Analysis of the Spatial Dispersion of Tomato Brown Rugose Fruit Virus on Surfaces in a Commercial Tomato Production Site
Abstract
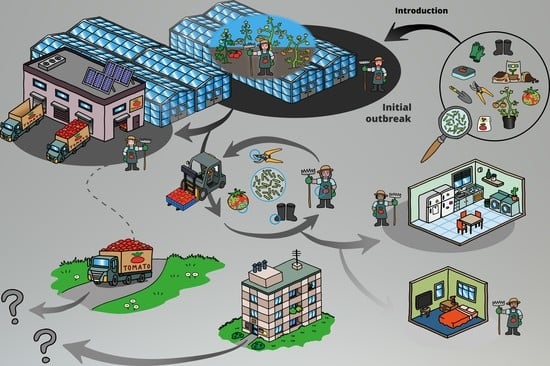
1. Introduction
2. Materials and Methods
2.1. Demarcated Production Site
2.2. Sampling in a Demarcated Tomato Farm
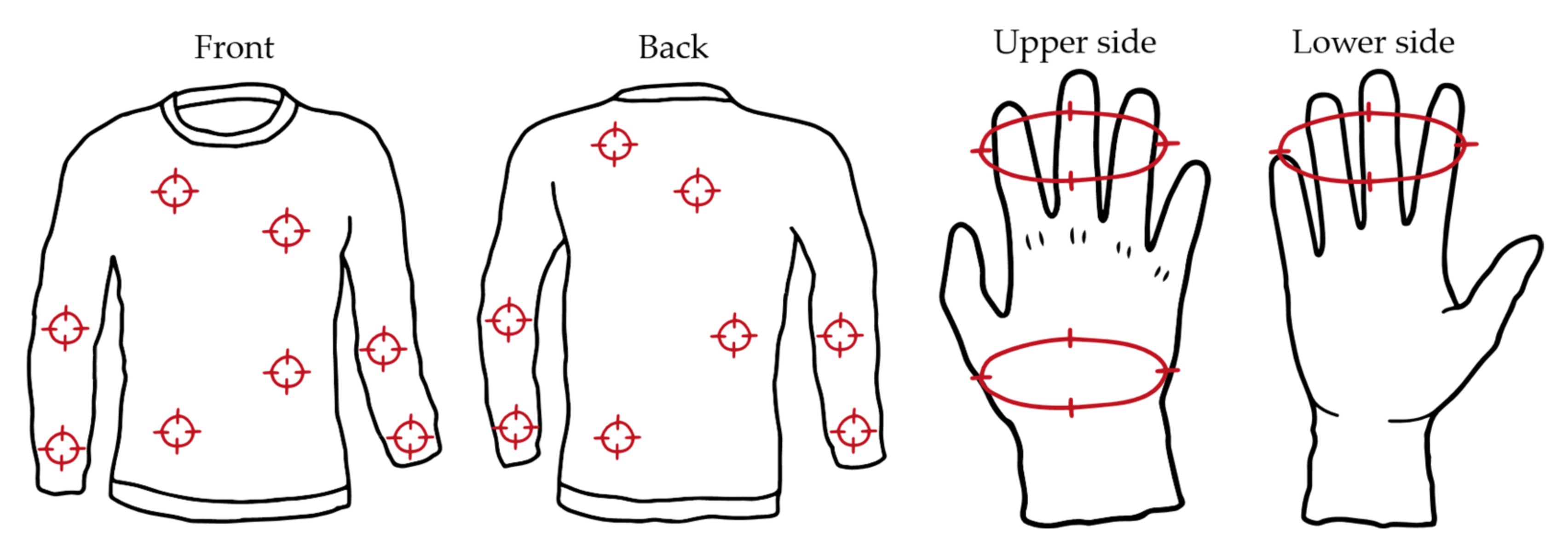
2.2.1. Sampling of Clothing, Protective Items and Other Fabrics
2.2.2. Sampling of Swab Samples
2.3. Detection of ToBRFV by Different Detection Methods
2.3.1. Detection of ToBRFV by RT-PCR
