Abstract
Herbaceous peony (Paeonia lactiflora Pall.) is a famous ornamental plant, and the study of its flower color is of great significance for cultivating new flower varieties. To explore the factors driving the formation and change of herbaceous peony flower color, we selected five herbaceous peony varieties at four flowering stages to determine the change in flower color, petal area, and microstructure. We also examined the composition and content of petal pigments, soluble sugar and soluble protein content, pH value of cell fluid, and water content. Finally, we analyzed the correlations between each factor. We found that Pn3G5G, Pg3G5G, and Cy3G5G were the main anthocyanin components in red and purple petals. Qu3G, Qu7G, Is3G, and lutein play important roles in yellow petal formation. The change in herbaceous peony flower color during the flowering process is directly caused by changes in the anthocyanin and carotenoid content in petals. In addition, changes in other physiological indices also influence the change in flower color. This study explored the physiological and biochemical factors affecting the color of herbaceous peony petals, which has an important practical significance for studying the physiological mechanism of herbaceous peony flower color formation and color breeding of new flowers.
1. Introduction
Color is an important characteristic of ornamental plants. The main compound groups of plant anthocyanins include flavonoids, carotenoids, chlorophyll, and alkaloids [1]. Carotenoids give plant organs their yellow or orange colors, chlorophyll their green colors, and beet pigments their red or yellow colors [2]. Flavonoids are the most extensive and diversified pigments in angiosperms; they are divided into anthocyanins, flavonoids, and flavonols, among others [3]. Anthocyanins make the color of plants show changes from red to blue and purple [4]. Flavanones and flavonols cause the color to appear white or light yellow to yellow, or through intermolecular interaction with anthocyanins, cause color changes [5].
During flower opening, there is a continuous expansion of the petal area, and a variety of physiological changes occur inside the petal. The physicochemical properties of cells inside the petal, the pH value in the vacuole, the shape of epidermal cells, and other factors cause changes in flower color [6]. Notably, changes in cellular pH have a significant influence on the stability of anthocyanins. It is generally believed that anthocyanins have a stable structure and are conducive to the formation of red flowers under acidic environments, while under alkaline environments, they have an unstable structure and are conducive to the formation of blue flowers [7]. In addition, soluble sugar provides energy and a substrate for anthocyanin synthesis; in the anabolic pathway, different soluble proteins and soluble sugars result in carbohydrate content changes, resulting in a significant but indirect role in color presentation [8].
The herbaceous peony (Paeonia lactiflora Pall.) is a perennial herb in the Peony family and is grown worldwide. It has rich colors, including white, yellow, pink, red, purple, and mixed colors. Herbaceous peony usually flowers from April to May; they have large and diverse flower shapes and a high ornamental value. They are widely used as freshly cut flowers in pots and landscaping, among others. Flower color not only affects the ornamental value but also the economic value of herbaceous peony. Currently, research on herbaceous peony flower color mainly focuses on the classification and identification of anthocyanins, the analysis of the anthocyanin synthesis pathway, and the analysis of anthocyanin stability [8,9]. There are no reports on the combination of flower color and petal structure, anthocyanin content, and the intracellular environment of the herbaceous peony in different flowering periods. In this study, representative varieties of herbaceous peony with different flower colors were selected. We explored the relationship between flower color, pigment type and content in petals, pH, moisture content, petal structure, soluble sugar, and soluble protein content. The changing trend of flower color and related physiological indexes in different flowering stages were analyzed, revealing the formation and change mechanisms of flower color in herbaceous peony. Our results provide a reference for the genetic study on herbaceous peony flower color.
2. Materials and Methods
2.1. Test Materials and Sampling
The sampling period was from 19 April 2019 to 7 May 2019. Samples were collected in the herbaceous peony resource nursery and horticultural test center of the Horticultural Test Station at Shandong Agricultural University from 9:00 a.m. to 10:00 a.m. on a sunny day. The wild herbaceous peony species, ‘Jinzanciyu,’ ‘Foguangzhuying,’ ‘Xuanliduocai,’ and ‘Jinguangshanshuo,’ were selected as sample varieties.
Fresh petals of herbaceous peony at the bud (S1), opening (S2), blooming (S3), and decay (S4) stages were selected as test materials. For the varieties with obvious differences between the inner and outer petal shapes (‘Jinzanciyu’, ‘Foguangzhuying’, ‘Xuanliduocai’, and ‘Jinguangshanshuo’), the inner and outer petals were sampled, respectively. Parts of the fresh samples were used for paraffin sectioning and to determine the pH value and relative water content of the cell fluid, while the remainder of the samples were frozen in liquid nitrogen and stored at −80 °C for future use.
2.2. Petal Color Measurement
According to the method of Li et al. [10], color comparisons were conducted on the middle parts of fresh herbaceous peony petals using the 6th edition of the Royal Horticultural Society Colorimetric Card (RHSCC). In order to improve variety representativeness and reduce the errors caused by differences in the growth environment, three different individual plants of each variety were selected for sampling. Each individual plant was measured five times, and the middle part of the petals with uniform coloring was selected for color measurement. According to the method of Zhong [11], the brightness L*, red/green value a*, and yellow/blue value b* of the petals were measured using a color chromatic meter (CR-400, Konica Minolta), and the chroma C* and hue angle h° were calculated according to the formulas C* = (a*2 + b*2)1/2 and h° = arctan (b*/a*). The color difference meter was calibrated with standard whiteness (L* = 97.06, a* = 0.04, b* = 2.01) before each measurement, and the average value was calculated to represent the color of the petals after measurement.
