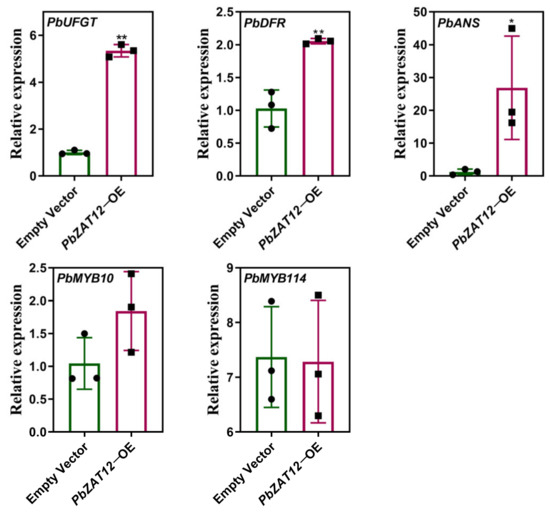
Figure 3.
The expression of anthocyanin-biosynthesis-related genes and MYB transcription factors in overexpressed PbZAT12 fruitlets. The promoters of expression analyses are listed in Supplementary Table S1. The accession numbers of genes for NCBI are presented in Supplementary Table S2. Statistical analysis was performed using Student’s t-test, and significance was indicated as follows: * p < 0.05, ** p < 0.01.
3.4. Subcellular Localization of PbZAT12
According to Uniprot (http://www.uniprot.org/, accessed on 23 August 2022), the subcellular localization prediction suggests that AtZAT12 is predominantly localized in the nucleus. To determine the localization of PbZAT12, localization analyses were performed using transient assays in Nicotiana benthamiana leaf cells. The results revealed that the GFP fluorescence of 35S: PbZAT12-GFP was localized to the nucleus of Nicotiana benthamiana leaf cells (Figure 4), indicating that PbZAT12 is also a nuclear protein. In contrast, the positive control 35S: GFP exhibited GFP fluorescence in both the cytomembrane and nucleus of Nicotiana benthamiana leaf cells (Figure 4).
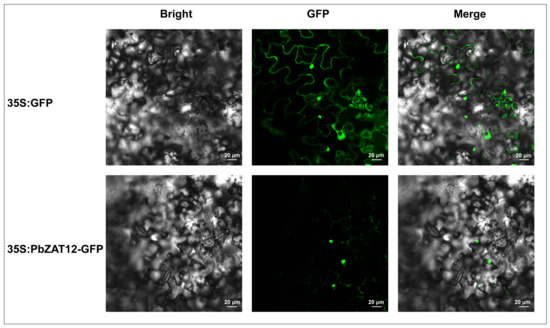
Figure 4.
Subcellular location of PbZAT12. The localization of PbZAT12 protein in Nicotiana benthamiana leaf cell. Bars = 0.01.
3.5. PbZAT12 Regulates the Promoter Activities of PbDFR, PbANS, and PbUFGT in Anthocyanin Biosynthetic Pathway
To confirm the impact of PbZAT12 on the promoters of anthocyanin synthesis structural genes, we performed both a Y1H assay and dual-luciferase analysis. The Y1H assay revealed that PbZAT12 was capable of directly binding to the promoters of PbDFR, PbANS, and PbUFGT (Figure 5a). We then analyzed the activation of PbZAT12 on the promoters of anthocyanin-related structural genes in leaves of Nicotiana benthamiana. Co-infiltration of PbZAT12 with PbDFR, PbANS, or PbUFGT in Nicotiana benthamiana leaves resulted in a significant increase in luciferase activities compared to the control group (Figure 5b), indicating that PbZAT12 is capable of both binding directly and activating proPbDFR, proPbANS, and proPbUFGT.
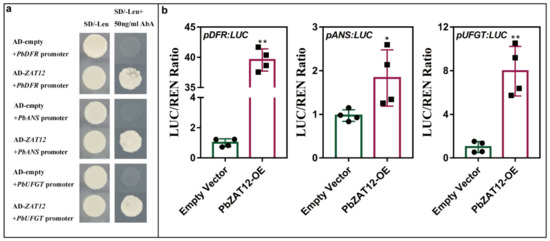
Figure 5.