2.3.2. Detection of ToBRFV and Proof of Infectiosity by Bioassay
2.3.3. Detection of ToBRFV by DAS-ELISA
2.4. Statistical Analysis
3. Results
3.1. Detection of ToBRFV by RT-PCR
3.2. Frequency of ToBRFV-Contamination on Surfaces in Different Farm Locations
3.3. Effect of Surface Material on the Frequency of ToBRFV Contamination
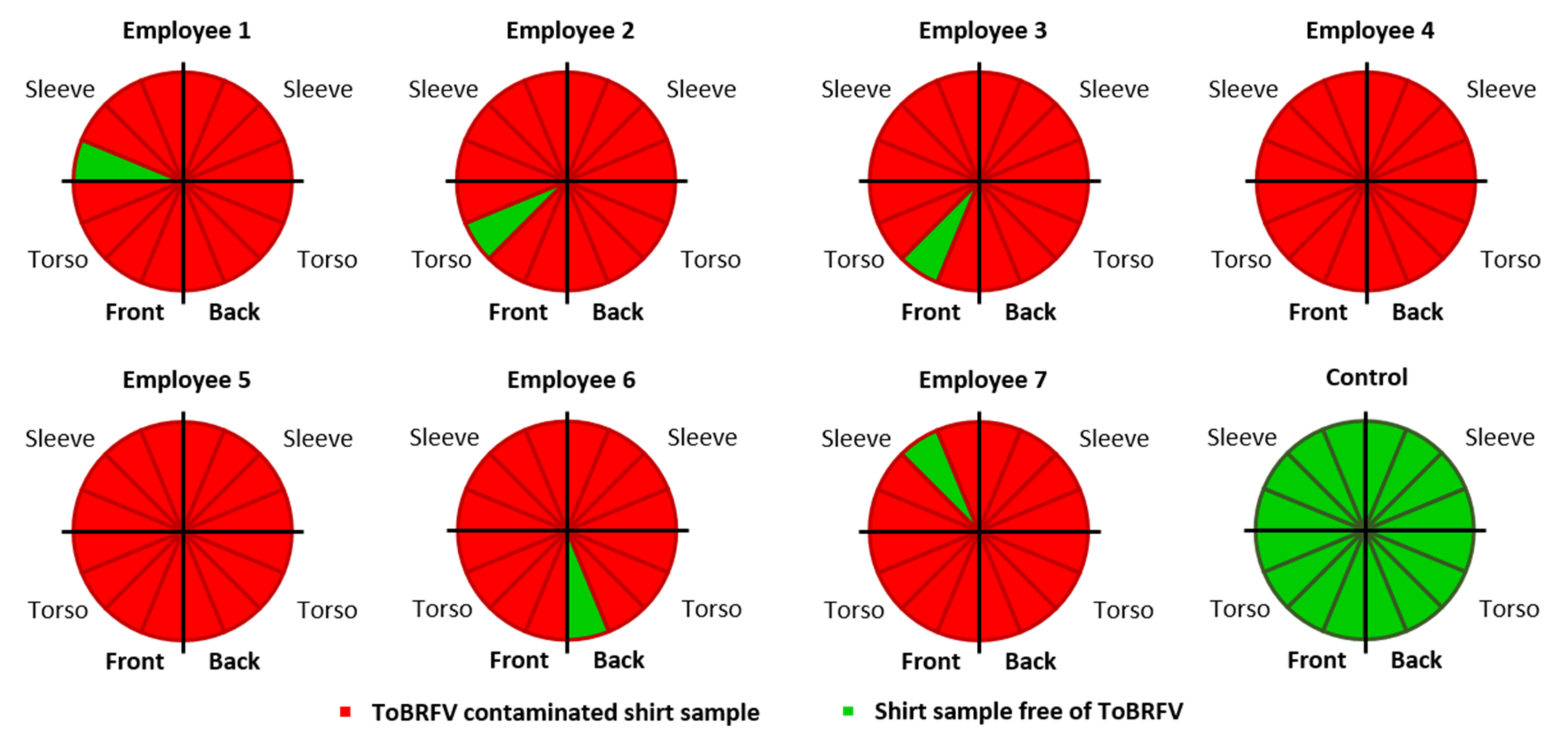
3.4. ToBRFV Contamination on Work Clothing
3.5. ToBRFV Contamination on Protective Items
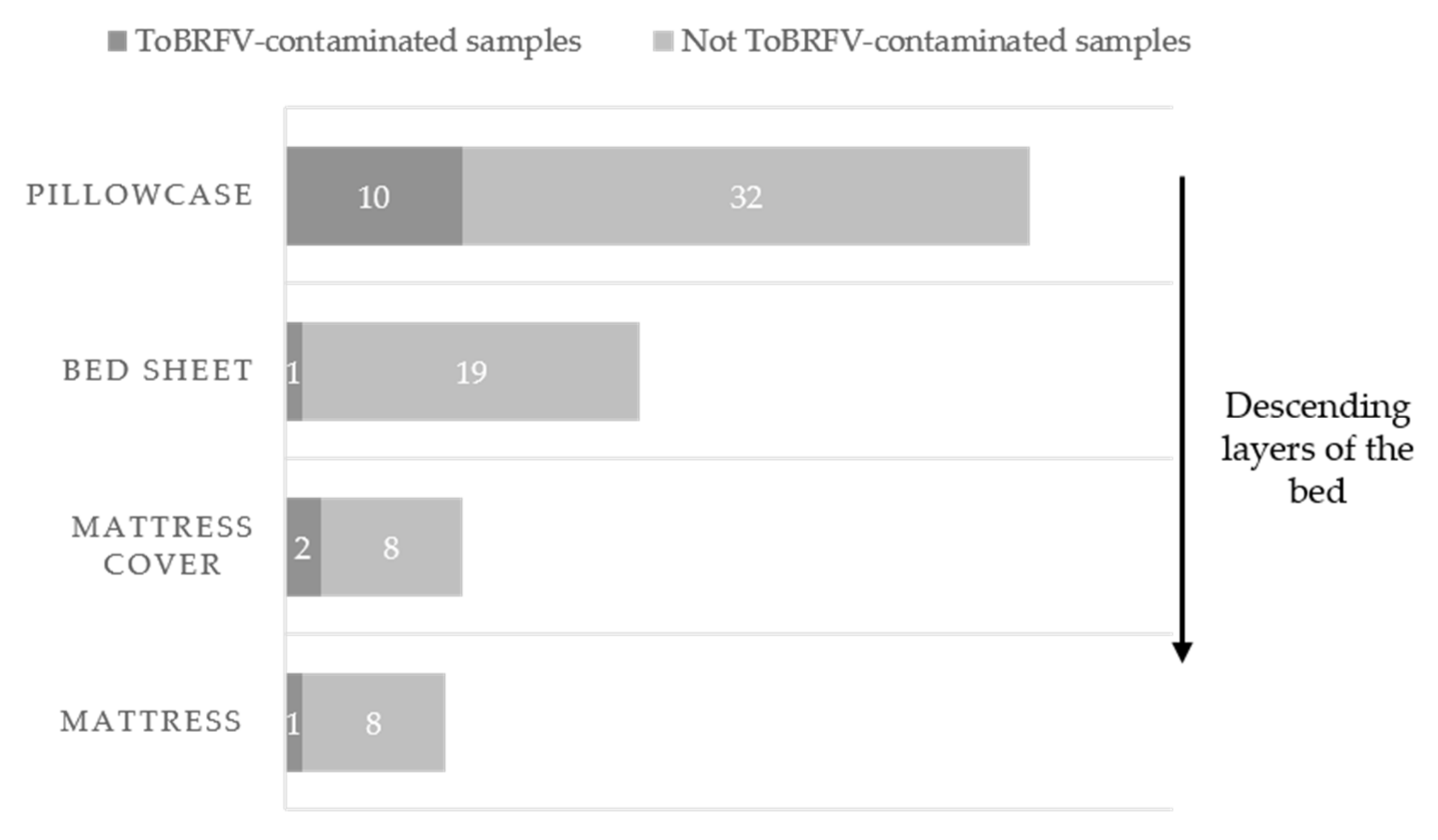
3.6. ToBRFV Contamination on Bed and Linen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Griffiths, J.S.; Marchand, G.; Bernards, M.A.; Wang, A. Tomato brown rugose fruit virus: An emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol. Plant Pathol. 2022, 23, 1262–1277. [Google Scholar] [CrossRef] [PubMed]
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep. 2019, 39, 1. [Google Scholar] [CrossRef]
- Anonymous. Tomato Brown Rugose Fruit Virus (ToBRFV). Auftretensmeldungen. Available online: https://pflanzengesundheit.julius-kuehn.de/tomato-brown-rugose-fruit-virus.html (accessed on 20 February 2023).
