Avermectin Trunk Injections: A Promising Approach for Managing the Walnut Husk Fly (Rhagoletis completa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Injection Method
2.3. Sampling
2.4. Insecticidal Effect and Rating of the Damage
2.5. Short- and Long-Term Efficacy of the Endotherapy
2.6. Chemical Analysis
2.7. Statistical Analysis
3. Results
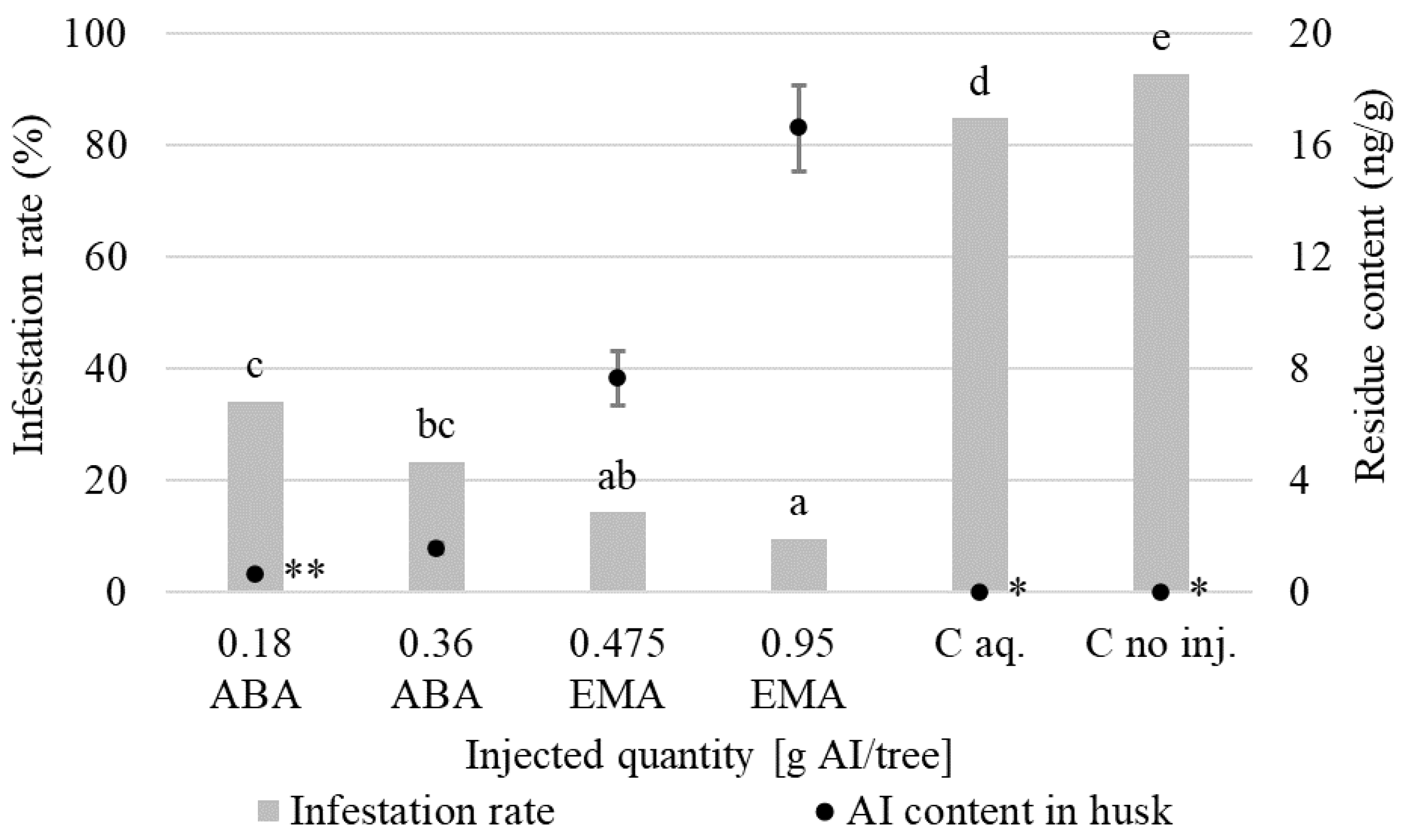
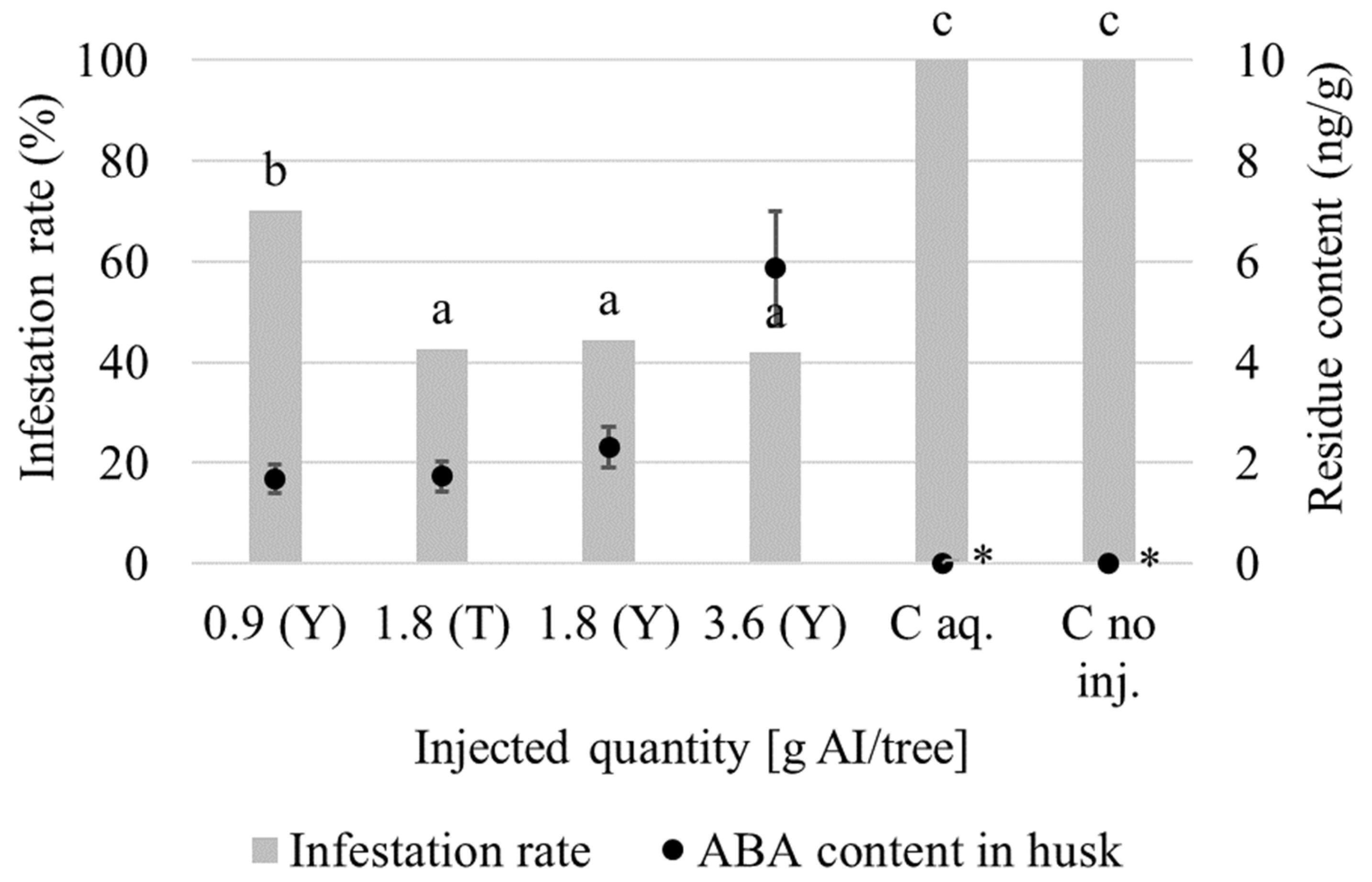
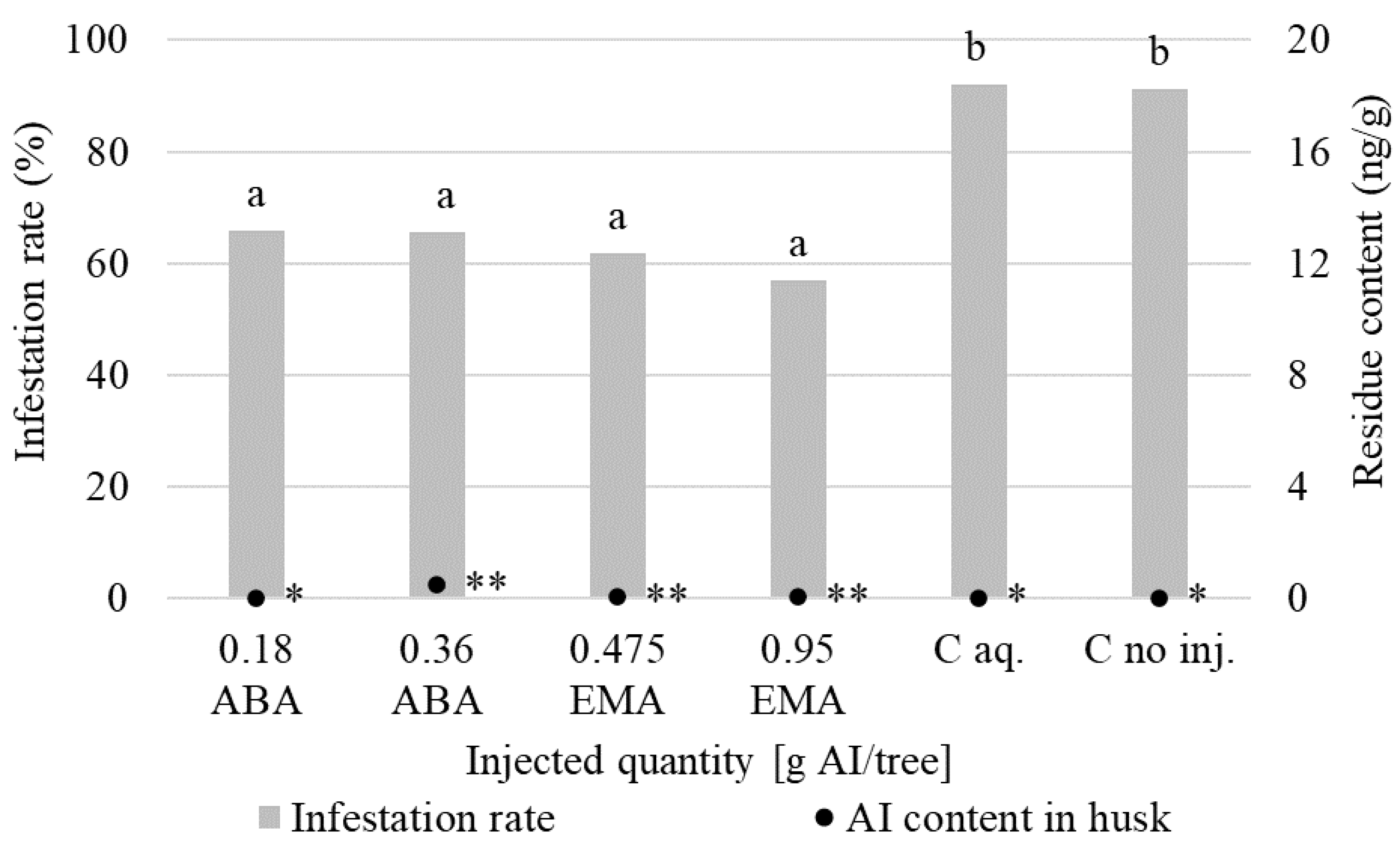
3.1. Insecticidal Effect
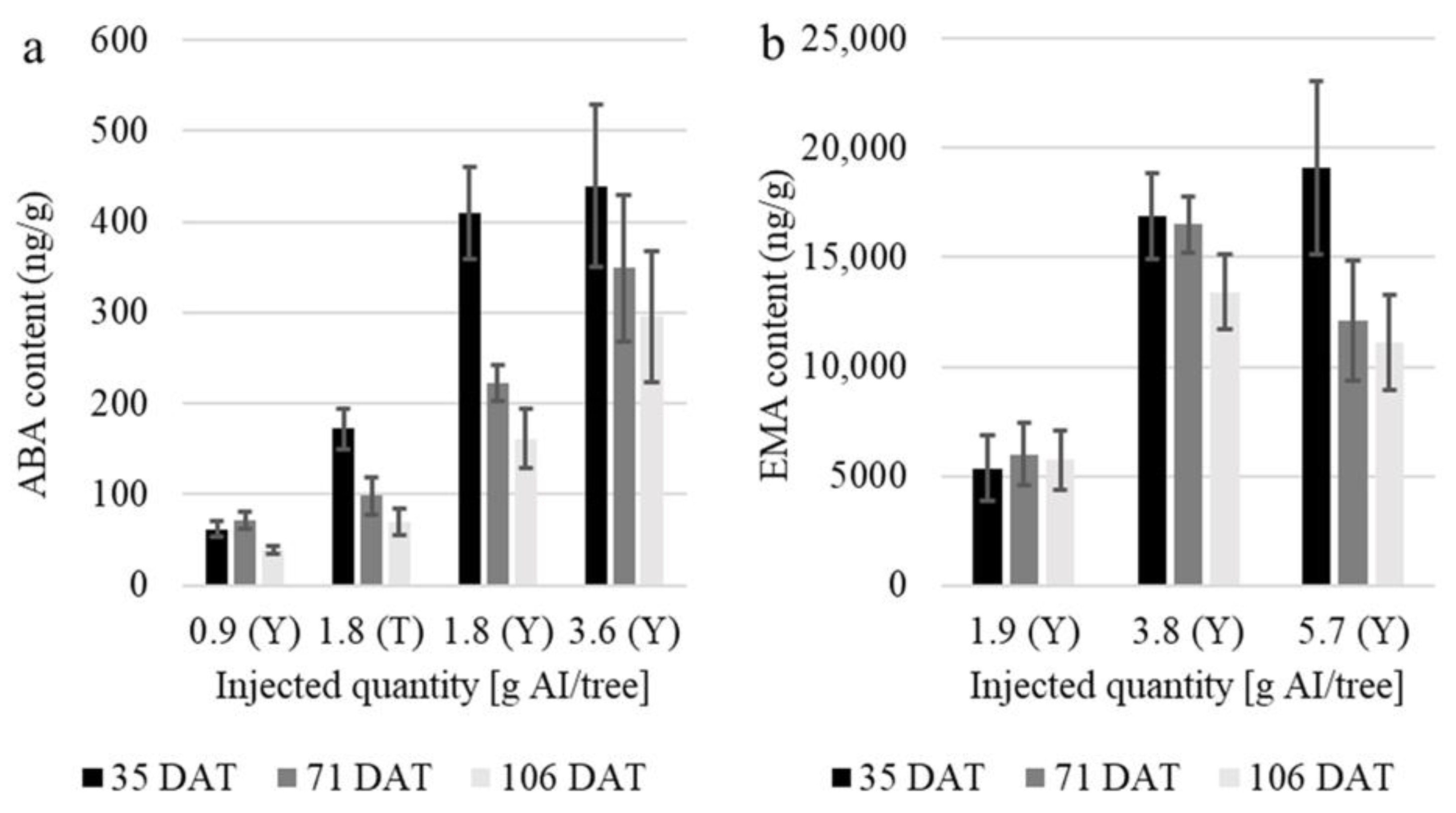
3.2. Short-Term Residue Monitoring in the Leaves
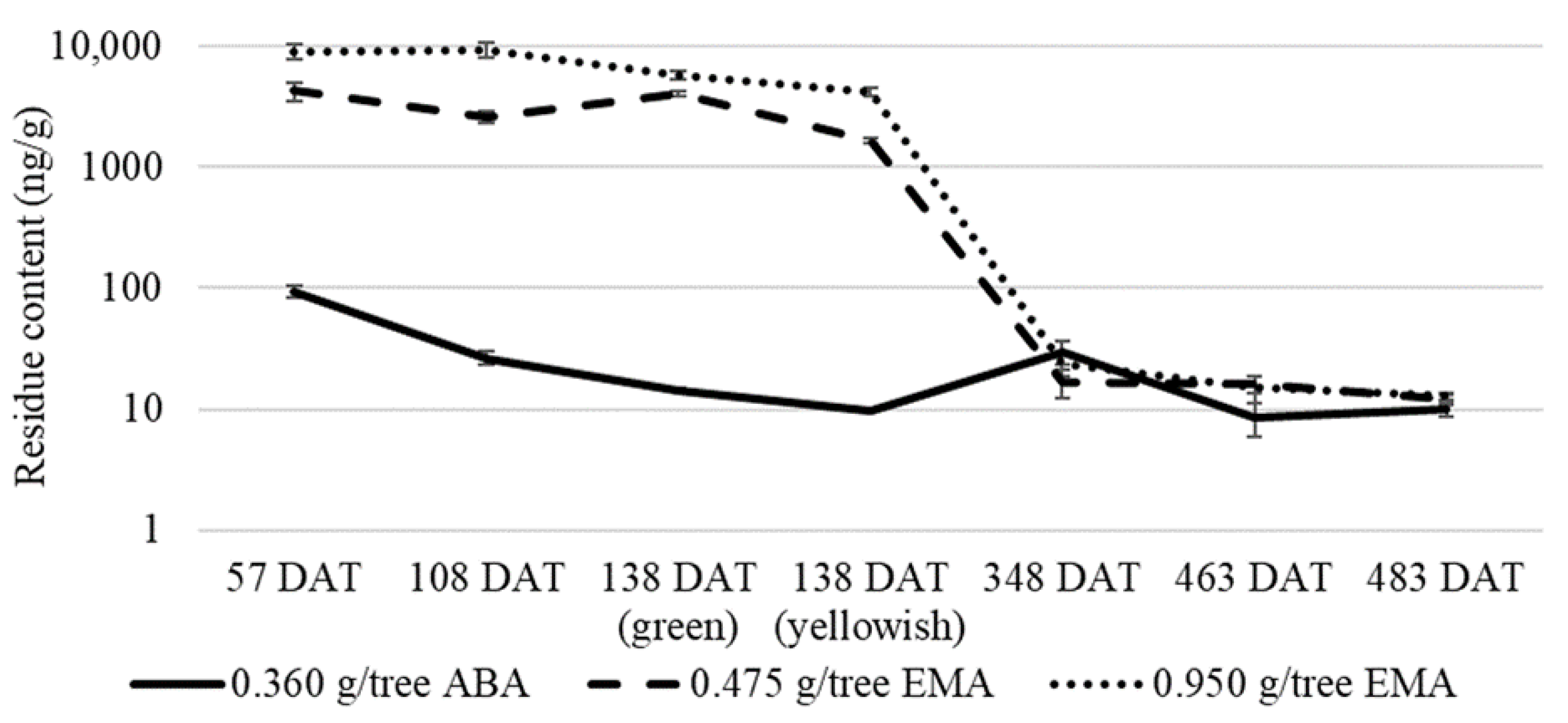
3.3. Long-Term Efficacy of the Endotherapy
3.4. Residue in the Flowers
3.5. Residue in the Kernels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en (accessed on 7 May 2022).
- FruitVeb. Október 16 Diópiaci Helyzetkép. 2019. Available online: https://fruitveb.hu/diopiaci-helyzetkep/ (accessed on 30 May 2022).
- Chen, Y.H.; Opp, S.B.; Berlocher, S.H.; Roderick, G.K. Are bottlenecks associated with colonization? Genetic diversity and diapause variation of native and introduced Rhagoletis completa populations. Oecologia 2006, 149, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Merz, B. Rhagoletis completa Cresson und Rhagoletis indifferens Curran zwei wirtschaftlich bedeutende nordamerikanische Fruchtfliegen, neu für Europa (Diptera: Tephritidae). Mitt. Der Schweiz. Entomol. Ges. 1991, 64, 55–57. [Google Scholar]
- Smith, J.J.; Bush, G.L. Phylogeny of the subtribe Carpomyina (Trypetinae), emphasizing relationships of the genus Rhagoletis. In Fruit Flies (Tephritidae), 1st ed.; Aluja, M., Norrbom, A., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 205–236. [Google Scholar]
- Duso, C. Sulla comparsa in Italia di un Tefritide neartico del noce: Rhagoletis completa Cresson (Diptera: Tephritidae). Boll. Di Zool. Agrar. Bachic. 1991, 23, 203–209. [Google Scholar]