2.3. Determination of the pH Value and Moisture Content of Cell Fluid
According to the method of Xue et al. [12], fresh petals were ground into a homogenate with quartz sand. Next, an acidity meter (PB-10; Sartorius, Goettingen, Germany) was used to determine the pH value. Quantitative fresh petals were weighed and dried at 80 °C to a constant weight in a DHG-9145A thermostatic air-blast drying oven (Shanghai Yiheng Technology Co., Ltd., Shanghai, China). The moisture content was calculated according to the formula: moisture content of petals = (Wf − Wd)/Wf × 100%, where Wf represents fresh weight (g), and Wd represents dry weight (g).
2.4. Observation of the Petal Microstructure
Paraffin-embedded sections were prepared according to the method described by Cheng [13]. The sections were observed using a Nikon Eclipse E100 ortho-optical microscope, and the images were collected and analyzed using a Nikon DS-U3 imaging system. The cross-sectional thickness of the petals, the thickness of the superficial parenchyma tissue, and the length and width of epidermal cells were measured using an eyepiece micrometer and Image-Pro Plus 6.0 software.
2.5. Determination of Nutrients and Pigments in Petals
The soluble sugar content was determined using the anthrone colorimetric method [14]. Soluble protein content was determined by the Coomassie bright-blue method [15]. Total proanthocyanidins were determined using the vanillin–hydrochloric acid method [16]. Anthocyanins were determined using the pH differential method, and total flavonoid content was determined using spectrophotometry [17]. Total carotenoid content was determined by spectrophotometry [18].
2.6. Determination of Flavonoids and Carotenoids
According to previous studies [11,19,20] and our preliminary test results, eight anthocyanins were selected: cyanidin-3-O-glucoside (Cy3G), cyanidin-3,5-O-diglucoside (Cy3G5G), paeoniflorin-3-O-glucoside (Pn3G), paeoniflorin-3,5-O-diglucoside (Pn3G5G), geranidin-3-O-glucoside (Pg3G), geranidin-3,5-O-diglucoside (Pg3G5G), delphinin-3-O-glucoside (Dp3G), and delphinidin-3,5-O-diglucoside (Dp3G5G).
Seven flavonol glycosides were selected: kaempferin-3-O-glucoside (Km3G), kaempferin-7-O-glucoside (Km7G), isorhamnein-3-O-glucoside (Is3G), isorhamnein-3-O-galactoside (Is3Gal), quercetin-3-O-glucoside (Qu3G), quercetin-7-O-glucoside (Qu7G), and bayberry glycoside (My3R).
One flavonoid glycoside was selected: luteolin (Lu7G). Two carotenoids were selected: β-carotene and lutein. All contents were analyzed and determined by HPLC. Anthocyanins were determined using a Shimadzu LC 20-A high-performance liquid chromatography (Shimadzu Corporation. Shanghai, China) and a Venusl XBP C18 column (4.6 × 100 mm, 5 μm). Flavonoids were determined using a Shimadzu LC 20-A high-performance liquid chromatography (Shimadzu Corporation. Shanghai, China) and an Inertsil ODS-3 column. Carotenoids were determined using a Shimadzu LC 20-A high-performance liquid chromatography (Shimadzu Corporation. Shanghai, China) and a Venusl XBP C18 column (100 mm × 4.6 mm, 5 μm).
2.7. Data Analysis
All indices were repeated three times. SPSS Statistics V 19.0 software was used for one-way analysis of variance and correlation analysis. Differences between the mean values of samples were tested using Duncan’s multiple comparison and the least significant difference (LSD) tests. Microsoft Excel and Origin 2016 were used for drawing.
3. Results
3.1. Phenotypic Characteristics of Flower Colors
The RHSCC colorimeter and CIE L*a*b* color system were used to describe the color changes of herbaceous peony petals during flowering (Figure 1A,B). Based on the description results of the RHSCC colorimetric card and naked-eye observations, the tested materials were divided into six color systems: white, pinkish-white, yellow, purplish-red, reddish-purple, and crimson.
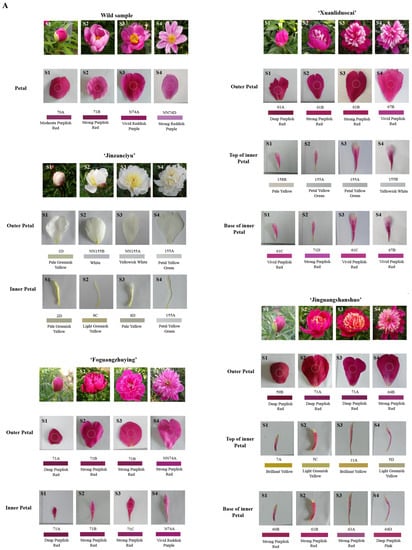
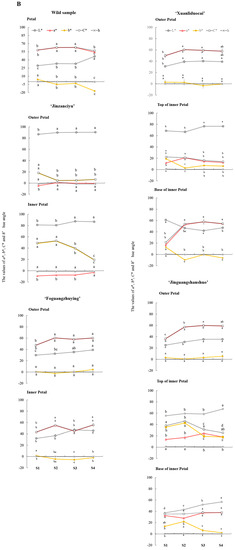
Figure 1.
