Agronomic Performance and Nutraceutical Quality of a Tomato Germplasm Line Selected under Organic Production System
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Plant Material
2.3. Experimental Design
2.4. Sample of Fruit
2.5. Agronomical and Physicochemical Measurements
2.6. Lycopene and β-Carotene Determinations
2.7. Total Phenols and Flavonoid Determinations
2.8. Vitamin C Determinations
2.9. Vitamin E Determinations
2.10. Radical Scavenging Activity Measurements
2.11. Statistical Analysis
3. Results
3.1. Agronomical and Physicochemical Characteristics
3.2. Nutraceutical Properties
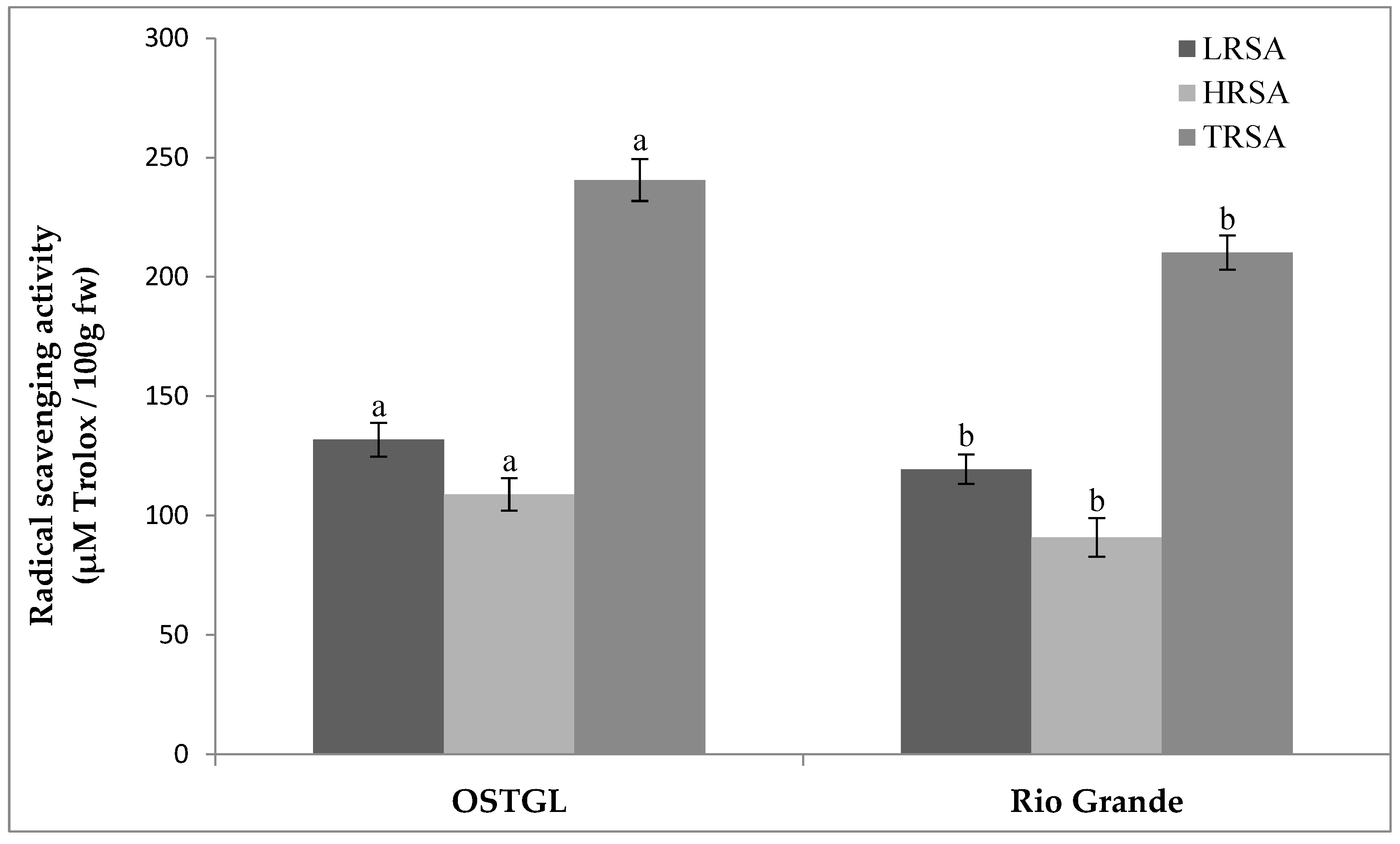
3.3. Radical Scavenging Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Harvested Area, Production and Average Yield of Tomato Culture in Tunisia. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 February 2023).