Influence of PbZAT12 on transcriptional activity of anthocyanin biosynthetic structural genes. (a) The direct binding between PbZAT12 and structural genes in yeast one-hybrid assays. (b) The activation effect of PbZAT12 on proPbDFR, proPbANS, and proPbUFGT was detected using dual luciferase assay in N. benthamiana leaves. The ratio of luciferase activity of the structural gene (LUC) to that of 35S Renilla (REN) was normalized using the LUC/REN value obtained from the empty vector. The results are presented as the means of five biological replicates, and significant differences were determined by comparing with the control. Statistical analysis was performed using Student’s t-test, and significance was indicated as follows: * p < 0.05, ** p < 0.01.
4. Discussion
The accumulation of anthocyanins is regulated by light activation through the light signaling pathway [37,38,39,40]. The BBX subfamily of zinc finger proteins can respond to the light pathway, activate the expression of downstream genes, and thus promote the biosynthesis of anthocyanins [29,30]. As a member of zinc finger proteins, the ZAT gene family has been studied in Arabidopsis in previous studies [38]. In this study, the obtained PbZAT12 showed high similarity to its homologous genes (Figure 1b,c) [41]. ZAT12 has been reported to assist plants in stress responses across various species, including abiotic stresses such as salt stress and high light stress, as well as biotic stresses [42]. Although the involvement of ZAT12 in stress responses is often associated with anthocyanin accumulation, the specific mechanisms underlying this process are not yet well understood. Overexpression of AtZAT12 enhances anthocyanin accumulation and improves plant tolerance to biotic and abiotic stresses [31,32]. RHL41 (also known as ZAT12) can promote anthocyanin biosynthesis when overexpressed in Arabidopsis, which is related to its induction by high light, and has thick and dark green leaves [31,43]. In the comparative transcriptomic data of two tropical water lilies under cold stress, it was observed that the expression level of NlZAT12 is highly correlated with anthocyanin accumulation. However, the study did not describe the expression changes of regulatory genes involved in anthocyanin synthesis in NlZAT12-OE [44]. These findings are consistent with the results of our study, where overexpression of PbZAT12 promotes anthocyanin accumulation in pear peel (Figure 2a,b). However, previous studies did not specifically elucidate the molecular pathways involved in anthocyanin biosynthesis during this process.
Studies on anthocyanin biosynthesis focus on both the structural genes and regulatory genes, as well as the MYB (MYB10)-bHLH-WD40 (MBW) complex [23,45,46,47]. PyPIF5, a light-responsive gene, is involved in anthocyanin biosynthesis through the PymiR156a-PySPL9-PyMYB114/MYB10 module [48]. Even though transcription factor PbWRKY75 can directly regulate the structural genes involved in anthocyanin biosynthesis, they also have an impact on PbMYB10b (also named PbMYB114) [49]. Some external factors, such as H2S, can induce the persulfidation of PyMYB10 at Cys218, thereby affecting the biosynthesis of anthocyanins [19]. The pathway through which zinc finger proteins promote anthocyanin biosynthesis was primarily focused on MYB10 too. As a member of the zinc finger structure protein family, BBX proteins 1, 17, 15, 35, 51, and 54 were found to directly act as activators of the MYB10 promoter in apple [29]. In pear peel, PpBBX16 is a positive regulator of light-induced anthocyanin accumulation by activating MYB10 [50]. PpBBX18 was found to form a heterodimer with PpHY5 through two B-box domains, with PpHY5 binding to the G-box motif of PpMYB10 and PpBBX18 inducing its transcription, while PpZAT5 represses PpBBX18 expression and suppresses anthocyanin biosynthesis by binding to its CAAT motif [51,52]. Zinc finger transcription factors were found to activate the expression of MYB10, rather than directly activating the expression of genes involved in anthocyanin biosynthesis in previous studies. However, in this study, there was no significant difference in the expression levels of PbMYB10 and PbMYB114 genes between PbZAT12-OE and WT, indicating that PbZAT12 might not regulate anthocyanin biosynthesis in pear peel through PbMYB10 (Figure 3), which is different from other zinc finger proteins that regulate anthocyanin biosynthesis in pear or apple. No significant inhibition of PbMYB10 and PbMYB114 expression was observed in our experiments.
