The Impact of High Temperatures in the Field on Leaf Tissue Structure in Different Grape Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Grapevine Resources and Cultivation Conditions
2.2. Temperature Measurement Tested
2.3. Leaf Blade Morphology Observation
2.4. Sampling for Leaf Structure Observation
2.5. Data Analysis
3. Results
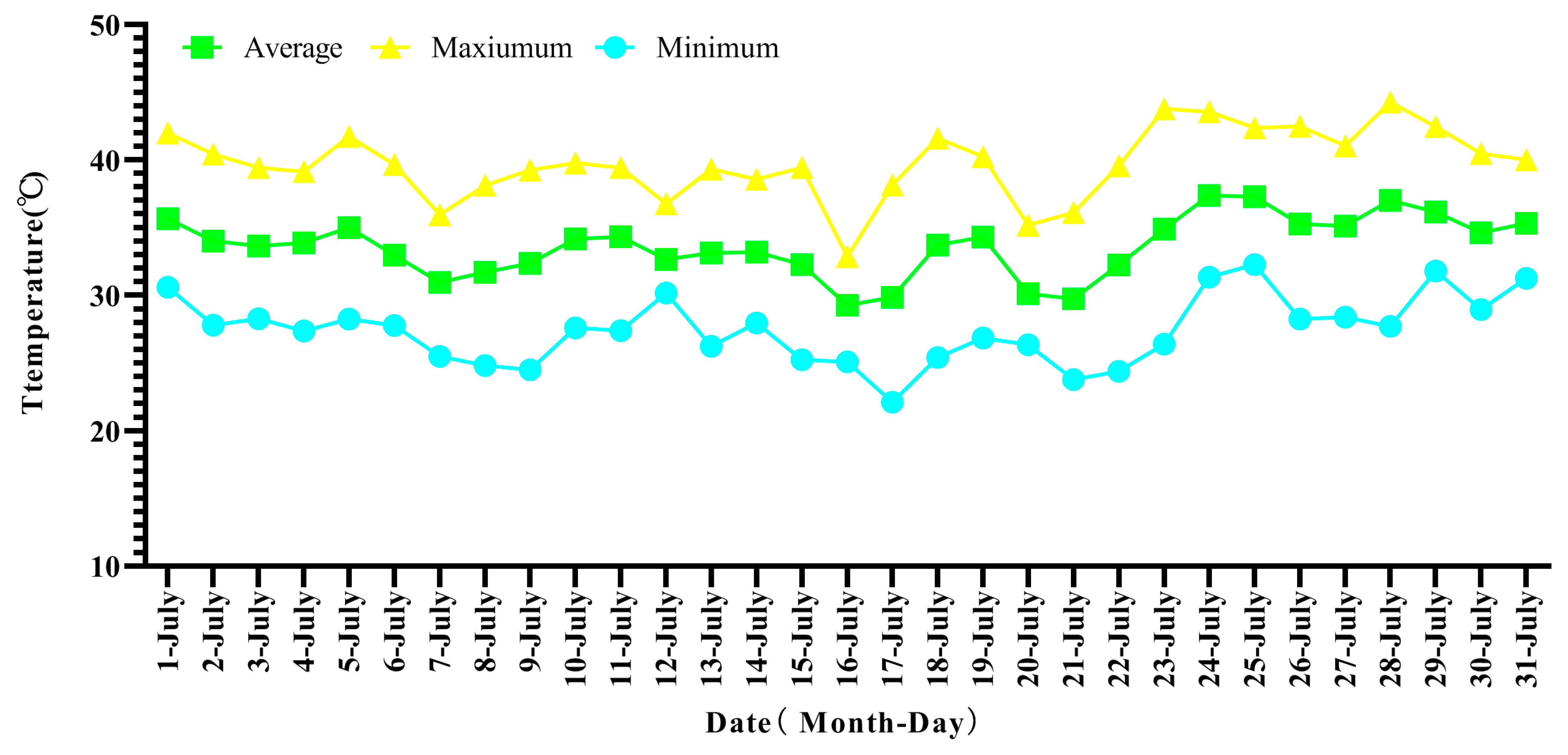
3.1. Temperature Dynamics in the Field
3.2. Phenotypic Observation of Leaves
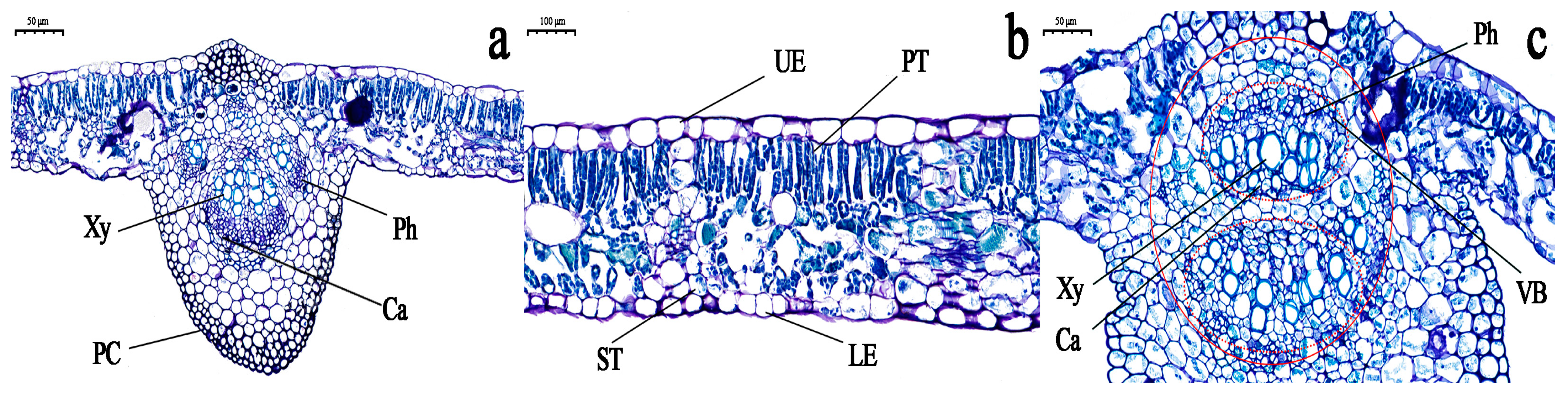
3.3. Observation of the Cell Structure of Grapevine Leaves
3.4. Leaf Thickness and Epidermal Cells
3.5. Palisade Tissue, Spongy Tissue and Palisade Tissue/Spongy Tissue
3.6. Stomata, Guard Cells and Cuticle
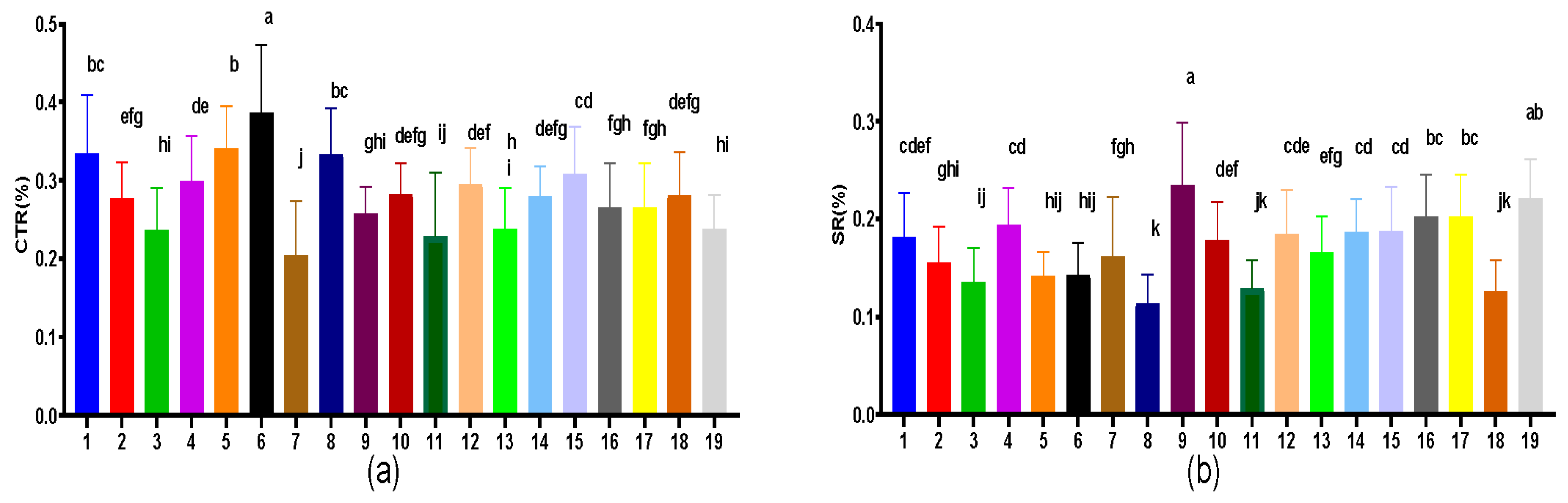
3.7. Leaf Vein, CTR and SR
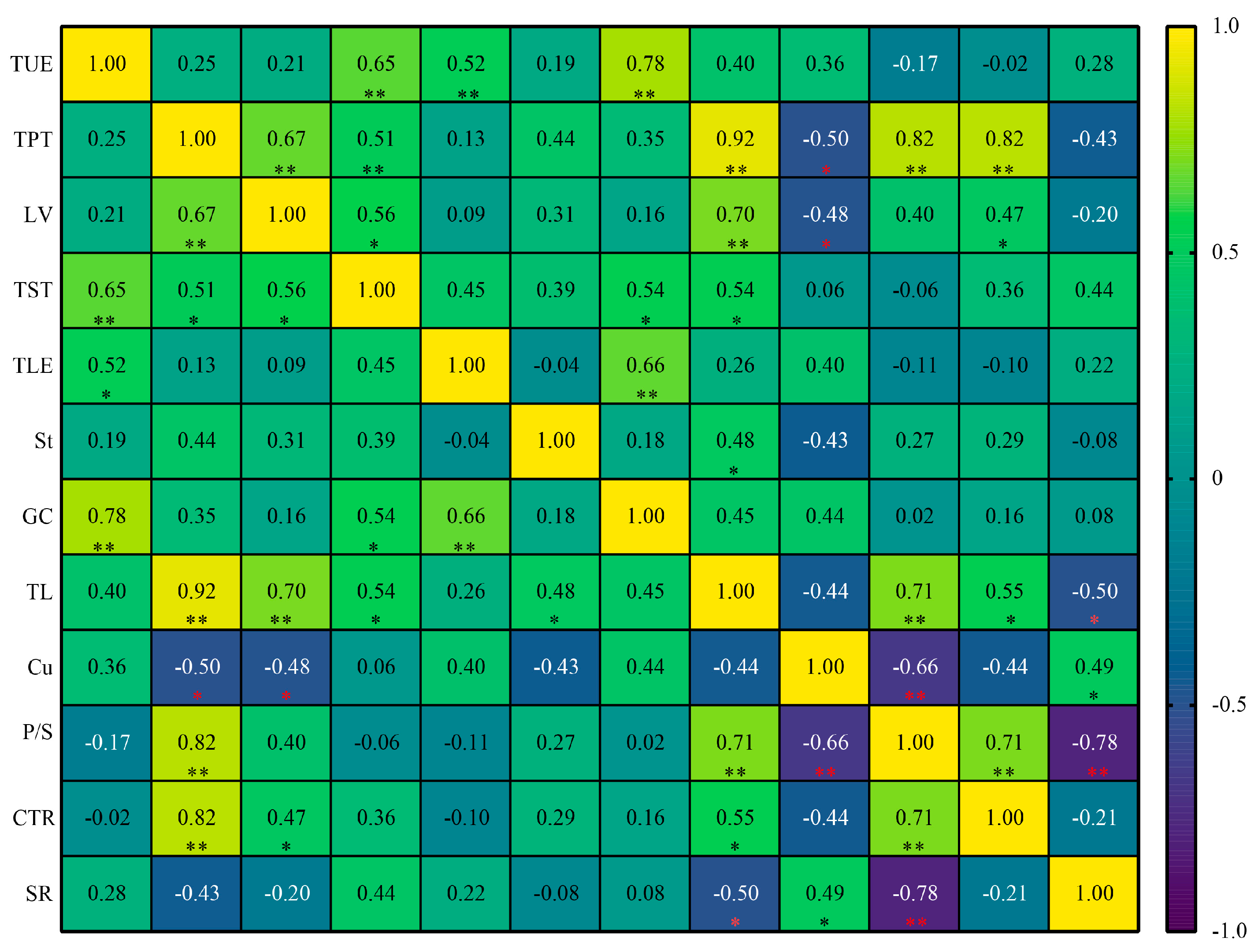
3.8. Correlation Analysis
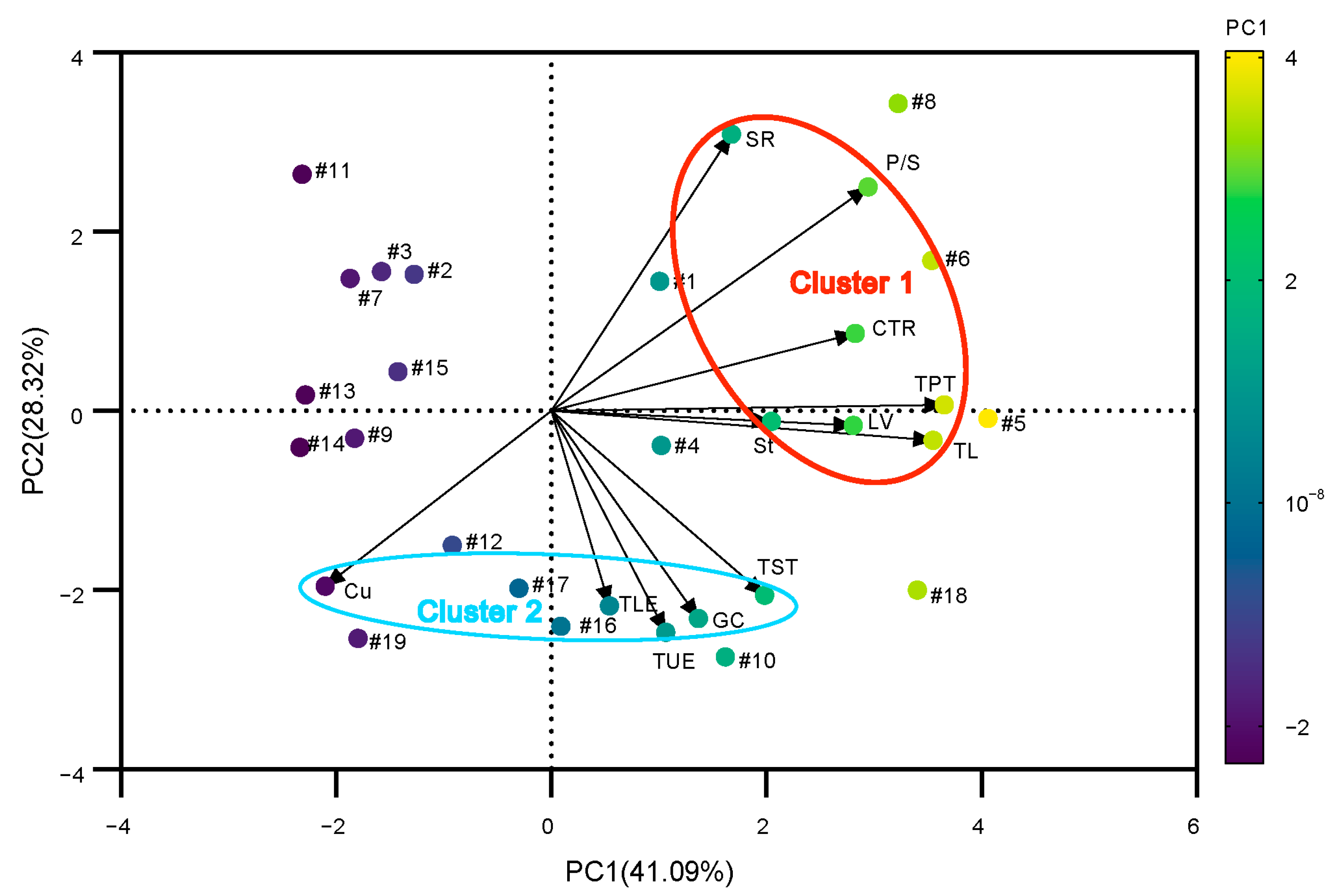
3.9. Principal Component Analysis and Heat Tolerance Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; Jeon, B.W.; Kim, J. Signaling Peptides Regulating Abiotic Stress Responses in Plants. Front. Plant Sci. 2021, 12, 704490. