Abstract
Volatiles are important quality components in tea (Camellia sinensis) flowers. Albino tea plants are mutant tea plants with diverse abnormal metabolisms. However, whether the metabolisms of volatiles in tea flowers from albino cultivars are abnormal remains unclear. In this study, headspace solid-phase microextraction coupled to gas chromatography-mass spectrometry and aroma evaluation were conducted to investigate the volatile composition and aroma of tea flowers from three albino cultivars (i.e., Baiye No.1, Huangjinya, and Yujinxiang) and one non-albino cultivar (i.e., Jiukeng). The results indicated that tea flowers shared the majority of volatiles but their relative abundances were different. Twelve differential compounds were screened out by partial least squares discriminant analysis. Linalool was the one with the highest relative abundance in three out of the four tea flowers, while acetophenone was the one with the highest relative abundance in tea flowers from Huangjinya. Aroma evaluation indicated that tea flowers from Huangjinya smelt sweetest among them. Partial least squares regression analysis revealed that acetophenone and (R)-1-phenylethanol were positively associated with the sweet smell, while methyl salicylate, 2-heptanol, (E)-2-hexenal, nonanal, and 2-pentanol were positively associated with the green smell. The results enhance our understanding of the volatiles and aroma of tea flowers from albino cultivars.
1. Introduction
Camellia sinensis leaves are raw materials of tea, a beverage consumed worldwide. Recently, albino tea has become popular because of its unique sensory quality. Albino tea is made of leaves from albino tea cultivars, which are special mutants of tea plants with a white or yellow leaf color [1]. There are two main types of albino tea plants. Temperature-sensitive albino tea plants grow albino leaves with a white mesophyll and greenish vein as the environmental temperature is below 20 °C, while light-sensitive albino tea plants grow yellow leaves under a high light intensity [2]. The albino of the leaves is attributed to a lack of chlorophylls [2]. Accumulations of violaxanthin, lutein, and neoxanthin also contribute to the yellow leaf color [2]. Thousands of differentially expressed genes were identified between albino and non-albino tea cultivars by transcriptome analyses, most of which were related to amino acid metabolism, photosynthesis, pigment metabolism, and flavonoid biosynthesis [3]. Li et al. measured the metabolite profiles between the leaves of three albino tea cultivars and two non-albino tea cultivars, and found differences in the galactose metabolism, tryptophan metabolism, phenylpropanoid biosynthesis, and flavonoid biosynthesis [1]. Enrichment of free amino acids is widely-observed in albino tea plants. The content of free amino acids in leaves ranges from 1–4% in non-albino tea plants, while it ranges from 4–11% in albino tea plants [4]. Compared with non-albino tea, albino tea tastes more umami and less bitter and astringent, which remarkably increases its acceptability. Due to the expanding customer demand and their high economic value, an increasing number of tea gardeners have started growing albino tea plants. Together with this, there is increased production of flowers from albino tea plants. As by-products of tea production, the disposal/utilization of Camellia sinensis flowers (tea flowers) is always challenging. At present, tea flowers are applied in the food and cosmetic industries. For example, dried tea flowers are brewed to make beverages or served as additives to improve the flavor and health-benefits of food [5]. Ground tea flower is added in soap to increase its foamability as tea flowers have abundant saponins [5]. However, the market of tea flower products is quite small. Most tea flowers are still regarded as wastes. It is important and urgent to find novel ways to promote the utilization of tea flowers.
Tea flowers usually bloom in autumn and early winter. The yield of tea flowers in mature tea gardens reaches 2.86–4.29 tons/acre [6]. As entomophilous flowers, tea flowers are fragrant, indicating the possibility in the utilization of volatiles. Wu et al. demonstrated that the aroma of tea flowers was strongly attractive to Chinese honeybees and had the potential to be applied in attractants [7]. Bai et al. demonstrated that tea flower essential oil prepared using subcritical water extraction significantly improved the aroma quality of cigarettes [8]. Chen et al. proved that tea flower essential oil prepared by supercritical CO2 extraction possessed free radical scavenging activity and had the potential to be used as antioxidants [9].
The content and composition of volatiles in tea flowers change during floral development [6,10]. Volatiles in tea flowers are present in the highest amount at the half-open period and then gradually decrease. Green volatiles, such as 2-pentanol and 2-heptanol, are enriched in the budding period [6]. These volatiles are largely replaced by floral volatiles in the half-open period, most of which belong to aromatic alcohols, ketones, and aldehydes [6,10]. The cultivar also has an impact on volatiles. Wang measured the volatile components in tea flowers from eight cultivars and concluded that the main volatile constituents of tea flowers were similar among cultivars, but their relative abundances were different [6]. Han et al. analyzed the volatile composition of tea flowers from different cultivars and the results suggested that some volatiles were only detected in certain cultivars [11]. A previous study suggested that tea made of albino leaves showed weak aroma properties because the levels of many key aroma precursors and aroma compounds in albino tea leaves were lower than those in non-albino tea leaves [12]. It implies that there may be differences in the volatile composition between tea flowers from albino cultivars and from non-albino cultivars.