- Verheggen, F.; Verhaeghe, A.; Giordanengo, P.; Tassus, X.; Escobar-Gutiérrez, A. Walnut husk fly, Rhagoletis completa (Diptera: Tephritidae), invades Europe: Invasion potential and control strategies. Appl. Entomol. Zool. 2017, 52, 1–7. [Google Scholar] [CrossRef]
- Oláh, R.; Vétek, G.; Orosz, S. A nyugati dióburok-fúrólégy (Rhagoletis completa Cresson. 1929) Magyarországi elterjedése (2012–2017). Növényvédelem 2017, 78, 11. [Google Scholar]
- Coates, W.W. Walnut Husk Fly: Varietal susceptibility and quality observations. In Walnut Research Reports; University of California: Berkeley, CA, USA, 2004; pp. 179–181. [Google Scholar]
- Guillén, L.; Aluja, M.; Rull, J.; Höhn, H.; Schwizer, T.; Samietz, J. Influence of walnut cultivar on infestation by Rhagoletis completa: Behavioural and management implications. Entomol. Exp. Appl. 2011, 140, 207–217. [Google Scholar] [CrossRef]
- Shelton, M.D.; Anderson, L. Walnut cultivars: Evidence for differential susceptibility to insect pests. Fruit Var. J. 1990, 44, 179–182. [Google Scholar]
- Roques, A.; Kenis, M.; Lees, D.; Lopez-Vaamonde, C.; Rabitsch, W.; Rasplus, J.Y.; Roy, D.B. Alien Terrestrial Arthropods of Europe. BioRisk Biodivers. Ecosyst. Risk Assess. 2010, 4, 918–919. [Google Scholar]
- Voigt, E.; Tóth, M. Dió buroklégy magyarországi elterjedése 2013 tavaszán. Növényvédelem 2013, 49, 341–345. [Google Scholar]
- Tuba, K.; Schuler, H.; Stauffer, C.; Lakatos, F. A nyugati dióburok-furólégy (Rhagoletis completa Cresson 1929—Diptera: Tephritidae) megjelenése Magyarországon. Növényvédelem 2012, 48, 419–423. [Google Scholar]
- Barić, B.; Pajač Živković, I.; Matošević, D.; Šubić, M.; Voigt, E.; Tóth, M. Rhagoletis completa (Diptera; Tephritidae) distribution, flight dynamics and influence on walnut kernel quality in the continental Croatia. Poljoprivreda 2015, 21, 53–58. [Google Scholar] [CrossRef]
- Duso, C.; Dal Lago, G. Life cycle, phenology and economic importance of the walnut husk fly Rhagoletis completa Cresson (Diptera: Tephritidae) in northern Italy. Ann. Société Entomol. Fr. 2006, 42, 245–254. [Google Scholar] [CrossRef]
