Selection of a Proper Maturity Index for the Mechanical Harvesting of ‘Mihong’ Peach Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Measurement of Physicochemical Properties
2.2.1. Fruit Size
2.2.2. Color Measurements
2.2.3. Firmness
2.2.4. Soluble Solids Content
2.2.5. Titratable Acidity
2.3. Determination of Ethylene Production
2.4. Statistical Analysis
3. Results and Discussion
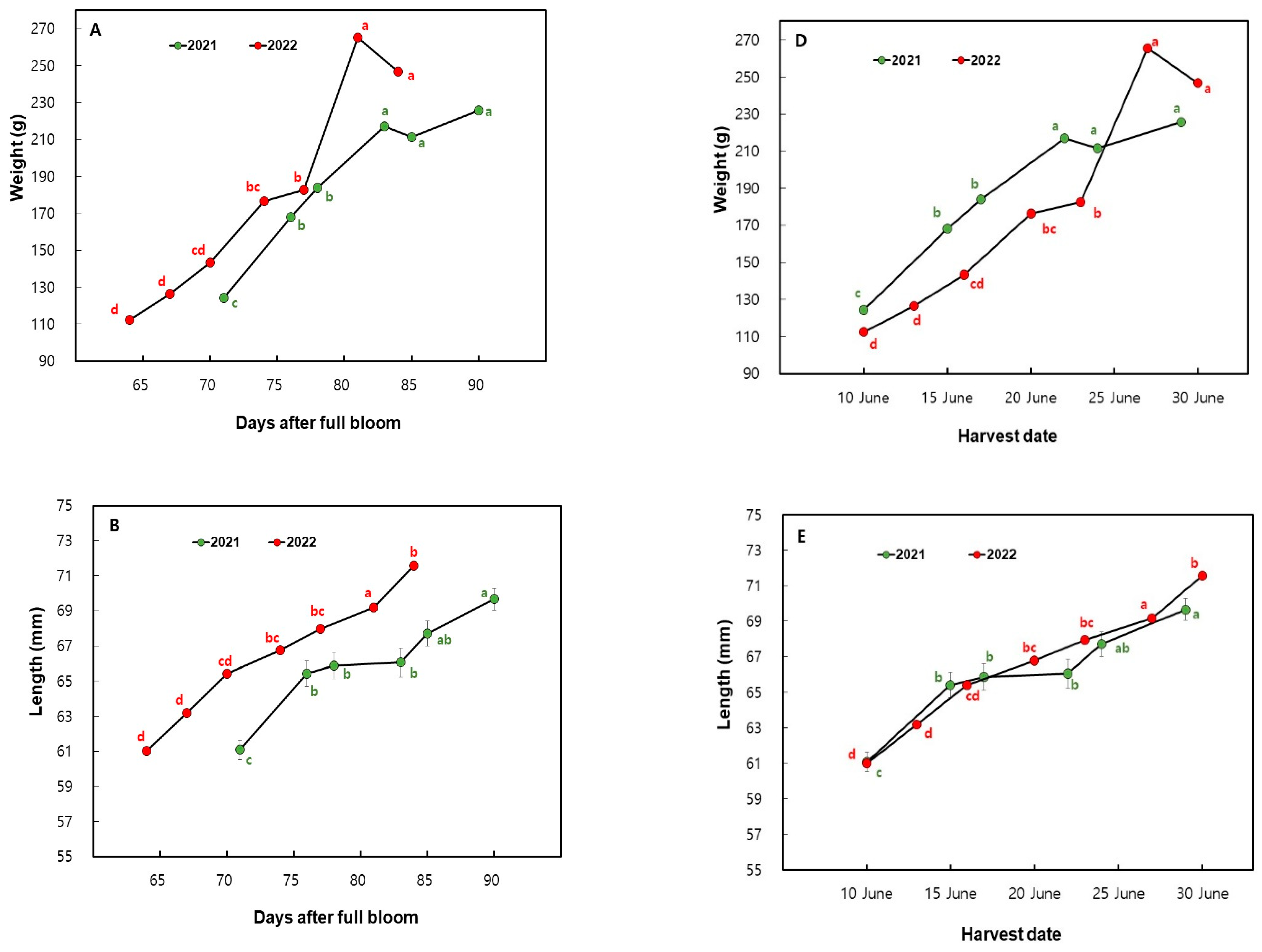
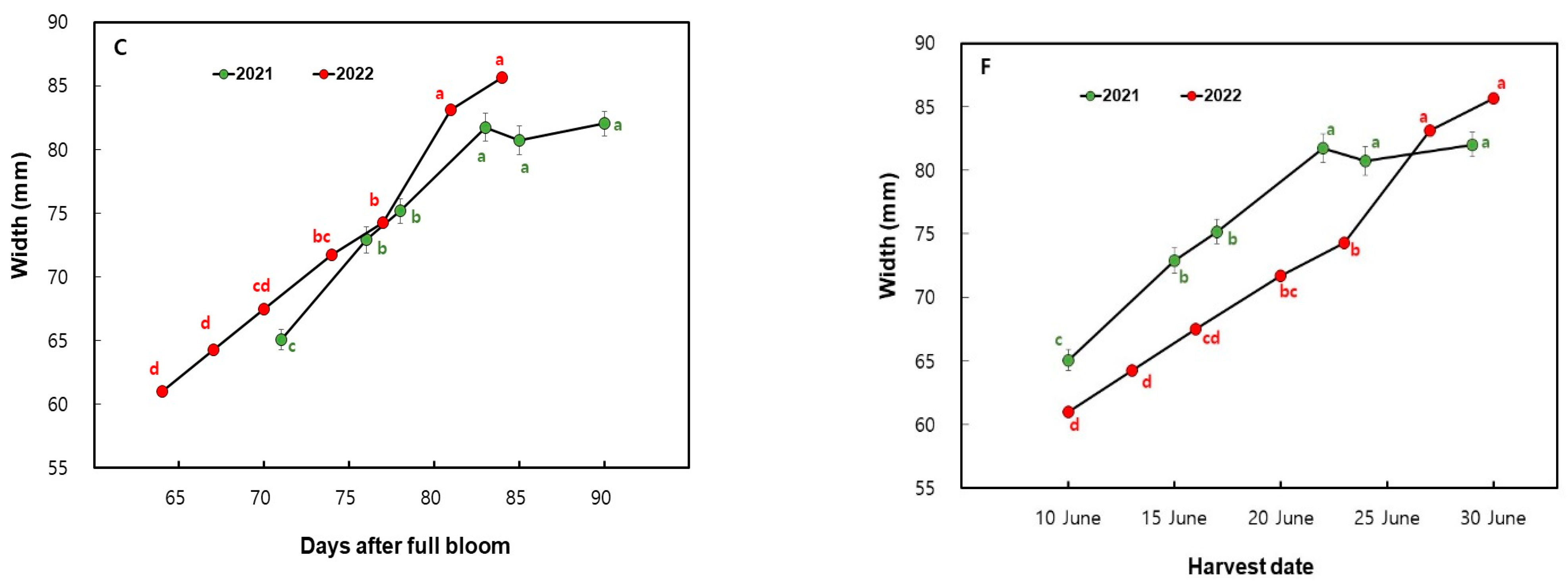
3.1. Changes in Fruit Size Measurements in ‘Mihong’ Peaches
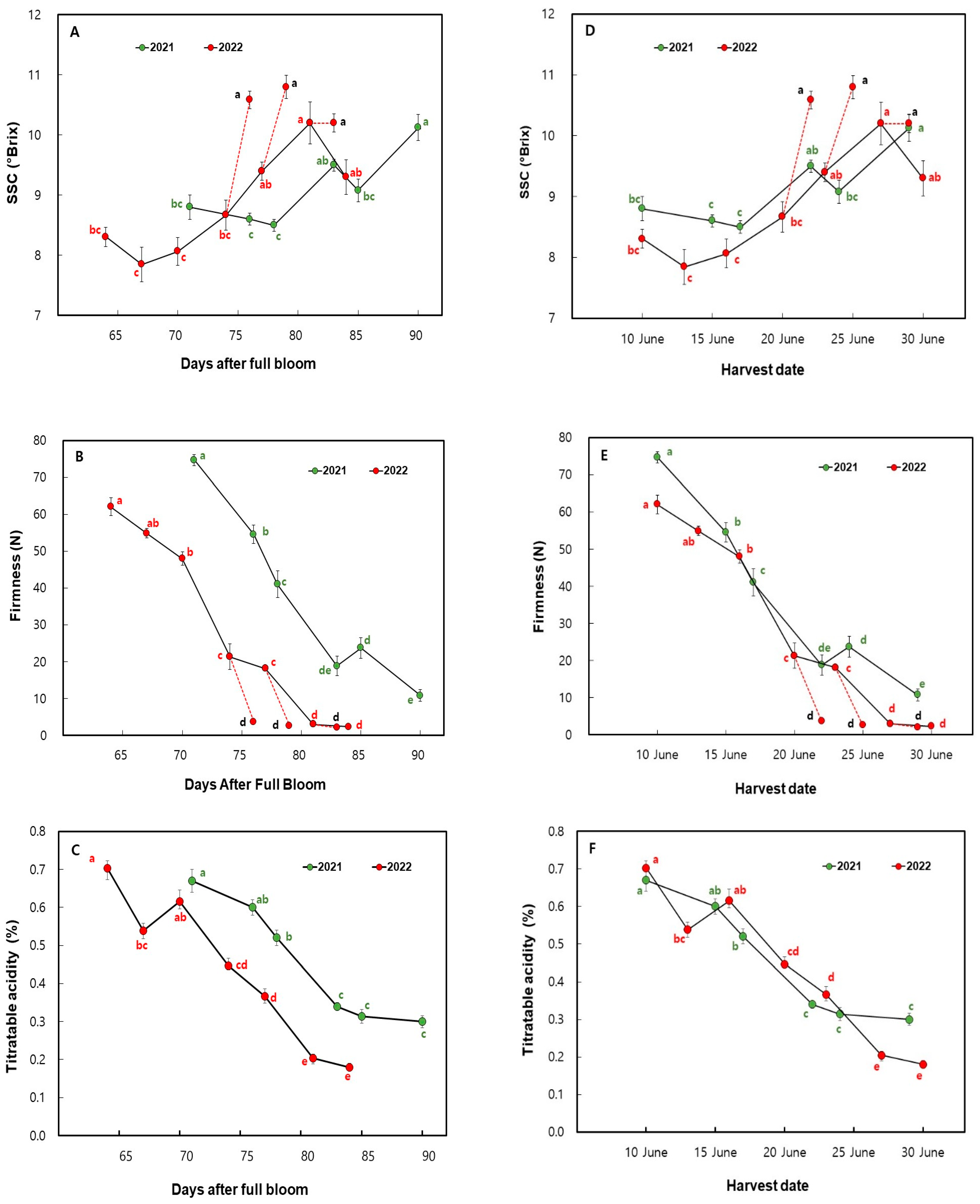
3.2. Changes in the Fruit SSC, Firmness, and TA in ‘Mihong’ Peaches
3.3. Changes in the Color Values of Fruit Skin in ‘Mihong’ Peaches
3.4. Changes of Ethylene Production in ‘Mihong’ Peaches by Harvest Date
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tilahun, S.; Jeong, M.J.; Choi, H.R.; Baek, M.W.; Hong, J.S.; Jeong, C.S. Prestorage high CO2 and 1-MCP treatment reduce chilling injury, prolong storability, and maintain sensory qualities and antioxidant activities of “Madoka” peach fruit. Front. Nutr. 2022, 9, 903352. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Y.; Xu, F.; Wang, H.; Chen, M.; Shao, X. Changes in the chlorophyll absorbance index (I AD) are related to peach fruit maturity. N. Z. J. Crop Hortic. Sci. 2020, 48, 34–46. [Google Scholar] [CrossRef]
- Crisosto, C.H. Stone fruit maturity indices: A descriptive. Postharvest News Inf. 1994, 5, 65N–68N. [Google Scholar]
- Minas, I.S.; Blanco-Cipollone, F.; Sterle. Accurate non-destructive prediction of peach fruit internal quality and physiological maturity with a single scan using near-infrared spectroscopy. Food Chem. 2021, 335, 127626. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, B.; Zhang, C.; Song, Z.; Ma, R. Determination of fruit maturity and its prediction model based on the pericarp index of absorbance difference (IAD) for peaches. PLoS ONE 2017, 12, e0177511. [Google Scholar] [CrossRef]