Currently, little is known about the volatile composition and aroma attributes of tea flowers from albino cultivars. Based on previous findings, we hypothesized that the differences in the volatile profile and aroma between tea flowers from albino and non-albino cultivars might be greater than those between tea flowers from different albino cultivars. To figure out whether there were significant differences in the volatile profile and aroma between flowers from albino and non-albino tea cultivars and whether flowers from different albino tea cultivars shared similar volatile profile and aroma, three albino cultivars (Baiye No.1, Huangjinya, and Yujinxiang) and one non-albino cultivar (Jiukeng) were selected. Baiye No.1 is a temperature-sensitive albino tea cultivar [2]. The new shoots of Baiye No.1 are white in spring and they are raw materials of the famous “Anji albino tea”. Huangjinya and Yujinxiang both belong to light-sensitive albino tea cultivars [13,14]. The new shoots of Huangjinya are yellow in spring, summer, and autumn [13], whereas the new shoots of Yujinxiang are yellow in spring and autumn, but green in summer [14]. Jiukeng is a representative non-albino cultivar. The new shoots of Jiukeng are always green. The four cultivars are widely planted in Zhejiang Province, a major tea-producing area in China. Headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (HS–SPME–GC–MS) was applied to analyze volatiles in tea flowers. Headspace solid-phase microextraction (HS–SPME) is a solvent-free method and does not interfere with the sample. It integrates the sampling, extraction, and concentration of the analytes [15]. Compared with the traditional solvent extraction, it has the advantages of portability and high degree of sensitivity [16]. It has been universally used in food, environmental, and even biomedical analysis [17,18]. Aroma evaluation by qualified panelists was conducted to describe the aroma attributes of these tea flowers. The results enhance our understanding of the volatile composition and aroma of tea flowers from albino cultivars and may guide manufacturers in selecting suitable tea flowers for specific utilization.
2. Materials and Methods
2.1. Tea Flower Samples
Dried tea flower samples were provided by Yuyao Sichuangyan Tea Co. Ltd. (Zhejiang, China). The tea flowers were from four cultivars, including one temperature-sensitive albino cultivar (Baiye No.1), two light-sensitive albino cultivars ((Huangjinya and Yujinxiang), and one non-albino cultivar (Jiukeng), respectively. They were collected from the same tea garden in Yuyao City, Zhejiang Province, China, in November. Half-open tea flowers were manually plucked, withered for 4 h, dried by hot air at 40 °C for 1 h, cooled for 30 min, and dried by hot air at 55 °C for 1h and at 70 °C for 1 h to obtain dried tea flowers. The dried tea flowers were stored at 4 °C. The weight of fresh tea flowers in each batch was 50 kg. Three batches of samples were prepared for each cultivar.
2.2. HS–SPME–GC–MS Analysis
HS–SPME–GC–MS was conducted according to a previously published method [19]. A divinylbenzene/carboxen/polydimethylsiloxane SPME needle (Product No. 57348-U, Supelco, Bellefonte, PA, USA) was used for the extraction. An Agilent 6890 gas chromatograph equipped with a DB-5MS capillary column (30 m × 250 μm × 0.25 μm) and coupled with an Agilent HP 5973 mass selective detector (Agilent, Wilmington, DE, USA) was used for the determination.
A 50 mL glass vial was quickly sealed and water-bathed at 60 °C after adding 0.5 g of tea flower powder, 5 mL of boiling water, and 30 μL of ethyl caprate into it. The SPME needle, which was pretreated by heating at 250 °C for 10 min to remove residual volatiles, was inserted into the glass vial through the cap to absorb volatiles for 60 min and then inserted into the injection port of GC to desorb volatiles at 250 °C for 5 min. The GC inlet temperature was 250 °C, the split ratio was 15:1, the carrier gas (high purity helium) flow was 1.0 mL/min, and the linear flow velocity of carrier gas was 40 cm/s. The gradient changes in the column temperatures were 0–2 min, 40 °C; 2–24.5 min, 40–85 °C; 24.5–26.5 min, 85 °C; 26.5–64.5 min, 85–180 °C; 64.5–66.5 min, 180 °C; 66.5–71.5 min, 180–230 °C; and 71.5–73.5 min, 230 °C. The MS conditions were as follows: the temperature of the ion source of 230 °C, the voltage of 70 eV, and the scan ranging from m/z 40 to 400. Tentative identification was made with the National Institute for Standards and Technology database (NIST 08, match percentage >80%) and the retention index of n-alkanes (C6-C15). The relative abundance of a specific compound was determined by comparing the peak area between the compound and total compounds.