- Ohlendorf, B. Walnut husky fly: Integrated Pest Management in the home garden. Pest Notes Fla. Entomol. 2000, 90, 626–634. [Google Scholar]
- Hislop, R.; Riedl, H.; Joos, J. Control of the walnut husk fly with pyrethroids and bait. Calif. Agric. 1981, 35, 23–26. [Google Scholar]
- Nickel, J.L.; Wong, T.T. Control of the walnut husk fly, Rhagoletis completa Cresson. with systemic insecticides. J. Econ. Entomol. 1966, 59, 1079–1082. [Google Scholar] [CrossRef]
- Nomoto, R.M.; Coates, W.W.; Hasey, J.K.; Elkins, R.B.; Grant, J.A.; Van Steenwyk, R.A.; Zolbrod, S.K. Walnut husk fly control with reduced risk insecticides. In ISHS Acta Horticulturae 861; McNeil, D.L., Ed.; VI International Walnut Symposium: Melbourne, Australia, 2010; pp. 375–382. [Google Scholar] [CrossRef]
- Van Steenwyk, R.A.; Choi, J.; Kim, A. Control of Walnut Husk Fly in Walnut, 2018. Arthropod Manag. Tests 2019, 44, 22. [Google Scholar] [CrossRef]
- Verhaeghe, A.; Chalaye, C.; Weydert, C. Control of walnut husk fly using alternative methods. In ISHS Acta Horticulturae 861; McNeil, D.L., Ed.; VI International Walnut Symposium: Melbourne, Australia, 2010; pp. 395–398. [Google Scholar] [CrossRef]
- Costonis, A.C. Tree Injection: Perspective macro-injection/micro-injection. J. Arboric. 1981, 7, 275–277. [Google Scholar] [CrossRef]
- Jones, T.W.; Gregory, G.F. An Apparatus for Pressure Injection of Solutions into Trees; Northeastern Forest Experiment Station, Forest Service, US Department of Agriculture: Radnor, PA, USA, 1971; pp. 1–8.
- Roach, W.A. Plant injection as a physiological method. Ann. Bot. 1939, 3, 155–226. [Google Scholar] [CrossRef]
- Doccola, J.J.; Wild, P.M. Tree injection as an alternative method of insecticide application. In Insecticides—Basic and Other Applications, 1st ed.; Soloneski, S., Larramendy, M., Eds.; InTech: Rijeka, Croatia, 2012; pp. 61–78. [Google Scholar]
- Berger, C.; Laurent, F. Trunk injection of plant protection products to protect trees from pests and diseases. Crop Prot. 2019, 124, 104831. [Google Scholar] [CrossRef]