- Pinto, C.; Reginato, G.; Shinya, P.; Mesa, K.; Díaz, M.; Atenas, C.; Infante, R. Skin color and chlorophyll absorbance: Indices for establishing a harvest date on non-melting peach. Sci. Hortic. 2015, 192, 231–236. [Google Scholar] [CrossRef]
- Lechaudel, M.; Urban, L.; Joas, J. Chlorophyll fluorescence, a nondestructive method to assess maturity of mango fruits (Cv.‘Cogshall’) without growth conditions bias. J. Agric. Food Chem. 2010, 58, 7532–7538. [Google Scholar] [CrossRef]
- Prinsi, B.; Negri, A.S.; Fedeli, C.; Morgutti, S.; Negrini, N.; Cocucci, M.; Espen, L. Peach fruit ripening: A proteomic comparative analysis of the mesocarp of two cultivars with different flesh firmness at two ripening stages. Phytochemistry 2011, 72, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Moore, J.N.; Murphy, J.B. Quantitative and qualitative changes in sugar content of peach genotypes [Prunus persica (L.) Batsch.]. J. Am. Soc. Hortic. Sci. 1993, 118, 97–100. [Google Scholar] [CrossRef]
- Wakabayashi, K. Changes in cell wall polysaccharides during fruit ripening. J. Plant Res. 2000, 113, 231. [Google Scholar] [CrossRef]
- Liu, T.; Song, S.; Yuan, Y.; Wu, D.; Chen, M.; Sun, Q.; Zhang, B.; Xu, C.; Chen, K. Improved peach peel color development by fruit bagging. Enhanced expression of anthocyanin biosynthetic and regulatory genes using white non-woven polypropylene as replacement for yellow paper. Sci. Hortic. 2015, 184, 142–148. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G.; Islam, M.S.; Goodwin, I. A fruit colour development index (CDI) to support harvest time decisions in peach and nectarine orchards. Horticulturae 2022, 8, 459. [Google Scholar] [CrossRef]