2.3. Aroma Evaluation
The aroma quality of each tea flower sample was assessed according to the method described in the national standard GB/T 23776-2018 with some modifications. Briefly, 1.5 g tea flowers were brewed in 150 mL of boiling water for 5 min and then the aroma was evaluated by seven qualified panelists. The panelists had been issued certificates for tea-quality evaluation from the Tea Scientific Society of China and had extensive experience in sensory evaluation. The intensities of the aroma attributes were scored. The score ranged from 0 to 10, indicating “extremely weak” to “extremely strong” intensities. Each evaluation was replicated thrice on different days with a randomized order of samples.
2.4. Statistical Analysis
The data are presented as the mean ± standard error of the mean (SEM). All the experiments were carried out in triplicate and repeated in three independent sets of experiments. The partial least squares discriminant analysis (PLS-DA), hierarchical clustering analysis, and partial least squares regression (PLSR) were performed using the SIMCA-P 13.0 software (Umetric, Umea, Sweden). The results were analyzed with SPSS Version 18.0 for Windows using the one-way analysis of variance with the 2-sided Dunnett’s post hoc test to assess differences among groups. p-values of <0.05 were considered to be statistically significant.
3. Results and Discussion
3.1. Volatile Composition of Tea Flowers from Different Cultivars
The diversity of volatiles in Camellia sinensis flowers was high compared with that in flowers of some other Camellia plants. Gan et al. compared the volatile composition of flowers from three members in genus Camellia, including Camellia japonica, Camellia oleifera Abel., and Camellia sinensis, by the HS–SPME–GC–MS method [20]. The numbers of volatiles observed in the three plants were 26, 36, and 48, respectively [20]. According to our results of HS–SPME–GC–MS (Table 1), the numbers of volatiles in tea flowers from Baiye No.1, Huangjinya, Yujinxiang, and Jiukeng were 45, 44, 44, and 48, respectively, indicating that tea flowers from the three albino cultivars contained less diverse volatiles than tea flowers from the non-albino cultivar Jiukeng. Thirty-six volatiles were found in all the four samples. Tea flowers from Yujinxiang had more types of unique volatiles than other samples. The above results were consistent with previous findings in non-albino tea flowers that tea flowers from different cultivars shared multiple common volatiles, but some volatiles were cultivar-specific [6,11].

Table 1.
Volatile compounds in tea flowers determined by GC-MS.
Previous researches suggested that categories of volatiles observed in tea flowers were dependent on the extraction methods. The composition of the volatile components extracted by simultaneous distillation extraction, supercritical fluid extraction, and HS–SPME differed considerably [21]. There were significant losses of heat-sensitive volatiles in tea flowers during simultaneous distillation extraction [21]. Alkanes and acids were the main components in tea flower essential oil prepared with petroleum ether by simultaneous distillation extraction [22]. Alkanes (45.4%), esters (10.5%), and ketones (7.1%) made up about 60% of the total volatiles in tea flower essential oil prepared by supercritical fluid extraction [9]. Volatile analysis using the HS–SPME revealed that alcohols, ketones, and aldehydes occupied 43.47%, 24.64%, and 22.95% of total volatiles in tea flowers [23]. In this research, HS–SPME was chosen because of its high degree of sensitivity and convenience. Our results were partially consistent with Chen et al.’s results [23]. The volatiles observed in the four tea flower samples belonged to alcohols, ketones, aldehydes, terpenes, esters, S-containing compounds, and some other categories (Table 2). Alcohols and ketones were the main classes. Alcohols were the most abundant class of volatiles in tea flowers from Baiye No.1, Yujinxiang, and Jiukeng. The relative abundance of alcohols accounted for over 50% in each of the three samples. In contrast, ketones were the most abundant class, accounting for 44% of total volatiles in tea flowers from Huangjinya. Notably, although the relative abundance of ketones was high in tea flowers, the type of ketones was rather limited (Table 2). In contrast to ketones, the variety of aldehydes was much richer. As the third abundant class of volatiles in tea flowers, the relative abundance of aldehydes ranged from 8.75% to 16.54%, with the lowest relative abundance detected in tea flowers from Yujinxiang. Tea flowers from Yujinxiang also possessed the lowest relative abundances of S-containing compounds and esters, while they possessed the highest relative abundance of terpenes among the four samples. These data revealed that the relative abundance of each category of volatiles varied among the four samples.

Table 2.