- Li, M.; Nangong, Z. Precision trunk injection technology for treatment of huanglongbing (HLB)-affected citrus trees—A review. J. Plant Dis. Prot. 2022, 129, 15–34. [Google Scholar] [CrossRef]
- Prasad, R.; Travnick, D. Translocation of benomyl in elm (Ulmus americana L.) Distribution patterns in mature trees following trunk injection under high pressures. Environ. Chem. Control 1973, 114, 18. [Google Scholar]
- Seyahooei, M.A.; Bagheri, A.; Morshedi, S.; Fallahzadeh, M.; Amiri, S.; Shahi, M. Trunk Injection a Promising Approach for Long-Lasting Suppression of Mango Leaf Hopper, Idioscopus clypealis. Egypt. Acad. J. Biol. Sci. Fr. Toxicol. Pest Control 2019, 11, 123–129. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Cregg, B.M.; Sundin, G.W.; Wise, J.C. Comparison of drill-and needle-based tree injection technologies in healing of trunk injection ports on apple trees. Urban For. Urban Green. 2016, 19, 151–157. [Google Scholar] [CrossRef]
- Archer, L.; Crane, J.H.; Albrecht, U. Trunk injection as a tool to deliver plant protection materials—An overview of basic principles and practical considerations. Horticulturae 2022, 8, 552. [Google Scholar] [CrossRef]
- Holderness, M. Comparison of metalaxyl/cuprous oxide sprays and potassium phosphonate as sprays and trunk injections for control of Phytophthora palmivora pod rot and canker of cocoa. Crop Prot. 1992, 11, 141–147. [Google Scholar] [CrossRef]
- Percival, G.C.; Boyle, S. Evaluation of microcapsule trunk injections for the control of apple scab and powdery mildew. Ann. Appl. Biol. 2005, 147, 119–127. [Google Scholar] [CrossRef]
- Ying, W.; Yu, Z.; Tang, G.; Peng, S.; Zhai, M. Control of fruit pests and diseases of walnut with trunk injection. J. Fruit Sci. 2014, 31, 454–459. [Google Scholar]
- Mokhtaryan, A.; Sheikhigarjan, A.; Arbab, A.; Mohammadipour, A.; Ardestanirostami, H. The efficiency of systemic insecticides and complete fertilizer by trunk injection method against leopard moth in infested walnut trees. J. Basic Appl. Zool. 2021, 82, 1–5. [Google Scholar] [CrossRef]