- Van der Vlugt, R.; Stijger, C.; Verhoeven, J.; Lesemann, D. First report of Pepino mosaic virus on tomato. Plant Dis. 2000, 84, 103. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlugt, R.A.; Verbeek, M.; Dullemans, A.M.; Wintermantel, W.M.; Cuellar, W.J.; Fox, A.; Thompson, J.R. Torradoviruses. Annu. Rev. Phytopathol. 2015, 53, 485–512. [Google Scholar] [CrossRef]
- Li, R.; Gao, S.; Fei, Z.; Ling, K.-S. Complete genome sequence of a new tobamovirus naturally infecting tomatoes in Mexico. Genome Announc. 2013, 1, e00794-13. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.G.; Bertacca, S.; Parrella, G.; Rizzo, R.; Davino, S.; Panno, S. Tomato brown rugose fruit virus: A pathogen that is changing the tomato production worldwide. Ann. Appl. Biol. 2022, 181, 258–274. [Google Scholar] [CrossRef]
- Roselló, S.; Díez, M.J.; Nuez, F. Viral diseases causing the greatest economic losses to the tomato crop. I. The Tomato spotted wilt virus—A review. Sci. Hortic. 1996, 67, 117–150. [Google Scholar] [CrossRef]
- Polston, J.E.; Anderson, P.K. The emergence of whitefly-transmitted geminiviruses in tomato in the western hemisphere. Plant Dis. 1997, 81, 1358–1369. [Google Scholar] [CrossRef]
- Spence, N.; Basham, J.; Mumford, R.; Hayman, G.; Edmondson, R.; Jones, D. Effect of Pepino mosaic virus on the yield and quality of glasshouse-grown tomatoes in the UK. Plant Pathol. 2006, 55, 595–606. [Google Scholar] [CrossRef]
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A. A new Israeli Tobamovirus isolate infects tomato plants harboring Tm-22 resistance genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef]
- González-Concha, L.F.; Ramírez-Gil, J.G.; Mora-Romero, G.A.; García-Estrada, R.S.; Carrillo-Fasio, J.A.; Tovar-Pedraza, J.M. Development of a scale for assessment of disease severity and impact of tomato brown rugose fruit virus on tomato yield. Eur. J. Plant Pathol. 2023, 165, 579–592. [Google Scholar] [CrossRef]
- Alfaro-Fernández, A.; Castillo, P.; Sanahuja, E.; Rodríguez-Salido, M.; Font, M.I. First report of Tomato brown rugose fruit virus in tomato in Spain. Plant Dis. 2021, 105, 515. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.; Davino, S. First report of tomato brown rugose fruit virus on tomato crops in Italy. Plant Dis. 2019, 103, 1443. [Google Scholar] [CrossRef]
- Eppo. Tomato Brown Rugose Fruit Virus (ToBRFV) Distribution. Available online: https://gd.eppo.int/taxon/TOBRFV/distribution (accessed on 16 February 2023).