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyay, A.K. Global transcriptome analysis of heat stress response of grape variety ‘Fantasy Seedless’ under different irrigation regimens. Vitis 2021, 143–151. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.J.; He, Y.N.; Jiang, A.L. Comprehensive evaluation of heat resistance in 68 Vitis germplasm resources. Vitis 2018, 57, 75–81. [Google Scholar]
- Liu, M.; Fang, Y.L. Effects of heat stress on physiological indexes and ultrastructure of grapevines. Sci. Agric. Sin. 2020, 53, 1444–1458. [Google Scholar]
- Coneva, V.; Frank, M.H.; Balaguer, M.A.D.L.; Li, M.; Sozzani, R.; Chitwood, D.H. Genetic Architecture and Molecular Networks Underlying Leaf Thickness in Desert-Adapted Tomato Solanum pennellii. Plant Physiol. 2017, 175, 376–391. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. Chapter 4—The Ecological Water-Use Strategies of Succulent Plants. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2010; Volume 55, pp. 179–225. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Smith, S.D.; Monson, R.K.; Anderson, J.E. Physiological Ecology of North American Desert Plants; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar] [CrossRef]
- Szenteleki, K.; Ladanyi, M.; Gaal, M.; Zanathy, G.; Bisztray, G. Climatic risk factors of central hungarian grape growing regions. Appl. Ecol. Environ. Res. 2012, 10, 87–105. [Google Scholar] [CrossRef]
- Asproudi, A.; Petrozziello, M.; Cavalletto, S.; Guidoni, S. Grape aroma precursors in cv. Nebbiolo as affected by vine microclimate. Food Chem. 2016, 211, 947–956. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.J.; He, Y.N.; Jiang, A.L. Research on high temperature stress response of different table grape cultivars. Acta Agric. Shanghai 2018, 34, 77–83. [Google Scholar] [CrossRef]
- Xu, H.; Song, B.; Gu, Z.F.; Bi, Y.F.; Wei, B. Advances in heat tolerance mechanisms of plants. Jiangsu J. Agr. Sci. 2020, 36, 43–50. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef]
- Champa, W.H.; Gill, M.; Mahajan, B.; Aror, N.; Bedi, S. Brassinosteroids improve quality of table grapes (Vitis vinifera L.) cv. flame seedless. Trop. Agric. Res. 2015, 26, 368. [Google Scholar] [CrossRef]