Changes of petals colorimetric results (A) and color values (B) in different flowering stages of herbaceous peony. Notes: The data sets of the same variety and the same petal position at different flowering stages were analyzed. Different lowercase letters indicate significant differences at p < 0.05 level. Abbreviations: S1, bud stage; S2, opening stage; S3, blooming stage; S4, decay stage; L*, brightness; a*, red/green value; b*, yellow/blue value; C* = (a*2 + b*2)1/2, chroma; h° = arctan (b*/a*), hue angle.
During the flowering process, the L* values of most of the tested materials showed a generally increasing trend. Among all the tested materials, the L* value of the outer petal of the white ‘Jinzanciyu’ was the highest in each period. From S1 to S2, except for the purplish-red ‘Jinguangshanshuo’ inner petal base, the a* values of all other tested materials showed an increasing trend. The a* values of the wild herbaceous peony and ‘Xuanliduocai’ outer petals were high in all periods. The b* value of ‘Jinzanciyu’ inner and outer petals, ‘Xuanliduocai’ inner petal, ‘Jinguangshanshuo’ inner petal, and the wild herbaceous peony showed an obvious general declining trend. Among all the tested materials, the b* value of the inner petal of the yellow ‘Jinzanciyu’ was the highest during all periods. The C* value rises first, then falls, and then rises in both the inner and outer petal of the reddish-purple ‘Foguangzhuying,’ decreases first and then rises in the white ‘Jinzanciyu’ outer petal; decreases continuously in the top of the pinkish-white ‘Xuanliduocai’ inner petal; increases continuously in the base of the purplish-red ‘Jinguangshanshuo’ inner petal. The C* values of the other six tested materials showed an increasing trend followed by a decreasing one. The h° values of four test materials: the wild herbaceous peony species in reddish-purple, the inner petal of ‘Jinzanciyu’ in yellow, the top of the pinkish-white ‘Xuanliduocai’ inner petal, and the base of the purplish-red ‘Xuanliduocai’ inner petal, decreased first, then increased, and finally decreased. The h° value of the base of the purplish-red ‘Jinguangshanshuo’ inner petals increased first and then decreased. The h° value of the outer petals of the white ‘Jinzanciyu’ increased first, then decreased, and then increased. The h° values of the other five tested materials initially decreased and then increased.
3.2. Determination of Pigments
As shown in Table 1, Pn3G5G and Pg3G5G are the main anthocyanin components in the yellow inner petal of ‘Jinzanciyu’, while Pn3G5G, Cy3G5G, and Pg3G5G are the main anthocyanin components in the other ten tested materials. Dp3G was detected only in the ‘Xuanliduocai’ outer petal at the S4 stage. The variation trend of anthocyanins in herbaceous peony petals differed significantly. Although the variation trends of yellow and white materials were similar, there were large differences in the other colors. The variation trend of the anthocyanin content in materials with similar colors was highly similar. Proanthocyanidins were detected in six test materials, and the purplish-red ‘Jinguangshanshuo’ outer petals had the highest content of proanthocyanidins at 1.33–0.68 mg/g. In the flowering process, the total amount of proanthocyanins decreased first and then increased in the inner petal of the reddish-purple ‘Foguangzhuying’ and the outer petal of the purplish-red ‘Jinguangshanshuo.’ In the reddish-purple ‘Foguangzhuying’ outer petal and the purplish-red ‘Jinguangshanshuo’ outer petal, there was an initial falling trend, followed by a rising trend, and finally a falling trend. There was a continuous decline in the reddish-purple wild herbaceous peony and in the reddish-purple ‘Jinguangshanshuo’ inner petal base. Proanthocyanidins were not detected in any of the other varieties.

Table 1.
Composition and content of anthocyanins in herbaceous peony petals.
As shown in Table 2, seven test materials were mainly composed of Qu3G, Km3G, and Is3G: the reddish-purple outer petal of ‘Foguangzhuying’, the yellow inner petal of ‘Jinzanciyu’, the top of the inner petal of ‘Jinguangshanshuo’, the purplish-red ‘Xuanliduocai’ outer and inner petal bases, and the ‘Jinguangshanshuo’ outer and inner petal bases. Two test materials have Qu3G, Km3G, and Is3Gal as the main flavonoids: the white outer petal of ‘Jinzanciyu’ and the reddish-purple inner petal of ‘Foguangzhuying’. Qu3G, Is3Gal, and Is3G were the main flavonoids at the top of the reddish-purple wild herbaceous peony and pinkish-white ‘Xuanliduocai’ inner petals. The change trends were similar in the total amount of flavonoids in the inner and outer petals of the darker ‘Foguangzhuying’ and the base of the outer and inner petals of the ‘Jinguangshanshuo’.

Table 2.
Composition and content of flavonoids in herbaceous peony petals.
As shown in Table 3, the total amount of carotenoids was highest at the top of the yellow ‘Jinguangshanshuo’ inner petal, at 0.082–0.032 mg/g, and lowest in the reddish-purple wild herbaceous peony, at 0.003–0.001 mg/g. Lutein was the main carotenoid component in ten tested varieties, and β-carotenoid was the main carotenoid component in the inner petal of yellow ‘Jinzanciyu.’ In the flowering process, the contents of lutein and β-carotene increased initially and then decreased in the base of the yellow ‘Jinzanciyu’ inner petal, the purplish-red ‘Xuanliduocai’ outer petal, and the ‘Jinguangshanshuo’ inner petal. In addition, β-carotene initially increased, then decreased, and finally increased in the top of the yellow ‘Jinguangshanshuo’ inner petal. The main carotenoid components at the top of the inner petal of yellow ‘Jinguangshanshuo’ were different from those of ‘Jinzanciyu’.

Table 3.