- Wheeler, C.E.; Vandervoort, C.; Wise, J.C. Organic Control of Pear Psylla in Pear with Trunk Injection. Insects 2020, 11, 650. [Google Scholar] [CrossRef]
- Dal Maso, E.; Linaldeddu, B.T.; Fanchin, G.; Faccoli, M.; Montecchio, L. The potential for pesticide trunk injections for control of thousand cankers disease of walnut. Phytopathol. Mediterr. 2019, 58, 73–79. [Google Scholar]
- McCullough, D.G.; Poland, T.M.; Tluczek, A.R.; Anulewicz, A.; Wieferich, J.; Siegert, N.W. Emerald ash borer (Coleoptera: Buprestidae) densities over a 6-yr period on untreated trees and trees treated with systemic insecticides at 1-, 2-, and 3-yr intervals in a Central Michigan Forest. J. Econ. Entomol. 2019, 112, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Smitley, D.R.; Doccola, J.J.; Cox, D.L. Multiple-year protection of ash trees from emerald ash borer with a single trunk injection of emamectin benzoate, and single-year protection with an imidacloprid basal drench. J. Arboric. 2010, 36, 206. [Google Scholar] [CrossRef]
- Lasota, J.A.; Dybas, R.A. Avermectins. a novel class of compounds: Implications for use in arthropod pest control. Annu. Rev. Entomol. 1991, 36, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Dybas, R.A. Abamectin use in crop protection. In Ivermectin and Abamectin; Campbell, W.C., Ed.; Springer: New York, NY, USA, 1989; pp. 287–310. [Google Scholar]
- Escalada, J.P.; Gianotti, J.; Pajares, A.; Massad, W.A.; Amat-Guerri, F.; García, N.A. Photodegradation of the acaricide abamectin: A kinetic study. J. Agric. Food Chem. 2008, 56, 7355–7359. [Google Scholar] [CrossRef] [PubMed]
- Jansson, R.K.; Dybas, R.A. Avermectins: Biochemical mode of action, biological activity and agricultural importance. In Insecticides with Novel Modes of Action; Ishaaya, I., Degheele, D., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 152–170. [Google Scholar]