- Divi, U.K.; Rahman, T.; Krishna, P. Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol. J. 2015, 14, 419–432. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.J.; He, Y.N.; Jiang, A.L. High temperature in field: Effect on the leaf tissue structure of grape varieties. Chin. Agric. Sci. Bull. 2019, 35, 74–77. [Google Scholar] [CrossRef]
- Qiu, Y.M.; Wang, W.; Hu, X.; Sun, M.M.; Ni, J.Z. Realationship between leaf anatomical structure and heat resistance of Rhododendron simsii. For. Enviromental Sci. 2021, 37, 69–81. [Google Scholar]
- Bueno, A.; Alfarhan, A.; Arand, K.; Burghardt, M.; Deininger, A.-C.; Hedrich, R.; Leide, J.; Seufert, P.; Staiger, S.; Riederer, M. Effects of temperature on the cuticular transpiration barrier of two desert plants with water-spender and water-saver strategies. J. Exp. Bot. 2019, 70, 1613–1625. [Google Scholar] [CrossRef]
- Ding, X.; Zhong, H.X.; Wang, X.P.; Song, J.Y.; Wu, J.Y.; Liu, G.H.; Zhang, F.C.; Hu, X.; Pan, M.Q.; Wu, X.Y. Observation on leaf anatomical structure and evaluation of drought resistance of grape rootstocks in Xinjiang. Mol. Plant Breed. 2022, 1–18. Available online: https://kns.cnki.net/kcms/detail/46.1068.S.20220429.1131.010.htm (accessed on 16 September 2022).