Composition and content of carotenoids in herbaceous peony petals.
3.3. Changes in Soluble Sugar and Soluble Protein Content
As shown in Figure 2, the content of soluble sugar was the highest at the top of the pinkish-white ‘Xuanliduocai’ inner petal, which was 26.77–101.76 mg/g, and the lowest in the base of reddish-purple ‘Xuanliduocai’ inner petal, which was 11.36–38.19 mg/g. From S1 to S4, the soluble sugar content in four test materials initially increased, then decreased, and finally increased: the reddish-purple ‘Foguangzhuying’ outer petal, the purplish-red ‘Xuanliduocai’ inner petal base, the ‘Jinguangshanshuo’ outer petal, and the yellow ‘Jinguangshanshuo’ inner petal top. The soluble sugar content initially fell and then rose in three test materials: the reddish-purple wild herbaceous peony, the white ‘Jinzanciyu’ outer petal, and the yellow ‘Jinzanciyu’ inner petal. In two test materials, the reddish-purple ‘Foguangzhuying’ inner petal and the purplish-red ‘Xuanliduocai’ outer petal, the soluble sugar content rose initially and then fell. There was a continuous rise in soluble sugar content in the purplish-red ‘Xuanliduocai’ inner petal base and the ‘Jinguangshanshuo’ inner petal base.
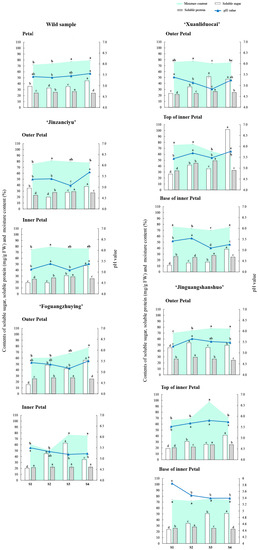
Figure 2.
Changes in the related physiological indexes in different flowering stages of the herbaceous peony. Notes: The data sets of the same variety and the same petal position at different flowering stages were analyzed. Different lowercase letters indicate significant differences at p < 0.05 level.
The soluble protein content was the highest at the top of the pinkish-white ‘Xuanliduocai’ inner petal (32.27–32.93 mg/g) and the lowest in the reddish-purple ‘Foguangzhuying’ inner petal (21.45–22.06 mg/g). During flowering, soluble protein content increased continuously at the top of the yellow ‘Jinguangshanshuo’ inner petal. In the reddish-purple ‘Foguangzhuying’ inner petal, the soluble protein content initially fell and then rose. In the purplish-red ‘Xuanliduocai’ outer petal, the soluble protein content initially fell, then rose, and finally fell again. For the other eight test materials, the soluble protein content increased initially and then decreased.
3.4. Changes in pH Value and Moisture Content of Petal Cell Fluid
The pH of petal cell fluid was highest at the top of the yellow ‘Jinguangshanshuo’ inner petal (5.52–5.73) and lowest in the purplish-red ‘Xuanliduocai’ outer petal (5.38–5.25). From stage S1 to S4, the pH value of the petal cell liquid initially increased, then decreased, and finally increased again in four test materials: the white outer petal of ‘Jinzanciyu,’ the yellow inner petal of ‘Jinzanciyu,’ the pinkish-white top of the ‘Xuanliduocai’ inner petal, and the purplish-red ‘Jinguangshanshuo’ inner petal base. In the purplish-red ‘Jinguangshanshuo’ inner petal top, the pH initially decreased and then increased. There was a continuous decrease in pH in the base of the purplish-red ‘Jinguangshanshuo’ inner petal.
The mean moisture content of petals was the highest in the outer petal of white ‘Jinzanciyu’ at 76–78% and the lowest in the inner petal of reddish-purple ‘Foguangzhuying’ at 61–75%. In the flowering process, the mean moisture content dropped first and then increased at the base of the purplish-red ‘Xuanliduocai’ inner petal. In the reddish-purple wild peony species and the outer petal of ‘Foguangzhuying,’ the mean moisture content continuously increased. The mean moisture content in the yellow ‘Jinzanciyu’ inner petal and the purplish-red ‘Xuanliduocai’ outer petal initially increased, then decreased, and finally increased again. The red-purple ‘Foguangzhuying’ inner petal and the purplish-red ‘Jinguangshanshuo’ inner petal base initially increased and then decreased. In the other four test materials, the mean moisture content initially increased and then decreased.
3.5. Changes in Petal Area and Internal Microstructure
The microstructure of herbaceous peony petals mainly consisted of two layers of epidermal cells and parenchyma tissue, but palisade tissue and spongy tissue were not observed (Table 4). The proportion of parenchymal tissue in wild peony species was the smallest at 51%. The proportion of parenchyma tissue at the base of the purplish-red ‘Jinguangshanshuo’ inner petal was the largest, accounting for 72% of the total petal thickness. Based on the observed shape of epidermal cells and the ratio of length (measured parallel to the petal) to width (measured perpendicular to the petal), they were divided into four categories: long arch (length/width ratio of 0.579–0.740), round arch (length/width ratio of 0.778–1.456), mastoid shape (length/width ratio of 0.549–0.713), and flat shape (length/width ratio of 1.332–3.959).

Table 4.
Physical parameters of herbaceous peony petals.