- Bai, S.H.; Ogbourne, S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere 2016, 154, 204–214. [Google Scholar] [CrossRef]
- Ferracini, C.; Alma, A. How to preserve horse chestnut trees from Cameraria ohridella in the urban environment. Crop Prot. 2008, 27, 1251–1255. [Google Scholar] [CrossRef]
- Kiss, M.; Hachoumi, I.; Nagy, V.; Ladanyi, M.; Gutermuth, A.; Szabo, A.; Sörös, C. Preliminary results about the efficacy of abamectin trunk injection against the walnut husk fly (Rhagoletis completa). J. Plant Dis. Prot. 2021, 128, 333–338. [Google Scholar] [CrossRef]
- EN 15662; Foods of Plant Origin—Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-up by Dispersive SPE—Modular QuEChERS-Method. ISO: Geneva, Switzerland, 2018. Available online: https://www.en-standard.eu/ (accessed on 30 March 2022).
- Kmellár, B.; Abrankó, L.; Fodor, P.; Lehotay, S.J. Routine approach to qualitatively screening 300 pesticides and quantification of those frequently detected in fruit and vegetables using liquid chromatography tandem mass spectrometry (LC-MS/MS). Food Addit. Contam. 2010, 27, 1415–1430. [Google Scholar] [CrossRef]
- Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Abamectin, Beer, Fluopyram, Fluxapyroxad, Maleic Hydrazide, Mustard Seeds Powder and Tefluthrin in or on Certain Products. (Text with EEA Relevance). Available online: https://eurlex.europa.eu/search.html?scope=EURLEX&text=2018%2F685&lang=en&type=quick&qid=1648636986231 (accessed on 30 March 2022).
- Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Acequinocyl. Bacillus Subtilis Strain IAB/BS03, Emamectin, Flutolanil and Imazamox in or on Certain Products. (Text with EEA Relevance). Available online: https://eurlex.europa.eu/search.html?scope=EURLEX&text=2021%2F2022&lang=en&type=quick&qid=1648637488554 (accessed on 30 March 2022).
- SANTE/12682/2019; Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission: Brussels, Belgium, 2019. Available online: https://ec.europa.eu/ (accessed on 30 March 2022).