- Zha, Q.; Xi, X.; He, Y.; Yin, X.; Jiang, A. Effect of Short-Time High-Temperature Treatment on the Photosynthetic Performance of Different Heat-Tolerant Grapevine Cultivars. Photochem. Photobiol. 2021, 97, 763–769. [Google Scholar] [CrossRef]
- Cramer, G. Abiotic stress and plant responses from the whole vine to the genes. Aust. J. Grape Wine Res. 2010, 16, 86–93. [Google Scholar] [CrossRef]
- Jiao, S.-Z.; Guo, C.; Yao, W.-K.; Zhang, N.-B.; Zhang, J.-Y.; Xu, W.-R. An Amur grape VaHsfC1 is involved in multiple abiotic stresses. Sci. Hortic. 2021, 295, 110785. [Google Scholar] [CrossRef]
- Yan, T.T. Evaluation of heat tolerance in different grape cultivars and proteomic analysis of heat tolerance difference between cultivars. Master’s Thesis, South China Agricultural University, Guangzhou, China, 16 July 2020. [Google Scholar]
- Wu, J.Y.; Lian, W.J.; Zeng, X.Y.; Liu, Z.G.; Mao, L.; Liu, Y.X.; Jiang, J.F. Physiological response to high temperature and heat tolerance evaluation of different grape cultivars. Acta Bot. Boreali-Occident. Sin. 2019, 39, 1075–1084. Available online: https://www.proquest.com/docview/2653586083?pq-origsite=gscholar (accessed on 15 June 2023).
- Wu, J.Y.; Lian, W.J.; Liu, Z.G.; Zeng, X.Y.; Jiang, J.F.; Wei, Y.N. High temperature response of chlorophyll fluorescence parameter and heat tolerance evaluation of different grape cultivars. J. Northwest AF Univ. (Nat. Sci. Ed.) 2019, 47, 80–88. [Google Scholar]
- Li, M.H.; Cherubini, P.; Dobbertin, M.; Arend, M.; Xiao, W.F.; Rigling, A. Responses of leaf nitrogen and mobile carbohydrates in different Quercus species/provenances to moderate climate changes. Plant Biol. 2013, 15, 177–184. [Google Scholar] [CrossRef]
- Yang, J.; Chong, P.; Chen, G.; Xian, J.; Liu, Y.; Yue, Y. Shifting plant leaf anatomical strategic spectra of 286 plants in the eastern Qinghai-Tibet Plateau: Changing gears along 1050–3070 m. Ecol. Indic. 2023, 146, 109741. [Google Scholar] [CrossRef]
- Jin, J.; Yang, L.; Fan, D.; Liu, X.; Hao, Q. Comparative transcriptome analysis uncovers different heat stress responses in heat-resistant and heat-sensitive jujube cultivars. PLoS ONE 2020, 15, e0235763. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Jiang, J.F.; Zhang, Y.; Tian, Z.S.; Fan, X.C.; Liu, C.H. Observation on microstructure and ultrastucture of leaves in Vitis L. Acta Bot. Boreali-Occident. Sin. 2012, 32, 1365–1371. [Google Scholar]
- Fu, L.J.; Li, C.X.; Su, S.Y.; Li, Y.H.; Zhou, Y. Screening of cucumber germplasms in seedling stage and the construction of evaluation system for heat tolerance. Plant Physiol. J. 2020, 56, 1593–1604. [Google Scholar] [CrossRef]
- Li, M.; Su, H.; Li, Y.Y.; Li, J.P.; Li, J.C.; Zhu, Y.L.; Song, Y.H. Analysis of heat tolerance of wheat with different genotypes and screening of identification indexes in Huang-huai-hai region. Sci. Agric. Sin. 2021, 54, 3381–3393. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S.H.; Li, Y.; Zhang, X.F.; Wang, G.P. Leaf morphology, anatomical structure and drought resistance evaluation of 238 Chinese chestnut cultivars (lines). Acta Hortic. Sin. 2020, 47, 1033–1046. [Google Scholar] [CrossRef]