- Amer, M.; Mahmoud, S. First report of Tomato brown rugose fruit virus on tomato in Egypt. New Dis. Rep. 2020, 41, 24. [Google Scholar] [CrossRef]
- Hasan, Z.; Salem, N.; Ismail, I.; Akel, E.; Ahmad, A. First report of Tomato brown rugose fruit virus on greenhouse tomato in Syria. Plant Dis. 2022, 106, 772. [Google Scholar] [CrossRef]
- Ghorbani, A.; Rostami, M.; Seifi, S.; Izadpanah, K. First report of Tomato brown rugose fruit virus in greenhouse tomato in Iran. New Dis. Rep. 2021, 44, e12040. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Ma, H.-Y.; Han, S.-L.; Geng, C.; Tian, Y.-P.; Li, X.-D. First report of tomato brown rugose fruit virus infecting tomato in China. Plant Dis. 2019, 103, 2973. [Google Scholar] [CrossRef]
- Sabra, A.; Al-Saleh, M.A.; Al-Shahwan, I.M.; Amer, M.A. First report of Tomato brown rugose fruit virus infecting tomato crop in Saudi Arabia. Plant Dis. 2021, 106, 1310. [Google Scholar] [CrossRef]
- Fidan, H.; Sarikaya, P.; Calis, O. First report of Tomato brown rugose fruit virus on tomato in Turkey. New Dis. Rep. 2019, 39, 18. [Google Scholar] [CrossRef]
- Sarkes, A.; Fu, H.; Feindel, D.; Harding, M.; Feng, J. Development and evaluation of a loop-mediated isothermal amplification (LAMP) assay for the detection of Tomato brown rugose fruit virus (ToBRFV). PLoS ONE 2020, 15, e0230403. [Google Scholar] [CrossRef]
- Camacho-Beltrán, E.; Pérez-Villarreal, A.; Leyva-López, N.; Rodríguez-Negrete, E.; Ceniceros-Ojeda, E.; Méndez-Lozano, J. Occurrence of Tomato brown rugose fruit virus Infecting Tomato Crops in Mexico. Plant Dis. 2019, 103, 1440. [Google Scholar] [CrossRef]
- Ling, K.-S.; Tian, T.; Gurung, S.; Salati, R.; Gilliard, A. First report of tomato brown rugose fruit virus infecting greenhouse tomato in the United States. Plant Dis. 2019, 103, 1439. [Google Scholar] [CrossRef]
- Abrahamian, P.; Cai, W.; Nunziata, S.O.; Ling, K.-S.; Jaiswal, N.; Mavrodieva, V.A.; Rivera, Y.; Nakhla, M.K. Comparative Analysis of Tomato Brown Rugose Fruit Virus Isolates Shows Limited Genetic Diversity. Viruses 2022, 14, 2816. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products. Available online: fao.org/faostat/en/#data/QCL (accessed on 16 February 2023).
- Nowicki, M.; Kozik, E.U.; Foolad, M.R. Late blight of tomato. Transl. Genom. Crop Breed. Biot. Stress 2013, 1, 241–265. [Google Scholar] [CrossRef]
- Avni, B.; Gelbart, D.; Sufrin-Ringwald, T.; Zemach, H.; Belausov, E.; Kamenetsky-Goldstein, R.; Lapidot, M. ToBRFV Infects the Reproductive Tissues of Tomato Plants but Is Not Transmitted to the Progenies by Pollination. Cells 2022, 11, 2864. [Google Scholar] [CrossRef]
- Chanda, B.; Gilliard, A.; Jaiswal, N.; Ling, K.-S. Comparative analysis of host range, ability to infect tomato cultivars with Tm-22 gene and real-time RT-PCR detection of tomato brown rugose fruit virus. Plant Dis. 2021, 105, 3643–3652. [Google Scholar] [CrossRef]
- Fraser, R. The genetics of resistance to plant viruses. Annu. Rev. Phytopathol. 1990, 28, 179–200. [Google Scholar] [CrossRef]
- Creager, A.N.; Scholthof, K.-B.G.; Citovsky, V.; Scholthof, H.B. Tobacco mosaic virus: Pioneering research for a century. Plant Cell 1999, 11, 301–308. [Google Scholar] [CrossRef]
- Castello, J.D.; Rogers, S.O.; Starmer, W.T.; Catranis, C.M.; Ma, L.; Bachand, G.D.; Zhao, Y.; Smith, J.E. Detection of tomato mosaic tobamovirus RNA in ancient glacial ice. Polar Biol. 1999, 22, 207–212. [Google Scholar] [CrossRef]