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC− MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Marascuilo, L.A. Large-sample multiple comparisons. Psychol. Bull. 1966, 65, 280–290. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Cregg, B.M.; McCullough, D.G.; Poland, T.M.; Hollingworth, R.M. Distribution of trunk-injected 14C-imidacloprid in ash trees and efects on emerald ash borer (Coleoptera: Buprestidae) adults. Crop Prot. 2009, 28, 655–661. [Google Scholar] [CrossRef]
- Fishel, F.M. Pesticide Injection and Drenching: PI274. 1/2018. EDIS. 2018. Available online: http://edis.ifas.ufl.edu (accessed on 30 March 2022).
- Wang, J.H.; Che, S.C.; Qiu, L.F.; Li, G.; Shao, J.L.; Zhong, L.; Xu, H. Efficacy of emamectin benzoate trunk injection against the asian long-horned beetle [Anoplophora glabripennis (Coleoptera: Cerambycidae)]. J. Econ. Entomol. 2020, 113, 340–347. [Google Scholar] [CrossRef]
- Anulewicz, A.C.; McCullough, D.G.; Poland, T.M.; Cappaert, D.; Lewis, P.A.; Molongoski, J. Evaluation of multi-year application of neonicotinoid insecticides for EAB control. In Proceedings of the Forest Health Technology Enterprise Team, Emerald Ash Borer Research and Technology Development Meeting, Pittsburgh, PA, USA, 20–21 October 2009; pp. 67–68. [Google Scholar]
- Eisenbach, B.M.; Salom, S.M.; Kok, L.T.; Lagalante, A.F. Impacts of Trunk and Soil Injections of Low Rates of Imidacloprid on Hemlock Woolly Adelgid (Hemiptera: Adelgidae) and Eastern Hemlock (Pinales: Pinaceae) Health. J. Econ. Entomol. 2014, 107, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Doccola, J.J.; Smitley, D.R.; Davis, T.W.; Aiken, J.J.; Wild, P.M. Tree wound responses following systemic injection treatments in Green ash (Fraxinus pennsylvanica Marsh) as determined by destructive autopsy. Arboric. Urban For. 2011, 37, 6–12. [Google Scholar] [CrossRef]
- Coley, D. Spotted Lanternfly Control Program in the Mid-Atlantic Region, North Carolina, Ohio and Kentucky; Environmental Assessment; United States Department of Agriculture: Washington, DC, USA, 2020. [Google Scholar]
- Cowles, R.S. Optimizing a basal bark spray of dinotefuran to manage armored scales (Hemiptera: Diaspididae) in Christmas tree plantations. J. Econ. Entomol. 2010, 103, 1735–1743. [Google Scholar] [CrossRef]
- Horner, I. Trunk Sprays and Lower Phosphite Injection Rates for Kauri Dieback Control–Brief Update October 2018; The New Zealand Institute for Plant & Food Research: Auckland, New Zealand, 2018. [Google Scholar]
- McCullough, D.G.; Cappaert, D.A.; Poland, T.M.; Lewis, P.; Molongowski, J. Evaluation of neo-nicotinoid insecticides applied as non-invasive trunk sprays. In Proceedings of the Forest Health Technology Enterprise Team, Emerald Ash Borer Research and Technology Development Meeting, Cincinatti, OH, USA, 29 October–2 November 2006; pp. 52–54. [Google Scholar]
- McCullough, D.G.; Poland, T.M.; Anulewicz, A.C.; Lewis, P.; Cappaert, D. Evaluation of Agrilus planipennis (Coleoptera: Buprestidae) control provided by emamectin benzoate and two neonicotinoid insecticides. one and two seasons after treatment. J. Econ. Entomol. 2011, 104, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.J.; Martin, K.; Sauerborn, J. Effects of azadirachtin injection in litchi trees (Litchi chinensis Sonn.) on the litchi stink bug (Tessaratoma papillosa Drury) in northern Thailand. J. Pest Sci. 2006, 79, 241–250. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Acimovic, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C. Trunk injection: A discriminating delivering system for horticulture crop IPM. Entomol. Ornithol. Herpetol. 2014, 3, 1. [Google Scholar] [CrossRef]
- Burkhard, R.; Binz, H.; Roux, C.A.; Brunner, M.; Ruesch, O.; Wyss, P. Environmental fate of emamectin benzoate after tree micro injection of horse chestnut trees. Environ. Toxicol. Chem. 2015, 34, 297–302. [Google Scholar] [CrossRef]
- Fantke, P.; Juraske, R. Variability of pesticide dissipation half-lives in plants. Environ. Sci. Technol. 2013, 47, 3548–3562. [Google Scholar] [CrossRef] [PubMed]