Relative abundances and numbers of each category of volatiles in tea flowers.
A PLS-DA model based on the volatile composition of the four samples was established. The R2X (cum), R2Y (cum), and Q2(cum) of the PLS-DA model were 0.963, 0.972, and 0.914, respectively, suggesting a good fit for the model. The PLS-DA score plot (Figure 1A) presented the differences of the four samples on the volatile profile in a visual way. Points representing different tea flower samples lay in different quadrants, suggesting that the volatile profile of each sample was distinctive. To classify the four samples, hierarchical clustering analysis was conducted. The results revealed that the volatile profile of tea flowers from Huangjinya, locating in an isolated cluster, was more distinctive than that of the other three samples (Figure 1B). It hinted that the difference in the volatile composition between tea flowers from the three albino cultivars and from the non-albino Jiukeng cultivar was not bigger than that inside the three albino cultivars. A previous study revealed that there was a significant difference in the aroma composition between tea leaves from albino and non-albino cultivars [24]. The accumulation of carotenoids in albino tea leaves led to an increase of flowery-fruity-like volatiles derived from carotenoid degradation [24]. Another study revealed that purple tea flowers contained higher contents of volatile benzenoid-phenylpropanoids than white tea flowers because of the enhanced shikimate pathway [25]. However, in this study, the difference in the volatile composition between tea flowers from albino and non-albino cultivars was not as obvious as we assumed. One of the reasons might be that the flowers used for this work were collected in the peak flowering stage (November), when the leaves of albino tea plants were green and the metabolisms inside the plants were less abnormal. The metabolisms which were associated with the biosynthesis of key volatiles in tea flowers, such as the metabolisms of amino acids, changed in different stages of the albino [26]. Shen et al. confirmed that the volatile composition of leaves collected from different albino stages was varied [27]. Further research studies are needed to verify whether a similar phenomenon happens or not in tea flowers.
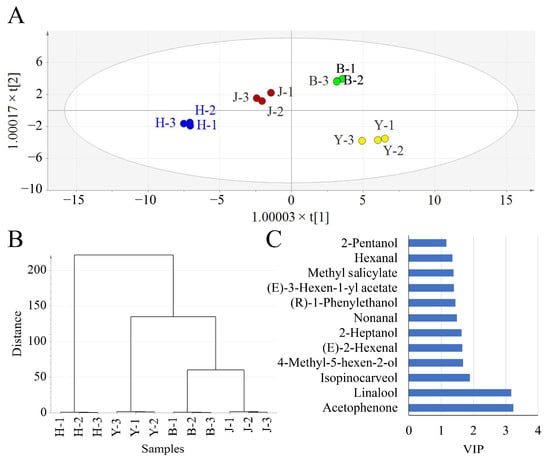
Figure 1.
Partial least squares discriminant analysis (PLS-DA) and hierarchical clustering analysis. (A) The PLS-DA plot. (B) The hierarchical clustering analysis dendrogram. (C) Compounds with variable importance in projection (VIP) > 1. B, H, Y, J are short for Baiye No.1, Huangjinya, Yujinxiang, and Jiuekeng, respectively.
To explore the differential volatiles, compounds with variable importance in projection (VIP) > 1 were screened out (Figure 1C). A total of 12 volatiles were screened as differential volatiles. Among these compounds, six compounds belonged to green/grass volatiles and three compounds belonged to floral volatiles, implying that the four samples might have differences in terms of the green and floral aroma attributes. It was noteworthy that the VIP values of linalool and acetophenone were much higher than for the others.
Linalool, a key active odorant of several aromatic species such as rose and lavender [28] was identified as a predominant volatile in tea flowers [10]. Linalool has a pleasant floral scent and is widely used in perfumes, cleaning agents, skincare/cosmetic products, and food to improve the aroma. Previous research revealed that the level of linalool was much lower in albino tea leaves than that in non-albino tea leaves due to the shortage of geranyl diphosphate, which was a precursor of linalool [12]. In this study, the relative abundance of linalool in tea flowers from the non-albino Jiukeng was not higher than that in tea flowers from the three albino cultivars (Table 1), implying differential metabolisms between flowers and leaves of albino tea plants. The relative abundance of linalool varied from 18.50% to 55.08% among samples. The relative abundance of linalool in tea flowers from Yujinxiang was almost thrice as much as that from Huangjinya, which might lead to differences in the aroma.