- CTAB. Available online: http://www.ctab.nat.tn/index.php/fr-fr/situation-du-secteur/tunisie/statistiques (accessed on 5 January 2023).
- Stoleru, V.; Munteanu, N.; Istrate, A. Perception Towards Organic vs. Conventional Products in Romania. Sustainability 2019, 11, 2394. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and beyond color: Comparative overview of functional quality of tomato and watermelon fruits. Front. Plant Sci. 2019, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Solis, E.S. Performance Evaluation of Different Tomato Lines Grown Organically in Catarman, Camiguin, Philippines. Am. J. Agric. Sci. Eng. Technol. 2022, 6, 18–24. [Google Scholar] [CrossRef]
- Mazon, S.; Brunetto, C.A.; Woyann, L.G.; Finatto, T.; Andrade, G.S.; Vargas, T.D.O. Agronomic performance and physicochemical quality of tomato fruits under organic production system. Rev. Ceres 2022, 69, 236–245. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Ponisio, L.C.; M’Gonigle, L.K.; Mace, K.C.; Palomino, J.; de Valpine, P.; Kremen, C. Diversification practices reduce organic to conventional yield gap. Proc. R. Soc. B 2015, 282, 20141396. [Google Scholar] [CrossRef]
- Fibiani, M.; Paolo, D.; Leteo, F.; Campanelli, G.; Picchi, V.; Bianchi, G.; Scalzo, R.L. Influence of year, genotype and cultivation system on nutritional values and bioactive compounds in tomato (Solanum lycopersicum L.). Food Chem. 2022, 389, 133090. [Google Scholar] [CrossRef]
- Crespo-Herrera, L.A.; Ortiz, R. Plant breeding for organic agriculture: Something new? Agric. Food Secur. 2015, 4, 25. [Google Scholar] [CrossRef]
- Kirk, A.P.; Fox, S.L.; Entz, M.H. Comparison of organic and conventional selection environments for spring wheat. Plant Breed. 2012, 131, 687–694. [Google Scholar] [CrossRef]
- Horneburg, B.; Becker, H.C. Selection for Phytophthora field resistance in the F2 generation of organic outdoor tomatoes. Euphytica 2011, 180, 357–367. [Google Scholar] [CrossRef]
- Van Bueren, E.L.; Jones, S.S.; Tamm, L.; Murphy, K.M.; Myers, J.R.; Leifert, C.; Messmer, M.M. The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: A review. NJAS Wagening. J. Life Sci. 2011, 58, 193–205. [Google Scholar] [CrossRef]
- Campanelli, G.; Sestili, S.; Acciarri, N.; Montemurro, F.; Palma, D.; Leteo, F.; Beretta, M. Multi-parental advances generation inter-cross population, to develop organic tomato genotypes by participatory plant breeding. Agronomy 2019, 9, 119. [Google Scholar] [CrossRef]
- Tripodi, P.; Soler, S.; Campanelli, G.; Díez, M.J.; Esposito, S.; Sestili, S.; Figàs, M.R.; Leteo, F.; Casanova, C.; Platani, C.; et al. Genome wide association mapping for agronomic, fruit quality, and root architectural traits in tomato under organic farming conditions. BMC Plant Biol. 2021, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Riahi, A.; Hdider, C.; Tarchoun, N.; Ben Khedher, M.; Guezel, I. Behaviour of different processing tomato cultivars grown organically in Tunisia. Acta Hortic. 2007, 758, 327–332. [Google Scholar] [CrossRef]
- Riahi, A.; Hdider, C.; Sanaa, M.; Tarchoun, N.; Ben Kheder, M.; Guezal, I. Effect of conventional and organic production systems on the yield and quality of field tomato cultivars grown in Tunisia. J. Sci. Food Agric. 2009, 89, 2275–2282. [Google Scholar] [CrossRef]
- Ilahy, R.; Siddiqui, M.W.; Tlili, I.; Piro, G.; Lenucci, M.S.; Hdider, C. Functional quality and colour attributes of two high-lycopene tomato breeding lines grown under greenhouse conditions. Turk. J. Agric.-Food Sci. Technol. 2016, 4, 365–373. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liv, H.L. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzè, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Daood, H.G.; Czinkotal, B.; Hoschke, Á.; Biacs, P. High-performance liquid chromatography of chlorophylls and carotenoids from vegetables. J. Chromatogr. A 1989, 472, 296–302. [Google Scholar] [CrossRef]