The coloring mechanism of ‘Red Zaosu’ pear, an oriental red pear, has been reported. The accumulation and coloration of anthocyanins in the red stripes of ‘Red Zaosu’ pear under light conditions are known to be influenced by various genes, such as PpPIF8 [53], PpHY5 [54], and PpBBX14 [55]. There are also some studies on the reasons for the formation of red and green stripes in ‘Red Zaosu’. For example, the demethylation of the PpMYB10 promoter region has been implicated in the development of red coloration and red stripes in ‘Red Zaosu’ pears [56]. Furthermore, the methylation level of the PbGA2ox8 promoter region affects the expression of PbMYB10, PbUFGT1, and PbGSTF12, revealing the reason for the red and green stripes in ‘Red Zaosu’ pear [57]. However, our findings demonstrate that overexpression of PbZAT12 in pear peel significantly increased the expression levels of PbDFR, PbANS, and PbUFGT (Figure 3). Our study confirms the correlation between PbZAT12 and anthocyanin biosynthesis through direct regulation of the structural genes involved in the pathway. But our study revealed that overexpression of PbZAT12 did not affect the expression of PbMYB10, which is different to transcriptomic data, indicating that the underlying mechanism of red and green stripes in ‘Red Zaosu’ pear is complex. This pathway provides another possibility for the formation of red and green stripes in ‘Red Zaosu’ pear (Figure 6).
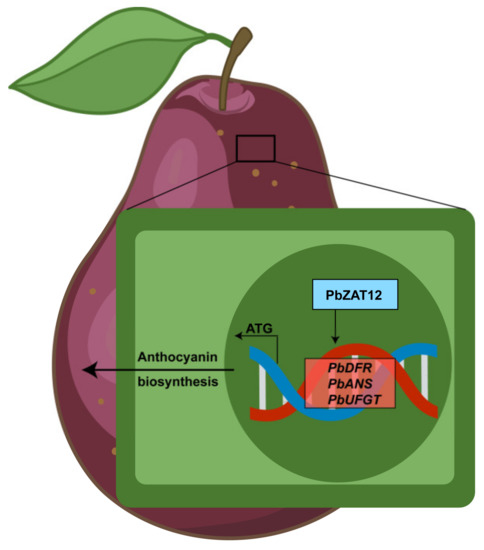
Figure 6.
Model of the molecular mechanism by which PbZAT12 promotes anthocyanin biosynthesis. PbZAT12 directly binds to the promoter regions of structural genes (PbDFR, PbANS, and PbUFGT) involved in anthocyanin biosynthesis, thereby enhancing their transcriptional activity and promoting anthocyanin biosynthesis in pear peel.
5. Conclusions
In this study, PbZAT12 was found to regulate anthocyanin accumulation by directly binding to and activating proPbDFR, proPbANS, and proPbUFGT. Overall, zinc-finger transcription factor PbZAT12 can enhance anthocyanin biosynthesis by increasing the expression levels of PbDFR, PbANS, and PbUFGT in pear.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9070775/s1, Table S1: List of primers of PbZAT12 and FPKM of PbZAT12 in the transcriptome. Table S2: Gene/protein name and accession number.
Author Contributions
L.X. (Lingfei Xu) and Z.W. designed the experiments. Y.L., H.C. and X.L. performed the experiments. R.Z., C.Y., L.X. (Lijuan Xiao) and Y.X. analyzed the data. Z.W., L.X. (Lingfei Xu), Z.Z. and H.C. wrote and revised this manuscript. Z.W. and H.C. participated in the research and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31972372).
Data Availability Statement
Data will be made available on request.