In contrast, the relative abundance of acetophenone in tea flowers from Yujinxiang was much lower than that in tea flowers from Huangjinya (Table 1). Acetophenone is derived from L-phenylalanine and regarded as an important endogenous volatile in tea flowers [29]. It smells sweet, with an almond and hawthorn scent. Based on our results, acetophenone was the major volatile ketone in tea flowers. The relative abundances of acetophenone in the four flower samples was 17.37–42.99%, which was similar to previous recordings in non-albino tea flowers [6]. Unlike the other three tea flowers, acetophenone rather than linalool was the one with the highest relative abundance in tea flowers from Huangjinya. And the relative abundance of acetophenone in tea flowers from Huangjinya was higher than that in the other three samples.
Apart from being an important volatile in tea flowers, acetophenone plays a role as the precursor of (R)-1-phenylethanol [29]. Like acetophenone, (R)-1-phenylethanol is also regarded as one of the key volatiles in tea flowers [21,29]. (R)-1-phenylethanol smells like honeysuckle. The relative abundance of (R)-1-phenylethanol in tea flowers was much lower than that of acetophenone, with a range of 0.64–6.98%. In particular, its relative abundance in tea flowers from Yujinxiang was merely 10% of that in tea flowers from Huangjinya. Previous research indicated that a considerable amount of (R)-1-phenylethanol was modified into nonvolatile glycosides in tea flowers, leading to a reduction of free (R)-1-phenylethanol [29]. The accumulation of (R)-1-phenylethanol was also affected by the availability of acetophenone and the activity of 1-phenylethanol synthase, an enzyme which facilitated the conversion of (R)-1-phenylethanol from acetophenone [30]. Tea flowers from Huangjinya contained abundant acetophenone. Therefore, it was not surprising that they also had abundant (R)-1-phenylethanol. Tea flowers from Yujinxiang did not lack acetophenone. The low level of (R)-1-phenylethanol in tea flowers from Yujinxiang might be caused by the disabled 1-phenylethanol synthase or the over-activity of enzymes which were related to the formation of (R)-1-phenylethanol glycosides.
Taken together, the types of volatiles in tea flowers from different cultivars were similar. Only a very small number of volatiles were present in samples from specific cultivars. However, the relative abundances of volatiles were different, leading to a distinctive volatile profile of each tea flower sample. In the next section, sensory evaluation was conducted to assess whether the differential volatile profiles led to differences in the aroma quality.
3.2. Aroma Attributes of Tea Flowers from Different Cultivars
Aroma, being a sensation associated with smell, requires the presence of volatiles. Different combinations of volatiles form different aromas. To compare the aroma attributes among the four tea flower samples, sensory evaluation was carried out. Sweet, flora, and green were the three main aroma attributes of tea flowers (Figure 2A). Tea flowers from Huangjinya smelt really sweet and had a honey-like aroma. Tea flowers from Yujinxiang smelt less sweet but were more floral than tea flowers from Huangjinya. The other two tea flowers not only had typical sweet and floral aroma but also a slightly refreshing tone. Ji et al. found that the aroma of green tea made of the non-albino cultivar (Longjing No. 43) was more green than the aroma of green teas made of albino cultivars (Yujinxiang and Huangjinya) [31]. Compared with the aroma of Huangjinya leaves, the aroma of Yujinxiang leaves smelt brisker and more tender [31]. Therefore, it was not surprising that tea flowers from Huangjinya and Yujinxiang smelt less green than tea flowers from Jiukeng. Linalool was considered as a key volatile related to the tender aroma of green tea [32]. Although tea flowers did not have a tender aroma, the relative abundance of linalool in Yujinxiang flowers was high (Table 1), indicating that the enrichment of linalool might not only exist in the leaves but also in the flowers of Yujinxiang tea plants.
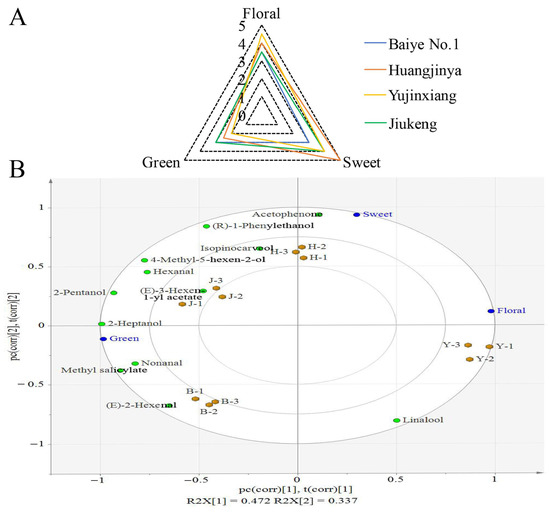
Figure 2.
Aroma evaluation and partial least squares regression (PLSR) analysis. (A) Aroma attributes of tea flowers. (B) The PLSR correlation loading plot. The green dots represent the differential compounds, the blue dots represent aroma attributes, and the yellow dots represent tea flower samples. B, H, Y, J are short for Baiye No.1, Huangjinya, Yujinxiang, and Jiuekeng, respectively.