- Kumarihami, H.P.C.; Kim, J.G.; Kim, Y.H.; Lee, M.; Lee, Y.S.; Kwack, Y.B.; Kim, J. Preharvest application of chitosan improves the postharvest life of ‘Garmrok’ kiwifruit through the modulation of genes related to ethylene biosynthesis, cell wall modification and lignin metabolism. Foods 2021, 10, 373. [Google Scholar] [CrossRef]
- Kumarihami, H.P.C.; Cha, G.H.; Kim, J.G.; Kim, H.L.; Lee, M.; Kwack, Y.B.; Cho, J.G.; Kim, J. Effect of preharvest Ca-Chitosan application on postharvest quality of ‘Garmrok’ kiwifruit during cold storage. Hortic. Sci. Technol. 2020, 38, 239–248. [Google Scholar]
- Tonutti, P.; Casson, P.; Ramina, A. Ethylene biosynthesis during peach fruit development. J. Am. Soc. Hortic. Sci. 1991, 116, 274–279. [Google Scholar] [CrossRef]
- Callahan, A.M.; Dardick, C.; Scorza, R. Characterization of ‘Stoneless’: A naturally occurring, partially stoneless plum cultivar. J. Am. Soc. Hortic. Sci. 2009, 134, 120–125. [Google Scholar] [CrossRef]
- Dardick, C.D.; Callahan, A.M.; Chiozzotto, R.; Schaffer, R.J.; Piagnani, M.C.; Scorza, R. Stone formation in peach fruit exhibits spatial coordination of the lignin and flavonoid pathways and similarity to Arabidopsisdehiscence. BMC Boil. 2010, 8, 13. [Google Scholar] [CrossRef]
- Bonghi, C.; Trainotti, L.; Botton, A.; Tadiello, A.; Rasori, A.; Ziliotto, F.; Zaffalon, V.; Casadoro, G.; Ramina, A. A microarray approach to identify genes involved in seed-pericarp cross-talk and development in peach. BMC Plant Biol. 2011, 11, 107. [Google Scholar] [CrossRef]
- Lara, M.V.; Borsani, J.; Budde, C.O.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F. Biochemical and proteomic analysis of ‘Dixiland’peach fruit (Prunus persica) upon heat treatment. J. Exp. Bot. 2009, 60, 4315–4333. [Google Scholar] [CrossRef] [PubMed]