Acknowledgments
We would like to express our sincere gratitude to the Horticultural Scientific Research Center of the College of Horticulture at NWAFU for their valuable technical support throughout this study.
Conflicts of Interest
The authors confirm that there are no known conflicts of interest associated with this publication.
References
- Tanaka, Y.; Ohmiya, A. Seeing is believing: Engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Regan, B.C.; Julliot, C.; Simmen, B.; Vienot, F.; Charles-Dominique, P.; Mollon, J.D. Fruits, foliage and the evolution of primate colour vision. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 229–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Pilu, R.; Tonelli, C. Anthocyanins in corn: A wealth of genes for human health. Planta 2014, 240, 901–911. [Google Scholar] [CrossRef]
- Azuma, A.; Ito, A.; Moriguchi, T.; Yakushiji, H.; Kobayashi, S. Light Emitting Diode Irradiation at Night Accelerates Anthocyanin Accumulation in Grape Skin. Acta Hortic. 2012, 956, 341–347. [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low night temperature at veraison enhances the accumulation of anthocyanins in Corvina grapes (Vitis vinifera L.). Sci. Rep. 2018, 8, 8719. [Google Scholar] [CrossRef]
- Fang, H.; Dong, Y.; Yue, X.; Hu, J.; Jiang, S.; Xu, H.; Wang, Y.; Su, M.; Zhang, J.; Zhang, Z.; et al. The B-Box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019, 42, 2090–2104. [Google Scholar] [CrossRef]
- Gu, K.D.; Wang, C.K.; Hu, D.G.; Hao, Y.J. How do anthocyanins paint our horticultural products? Sci. Hortic. 2019, 249, 257–262. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Q.; Xia, L.; Hui, J.; Li, J.; Feng, Y.; Chen, Y. Preliminarily exploring of the association between sugars and anthocyanin accumulation in apricot fruit during ripening. Sci. Hortic. 2019, 248, 112–117. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffe, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef]
- Allan, A.C.; Hellens, R.P.; Laing, W.A. MYB transcription factors that colour our fruit. Trends Plant Sci. 2008, 13, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis–related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Shin, J.; Park, E.; Choi, G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 2007, 49, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I.; Tonelli, C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef]
- Li, Y.Y.; Mao, K.; Zhao, C.; Zhao, X.Y.; Zhang, H.L.; Shu, H.R.; Hao, Y.J. MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol. 2012, 160, 1011–1022. [Google Scholar] [CrossRef]
- Yao, G.; Ming, M.; Allan, A.C.; Gu, C.; Li, L.; Wu, X.; Wang, R.; Chang, Y.; Qi, K.; Zhang, S.; et al. Map-based cloning of the pear gene MYB114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis. Plant J. 2017, 92, 437–451. [Google Scholar] [CrossRef]
- Yao, G.; Gou, S.; Zhong, T.; Wei, S.; An, X.; Sun, H.; Sun, C.; Hu, K.; Zhang, H. Persulfidation of transcription factor MYB10 inhibits anthocyanin synthesis in red-skinned pear. Plant Physiol. 2023, 192, 2185–2202. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and Biochemistry of Anthocyanin Biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef]
- Broun, P. Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 2005, 8, 272–279. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, X.T.; Feng, W.T.; Chen, S.S.; Xu, L.F.; Wang, Z.G.; Zhang, J.L.; Li, P.M.; Ma, F.W. Different biosynthesis patterns among flavonoid 3-glycosides with distinct effects on accumulation of other flavonoid metabolites in pears (Pyrus bretschneideri Rehd.). PLoS ONE 2014, 9, e91945. [Google Scholar] [CrossRef]