- Dombrovsky, A.; Mor, N.; Gantz, S.; Lachman, O.; Smith, E. Disinfection Efficacy of Tobamovirus-Contaminated Soil in Greenhouse-Grown Crops. Horticulturae 2022, 8, 563. [Google Scholar] [CrossRef]
- Ehlers, J.; Nourinejhad Zarghani, S.; Kroschewski, B.; Büttner, C.; Bandte, M. Cleaning of Tomato brown rugose fruit virus (ToBRFV) from Contaminated Clothing of Greenhouse Employees. Horticulturae 2022, 8, 751. [Google Scholar] [CrossRef]
- Ehlers, J.; Nourinejhad Zarghani, S.; Kroschewski, B.; Büttner, C.; Bandte, M. Decontamination of Tomato Brown Rugose Fruit Virus-Contaminated Shoe Soles under Practical Conditions. Horticulturae 2022, 8, 1210. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Schmidt, U.; Büttner, C.; Bandte, M. Electrolytic Disinfection of Irrigation Water for Intensive Crop Production in Greenhouses as Demonstrated on Tomatoes (Solanum lycopersicum Mill). Horticulturae 2022, 8, 414. [Google Scholar] [CrossRef]
- Klap, C.; Luria, N.; Smith, E.; Bakelman, E.; Belausov, E.; Laskar, O.; Lachman, O.; Gal-On, A.; Dombrovsky, A. The potential risk of plant-virus disease initiation by infected tomatoes. Plants 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef]
- Salem, N.M.; Sulaiman, A.; Samarah, N.; Turina, M.; Vallino, M. Localization and mechanical transmission of tomato brown rugose fruit virus in tomato seeds. Plant Dis. 2022, 106, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Davino, S.; Caruso, A.G.; Bertacca, S.; Barone, S.; Panno, S. Tomato brown rugose fruit virus: Seed transmission rate and efficacy of different seed disinfection treatments. Plants 2020, 9, 1615. [Google Scholar] [CrossRef]
- Ellouze, W.; Mishra, V.; Howard, R.J.; Ling, K.-S.; Zhang, W. Preliminary study on the control of cucumber green mottle mosaic virus in commercial greenhouses using agricultural disinfectants and resistant cucumber varieties. Agronomy 2020, 10, 1879. [Google Scholar] [CrossRef]
- Van de Vossenberg, B.T.; Dawood, T.; Woźny, M.; Botermans, M. First expansion of the public Tomato brown rugose fruit virus (ToBRFV) Nextstrain build; inclusion of new genomic and epidemiological data. PhytoFrontiers 2021, 1, 359–363. [Google Scholar] [CrossRef]
- Nourinejhad Zarghani, S.; Ehlers, J.; Monavari, M.; von Bargen, S.; Hamacher, J.; Büttner, C.; Bandte, M. Applicability of Different Methods for Quantifying Virucidal Efficacy Using MENNO Florades and Tomato Brown Rugose Fruit Virus as an Example. Plants 2023, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- DSMZ. Double Antibody Sandwich ELISA (DAS-ELISA). Available online: https://www.dsmz.de/fileadmin/_migrated/content_uploads/DAS-ELISA_01.pdf (accessed on 15 March 2022).
- Nourinejhad Zarghani, S.; Monavari, M.; Ehlers, J.; Hamacher, J.; Büttner, C.; Bandte, M. Comparison of Models for Quantification of Tomato Brown Rugose Fruit Virus Based on a Bioassay Using a Local Lesion Host. Plants 2022, 11, 3443. [Google Scholar] [CrossRef]
- Samarah, N.; Sulaiman, A.; Salem, N.; Turina, M. Disinfection treatments eliminated tomato brown rugose fruit virus in tomato seeds. Eur. J. Plant Pathol. 2021, 159, 153–162. [Google Scholar] [CrossRef]