- Génard, M.; Lescourret, F.; Gomez, L.; Habib, R. Changes in fruit sugar concentrations in response to assimilate supply, metabolism and dilution: A modeling approach applied to peach fruit (Prunus persica). Tree Physiol. 2003, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Hussain, K.; Shah, H.M.S.; Malik, A.U.; Anwar, R.; Rehman, R.N.U.; Bakhsh, A. Cold storage influences postharvest chilling injury and quality of peach fruits. J. Hortic. Sci. Technol. 2018, 1, 28–34. [Google Scholar] [CrossRef]
- Layne, D.R.; Bassi, D. The Peach: Botany, Production and Uses; Cabi: Wallingford, UK, 2008. [Google Scholar]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC−DAD−ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Ciaccheri, L.; Ieri, F.; Ganino, T.; Faraloni, C. Evaluation of the fruit quality and phytochemical compounds in peach and nectarine cultivars. Plants 2023, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Heritable pleiotropy of glabrous and saucer shape gene loci from peach and their breeding value. J. Fruit Sci. 2009, 26, 692–698. [Google Scholar]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G.E.; Goulas, V.; Manganaris, G.A.; Ziogas, V.; Manganaris, A. The appraisal of qualitative parameters and antioxidant contents during postharvest peach fruit ripening underlines the genotype significance. Postharvest Biol. Technol. 2016, 115, 142–150. [Google Scholar] [CrossRef]
- Reig, G.; Iglesias, I.; Gatius, F.; Alegre, S. Antioxidant capacity, quality, and anthocyanin and nutrient contents of several peach cultivars [Prunus persica (L.) Batsch] grown in Spain. J. Agric. Food Chem. 2013, 61, 6344–6357. [Google Scholar] [CrossRef]