- Huang, L.; Sun, Y.Q.; Hao, L.H.; Dang, C.H.; Zhu, Y.; Wang, H.X.; Cheng, D.J.; Zhang, Y.X.; Zheng, Y.P. Effects of high temperatures on leaf structures and physiological metabolism of north highbush blueberry. Acta Hortic. Sin. 2016, 43, 1044–1056. [Google Scholar] [CrossRef]
- Ma, W.; Liang, W.; Zhao, B. Effect of Relative Air Humidity and High Temperature on the Physiological and Anatomical Responses of Two Rhododendron Cultivars. Hortscience 2019, 54, 1115–1123. [Google Scholar] [CrossRef]
- Tian, M.; Yu, G.; He, N.; Hou, J. Leaf morphological and anatomical traits from tropical to temperate coniferous forests: Mechanisms and influencing factors. Sci. Rep. 2016, 6, 19703. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, G.; Kim, S.; Thi, T.N.; Kim, H.; Jeong, J.; Kim, J.; Kim, J.; Choi, G.; Oh, E. The epidermis coordinates thermoresponsive growth through the phyB-PIF4-auxin pathway. Nat. Commun. 2020, 11, 1053. [Google Scholar] [CrossRef]
- Xie, Z.S.; Guo, X.J.; Cao, H.M. Effect of root restriction on vegetative growth and leaf anatomy of Kyoho grapevines cultivar. Afr. J. Agric. Res. 2013, 8, 1304–1309. [Google Scholar] [CrossRef]
- Wu, J.; Xu, G.; Lian, W.; Chen, Y.; Li, H.; Liu, Y.; Jiang, J.; Wen, J. Physiological response to high temperature and evaluation of heat tolerance of different grape cultivars. Agric. Biotechnol. 2022, 11, 47–54+85. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.; Fan, X.; Zhang, Y.; Wu, J.; Wang, L.; Liu, C. Identification of Heat Tolerance in Chinese Wildgrape Germplasm Resources. Horticulturae 2020, 6, 68. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in Plant Tolerance to Abiotic Stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.; Jiang, A.; Tian, Y. High Temperature Affects Photosynthetic and Molecular Processes in Field-Cultivated Vitis vinifera L. × Vitis labrusca L. Photochem. Photobiol. 2016, 92, 446–454. [Google Scholar] [CrossRef]

| No. | Cultivar | Species | Parental Origin (Female/Male) |
|---|---|---|---|
| 1 | Golden Finger | V. vinifera × V. labrusca | Manicure Finger × Seneca |
| 2 | Zhengyan Wuhe | V. vinifera | Jingxiu × Bronx Seedless |
| 3 | Flame Seedless | V. vinifera | Unknown |
| 4 | Jumeigui | V. vinifera × V. labrusca | Shenyang Meigui × Kyoho |
| 5 | Kyoho | V. vinifera × V. labrusca | Ishiharawase × Centennial |
| 6 | Cardinal | V. vinifera | Flame Tokay × Ribier |
| 7 | Bixiang Wuhe | V. vinifera | Zhengzhou Zaoyu × Pearlof Csaba |
| 8 | Qingfeng | V. vinifera × V. labrusca | Jingxiu × Bronx Seedless |
| 9 | Jintian Meigui | V. vinifera | Muscat Hamburg × Red Globe |
| 10 | Centennial Seedless | V. vinifera | Gold × Q25-6 |
| 11 | Thompson Seedless | V. vinifera | Unknown |
| 12 | Summer Black | V. vinifera × V. labrusca | Kyoho × Thompson Seedless |
| 13 | Xinyu | V. vinifera | Red Globe × Rizamat |
| 14 | Shine Muscat | V. vinifera × V. labrusca | Akitsu21 × Hakunan |
| 15 | Zhengmei | V. vinifera | Manicure Finger × Zhengzhou Zaohong |
| 16 | Zitian Wuhe | V. vinifera | Niunai × Autumroyal |
| 17 | Zuijinxiang | V. vinifera × V. labrusca | 7601 × Kyoho |
| 18 | Zaoxia Wuhe | V. vinifera × V. labrusca | Summer Black Mutation |
| 19 | Brilliant Seedless | V. vinifera | Red Globe × Centennial Seedless |