At the early flowering stage, the epidermal cells of the tested materials were regular in shape and neatly arranged, and the parenchyma was evenly distributed and tightly arranged (Figure 3). When the flower opened, the petal area of the tested materials continued to increase, the petal surface gradually shrank, the tissue cells inside the petals were damaged to varying degrees, the epidermal cells were gradually deformed and broken, the parenchyma gradually loosened, and the cell space expanded. The epidermal cells of the reddish-purple wild herbaceous peony species, the ‘Foguangzhuying’ inner petal, the base of the purplish-red ‘Xuanliduocai’ inner petal, and the top of the yellow ‘Jinguangshanshuo’ inner petal were all mastoid in blooming or decaying stage.
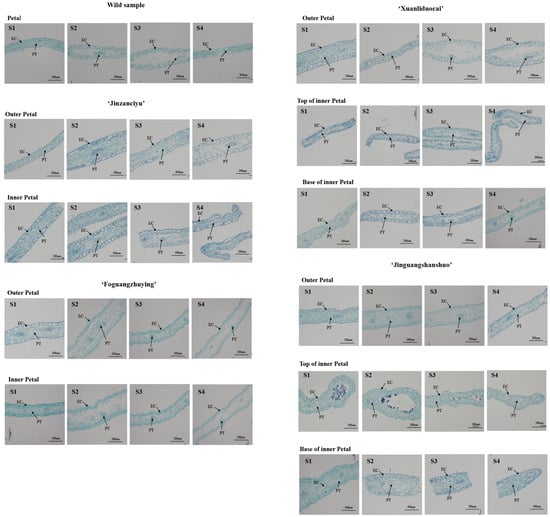
Figure 3.
Changes in petal microstructure in different flowering stages of the herbaceous peony. Abbreviations: S1, bud stage; S2, opening stage; S3, blooming stage; S4, decay stage; EC, epidermal cells; PT, parenchyma.
3.6. Correlation Analysis
The relationship between the pigment content and flower color of herbaceous peony during flowering is shown in Table 5. The L* value of petals was highly significant and negatively correlated with the contents of Cy3G5G, Pg3G5G, and Pn3G5G but significantly positively correlated with the content of Qu3G. The a* value was highly positively correlated with the contents of Cy3G5G, Pg3G5G, and Pn3G5G and negatively correlated with the contents of Qu3G and Km3G. The b* value was highly significantly negatively correlated with the Pg3G5G and Pn3G5G contents, highly positively correlated with the Qu7G content, and positively correlated with the lutein content. The C* value was highly positively correlated with the contents of Cy3G5G, Pg3G5G, and Pn3G5G and inversely correlated with the Qu3G content.

Table 5.
Correlation analysis of pigment content and L*a*b* values in petals.
Correlation analysis of the physiological indices of herbaceous peony petals in four periods (Table 6) showed that the L* value of tested materials was highly negatively correlated with the total anthocyanin content (TA), highly positively correlated with soluble protein content, and positively correlated with water content. The a* value was highly positively correlated with TA, highly negatively correlated with the total carotenoid content (TC), and positively correlated with the petal area. The b* value was highly positively correlated with TC, negatively correlated with petal area and soluble sugar content, and positively correlated with the total proanthocyanidin content. The C* value was highly positively correlated with TA and highly negatively correlated with the soluble protein content. The total flavonoid content (TF) was highly positively correlated with TC and total proanthocyanidins in the tested varieties. TC was highly positively correlated with proanthocyanidin content and negatively correlated with petal area. The thickness of the petals was highly positively correlated with the thickness of the parenchymal tissue.

Table 6.
Correlation analysis of physiological indexes in herbaceous peony petals.
4. Discussion
4.1. Comparison of Flower Color among Different Varieties
The type and concentration of pigments are the main factors that determine flower color [21]. Anthocyanin content is the main determinant of the white, pink, red, and purple petals of herbaceous peony [22]. Herbaceous peony petals were white when anthocyanin content was low, red when anthocyanin content was high, and pink when anthocyanin content was in between [23]. The types and concentrations of anthocyanins in the petals of deep purple and red herbaceous peony cultivars were much higher than those in purple cultivars [9]. The results of this study showed that the different shades of reddish-purple varied according to the average content of Pn3G5G in the base of the inner and outer petals of the wild herbaceous peony and ‘Foguangzhuying,’ as well as the inner and outer petals of ‘Xuanliduocai’ and ‘Jinguangshanshuo.’ Due to the accumulation of Pg3G5G and Cy3G5G in the ‘Jinguangshanshuo’ outer petal, the flower color is crimson.
In some herbaceous peony petals of similar colors, the composition of anthocyanins and flavonoids differs. Pg3G and Cy3G could be detected in the outer petal of ‘Jinzanciyu’ (white); however, myricetin and luteolin could not be detected. The detection result was the opposite at the top of the inner petal of ‘Xuanliduocai’ (pinkish-white). Cy3G5G, luteolin, and Is3Gal could be detected in the top of the ‘Jinguangshanshuo’ inner petal (yellow), but not could be detected in the ‘Jinzanciyu’ inner petal (yellow). Pg3G was detected in the outer petal (reddish-purple) of ‘Foguangzhuying,’ but Is3Gal could not be detected. The detection result was the opposite in the inner petals (reddish-purple).
The color of the top and base of the inner petal of ‘Xuanliduocai’ and ‘Jinguangshanshuo’ are different, and the composition of anthocyanins and carotenoids are the same; only the composition of flavonoids is different. Is3Gal was detected at the top of the ‘Xuanliduocai’ inner petal (pinkish-white) but could not be detected at the base of the inner petal (reddish-purple). Luteolin and Is3Gal were detected at the top of the inner petal of ‘Jinguangshanshuo’ (yellow) but could not be detected at the base of the inner petal (red). High concentrations of lutein, Qu3G, Qu7G, and Is3G accumulated at the top of the inner petals of ‘Jinguangshanshuo,’ and it was speculated that these pigments acted together to make the top of the inner petals appear yellow. These results were similar to the results of a study on flower color change in cinnamon buds [24].