- Baccichet, I.; Chiozzotto, R.; Bassi, D.; Gardana, C.; Cirilli, M.; Spinardi, A. Characterization of fruit quality traits for organic acids content and profile in a large peach germplasm collection. Sci. Hortic. 2021, 278, 109865. [Google Scholar]
- Zhuang, J.; Hou, C.; Tang, Y.; He, Y.; Guo, Q.; Miao, A.; Zhong, Z.; Luo, S. Assessment of external properties for identifying banana fruit maturity stages using optical imaging techniques. Sensors 2019, 19, 2910. [Google Scholar] [CrossRef]
- Urbonaviciene, D.; Viskelis, P.; Viskelis, J.; Jankauskiene, J.; Bobinas, C. Lycopene and β-carotene in non-blanched and blanched tomatoes. J. Food Agric. Environ. 2012, 10, 142–146. [Google Scholar]
- Arias, R.; Lee, T.C.; Logendra, L.; Janes, H. Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Scalisi, A.; Pelliccia, D.; O’Connell, M.G. Maturity prediction in yellow peach (Prunus persica L.) cultivars using a fluorescence spectrometer. Sensors 2020, 20, 6555. [Google Scholar] [CrossRef]
- Kim, C.Y.; Ahn, Y.O.; Kim, S.H.; Kim, Y.H.; Lee, H.S.; Catanach, A.S.; Jacobs, J.M.; Conner, A.J.; Kwak, S.S. The sweet potato IbMYB1 gene as a potential visible marker for sweet potato intragenic vector system. Physiol. Plant. 2010, 139, 229–240. [Google Scholar]
- Lin-Wang, K.; Bolitho, K.; Grafton, K.; Kortstee, A.; Karunairetnam, S.; McGhie, T.K.; Espley, R.V.; Hellens, R.P.; Allan, A.C. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol. 2010, 10, 50. [Google Scholar] [CrossRef]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef]
- Khan, I.A.; Rahman, M.U.; Sakhi, S.; Nawaz, G.; Khan, A.A.; Ahmad, T.; Adnan, M.; Khan, S.M. PpMYB39 activates PpDFR to modulate anthocyanin biosynthesis during peach fruit maturation. Horticulturae 2022, 8, 332. [Google Scholar] [CrossRef]