| No. | TUE (μm) | TPT (μm) | LV (μm) | TST (μm) | TLE (μm) | St (μm) | GC (μm) | Cu (μm) | TL (μm) | P/S |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14.24 ± 2.28 gh | 36.8 ± 7.64 cd | 18.01 ± 3.62 e | 20.12 ± 4.69 de | 10.19 ± 3.18 h | 7.34 ± 3.25 a | 6.66 ± 2.46 defgh | 2.89 ± 1.51 g | 111.33 ± 7.69 e | 1.91 ± 0.53 d |
| 2 | 13.4 ± 3.32 hi | 25.45 ± 2.84 f | 18.4 ± 3.49 e | 14.35 ± 3.33 f | 16.96 ± 3.4 bcde | 4.31 ± 1.74 fgh | 6.06 ± 2.41 fgh | 3.55 ± 1.03 defg | 93.34 ± 9.78 hi | 1.12 ± 0.31 h |
| 3 | 9.88 ± 3.32 j | 24.07 ± 4.01 fg | 14.14 ± 3.27 fg | 14.03 ± 4.07 f | 17.91 ± 3.48 bcd | 4.08 ± 2.06 ghi | 6.79 ± 3.19 defgh | 3.47 ± 1.23 defg | 103.77 ± 14.34 f | 1.84 ± 0.53 de |
| 4 | 17.12 ± 3.5 fg | 33.94 ± 6.14 d | 34.52 ± 7.92 a | 22.09 ± 4.25 bcd | 18.86 ± 4.81 bc | 5.57 ± 1.85 bcde | 6.68 ± 1.94 defgh | 3.15 ± 1.15 efg | 114.44 ± 10.5 e | 1.58 ± 0.37 defg |
| 5 | 30.39 ± 37.78 cd | 54.63 ± 8.27 a | 31.72 ± 5.81 b | 22.7 ± 3.82 abc | 13.45 ± 3.22 fg | 5.47 ± 3.24 cdef | 7.86 ± 2.8 bcd | 2.94 ± 1.14 g | 161.29 ± 11.03 b | 2.47 ± 0.56 c |
| 6 | 11.32 ± 3.52 hij | 57.66 ± 12 a | 26.94 ± 5.77 c | 21.07 ± 4.1 bcd | 17.86 ± 3.37 bcd | 4.53 ± 2.35 efgh | 6.47 ± 2.98 defgh | 2.87 ± 1.26 g | 149.98 ± 13.12 c | 2.85 ± 0.95 b |
| 7 | 16.37 ± 6.65 fg | 19.36 ± 3.94 h | 23.04 ± 4.22 d | 15.29 ± 3.85 f | 13.6 ± 3.34 fg | 4.8 ± 2.5 defgh | 5.5 ± 2.84 h | 3.05 ± 1.02 fg | 101.03 ± 21.9 fg | 1.35 ± 0.47 gh |
| 8 | 12.33 ± 3.74 hij | 47.92 ± 7.94 b | 23.26 ± 5.78 d | 16.26 ± 4.12 f | 16.3 ± 3.72 cde | 5.98 ± 2.12 bc | 5.66 ± 2.32 gh | 1.34 ± 1.34 h | 144.93 ± 13.14 cd | 3.14 ± 0.91 a |
| 9 | 22.08 ± 5.28 d | 23.6 ± 2.18 fg | 15.11 ± 2.86 f | 21.38 ± 5.43 bcd | 15.68 ± 5.04 def | 4.89 ± 1.6 cdefgh | 5.37 ± 1.82 h | 3.02 ± 1.79 fg | 92.89 ± 12.84 hi | 1.17 ± 0.3 h |
| 10 | 30.19 ± 9.08 a | 39.13 ± 5.11 c | 23.66 ± 5.52 d | 24.65 ± 5.03 a | 21.96 ± 5.61 a | 5.41 ± 1.5 cdef | 8.9 ± 2.22 ab | 3.96 ± 1.33 cd | 139.34 ± 11.73 d | 1.65 ± 0.38 defg |
| 11 | 10.55 ± 4.39 ij | 20.87 ± 7.18 gh | 12.08 ± 4.96 gh | 11.77 ± 2.38 g | 12.52 ± 3.49 g | 4.71 ± 1.38 defgh | 5.72 ± 2.08 gh | 3.8 ± 1.15 de | 92.2 ± 9.18 hi | 1.85 ± 0.84 de |
| 12 | 25.6 ± 7.27 bc | 29.47 ± 4.13 e | 18.92 ± 4.47 e | 18.41 ± 4.15 e | 19.22 ± 5.34 b | 3.14 ± 1.25 i | 8.39 ± 2.71 abc | 4.67 ± 0.99 bc | 100.61 ± 6.98 fg | 1.67 ± 0.4 def |
| 13 | 21.1 ± 5.69 de | 22.51 ± 4.73 fgh | 11.37 ± 2.62 hi | 15.51 ± 2.94 f | 16.44 ± 3.77 cde | 4.57 ± 1.32 efgh | 6.27 ± 1.88 efgh | 4.6 ± 1.37 bc | 95.54 ± 11.63 gh | 1.5 ± 0.39 fg |
| 14 | 19.04 ± 4.69 ef | 24.43 ± 2.39 f | 9.69 ± 2.15 i | 16.33 ± 2.91 f | 17.66 ± 4.39 bcd | 3.95 ± 0.82 hi | 7.1 ± 3.01 cdefg | 4.96 ± 1.11 ab | 87.87 ± 7.48 ij | 1.54 ± 0.31 efg |
| 15 | 17.37 ± 5.42 f | 25.45 ± 2.58 f | 12.39 ± 2.98 gh | 15.39 ± 2.3 f | 14.8 ± 3.49 efg | 5.22 ± 2.03 cdefg | 7.36 ± 2.24 cdef | 4.03 ± 1.42 cd | 85 ± 14.73 j | 1.68 ± 0.25 def |
| 16 | 26.84 ± 8.01 b | 34.58 ± 5.02 d | 15.47 ± 2.81 f | 20.57 ± 3.79 cd | 24.31 ± 8.19 a | 5.8 ± 1.39 bcd | 8.39 ± 2.47 abc | 4.15 ± 1.37 cd | 112.96 ± 17.16 e | 1.74 ± 0.42 def |
| 17 | 21.92 ± 6.62 de | 29.32 ± 4.48 e | 20.2 ± 3.75 e | 22.15 ± 3.2 bcd | 22.38 ± 4.65 a | 5.64 ± 1.55 bcde | 7.62 ± 1.68 bcde | 4.15 ± 1.37 cd | 112.96 ± 17.16 e | 1.35 ± 0.26 gh |
| 18 | 32.57 ± 5.46 a | 47.05 ± 8.92 b | 23 ± 3.59 d | 21.08 ± 4.9 bcd | 22.77 ± 4.34 a | 6.64 ± 1.35 ab | 9.51 ± 2.12 a | 3.74 ± 1.57 def | 168.26 ± 13.56 a | 2.35 ± 0.75 c |