- Kreutzweiser, D.; Thompson, D.; Grimalt, S.; Chartrand, D.; Good, K.; Scarr, T. Environmental safety to decomposer invertebrates of azadirachtin (neem) as a systemic insecticide in trees to control emerald ash borer. Ecotoxicol. Environ. Saf. 2011, 74, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Coslor, C.C.; Vandervoort, C.; Wise, J.C. Insecticide dose and seasonal timing of trunk injection in apples influence efficacy and residues in nectar and plant parts. Pest Manag. Sci. 2019, 75, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Mach, B.M.; Bondarenko, S.; Potter, D.A. Uptake and dissipation of neonicotinoid residues in nectar and foliage of systemically treated woody landscape plants. Environ. Toxicol. Chem. 2018, 37, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Prado, A.; Pioz, M.; Vidau, C.; Requier, F.; Jury, M.; Crauser, D.; Brunet, J.C.; Conte, J.L.; Alaux, C. Exposure to pollen-bound pesticide mixtures induces longer-lived but less efficient honey bees. Sci. Total Environ. 2019, 650, 1250–1260. [Google Scholar] [CrossRef]

| Location and Date of Injection | AI | Injection Parameters | Injection Method | Days after Treatment (DAT) | Numbering of the Trees | ||||
|---|---|---|---|---|---|---|---|---|---|
| Product (mL/Tree) | Volume (Diluted) (mL/Tree) | AI (g/Tree) | Leaf | Husk and Kernel | Flower | ||||
| Taksony 4 June 2020 (Trial I.) | ABA | 10 | 20 | 0.180 | T | 57; 108; 138 | 108; 483 | 348 | 1; 2;3 |
| ABA | 20 | 20 | 0.360 | T | 57; 108; 138; 348; 463; 483 | 4; 5; 6 | |||
| EMA | 5 | 10 | 0.475 | T | 7; 8; 9 | ||||
| EMA | 10 | 10 | 0.950 | T | 10; 11; 12 | ||||
| C aq. | - | 10 | - | T | 13; 14; 15 | ||||
| C no inj. | - | - | - | - | 16; 17; 18 | ||||
| Szelevény 28 May 2021 (Trial II.) | ABA | 50 | 100 | 0.900 | Y | 35; 71; 106 | 111 | 337 | 19; 20; 21 |
| ABA | 100 | 100 | 1.800 | T | 22; 23; 24 | ||||
| ABA | 100 | 200 | 1.800 | Y | 25; 26; 27 | ||||
| ABA | 200 | 200 | 3.600 | Y | 28; 29; 30 | ||||
| EMA | 20 | 60 | 1.900 | Y | 31; 32; 33 | ||||
| EMA | 40 | 60 | 3.800 | Y | 34; 35; 36 | ||||
| EMA | 60 | 60 | 5.700 | Y | 37; 38; 39 | ||||
| C aq. | - | 100 | - | Y | 40; 41; 42 | ||||
| C no inj. | - | - | - | - | 43; 44; 45 | ||||
| Frequency of Spotty Fruit | 0 | 1–10% | 11–50% | 50–74% | 75–99% | 100% |
| The extent of the largest spot visible on each nut, in the percentage of the husk | 0 | 1–10% | 11–50% | 50–74% | 75–89% | >90% |
| Rating (damage) | 0 | 1 | 2 | 3 | 4 | 5 |
| Economically acceptable (Yes/No) | Y | Y | Y | N | N | N |
| AI Type (Injection Tool) | Injected AI (g/Tree) | Average Rating (0–5) |
|---|---|---|
| ABA(Y) | 0.9 | 2.7 |
| ABA(T) | 1.8 | 2.3 |
| ABA(Y) | 1.8 | 2.0 |
| ABA(Y) | 3.6 | 2.0 |
| EMA(Y) | 1.9 | 1.0 |
| EMA(Y) | 3.8 | 0.7 |
| EMA(Y) | 5.7 | 1.0 |
| C aq. | - | 5.0 |
| C no inj. | - | 5.0 |
| Trial | Treatment (Tool) | Amount of Injected Active Ingredient (g/Tree) | Pesticide Residue in 2nd-Year Flowers (337–348 DAT) Min-Max (ng/g) |
|---|---|---|---|
| Trial I. | ABA (T) | 0.180 | <DL |
| ABA (T) | 0.360 | <DL | |
| EMA (T) | 0.475 | 1.46–2.31 | |
| EMA (T) | 0.950 | 1.84–2.01 | |
| Caq. (T) | - | <DL | |
| C no inj. | - | <DL | |
| Trial II. | ABA (Y) | 0.9 | <DL |
| ABA (T) | 1.8 | <DL | |
| ABA (Y) | 1.8 | <DL | |
| ABA (Y) | 3.6 | <DL | |
| EMA (Y) | 1.9 | 0.39–0.73 | |
| EMA (Y) | 3.8 | 0.64–1.46 | |
| EMA (Y) | 5.7 | 0.28–1.63 | |
| C aq. (Y) | - | <DL | |
| C no inj. | - | <DL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, M.; Sörös, C.; Gutermuth, Á.; Ittzés, A.; Szabó, Á. Avermectin Trunk Injections: A Promising Approach for Managing the Walnut Husk Fly (Rhagoletis completa). Horticulturae 2023, 9, 655. https://doi.org/10.3390/horticulturae9060655
Kiss M, Sörös C, Gutermuth Á, Ittzés A, Szabó Á. Avermectin Trunk Injections: A Promising Approach for Managing the Walnut Husk Fly (Rhagoletis completa). Horticulturae. 2023; 9(6):655. https://doi.org/10.3390/horticulturae9060655
Chicago/Turabian StyleKiss, Máté, Csilla Sörös, Ádám Gutermuth, András Ittzés, and Árpád Szabó. 2023. "Avermectin Trunk Injections: A Promising Approach for Managing the Walnut Husk Fly (Rhagoletis completa)" Horticulturae 9, no. 6: 655. https://doi.org/10.3390/horticulturae9060655
APA StyleKiss, M., Sörös, C., Gutermuth, Á., Ittzés, A., & Szabó, Á. (2023). Avermectin Trunk Injections: A Promising Approach for Managing the Walnut Husk Fly (Rhagoletis completa). Horticulturae, 9(6), 655. https://doi.org/10.3390/horticulturae9060655








