Isolation of FLOWERING LOCUS C and Preliminary Characterization in the Floral Transition of Xinjiang Precocious Walnut
Abstract
1. Introduction
2. Materials and Methods
2.1. Walnut Germplasm
2.2. Walnut Morphological Analysis
2.3. RNA Extraction
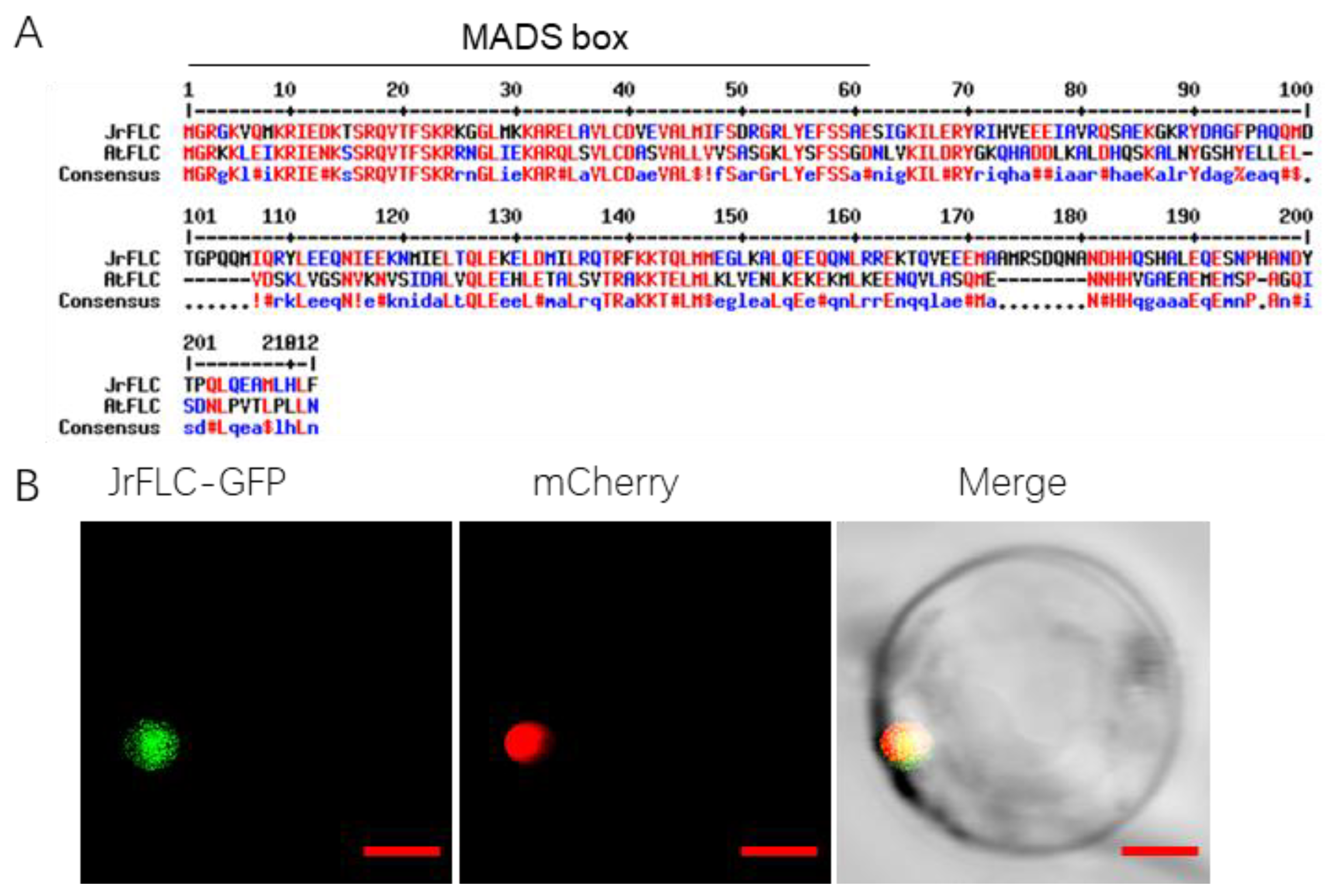
2.4. Identification of a Putative FLC Protein in Xingjiang Walnut
2.5. Subcellular Localization
2.6. Quantitative Reverse Transcription (qRT)-PCR
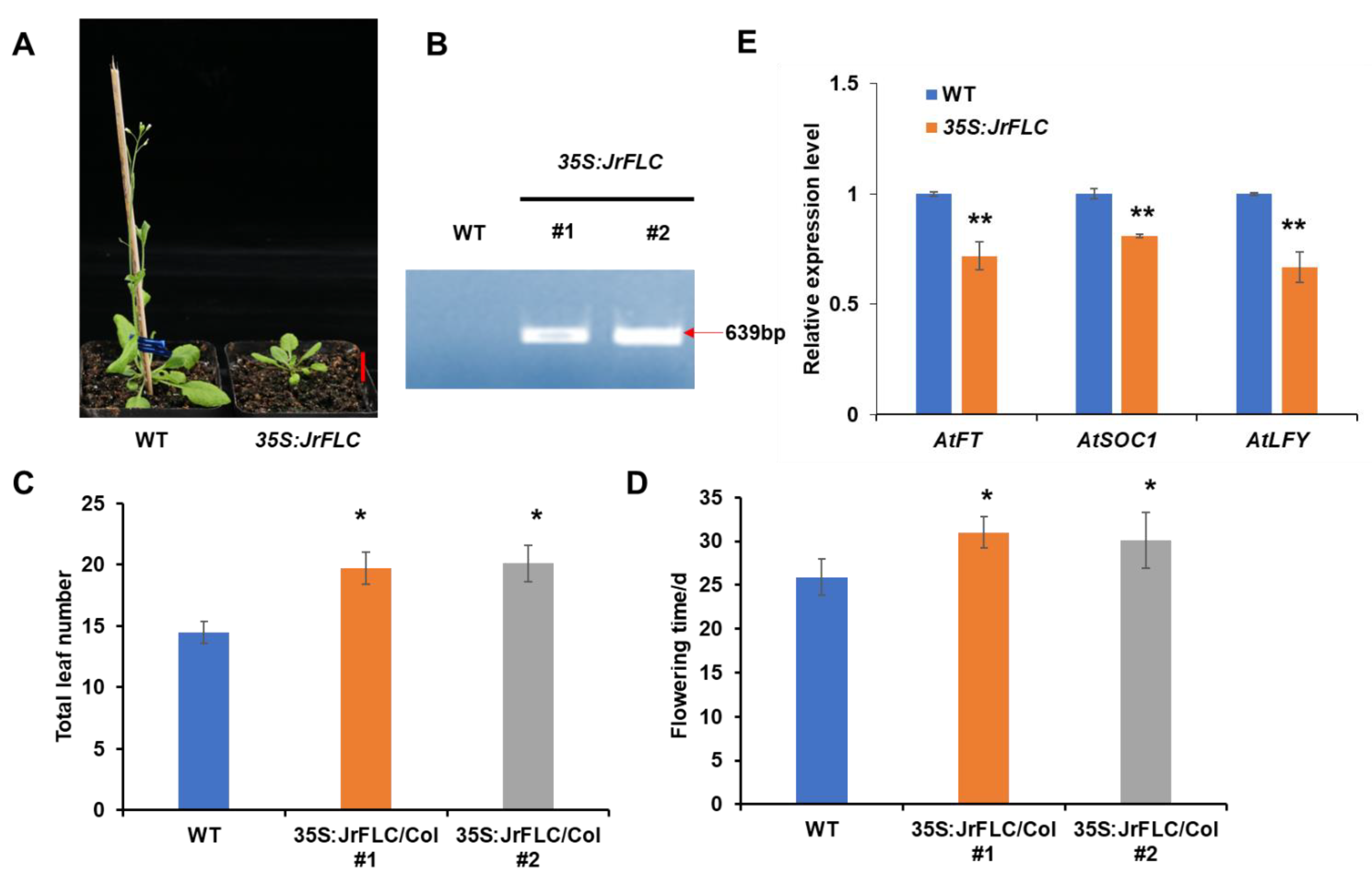
2.7. Plasmid Construction and Arabidopsis Transformation
3. Results and Discussion
3.1. JrFLC Isolation and Expression in Precocious Walnut
3.2. JrFLC Overexpression Delayed Flowering in Arabidopsis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and future of genetic improvement. Tree Genet. Genomes 2018, 14, 1–28. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Sheng, F.; Hu, B.; Jin, Q.; Wang, J.; Wu, C.; Luo, Z. The analysis of phenolic compounds in walnut husk and pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules 2021, 26, 3013. [Google Scholar] [CrossRef]
- Hardman, W.E. Walnuts have potential for cancer prevention and treatment in mice. J. Nutr. 2014, 144, 555S–560S. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Trandafir, I.; Cosmulescu, S.; Nour, V. Phenolic profile and antioxidant capacity of walnut extract as influenced by the extraction method and solvent. Int. J. Food Eng. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Y.; He, Y. Photoperiodic regulation of flowering time through periodic histone deacetylation of the florigen gene FT. PLoS Biol. 2013, 11, e1001649. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Jiang, D.; Wang, Y.; Bachmair, A.; He, Y. Repression of the floral transition via histone H2B monoubiquitination. Plant J. 2009, 57, 522–533. [Google Scholar] [CrossRef]
- Jiang, D.; Gu, X.; He, Y. Establishment of the winter-annual growth habit via FRIGIDA-mediated histone methylation at FLOWERING LOCUS C in Arabidopsis. Plant Cell 2009, 21, 1733–1746. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Amasino, R.M. Role of chromatin modification in flowering-time control. Trends Plant Sci. 2005, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.A.; Zhang, Q.; Huang, M.M.; Yakup, A. The breeding of six Xinjiang dwarf walnut cultivars. Acta Hortic. 2014, 1050, 151–160. [Google Scholar] [CrossRef]