- Zhai, R.; Wang, Z.; Zhang, S.; Meng, G.; Song, L.; Wang, Z.; Li, P.; Ma, F.; Xu, L. Two MYB transcription factors regulate flavonoid biosynthesis in pear fruit (Pyrus bretschneideri Rehd.). J. Exp. Bot. 2016, 67, 1275–1284. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ding, Y.; Cai, C.; Chen, Z.; Zhu, C. The role of C2H2 zinc finger proteins in plant responses to abiotic stresses. Physiol. Plant. 2019, 165, 690–700. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, S.; Li, J.; Pei, R.; Tian, L.; Qi, J.; Azam, M.; Agyenim-Boateng, K.G.; Shaibu, A.S.; Liu, Y.; et al. Dual-function C2H2-type zinc-finger transcription factor GmZFP7 contributes to isoflavone accumulation in soybean. New Phytol. 2023, 237, 1794–1809. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, B.J.; Henry-Kirk, R.; Friend, A.; Diack, R.; Helbig, S.; Mouhu, K.; Tomes, S.; Dare, A.P.; Espley, R.V.; Putterill, J.; et al. Apple B-box factors regulate light-responsive anthocyanin biosynthesis genes. Sci. Rep. 2019, 9, 17762. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Wang, X.F.; Espley, R.V.; Lin-Wang, K.; Bi, S.Q.; You, C.X.; Hao, Y.J. An apple B-Box protein MdBBX37 modulates anthocyanin biosynthesis and hypocotyl elongation synergistically with MdMYBs and MdHY5. Plant Cell Physiol. 2020, 61, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Kazuoka, T.; Torikai, S.; Kikuchi, H.; Oeda, K. A zinc finger protein RHL41 mediates the light acclimatization response in Arabidopsis. Plant J. 2000, 24, 191–203. [Google Scholar] [CrossRef]
- Davletova, S.; Schlauch, K.; Coutu, J.; Mittler, R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef]
- Wang, Z.; Du, H.; Zhai, R.; Song, L.; Ma, F.; Xu, L. Transcriptome Analysis Reveals Candidate Genes Related to Color Fading of ‘Red Bartlett’ (Pyrus communis L.). Front. Plant Sci. 2017, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, C.; Yan, D.; Wen, X.; Liu, Y.; Wang, H.; Dai, J.; Zhang, Y.; Liu, Y.; Zhou, B.; et al. MdHB1 down-regulation activates anthocyanin biosynthesis in the white-fleshed apple cultivar ‘Granny Smith’. J. Exp. Bot. 2017, 68, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, S.; Trainotti, L.; Casadoro, G. A simple protocol for transient gene expression in ripe fleshy fruit mediated by Agrobacterium. J. Exp. Bot. 2001, 52, 845–850. [Google Scholar] [CrossRef]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Xie, X.B.; Li, S.; Zhang, R.F.; Zhao, J.; Chen, Y.C.; Zhao, Q.; Yao, Y.X.; You, C.X.; Zhang, X.S.; Hao, Y.J. The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant Cell Environ. 2012, 35, 1884–1897. [Google Scholar] [CrossRef]
- Ahmed, N.U.; Park, J.I.; Jung, H.J. Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct. Integr. Genom. 2015, 15, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharvest Biol. Technol. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Xie, M.; Sun, J.; Gong, D.; Kong, Y. The Roles of Arabidopsis C1-2i Subclass of C2H2-type Zinc-Finger Transcription Factors. Genes 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Tsitsekian, D.; Daras, G.; Templalexis, D.; Avgeri, F.; Lotos, L.; Orfanidou, C.G.; Ntoukakis, V.; Maliogka, V.I.; Rigas, S. A subset of highly responsive transcription factors upon tomato infection by pepino mosaic virus. Plant Biol. 2023, 25, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, G.; Wei, Y.; Chan, Z. The zinc-finger transcription factor ZAT6 is essential for hydrogen peroxide induction of anthocyanin synthesis in Arabidopsis. Plant Mol. Biol. 2018, 97, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jin, Q.; Wang, Y.; Wang, X.; Wang, X.; Yang, M.; Ye, C.; Yang, Z.; Xu, Y. Comparative transcriptome analysis reveals the regulatory mechanisms of two tropical water lilies in response to cold stress. BMC Genom. 