The main flavonoid components of the inner petal of ‘Jinzanciyu’ were the same as those at the top of the inner petal of ‘Jinguangshanshuo’; however, the content of Km3G was higher, so its yellow color was slightly different from that of ‘Jinguangshanshuo’. The outer petal of ‘Jinzanciyu’ was white, and the total amounts of anthocyanins and flavonoids were the lowest among the tested varieties. The top of the ‘Xuanliduocai’ inner petal contained a small amount of anthocyanins at the beginning of flowering, appearing pinkish-white, which gradually turned white as the flower developed. The contents of each flavonoid component (except Km3G) in ‘Xuanliduocai’ were all higher than those in ‘Jinzanciyu’ outer petal, which may be the reason why the top of ‘Xuanliduocai’ inner petal showed dimmer brightness and more intense yellowness compared to those of the ‘Jinzanciyu’ outer petal. Km3G showed a subtractive effect on the color of the herbaceous peony, rendering the color lighter, similar to the results of Zhong [11].
Proanthocyanidins, as auxiliary pigments, can improve the stability of anthocyanins, thus affecting their color presentation. In this study, although there was no significant correlation between proanthocyanidin and anthocyanin contents, the color of the tested varieties with higher proanthocyanidin content was dark red or reddish-purple. This suggests that the accumulation of condensed tannins in the petals is conducive to the formation of deeper flower colors of the herbaceous peony, similar to the results of a study on purple sweet potatoes [25].
The vacuolar pH of plant organs can significantly affect the stability of anthocyanins. Our study found that the higher pH value of the cell fluid was conducive to the formation of yellow color, while lower pH values were conducive to the formation of red color. One study found that anthocyanin was stable at a low pH, while an increase in pH value promoted anthocyanin degradation [26]. In this study, the pH of the cell fluids of the tested varieties was slightly acidic. Although the top and base of the inner petals were different in color in ‘Xuanliduocai’ and ‘Jinguangshanshuo’, the anthocyanin and carotenoid components were the same, and the pH at the base of the inner petals was lower than that at the top, which may explain the red color at the base of inner petals. The top of the inner petal of ‘Jinguangshanshuo’ was yellow, and its pH value and carotenoid content were the highest among tested varieties, consistent with correlation analysis.
When the epidermal cells of herbaceous peony have a tapered structure, the proportion of incident light entering the cells increases, which is conducive to the development of pigments [27]. In this study, the epidermal cells of ‘Jinzanciyu’ and ‘Foguangzhuying’ had similar shapes, indicating that their light absorption characteristics were similar. The shape of epidermal cells of different colors in the inner petal of ‘Xuanliduocai’ and ‘Jinguangshanshuo’ showed obvious differences in the middle and late flowering.
4.2. Changes in Flower Color during Different Flowering Periods
At different flowering stages, the L* and b* values of pinkish-white and yellow varieties were all higher; the a* and C* values of reddish-purple and purplish-red materials were at high levels. This was consistent with the colorimetric results obtained using the RHSCC colorimetric card. With the gradual opening of herbaceous peony flowers, the colors of the flowers in most of the tested varieties faded. During the flowering process, the main components of the pigment in the petals of tested varieties did not change significantly, and the reason for the change in flower color was directly related to the total amount of anthocyanins and carotenoids. Because of the difference in the synthesis mechanism of flower pigments, the degree of variation in flower color differed.
At the beginning of flowering, the petals of most tested varieties were not fully unfolded, and the color was dark. The total amount of anthocyanins in the inner petal of ‘Foguangzhuying’, at the top of ‘Xuanliduocai’ inner petal, and the base of ‘Jinguangshanshuo’ inner petal was the highest in the bud stage. It is speculated that the anthocyanins needed for the color of these varieties accumulated before the flowers opened. Subsequently, anthocyanins gradually degrade due to the influence of the external environment, such as the enlargement of the petal area and light. The petals of the wild herbaceous peony, the inner petals of ‘Jinzanciyu’, the outer petals of ‘Xuanliduocai’, and the top of the outer and inner petals of ‘Jinguangshanshuo’ can continue to synthesize pigments after flowering. The precipitous decrease in pigment content at the beginning of flowering may be due to a rapid increase in the flower diameter. During flowering, the expansion speed of the petal area is faster than the speed of pigment synthesis, which leads to a decrease in the pigment content per unit area of petals [14].
In the middle and late flowering periods, the color of tested varieties showed fading or darkening due to pigment degradation in the petals. Among them, the total amount of anthocyanins in the outer petals of ‘Jinzanciyu’, the outer petals of ‘Foguangzhuying’, and the outer petals and the base of the inner petals of ‘Xuanliduocai’ all rose slightly during the period of decline. The total flavonoid and carotenoid contents of most tested varieties showed an upward trend during the period of decline. Studies have shown that flavonoids and phenols are the main components of antioxidants in peony petals, and their content changes significantly during petal aging [28]. This study speculated that the increased content of anthocyanins and flavones in herbaceous peony petals at the end of flowering might be related to the aging mechanism of flowers. The increase in anthocyanin and carotenoid content is the main reason for the gradual red or yellow color of herbaceous peony in the decay stage, which is similar to the results of research on chrysanthemums [29].