- González-Concha, L.F.; Ramírez-Gil, J.G.; García-Estrada, R.S.; Rebollar-Alviter, Á.; Tovar-Pedraza, J.M. Spatiotemporal analyses of tomato brown rugose fruit virus in commercial tomato greenhouses. Agronomy 2021, 11, 1268. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.G.; Barone, S.; Lo Bosco, G.; Rangel, E.A.; Davino, S. Spread of tomato brown rugose fruit virus in sicily and evaluation of the spatiotemporal dispersion in experimental conditions. Agronomy 2020, 10, 834. [Google Scholar] [CrossRef]
- Reingold, V.; Lachman, O.; Belausov, E.; Koren, A.; Mor, N.; Dombrovsky, A. Epidemiological study of Cucumber green mottle mosaic virus in greenhouses enables reduction of disease damage in cucurbit production. Ann. Appl. Biol. 2016, 168, 29–40. [Google Scholar] [CrossRef]
- Carrington, J.C.; Kasschau, K.D.; Mahajan, S.K.; Schaad, M.C. Cell-to-cell and long-distance transport of viruses in plants. Plant Cell 1996, 8, 1669. [Google Scholar] [CrossRef]
- Broadbent, L.; Fletcher, J. The epidemiology of tomato mosaic: IV. persistence of virus on clothing and glasshouse structures. Ann. Appl. Biol. 1963, 52, 233–241. [Google Scholar] [CrossRef]
- Broadbent, L. Epidemiology and control of tomato mosaic virus. Annu. Rev. Phytopathol. 1976, 14, 75–96. [Google Scholar] [CrossRef]
- Li, R.; Baysal-Gurel, F.; Abdo, Z.; Miller, S.A.; Ling, K.-S. Evaluation of disinfectants to prevent mechanical transmission of viruses and a viroid in greenhouse tomato production. Virol. J. 2015, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.-S.; Gilliard, A.C.; Zia, B. Disinfectants Useful to Manage the Emerging Tomato Brown Rugose Fruit Virus in Greenhouse Tomato Production. Horticulturae 2022, 8, 1193. [Google Scholar] [CrossRef]
- Chanda, B.; Shamimuzzaman, M.; Gilliard, A.; Ling, K.-S. Effectiveness of disinfectants against the spread of to bamo viruses: Tomato brown rugose fruit virus and Cucumber green mottle mosaic virus. Virol. J. 2021, 18, 7. [Google Scholar] [CrossRef]
- Darzi, E.; Lachman, O.; Smith, E.; Koren, A.; Klein, E.; Pass, N.; Frenkel, O.; Dombrovsky, A. Paths of cucumber green mottle mosaic virus disease spread and disinfectant-based management. Ann. Appl. Biol. 2020, 177, 374–384. [Google Scholar] [CrossRef]
- Coutts, B.; Kehoe, M.; Jones, R. Zucchini yellow mosaic virus: Contact transmission, stability on surfaces, and inactivation with disinfectants. Plant Dis. 2013, 97, 765–771. [Google Scholar] [CrossRef]
- Vasickova, P.; Pavlik, I.; Verani, M.; Carducci, A. Issues concerning survival of viruses on surfaces. Food Environ. Virol. 2010, 2, 24–34. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Holmes, F.O. Local lesions in tobacco mosaic. Bot. Gaz. 1929, 87, 39–55. [Google Scholar] [CrossRef]




| Type of Sample | Location | Sampled Object | No. of Samples |
|---|---|---|---|
| Carrier | Greenhouses | T-shirts, gloves, shoe covers | 178 |
| Private accommodation | mattresses, bed cover, pillowcase | 81 | |
| Swab | Greenhouses | Concrete floor, foil, foot mats, rails, pipes, tubes, etc. | 78 |
| Packaging | Foot mat, boxes, weight, blade, trolley, lift truck, etc. | 66 | |
| Shared accommodation | Remote control, fridge, kettle, light switch, cabinet knob, etc. | 114 | |
| Private accommodation | Remote control, door handle, washing basin, wall, etc. | 56 | |
| Vehicles | Car, forklift, tractor | 34 | |
| Office restricted access | Personal electronic devices | 8 |
| Location | Sampled Object | Total | ToBRFV-Contaminated Samples | |