- Duah, S.A.; e Souza, C.S.; Daood, H.G.; Pék, Z.; Neményi, A.; Helyes, L. Content and response to Ɣ-irradiation before over-ripening of capsaicinoid, carotenoid, and tocopherol in new hybrids of spice chili peppers. LWT 2021, 147, 111555. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. The relative contribution of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apples fruits juices and blackcurrant drinks. Food Chem. 1997, 60, 331–337. [Google Scholar] [CrossRef]
- Giordano, L.D.B.; Boiteux, L.S.; Quezado-Duval, A.M.; Fonseca, M.E.D.N.; Resende, F.V.; Reis, A.; González, M.; Nascimento, W.M.; Mendonça, J.L. “BRS Tospodoro”: A high lycopene processing tomato cultivar adapted to organic cropping systems and with multiple resistance to pathogens. Hortic. Bras. 2010, 28, 241–245. [Google Scholar] [CrossRef]
- Sidhu, V.; Nandwani, D. Cultivar Evaluation and Yield Performance of Tomato in an Organic Management System. J. Hortic. 2017, 4, 2376-0354. [Google Scholar] [CrossRef]
- Lahoz, I.; Leiva-Brondo, M.; Martí, R.; Macua, J.I.; Campillo, C.; Roselló, S.; Cebolla-Cornejo, J. Influence of high lycopene varieties and organic farming on the production and quality of processing tomato. Sci. Hortic. 2016, 204, 128–137. [Google Scholar] [CrossRef]
- Ficiciyan, A.M.; Loos, J.; Tscharntke, T. Better performance of organic than conventional tomato varieties in single and mixed cropping. Agroecol. Sustain. Food Syst. 2022, 46, 491–509. [Google Scholar] [CrossRef]
- Malundo, M.M.; Shewfelt, R.L.; Scott, J.W. Flavor quality of fresh tomato (Lycopersicon esculentum Mill.) as affected by sugar and acid levels. Postharvest Biol. Technol. 1995, 6, 103–110. [Google Scholar] [CrossRef]
- Murariu, O.C.; Brezeanu, C.; Jităreanu, C.D.; Robu, T.; Irimia, L.M.; Trofin, A.E.; Popa, L.-D.; Stoleru, V.; Murariu, F.; Brezeanu, P.M. Functional Quality of Improved Tomato Genotypes Grown in Open Field and in Plastic Tunnel under Organic Farming. Agriculture 2021, 11, 609. [Google Scholar] [CrossRef]
- De Sio, F.; Rapacciuolo, M.; De Giorgi, A.; Sandei, L.; Giuliano, B.; Sekara, A.; Tallarita, A.; Morano, G.; Cuciniello, A.; Cozzolino, E.; et al. Yield and quality performances of organic tomato as affected by genotype and industrial processing in southern Italy. Italus Hortus. 2020, 27, 85–99. [Google Scholar] [CrossRef]
- Ayuso-Yuste, M.C.; González-Cebrino, F.; Lozano-Ruiz, M.; Fernández-León, A.M.; Bernalte-García, M.J. Influence of Ripening Stage on Quality Parameters of Five Traditional Tomato Varieties Grown under Organic Conditions. Horticulturae 2022, 8, 313. [Google Scholar] [CrossRef]
- Petro-Turza, M. Flavor of tomato and tomato products. Food Rev. Int. 1987, 2, 309–351. [Google Scholar] [CrossRef]
- Dobrin, A.; Nedelus, A.; Bujor, O.; Mot, A.; Zugravu, M.; Badulescu, L. Nutritional quality parameters of the fresh red tomato varieties cultivated in organic system. Sci. Papers Ser. B. Hortic. 2019, 63, 439–443. [Google Scholar]
- Riahi, A.; Hdider, C. Bioactive compounds and antioxidant activity of organically grown tomato (Solanum lycopersicum L.) cultivars as affected by fertilization. Sci. Hortic. 2013, 151, 90–96. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid content of 50 watermelon cultivars. J. Agric. Food Chem. 2006, 54, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Fracchiolla, M.; Renna, M.; Durante, M.; Mita, G.; Serio, F.; Cazzato, E. Cover Crops and Manure Combined with Commercial Fertilizers Differently Affect Yield and Quality of Processing Tomato (Solanum lycopersicum L.) Organically Grown in Puglia. Agriculture 2021, 11, 757. [Google Scholar] [CrossRef]