2023, 24, 82. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Hu, G.B.; Hu, F.C.; Wang, H.C.; Yang, Z.Y.; Lai, B. The UDP glucose: Flavonoid-3-O-glucosyltransferase (UFGT) gene regulates anthocyanin biosynthesis in litchi (Litchi chinesis Sonn.) during fruit coloration. Mol. Biol. Rep. 2012, 39, 6409–6415. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Yao, G.F.; Zheng, D.M.; Zhang, S.L.; Wang, C.; Zhang, M.Y.; Wu, J. Expression differences of anthocyanin biosynthesis genes reveal regulation patterns for red pear coloration. Plant Cell Rep. 2015, 34, 189–198. [Google Scholar] [CrossRef]
- González, M.; Salazar, E.; Cabrera, S.; Olea, P.; Carrasco, B. Analysis of anthocyanin biosynthesis genes expression profiles in contrasting cultivars of Japanese plum (Prunus salicina L.) during fruit development. Gene Expr. Patterns 2016, 21, 54–62. [Google Scholar] [CrossRef]
- Liu, H.; Shu, Q.; Lin-Wang, K.; Allan, A.C.; Espley, R.V.; Su, J.; Pei, M.; Wu, J. The PyPIF5-PymiR156a-PySPL9-PyMYB114/MYB10 module regulates light-induced anthocyanin biosynthesis in red pear. Mol. Hortic. 2021, 1, 14. [Google Scholar] [CrossRef]
- Cong, L.; Qu, Y.; Sha, G.; Zhang, S.; Ma, Y.; Chen, M.; Zhai, R.; Yang, C.; Xu, L.; Wang, Z. PbWRKY75 promotes anthocyanin synthesis by activating PbDFR, PbUFGT, and PbMYB10b in pear. Physiol. Plant. 2021, 173, 1841–1849. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating, MYB10, in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, R.; Wang, S.; Gao, Y.; Wang, L.; Yang, S.; Zhang, X.; Yu, W.; Wu, X.; Li, K.; et al. PpZAT5 suppresses the expression of a B-box gene PpBBX18 to inhibit anthocyanin biosynthesis in the fruit peel of red pear. Front. Plant Sci. 2022, 13, 1022034. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Tao, R.; Yin, L.; Ni, J.; Yang, Q.; Yan, X.; Yang, F.; Guo, X.; Li, H.; Teng, Y. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019, 100, 1208–1223. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wei, C.; Cheng, Y.; Shang, Z.; Guo, X.; Guan, J. RNA-Seq Analysis Identifies Transcription Factors Involved in Anthocyanin Biosynthesis of ‘Red Zaosu’ Pear Peel and Functional Study of PpPIF8. Int. J. Mol. Sci. 2022, 23, 4798. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Bai, S.; Ni, J.; Yang, Q.; Zhao, Y.; Teng, Y. The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’pear. Planta 2018, 248, 37–48. [Google Scholar] [CrossRef]
- Ou, C.; Zhang, X.; Wang, F.; Zhang, L.; Zhang, Y.; Fang, M.; Wang, J.; Wang, J.; Jiang, S.; Zhang, Z. A 14 nucleotide deletion mutation in the coding region of the PpBBX24 gene is associated with the red skin of “Zaosu Red” pear (Pyrus pyrifolia White Pear Group): A deletion in the PpBBX24 gene is associated with the red skin of pear. Hortic. Res. 2020, 7, 39. [Google Scholar] [CrossRef]
- Qian, M.; Sun, Y.; Allan, A.C.; Teng, Y.; Zhang, D. The red sport of ‘Zaosu’pear and its red-striped pigmentation pattern are associated with demethylation of the PyMYB10 promoter. Phytochemistry 2014, 107, 16–23. [Google Scholar] [CrossRef]
- Zhai, R.; Wang, Z.; Yang, C.; Lin-Wang, K.; Espley, R.; Liu, J.; Li, X.; Wu, Z.; Li, P.; Guan, Q.; et al. PbGA2ox8 induces vascular-related anthocyanin accumulation and contributes to red stripe formation on pear fruit. Hortic. Res. 2019, 6, 137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).