To study the associations among the four samples, their aroma attribute, and their volatile composition, PLSR analysis was performed. The relative abundances of 12 differential volatiles among groups were defined as the X variables and the scores of aroma attributes were defined as the Y variables. The biplot based on the results of PLSR analysis gives a general description of their associations (Figure 2B). A short distance between dots indicates a close relationship. For example, the “sweet” dot was near the “Huangjinya” dots, indicating that “Huangjinya” smelt sweeter than the others. The “(E)-3-hexen-1-yl acetate” dot was closer to the “Jiukeng” dots than dots representing other tea flowers, indicating that the compound was a volatile featuring in “Jiukeng” tea flowers. The “linalool” dot was the only one which was located in the same quadrant with the “Yujinxiang” dots, indicating that linalool was a key differential volatile between “Yujinxiang” and the other tea flowers. The “acetophenone” dot was close to the “sweet” dot, indicating that acetophenone might contribute to the sweet aroma. In contrast, the “(E)-2-hexenal” dot was far from the “sweet” dot and located in the diagonally opposite quadrant, indicating that the compound might have an adverse impact on the sweet aroma. Although the biplot outlined the associations, it did not provide the details.
To screen out volatiles which might have a crucial impact on a specific aroma attribute, PLSR analysis between one specific aroma attribute and the relative abundances of the 12 differential volatiles was carried out. The VIP values were calculated (Table 3). Compounds with VIP > 1 were considered as key compounds. The coefficient between the key compound and the specific attribute was calculated to assess whether the compound was positively or negatively correlated to the specific attribute (Table 3). The results indicated that 2-heptanol, methyl salicylate, 2-pentanol, nonanal, 4-methyl-5-hexen-2-ol, and (E)-2-hexenal were positively associated with the green smell and negatively associated with the floral smell of tea flowers. Acetophenone and (R)-1-phenylethanol were positively associated with the sweet smell of tea flowers, while (E)-2-hexenal, linalool, and methyl salicylate were negatively associated with the sweet smell of tea flowers. It was unexpected that linalool, the floral volatile with a high relative abundance in tea flowers, did not show a significant correlation to the floral aroma. It was previously proved that the contribution of an aroma component to the odor intensity and quality in a mixture was not only related to the content of the aroma component but also related to its perceptual interactions with other aroma components [33,34,35]. Generally, compounds with similar structures or aroma were more likely to present a synergistic or additive effect, while compounds with different structures tended to have a weak additive or masking effect [35]. For example, methyl salicylate had a masking effect on floral volatiles, including linalool, phenyl acetaldehyde, geraniol, and phenethyl alcohol [33]. (E)-2-Hexenal had synergistic effects with (E)-2-heptanal and hexanal [35]. Some esters and alcohols, although they had little direct effect on the aroma, had an impact on the olfactory threshold of the fruity pool of red wine [36,37]. It is very likely that perceptive interactions are also present among volatiles in tea flowers. Further studies are required to investigate it.

Table 3.
The variable importance in projection (VIP) scores and coefficients for the partial least squares regression model based on the relative abundances of differential volatiles with scores of each aroma attribute.
4. Conclusions
In this study, the volatile composition and aroma attributes of tea flowers from three albino cultivars and one non-albino cultivar were compared. It turned out that the difference between tea flowers from the three albino cultivars and from the non-albino Jiukeng was not bigger than that within the three albino cultivars. Tea flowers from the three albino tea cultivars and from the non-albino Jiukeng cultivar shared the majority of volatiles but their relative abundances were different. Tea flowers from Yujinxiang had the highest relative abundance of linalool among the four samples. High relative abundances of acetophenone and (R)-1-phenylethanol were features of tea flowers from Huangjinya and were associated with the sweet aroma of tea flowers. Tea flowers from Baiye No.1 and Jiukeng contained higher relative abundances of several green/grassy volatiles, such as 2-heptanol, methyl salicylate, and (E)-2-hexenal, and smelt more refreshing than the other two. Our current results provide a preliminary understanding of the differences on the volatiles and aroma between tea flowers from different cultivars. More experiments are needed to verify the aroma-active compounds in tea flowers and explore the possible perceptual interactions between them in the future.