- Jin, Q.; Mo, R.; Chen, W.; Zhang, Q.; Sheng, F.; Wu, C.; Zhang, R.; Luo, Z. Identification and comparative analysis of genes and microRNAs involved in the floral transition of the Xinjiang early-flowering walnut (Juglans regia L.). Horticulturae 2022, 8, 136. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, J.; Zhou, L.; Quan, S.; Ma, L.; Xu, H. Evolutionary analysis of MIR156/157 family during floral induction in walnut (Juglans regia L.). J. Hortic. Sci. Biotechnol. 2022, 97, 697–707. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; You, F.M.; Rodriguez, J.C.; Deal, K.R.; Chen, L.; Li, J.; Chakraborty, S.; Balan, B.; Jiang, C.Z.; et al. Sequencing a Juglans regia × J. microcarpa hybrid yields high-quality genome assemblies of parental species. Hortic. Res. 2019, 6, 55. [Google Scholar] [CrossRef]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, C.; Geng, Y.; Wang, Y.; Yang, Y.; Liu, Q.; Guo, W.; Chachar, S.; Riaz, A.; Yan, S.; et al. Rice and Arabidopsis homologs of yeast chromosome transmission fidelity protein 4 commonly interact with Polycomb complexes but exert divergent regulatory functions. Plant Cell 2021, 33, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Wang, Y.; Guo, W.; Chachar, S.; Riaz, A.; Geng, Y.; Gu, X.; Yang, L. PRMT6 physically associates with nuclear factor Y to regulate photoperiodic flowering in Arabidopsis. aBIOTECH 2021, 2, 403–414. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, W.; He, Y.; Amasino, R.M. Arabidopsis relatives of the human lysine-specific demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell 2007, 19, 2975–2987. [Google Scholar] [CrossRef]