During flowering, a decrease in proanthocyanidin content reduces the stability of anthocyanidin and makes it easier to degrade. In the present study, the proanthocyanidin content in all tested varieties decreased to varying degrees. The increase of proanthocyanidin content in the inner petal of ‘Foguangzhuying’ and the outer petal of ‘Jinguangshanshuo’ in the decay stage may be related to its participation in the aging process of flowers as antioxidant substances.
Soluble sugar and soluble protein are energy sources and precursors for pigment synthesis, and changes in their content affect the accumulation of pigments in flower petals [30]. In this study, soluble sugar content in petals of tested varieties gradually accumulated during flowering. Most varieties showed a decrease in soluble sugar content at the S2 or S3 stage, which was speculated to be related to the simultaneous increase in anthocyanin content. The soluble protein content of most tested varieties initially increased and then decreased, indicating that the soluble protein content was mainly synthesized and accumulated in the early flowering period. The soluble protein content tended to decrease after the opening of flowers.
In this study, the pH of the cell fluid had no direct effect on the change in flower color, which is consistent with the results of Changling et al. [23]. In addition, the pH of the cell fluid of most tested varieties dropped sharply during the blooming stage and then rebounded. However, the pH value at the top of the outer and inner petals of Jinguangshanshuo initially increased and then decreased, while the pH value at the base of the inner petals continued to decrease. It is generally believed that the higher the moisture content of petals, the lighter the color [31]. The water content of petals in the decay stage of most tested varieties was higher than that in the bud stage; however, the water content of ‘Xuanliduocai’ outer petals and the top of inner petals and ‘Jinguangshanshuo’ in the decay stage was lower than that in the bud stage. The thickness of epidermal cells of petals showed a significant correlation with flower color, which may affect the appearance of flower color by changing the pigment content in epidermal cells.
5. Conclusions
In this study, the content and composition of pigments in the petals of herbaceous peony during the flowering process, as well as the tissue structure, cellular environment, cellular substances, and other indicators, were comprehensively analyzed to explore the physiological and biochemical factors affecting the color of herbaceous peony petals. The formation of herbaceous peony flower color is affected by various factors, and differences in pigment content and composition in petals are the main factor in determining petal color. Increasing the content of anthocyanins Cy3G5G, Pg3G5G, and Pn3G5G in herbaceous peony petals can reduce the brightness of the flower color and improve the redness and chroma. The accumulation of Qu3G and Km3G can lead to a decrease in the redness of the color, while the accumulation of Qu7G can improve the yellowness of the color. The increase of the lutein content in petals can reduce the redness of herbaceous peony color and promote the formation of yellow flowers. Furthermore, the accumulation of condensed tannins can enhance the yellowness of flower color and promote the formation of dark herbaceous peony flowers. In addition, the contents of condensed tannins, flavonoids, and carotenoids were positively correlated. The contents of soluble sugar, soluble protein, pH value of cell fluid, and water content can regulate anthocyanin synthesis and affect the stability of anthocyanin by participating in related physiological and metabolic activities or changing the light absorption characteristics of petals through the shape of epidermal cells, so as to cause a change in herbaceous peony flower color. The higher the content of soluble sugar in the petals, the lower the yellow color and the deeper the red and purple colors. When the soluble protein content is higher, the brightness of the flower color is improved, while the color is reduced, originating light-pink white and yellow petals. The cell fluid of herbaceous peony petals is weakly acidic, and the pH value of the cell fluid of yellow petals is the highest. It is speculated that a low pH value of the cell fluid of peony petals is conducive to the formation of red color. When the moisture content of petals is higher, the brightness of flower color increases, which is conducive to the formation of lighter flower color in herbaceous peony. In future studies, the number of varieties should be expanded. In addition, key genes related to the anthocyanin metabolism pathway should be further studied to provide a theoretical reference at the physiological and molecular levels for the study of herbaceous peony flower color and the breeding of new varieties.