|---|---|---|---|---|
| [n] | [%] | |||
| Greenhouses | Road surface | 18 | 10 | 55.6 |
| Foil | 12 | 8 | 66.7 | |
| Pipes | 6 | 3 | 50.0 | |
| Tracks | 4 | 3 | 75.0 | |
| Machines | 14 | 8 | 57.1 | |
| Foot mat | 4 | 4 | 100 | |
| Construction | 6 | 0 | 0 | |
| Hanging channel | 6 | 1 | 16.7 | |
| Other | 8 | 1 | 12.5 | |
| ∑ | 78 | 38 | 48.7 | |
| Packaging | Foot mat | 20 | 7 | 35.0 |
| Transport boxes | 10 | 2 | 20.0 | |
| Weight | 8 | 3 | 37.5 | |
| Pruning shears | 6 | 6 | 100 | |
| Personal electronic devices | 4 | 0 | 0 | |
| Machines | 18 | 10 | 55.6 | |
| ∑ | 66 | 28 | 40.9 | |
| Shared accommodation | Floor | 18 | 4 | 22.2 |
| Wall | 16 | 0 | 0 | |
| Handle | 30 | 7 | 23.3 | |
| Switches | 24 | 3 | 12.5 | |
| Seat | 10 | 1 | 10.0 | |
| Sink | 6 | 3 | 50.0 | |
| Fabrics | 4 | 0 | 0 | |
| Other | 6 | 3 | 50.0 | |
| ∑ | 114 | 21 | 18.4 | |
| Private accommodation | Switches | 12 | 0 | 0 |
| Handle | 18 | 0 | 0 | |
| Remote control | 6 | 2 | 33.3 | |
| Wall | 8 | 0 | 0 | |
| Sink | 12 | 0 | 0 | |
| ∑ | 56 | 2 | 3.6 | |
| Vehicle | Car | 20 | 1 | 5.0 |
| Forklift | 10 | 2 | 20.0 | |
| Tractor | 4 | 0 | 0 | |
| ∑ | 34 | 3 | 8.8 | |
| Office restricted access | Personal electronic devices | 8 | 0 | 0 |
| Whole farm | All | 356 | 92 | 25.8 |
| Location | Surface Material | ToBRFV-Contaminated Samples | ||
|---|---|---|---|---|
| Total | [n] | [%] | ||
| Greenhouse | Plastic | 26 | 15 | 57.7 |
| Stainless steel | 22 | 8 | 36,4 | |
| Others | 30 | 15 | 50.0 | |
| Chi2 = 2.20 p = 0.333 | ||||
| Packaging | Plastic | 22 | 7 | 31.8 |
| Stainless steel | 24 | 14 | 58,3 | |
| Others | 20 | 7 | 35.0 | |
| Chi2 = 3.95 p = 0.139 | ||||
| Shared accommodation | Plastic | 44 | 11 | 25.0 |
| Stainless steel | 26 | 5 | 19,2 | |
| Others | 44 | 5 | 11,4 | |
| Chi2 = 2.74 p = 0.255 | ||||
| Private accommodation | Plastic | 24 | 2 | 8.3 |
| Stainless steel | 18 | 0 | 0 | |
| Others | 14 | 0 | 0 | |
| Chi2 = 2.202 p = 0.333 | ||||
| ∑ | Plastic | 116 | 35 | 30.2 |
| Stainless steel | 90 | 27 | 30.0 | |
| Others | 108 | 27 | 25.0 | |
| Chi2 = 0.91 p = 0.635 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlers, J.; Nourinejhad Zarghani, S.; Liedtke, S.; Kroschewski, B.; Büttner, C.; Bandte, M. Analysis of the Spatial Dispersion of Tomato Brown Rugose Fruit Virus on Surfaces in a Commercial Tomato Production Site. Horticulturae 2023, 9, 611. https://doi.org/10.3390/horticulturae9050611
Ehlers J, Nourinejhad Zarghani S, Liedtke S, Kroschewski B, Büttner C, Bandte M. Analysis of the Spatial Dispersion of Tomato Brown Rugose Fruit Virus on Surfaces in a Commercial Tomato Production Site. Horticulturae. 2023; 9(5):611. https://doi.org/10.3390/horticulturae9050611
Chicago/Turabian StyleEhlers, Jens, Shaheen Nourinejhad Zarghani, Stefanie Liedtke, Bärbel Kroschewski, Carmen Büttner, and Martina Bandte. 2023. "Analysis of the Spatial Dispersion of Tomato Brown Rugose Fruit Virus on Surfaces in a Commercial Tomato Production Site" Horticulturae 9, no. 5: 611. https://doi.org/10.3390/horticulturae9050611
APA StyleEhlers, J., Nourinejhad Zarghani, S., Liedtke, S., Kroschewski, B., Büttner, C., & Bandte, M. (2023). Analysis of the Spatial Dispersion of Tomato Brown Rugose Fruit Virus on Surfaces in a Commercial Tomato Production Site. Horticulturae, 9(5), 611. https://doi.org/10.3390/horticulturae9050611