- Tsuda, T.; Yamaguchi, M.; Honda, C.; Moriguchi, T. Expression of anthocyanin biosynthesis genes in the skin of peach and nectarine fruit. J. Am. Soc. Hortic. Sci. 2004, 129, 857–862. [Google Scholar] [CrossRef]
- Kortei, N.K.; Akonor, P.T. Correlation between hue-angle and colour lightness of gamma irradiated mushrooms. Ann. Food Sci. Technol. 2015, 16, 98–103. [Google Scholar]
- Tadesse, T.; Hewett, E.W.; Nichols, M.A.; Fisher, K.J. Changes in physicochemical attributes of sweet pepper cv. Domino during fruit growth and development. Sci. Hortic. 2002, 93, 91–103. [Google Scholar] [CrossRef]
- Alba, R.; Payton, P.; Fei, Z.; McQuinn, R.; Debbie, P.; Martin, G.B.; Tanksley, S.D.; Giovannoni, J.J. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell 2005, 17, 2954–2965. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16 (Suppl. 1), S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Hayama, H.; Shimada, T.; Fujii, H.; Ito, A.; Kashimura, Y. Ethylene-regulation of fruit softening and softening-related genes in peach. J. Exp. Bot. 2006, 57, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
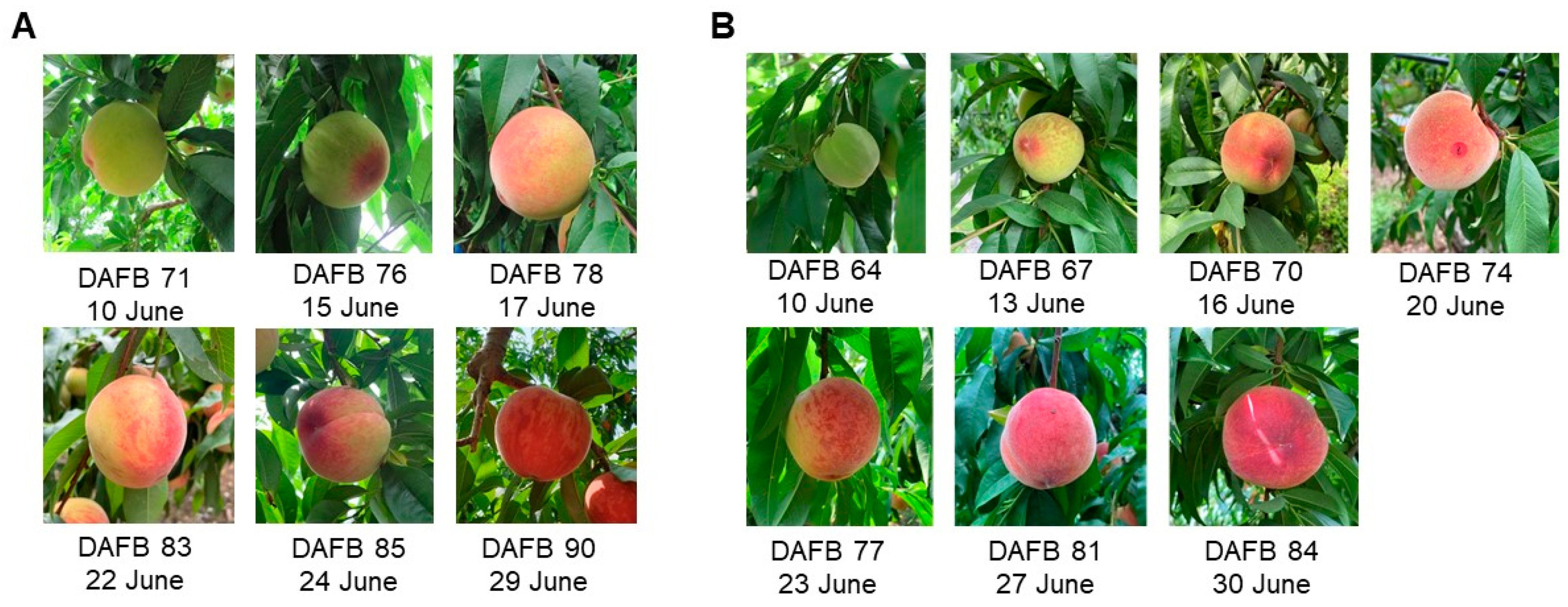


| Harvest Date /DAFB | L* Value | a* Value | b* Value | Chroma | Hue | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| 10 June (DAFB 71) | 66.4 ± 0.8 ab | 66.0 ± 0.8 a | −11.8 ± 0.6 c | −11.4 ± 0.7 c | 37.3 ± 0.5 a | 37.2 ± 0.4 a | 39.3 ± 0.5 a | 39.1 ± 0.5 a | 107.3 ± 1.0 a | 106.8 ± 1.0 a |
| 15 June (DAFB 76) | 69.2 ± 0.9 a | 68.8 ± 1.0 a | −8.3 ± 1.3 c | −7.8 ± 1.4 c | 37.1 ± 0.6 a | 36.9 ± 0.7 a | 38.8 ± 0.6 ab | 38.8 ± 0.5 a | 101.4 ± 2.1 a | 100.8 ± 2.5 a |
| 17 June (DAFB 78) | 70.7 ± 1.0 a | 69.8 ± 1.2 a | −7.3 ± 1.4 c | −4.8 ± 1.9 c | 37.9 ± 0.6 a | 36.5 ± 0.7 a | 39.5 ± 0.5 a | 38.6 ± 0.6 ab | 100.1 ± 2.2 a | 96.0 ± 3.1 a |