- Chen, W.; Tamada, Y.; Yamane, H.; Matsushita, M.; Osako, Y.; Gao-takai, M. H3K4me3 plays a key role in establishing permissive chromatin states during bud dormancy and bud break in apple. Plant J. 2022, 111, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Hassankhah, A.; Rahemi, M.; Ramshini, H.; Sarikhani, S.; Vahdati, K. Flowering in Persian walnut: Patterns of gene expression during flower development. BMC Plant Biol. 2020, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Vahdati, K.; Mohseniazar, M. Early bearing genotypes of walnut: A suitable material for breeding and high density orchards. Acta Hortic. 2016, 1139, 101–105. [Google Scholar] [CrossRef]
- Breton, C.; Cornu, D.; Chriqui, D.; Sauvanet, A.; Capelli, P.; Germain, E.; Jay-Allemand, C. Somatic embryogenesis, micropropagation and plant regeneration of “Early Mature” walnut trees (Juglans regia) that flower in vitro. Tree Physiol. 2004, 24, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Zarei, A.; Zamani Fardadonbeh, M.; Lawson, S. Evaluation of genetic variability among “Early Mature” Juglans regia using microsatellite markers and morphological traits. PeerJ 2017, 5, e3834. [Google Scholar] [CrossRef]
- He, Y. Chromatin regulation of flowering. Trends Plant Sci. 2012, 17, 556–562. [Google Scholar] [CrossRef]
- He, Y.; Li, Z. Epigenetic environmental memories in plants: Establishment, maintenance, and reprogramming. Trends Genet. 2018, 34, 856–866. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Q.; Zhang, R.; Chen, L.; Luo, Z. Isolation of FLOWERING LOCUS C and Preliminary Characterization in the Floral Transition of Xinjiang Precocious Walnut. Horticulturae 2023, 9, 582. https://doi.org/10.3390/horticulturae9050582
Jin Q, Zhang R, Chen L, Luo Z. Isolation of FLOWERING LOCUS C and Preliminary Characterization in the Floral Transition of Xinjiang Precocious Walnut. Horticulturae. 2023; 9(5):582. https://doi.org/10.3390/horticulturae9050582
Chicago/Turabian StyleJin, Qiang, Rui Zhang, Liping Chen, and Zhengrong Luo. 2023. "Isolation of FLOWERING LOCUS C and Preliminary Characterization in the Floral Transition of Xinjiang Precocious Walnut" Horticulturae 9, no. 5: 582. https://doi.org/10.3390/horticulturae9050582
APA StyleJin, Q., Zhang, R., Chen, L., & Luo, Z. (2023). Isolation of FLOWERING LOCUS C and Preliminary Characterization in the Floral Transition of Xinjiang Precocious Walnut. Horticulturae, 9(5), 582. https://doi.org/10.3390/horticulturae9050582