Author Contributions
Conceptualization, Y.G., Y.X. and J.Y.; methodology, Y.G.; formal analysis, Y.G. and Y.C.; investigation, Y.G., Y.C., F.W., J.C., G.C.; writing—original draft preparation, Y.G.; writing—review and editing, Y.X. and J.Y.; supervision, Y.X., and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32202114), the China Agriculture Research System of MOF and MARA (CARS-19), and the Innovation Project for the Chinese Academy of Agricultural Sciences.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Zhen Han from the Department of Research and Promotion, Agro-Technical Extension Station of Ningbo City for his help in preparing the tea flower samples for the study. We thank Jieqiong Wang from the Tea Research Institute Chinese Academy of Agricultural Sciences for her technical support on GC-MS. We also thank Sho Hirano and Yuta Jinguji, as their music and performance inspired the author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Li, C.F.; Ma, J.Q.; Huang, D.J.; Ma, C.L.; Jin, J.Q.; Yao, M.Z.; Chen, L. Comprehensive dissection of metabolic changes in albino and green tea cultivars. J. Agric. Food Chem. 2018, 66, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Yang, R.; Shi, Y.L.; Li, X.M.; Fu, Q.Y.; Lu, J.L.; Ye, J.H.; Wang, K.R.; Ma, S.C.; Zheng, X.Q.; et al. Light-sensitive albino tea plants and their characterization. HortScience 2018, 53, 144–147. [Google Scholar] [CrossRef]
- Wang, L.; Yue, C.; Cao, H.; Zhou, Y.; Zeng, J.; Yang, Y.; Wang, X. Biochemical and transcriptome analyses of a novel chlorophyll-deficient chlorina tea plant cultivar. BMC Plant Biol. 2014, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Mei, J.F.; Wang, J.Y.; Tang, R.J.; Chen, L.; Ma, C.L. Research progress on albino trait of tea plant. China Tea 2020, 42, 24–35. [Google Scholar]
- Yin, J.; Fu, Z.; Xu, Y. Tea as a Food Ingredient: Properties, Processing, and Health Aspects, 1st ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2022; 380p. [Google Scholar]
- Wang, L.L. Study on Aroma Components of Tea (Camellia Sinensis) Flowers; Zhejiang Gongshang University: Hangzhou, China, 2008. [Google Scholar]
- Wu, G.H.; Cui, L.; Wang, M.X.; Li, H.H.; Han, B.Y. Attraction of aroma from tea flowers and leaves to the chinese honeybees (Apiscerana cerana). Acta Ecol. Sin. 2020, 40, 4024–4031. [Google Scholar]
- Bai, X.L.; Kong, L.Y.; Gong, R.G.; Peng, G.G.; Zhang, Y.G.; Duan, K. Study on the antioxidant effects of the tea flower essential oils by different extraction and applied to the tobacco. Food Ind. 2013, 34, 110–113. [Google Scholar]
- Chen, Z.; Mei, X.; Jin, Y.; Kim, E.H.; Yang, Z.; Tu, Y. Optimisation of supercritical carbon dioxide extraction of essential oil of flowers of tea (Camellia sinensis L.) plants and its antioxidative activity. J. Sci. Food Agric. 2014, 94, 316–321. [Google Scholar] [CrossRef]
- Joshi, R.; Poonam; Gulati, A. Biochemical attributes of tea flowers (Camellia sinensis) at different developmental stages in the kangra region of india. Sci. Hortic. 2011, 130, 266–274. [Google Scholar] [CrossRef]
- Han, B.; Zhou, P.; Cui, L.; Fu, J.Y.; Jain, N.K. Characterization of the key aromatic constituents in tea flowers of elite chinese tea cultivars. Int. J. Tea Sci. 2007, 6, 31–36. [Google Scholar]
- Dong, F.; Zeng, L.; Yu, Z.; Li, J.; Tang, J.; Su, X.; Yang, Z. Differential accumulation of aroma compounds in normal green and albino-induced yellow tea (Camellia sinensis) leaves. Molecules 2018, 23, 2677. [Google Scholar] [CrossRef]
- Wang, K.R.; Li, M.; Liang, Y.R.; Zhang, L.J.; Shen, L.M.; Wang, S.B. Study on the breeding of a novel tea cultivar “huangjinya”. China Tea 2008, 4, 21–23. [Google Scholar]
- Wang, K.R.; Han, Z.; Liang, Y.R.; Zhang, L.J.; Li, M.; Wang, S.B. Study on the breeding of a novel light-sensitive albino tea cultivar “yujinxiang”. China Tea 2013, 6, 24–25. [Google Scholar]
- Gherghel, S.; Morgan, R.M.; Arrebola-Liebanas, J.; Romero-Gonzalez, R.; Blackman, C.S.; Garrido-Frenich, A.; Parkin, I.P. Development of a hs-spme/gc-ms method for the analysis of volatile organic compounds from fabrics for forensic reconstruction applications. Forensic. Sci. Int. 2018, 290, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Applications of solid phase microextraction. J. Agric. Food Chem. 1999, 46, 3721–3726. [Google Scholar]