| 22 June (DAFB 83) | 66.2 ± 1.7 ab | 65.4 ± 1.7 a | 11.6 ± 0.1 b | 10.8 ± 2.15 b | 31.9 ± 0.9 b | 32.1 ± 1.2 b | 37.1 ± 0.4 bc | 36.7 ± 0.5 bc | 70.3 ± 4.0 b | 70.6 ± 3.8 b |
| 24 June (DAFB 85) | 62.8 ± 1.7 bc | 66.1 ± 1.7 a | 18.1 ± 2.0 b | 13.5 ± 2.0 b | 27.9 ± 0.7 c | 29.4 ± 1.0 b | 35.4 ± 0.6 c | 34.9 ± 0.5 c | 58.5 ± 3.4 c | 65.4 ± 3.7 b |
| 29 June (DAFB 90) | 57.8 ± 1.4 c | 57.1 ± 1.3 b | 27.7 ± 1.3 a | 29.2 ± 1.0 a | 26.1 ± 0.7 c | 25.4 ± 0.8 c | 39.1 ± 0.5 ab | 39.3 ± 0.5 a | 43.9 ± 2.2 d | 41.2 ± 1.7 c |
| Harvest Date /DAFB | L* Value | a* Value | b* Value | Chroma | Hue | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Apex | Right | Left | Apex | Right | Left | Apex | Right | Left | Apex | Right | Left | Apex | |
| 10 June (DAFB 64) | 67.8 ± 0.8 a | 67.0 ± 0.9 a | 59.6 ± 1.0 a | −10.3 ± 0.6 e | −8.5 ± 1.4 d | 4.7 ± 1.6 d | 27.3 ± 0.5 a | 26.8 ± 0.6 ab | 18.9 ± 1.2 a | 29.2 ± 0.7 b | 28.7 ± 0.7 b | 21.0 ± 0.9 b | 110.4 ± 1.0 a | 106.9 ± 2.7 a | 72.4 ± 5.0 a |
| 13 June (DAFB 67) | 69.0 ± 0.9 a | 66.4 ± 1.1 ab | 54.9 ± 1.1 b | −10.2 ± 0.8 e | −8.0 ± 1.0 d | 14.3 ± 1.0 bc | 28.3 ± 0.7 a | 26.5 ± 0.8 ab | 14.1 ± 1.0 bc | 30.2 ± 0.8 b | 27.9 ± 0.9 b | 20.7 ± 0.8 b | 109.3 ± 1.3 a | 105.6 ± 1.9 a | 44.3 ± 3.2 b |
| 16 June (DAFB 70) | 69.5 ± 1.0 a | 70.1 ± 1.0 a | 62.2 ± 1.2 a | −8.8 ± 1.0 e | −7.6 ± 1.1 d | 8.9 ± 1.8 cd | 28.2 ± 0.7 a | 28.2 ± 0.5 a | 19.3 ± 1.1 a | 29.7 ± 0.8 b | 29.6 ± 0.7 b | 23.0 ± 0.6 b | 106.5 ± 1.7 a | 104.3 ± 2.0 a | 64.3 ± 5.0 a |
| 20 June (DAFB 74) | 68.1 ± 1.7 a | 68.2 ± 1.3 a | 51.2 ± 0.7 bc | 1.9 ± 2.1 d | 1.9 ± 1.9 c | 18.8 ± 0.8 b | 26.1 ± 1.3 a | 26.6 ± 1.2 ab | 10.4 ± 0.5 c | 28.0 ± 0.8 b | 28.1 ± 0.9 b | 21.5 ± 1.0 b | 83.2 ± 5.0 b | 83.8 ± 4.4 b | 28.9 ± 0.6 c |
| 23 June (DAFB 77) | 68.5 ± 1.6 a | 66.7 ± 1.2 a | 49.4 ± 1.0 cd | 6.8 ± 2.8 c | 9.6 ± 1.9 b | 19.3 ± 1.0 b | 24.9 ± 1.5 ab | 24.0 ± 0.9 b | 10.9 ± 0.9 c | 28.9 ± 0.9 b | 27.4 ± 0.5 b | 22.5 ± 1.1 b | 72.1 ± 6.1 b | 67.7 ± 4.3 c | 29.4 ± 2.0 c |