| 19 | 22.54 ± 5.44 d | 24.71 ± 3.94 f | 18.18 ± 2.79 e | 22.93 ± 4.46 ab | 19.46 ± 4.37 b | 4.65 ± 1.63 defgh | 7.42 ± 2 cdef | 5.5 ± 1.1 a | 104.71 ± 7.92 f | 1.89 ± 0.58 d |
| No. | Cultivars | F1 | Rank1 | F2 | Rank2 | F | Rank | Heat Tolerance |
|---|---|---|---|---|---|---|---|---|
| 1 | Golden Finger | 0.43701 | 7 | −0.76282 | 13 | −0.052 | 11 | Medium |
| 2 | Zhengyan Wuhe | −0.56488 | 11 | −0.82448 | 15 | −0.6747 | 16 | Weak |
| 3 | Flame Seedless | −0.6953 | 13 | −0.8409 | 16 | −0.7592 | 17 | Weak |
| 4 | Jumeigui | 0.46852 | 6 | 0.19565 | 8 | 0.3595 | 6 | Medium |
| 5 | Kyoho | 1.81753 | 1 | 0.07831 | 10 | 1.1159 | 2 | Strong |
| 6 | Cardinal | 1.61936 | 2 | −0.89711 | 17 | 0.5981 | 4 | Medium |
| 7 | Bixiang Wuhe | −0.8295 | 15 | −0.8212 | 14 | −0.8311 | 18 | Weak |
| 8 | Qingfeng | 1.47415 | 4 | −1.85897 | 19 | 0.1174 | 8 | Medium |
| 9 | Jintian Meigui | −0.82192 | 14 | 0.13828 | 9 | −0.4335 | 12 | Weak |
| 10 | Centennial Seedless | 0.69555 | 5 | 1.51558 | 1 | 1.0358 | 3 | Strong |
| 11 | Thompson Seedless | −1.01656 | 17 | −1.45255 | 18 | −1.2014 | 19 | Weak |
| 12 | Summer Black | −0.42692 | 10 | 0.78397 | 6 | 0.0666 | 9 | Medium |
| 13 | Xinyu | −1.03539 | 18 | −0.08435 | 11 | −0.652 | 15 | Weak |
| 14 | Shine Muscat | −1.05973 | 19 | 0.22746 | 7 | −0.5387 | 14 | Weak |
| 15 | Zhengmei | −0.65071 | 12 | −0.23027 | 12 | −0.4824 | 13 | Weak |
| 16 | Zitian Wuhe | 0.03903 | 8 | 1.29195 | 3 | 0.5526 | 5 | Medium |
| 17 | Zuijinxiang | −0.13273 | 9 | 1.05875 | 5 | 0.3547 | 7 | Medium |
| 18 | Zaoxia Wuhe | 1.51481 | 3 | 1.12429 | 4 | 1.364 | 1 | Strong |
| 19 | Brilliant Seedless | −0.83232 | 16 | 1.3584 | 2 | 0.0603 | 10 | Medium |
| Cluster Number | Error Function | Optimal Segmentation Results |
|---|---|---|
| 2 | 0.4129 | 1–11, 12–19 |
| 3 | 0.1355 | 1–3, 4–11, 12–19 |
| 4 | 0.0823 | 1–3, 4–7, 8–11, 12–19 |
| 5 | 0.0381 | 1–3, 4–7, 8–11, 12–18, 19 |
| 6 | 0.0225 | 1–3, 4–7, 8–11, 12–14, 15–18, 19 |
| 7 | 0.0141 | 1, 2–3, 4–7, 8–11, 12–14, 15–18, 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Abudureheman, R.; Zhong, H.; Yadav, V.; Zhang, C.; Ma, Y.; Liu, X.; Zhang, F.; Zha, Q.; Wang, X. The Impact of High Temperatures in the Field on Leaf Tissue Structure in Different Grape Cultivars. Horticulturae 2023, 9, 731. https://doi.org/10.3390/horticulturae9070731
Wu J, Abudureheman R, Zhong H, Yadav V, Zhang C, Ma Y, Liu X, Zhang F, Zha Q, Wang X. The Impact of High Temperatures in the Field on Leaf Tissue Structure in Different Grape Cultivars. Horticulturae. 2023; 9(7):731. https://doi.org/10.3390/horticulturae9070731
Chicago/Turabian StyleWu, Jiuyun, Riziwangguli Abudureheman, Haixia Zhong, Vivek Yadav, Chuan Zhang, Yaning Ma, Xueyan Liu, Fuchun Zhang, Qian Zha, and Xiping Wang. 2023. "The Impact of High Temperatures in the Field on Leaf Tissue Structure in Different Grape Cultivars" Horticulturae 9, no. 7: 731. https://doi.org/10.3390/horticulturae9070731
APA StyleWu, J., Abudureheman, R., Zhong, H., Yadav, V., Zhang, C., Ma, Y., Liu, X., Zhang, F., Zha, Q., & Wang, X. (2023). The Impact of High Temperatures in the Field on Leaf Tissue Structure in Different Grape Cultivars. Horticulturae, 9(7), 731. https://doi.org/10.3390/horticulturae9070731