Author Contributions
D.Z. co-designed and undertook most of the study, analyzed the data, and wrote the paper. A.X., X.Y., L.Y., Y.S., L.D. and F.L. participated in tests and data analysis. L.S. provided comments on the first draft of this paper. M.B. and X.S. co-designed the study and revised drafts of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Shandong Province Improved Seed Project (No. 2021S230304-02583).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Narbona, E.; del Valle, J.C.; Whittall, J.B. Painting the green canvas: How pigments produce flower colors. Biochemist 2021, 43, 6–12. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Arista, M.; Buide, M.L.; Ortiz, P.L. Major flower pigments originate from different color signals to pollinators. Front. Ecol. Evol. 2021, 9, 743850. [Google Scholar] [CrossRef]
- Iwashina, T. Contribution to flower colors of flavonoids, including anthocyanins: A review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, Betalains, and Carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, N.; Momonoi, K.; Kawachi, M.; Katou, K.; Okazaki, Y.; Uozumi, N.; Maeshima, M.; Kondo, T. Synchrony between flower opening and petal-color change from red to blue in morning glory, Ipomoea tricolor cv. Heavenly Blue. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 187–197. [Google Scholar] [CrossRef]
- Grotewold, E. Genetics and biochemistry of the floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Bao, M.Y.; Liu, M.M.; Zhang, Q.X.; Wang, T.L.; Sun, X.; Xu, J. Factors affecting the color of herbaceous peony. J. Am. Soc. Hortic. Sci. 2020, 145, 257–266. [Google Scholar] [CrossRef]
- Jia, N.; Shu, Q.Y.; Wang, L.S.; Du, H.; Xu, Y.J.; Liu, Z.A. Analysis of petal anthocyanins to investigate coloration mechanism in herbaceous peony cultivars. Sci. Hortic. 2008, 117, 167–173. [Google Scholar] [CrossRef]
- Li, Q.; Xu, J.G.; Gao, C.R.; Lu, J.; Du, G.C.; Sun, X. Taxonomic keys of 63 herbaceous peony cultivars. Chin. Agric. Sci. Bull. 2018, 34, 51–59. [Google Scholar]
- Zhong, P. The Flavonoids Composition in Herbaceous Peony Petals and Their Effects on the Coloration. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2012. [Google Scholar]
- Xue, Y.L.; Zhao, Q.S.; Huang, Y.; Liu, Z.; Wu, L.Q.; Xi, C.F.; Li, J.B.; Li, J.Y. Research on relationship between floral colors and intracellular environment of Camellia reticulata Lindl. J. Yunnan Agric. Univ. 2015, 30, 455–463. [Google Scholar]
- Cheng, M.L. Flower Bud Differentiation Process and Effects of Exogenous GA3 on the Flower Bud Development of Introduced Paeonia lactiflora. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2008. [Google Scholar]
- Cang, J.; Zhao, H.J. Determination of soluble sugar by anthranone colorimetry. In Experimental Course of Plant Physiology; Cang, J., Ed.; Higher Education Press: Beijing, China, 2013; pp. 85–158. [Google Scholar]
- Yu, Q.Y. (Ed.) Experiment for the determination of soluble protein in plant tissues. In Experimental Course of Plant Physiology; Beijing Institute of Technology Press: Beijing, China, 2014; pp. 17–19. [Google Scholar]
- Yao, S.P.; Ti, X.N. Optimization of assay conditions for condensed tannin in cotton. J. Xijiang Agric. Univ. 2010, 33, 339–342. [Google Scholar]
- Wang, X.Q. Phenolic Metabolism of Red-Fleshed Apples and Its Response to Stress. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2015. [Google Scholar]
- Li, H.S.; Sun, Q.; Zhao, S.J. Determination of chlorophyll content. In The Experiment Principle and Technique on Plant Physiology and Biochemistry; Li, H.S., Sun, Q., Eds.; Higher Education Press: Beijing, China, 2000; pp. 134–138. [Google Scholar]
- Zhao, D.Q.; Wei, M.R.; Liu, D.; Tao, J. Anatomical and biochemical analysis revealed the role of anthocyanins in flower coloration of herbaceous peony. Plant Physiol. Biochem. 2016, 102, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, D.Q.; Tao, J. Research progress on influential factors and regulation of tree peony flower color formation. Plant Physiol. J. 2019, 55, 925–931. [Google Scholar]
- Nakayama, M.; Tanikawa, N.; Morita, Y.; Ban, Y. Comprehensive analyses of anthocyanin and related compounds to understand flower color change in ion-beam mutants of cyclamen (Cyclamen spp.) and carnation (Dianthus caryophyllus). Plant Biotechnol. 2012, 29, 215–221. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F.; Kalc, G.; Senior, M.; Dyson, B.; Nakamura, N.; Katsumoto, Y.; Chandler, S. Flower color modification by engineering of the flavonoid biosynthetic pathway: Practical perspectives. Biosci. Biotechnol. Biochem. 2010, 74, 1760–1769. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, W.; Chen, J. Formation and regulation of flower color in higher plants. Chin. Bull. Bot. 2005, 22, 70–81. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Zhang, C.; Fu, J.; Hu, S.; Zhao, H. Flower color and pigment composition in the petals of bud mutation and its stock plant of Osmanthus fragrans ‘Jingui’. Acta Hortic. Sin. 2017, 44, 528–536. [Google Scholar]
- Zhu, H.; Han, Y.; Gu, Z.; Fan, G. Studies on the effects of tannins on purple sweet potato anthocyanins. J. Nanjing Agric. Univ. 2006, 29, 98–102. [Google Scholar] [CrossRef]
- Zhuang, W.; Liu, T.; Shu, X.; Qu, S.; Qu, H.; Wang, T.; Zhang, F.; Wang, Z. Molecular regulation mechanisms of anthocyanin biosynthesis and coloration in plants. Plant Physiol. J. 2018, 54, 1630–1644. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, L.; Xu, Z.; Yu, X. Transcriptome sequencing of Paeonia suffruticosa ‘Shima Nishiki’ to identify differentially expressed genes mediating double-color formation. Plant Physiol. Biochem. 2018, 123, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, M.; Fu, J.X.; Wang, Y.J.; Dong, L. Exogenous sugars involvement in senescence and ethylene production of tree peony Luoyang hong’ cut flowers. Kor. J. Hortic. Sci. Technol. 2012, 30, 718–724. [Google Scholar] [CrossRef]
- Chen, S.-M.; Li, C.-H.; Zhu, X.-R.; Deng, Y.-M.; Sun, W.; Wang, L.-S.; Chen, F.-D.; Zhang, Z. Identification of flavonoids and gene expression involved in anthocyanin biosynthesis in chrysanthemum flowers. Biol. Plant. 2012, 56, 458–464. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef]
- Jiang, L.N.; Li, J.Y.; Tong, R.; He, L.B.; Zhang, L.; Li, Z.H.; Huang, X.N. Relationship between flower color and important cellular environment elemental factors in yellow camellia. Guihaia 2019, 39, 1609–1612. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).