| 27 June (DAFB 81) | 65.4 ± 1.8 a | 60.8 ± 1.5 bc | 53.9 ± 1.3 bc | 17.7 ± 1.6 b | 20.6 ± 1.0 a | 17.9 ± 1.0 b | 20.3 ± 1.3 b | 17.5 ± 1.2 c | 10.9 ± 1.3 c | 28.2 ± 0.6 b | 27.6 ± 0.8 b | 21.2 ± 1.4 b | 48.7 ± 4.0 c | 47.9 ± 2.9 d | 29.9 ± 1.8 c |
| 30 June (DAFB 84) | 57.6 ± 2.1 b | 60.3 ± 2.2 c | 45.4 ± 1.2 d | 24.5 ± 1.6 a | 22.8 ± 1.4 a | 28.5 ± 2.6 a | 23.7 ± 1.6 ab | 25.3 ± 1.3 ab | 18.2 ± 1.1 ab | 35.0 ± 1.4 a | 34.8 ± 0.9 a | 34.1 ± 2.7 a | 43.6 ± 3.2 c | 39.9 ± 2.8 d | 33.3 ± 1.2 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasooriya, L.S.H.; Shin, M.H.; Wijethunga, W.M.U.D.; Lee, S.K.; Cho, J.G.; Jang, S.H.; Kim, J.G. Selection of a Proper Maturity Index for the Mechanical Harvesting of ‘Mihong’ Peach Fruit. Horticulturae 2023, 9, 730. https://doi.org/10.3390/horticulturae9070730
Jayasooriya LSH, Shin MH, Wijethunga WMUD, Lee SK, Cho JG, Jang SH, Kim JG. Selection of a Proper Maturity Index for the Mechanical Harvesting of ‘Mihong’ Peach Fruit. Horticulturae. 2023; 9(7):730. https://doi.org/10.3390/horticulturae9070730
Chicago/Turabian StyleJayasooriya, L. Sugandhi Hirushika, Mi Hee Shin, W. M. Upeksha Darshani Wijethunga, Seul Ki Lee, Jung Gun Cho, Si Hyeong Jang, and Jin Gook Kim. 2023. "Selection of a Proper Maturity Index for the Mechanical Harvesting of ‘Mihong’ Peach Fruit" Horticulturae 9, no. 7: 730. https://doi.org/10.3390/horticulturae9070730
APA StyleJayasooriya, L. S. H., Shin, M. H., Wijethunga, W. M. U. D., Lee, S. K., Cho, J. G., Jang, S. H., & Kim, J. G. (2023). Selection of a Proper Maturity Index for the Mechanical Harvesting of ‘Mihong’ Peach Fruit. Horticulturae, 9(7), 730. https://doi.org/10.3390/horticulturae9070730