- Roszkowska, A.; Miekus, N.; Baczek, T. Application of solid-phase microextraction in current biomedical research. J. Sep. Sci. 2019, 42, 285–302. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Microextraction (spme) in food and environmental analysis—A review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Q.Q.; Chen, Y.H.; Granato, D.; Wang, J.Q.; Yin, J.F.; Zhang, X.B.; Wang, F.; Chen, J.X.; Xu, Y.Q. Effects of the baking process on the chemical composition, sensory quality, and bioactivity of tieguanyin oolong tea. Front. Nutr. 2022, 9, 881865. [Google Scholar] [CrossRef]
- Gan, X.H.; Liang, Z.Y.; Wang, D.P.; Wang, R. Analysis of aroma components in flowers of three kinds of camellia by hs-spme/gc-ms. Food Sci. 2013, 34, 204–207. [Google Scholar]
- Joshi, R.; Poonam; Saini, R.; Guleria, S.; Babu, G.D.; Kumari, M.; Gulati, A. Characterization of volatile components of tea flowers (camellia sinensis) growing in kangra by gc/ms. Nat. Prod. Commun. 2011, 6, 1155–1158. [Google Scholar]
- Gu, Y.P.; Qian, H. Analysis and microencapsulation of the essential oil from the flowers of camellia sinensis. Food Res. Dev. 2008, 29, 187–190. [Google Scholar]
- Chen, L.H.; Lv, X.; Wei, H.; Mao, W.L.; Li, Y.R. Analysis of aroma components in flower tea of camellia sinensis by headspace solid phase microextraction combined with gas chromatography-mass spectrometry. J. Food Saf. Qual. 2021, 12, 115–121. [Google Scholar]
- Mei, X.; Lin, C.; Wan, S.; Chen, B.; Wu, H.; Zhang, L. A comparative metabolomic analysis reveals difference manufacture suitability in “yinghong 9” and “huangyu” teas (Camellia sinensis). Front. Plant Sci. 2021, 12, 767724. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wan, S.; Lin, C.; Zhou, C.; Hu, L.; Deng, C.; Zhang, L. Integration of metabolome and transcriptome reveals the relationship of benzenoid-phenylpropanoid pigment and aroma in purple tea flowers. Front. Plant Sci. 2021, 12, 762330. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Analysis of young shoots of ‘anji baicha’ (Camellia sinensis) at three developmental stages using nontargeted lc-ms-based metabolomics. J. Food Sci. 2019, 84, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, X.Q.; Liu, X.X.; Liu, Z.Y.; He, P.; Zheng, W.J. Aroma components analysis of zheng’an white tea in different stages of whitening process. Food Sci. Technol. 2021, 46, 276–282. [Google Scholar]
- Russo, E.B.; Marcu, J. Chapter three cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar]
- Dong, F.; Yang, Z.; Baldermann, S.; Kajitani, Y.; Ota, S.; Kasuga, H.; Imazeki, Y.; Ohnishi, T.; Watanabe, N. Characterization of l-phenylalanine metabolism to acetophenone and 1-phenylethanol in the flowers of camellia sinensis using stable isotope labeling. J. Plant Physiol. 2012, 169, 217–225. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Q.; Zeng, L.; Tang, J.; Li, J.; Dong, F.; Yang, Z. Study of the biochemical formation pathway of aroma compound 1-phenylethanol in tea (Camellia sinensis (L.) o. Kuntze) flowers and other plants. Food Chem. 2018, 258, 352–358. [Google Scholar] [CrossRef]
- Ji, W.B.; Cui, J.X.; Yin, J.; Wang, Z.; Yu, B. Introduction trials of four chlorosis-specific tea cultivars in maoshan area. China Tea 2021, 43, 49–55. [Google Scholar]
- Shu, C. Characterization of Aroma-Active Components of Longjing Tea; Shanghai Institute of Technology: Shanghai, China, 2016. [Google Scholar]
- Niu, Y.; Ma, Y.; Xiao, Z.; Zhu, J.; Xiong, W.; Chen, F. Characterization of the key aroma compounds of three kinds of chinese representative black tea and elucidation of the perceptual interactions of methyl salicylate and floral odorants. Molecules 2022, 27, 1631. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, J.; Niu, Y.; Wang, P.; Wang, R.; Sun, X. Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution. Food Res. Int. 2019, 116, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Xiao, Z. Evaluation of the synergism among volatile compounds in oolong tea infusion by odour threshold with sensory analysis and e-nose. Food Chem. 2017, 221, 1484–1490. [Google Scholar] [CrossRef]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J.C. Olfactory impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.C. Study of sensory interactions among red wine fruity esters in a model solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).