- Rodríguez-Ortiz, J.C.; Díaz-Flores, P.E.; Zavala-Sierra, D.; Preciado-Rangel, P.; Rodríguez-Fuentes, H.; Estrada-González, A.J.; Carballo-Méndez, F.J. Organic vs. Conventional Fertilization: Soil Nutrient Availability, Production, and Quality of Tomato Fruit. Water Air Soil Pollut. 2022, 233, 87. [Google Scholar] [CrossRef]
- Martí, R.; Leiva-Brondo, M.; Lahoz, I.; Campillo, C.; Cebolla-Cornejo, J.; Roselló, S. Polyphenol and L-ascorbic acid content in tomato as influenced by high lycopene genotypes and organic farming at different environments. Food Chem. 2018, 239, 148–156. [Google Scholar] [CrossRef]
- Zheng, X.; Gong, M.; Zhang, Q.; Tan, H.; Li, L.; Tang, Y.; Li, Z.; Peng, M.; Deng, W. Metabolism and Regulation of Ascorbic Acid in Fruits. Plants 2022, 11, 1602. [Google Scholar] [CrossRef]
- Abou Chehade, L.; Antichi, D.; Martelloni, L.; Frasconi, C.; Sbrana, M.; Mazzoncini, M.; Peruzzi, A. Evaluation of the agronomic performance of organic processing tomato as affected by different cover crop residues management. Agronomy 2019, 9, 504. [Google Scholar] [CrossRef]
- Hallmann, E.; Lipowski, J.; Marszałek, K.; Rembiałkowska, E. The Seasonal Variation in Bioactive Compounds Content in Juice from Organic and Non-organic Tomatoes. Plant Foods Hum. Nutr. 2013, 68, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.B.; Moura, C.F.; Gomes-Filho, E.; Marco, C.A.; Urban, L.; Miranda, M.R.A. The impact of organic farming on quality of tomatoes is associated to increased oxidative stress during fruit development. PLoS ONE 2013, 8, e56354. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.F.; Barreira, S.V.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Organic versus conventional tomatoes: Influence on physicochemical parameters, bioactive compounds and sensorial attributes. Food Chem. Toxicol. 2014, 67, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, A.; Riahi, A.; Ujj, A.; Ramos-Diaz, F.; Marjanović, J.; Hdider, C. Comparative Nutrient and Antioxidant Profile of High Lycopene Variety with hp Genes and Ordinary Variety of Tomato under Organic Conditions. Agronomy 2023, 13, 649. [Google Scholar] [CrossRef]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Rossi, M.; Kessler, F. Essential role for phytol kinase and tocopherol in tolerance to combined light and temperature stress in tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Daood, H.; Azam, M.; Siddiqui, M.W.; R’him, T.; Durante, M.; Lenucci, M.S.; et al. Effect of Individual and Selected Combined Treatments with Saline Solutions and Spent Engine Oil on the Processing Attributes and Functional Quality of Tomato (Solanum lycopersicon L.) Fruit: In Memory of Professor Leila Ben Jaballah Radhouane (1958–2021). Front. Nutr. 2022, 9, 844162. [Google Scholar] [CrossRef]
| Month | Tmin (°C) | Tmax (°C) | Humidity (%) | Rainfall (mm) | ||||
|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | |
| April | 15 | 14 | 22 | 20 | 78 | 79 | 50 | 45 |
| May | 18 | 17 | 24 | 23 | 73 | 70 | 78 | 51 |
| June | 23 | 24 | 29 | 31 | 68 | 62 | 07 | 26 |
| July | 28 | 27 | 34 | 34 | 61 | 60 | 02 | 04 |
| Varieties | |||
|---|---|---|---|
| Characteristics | OSTGL | Rio Grande | |
| Marketable yield (t/ha) | 73.2 ± 3.9 a | 66.1 ± 4.1 b | |
| Average fruit weight (g) | 77.1 ± 4.1 a | 72.3 ± 3.9 a | |
| Soluble solids (°Brix) | 5.3 ± 0.3 a | 5.2 ± 0.3 a | |
| pH | 4.41 ± 0.03 a | 4.39 ± 0.03 a | |
| Titratable acidity (% citric acid) | 0.34 ± 0.01 a | 0.36 ± 0.01 a | |
| Firmness (kg/cm2) | 4.30 ± 0.31 a | 4.10 ± 0.21 a | |
| Color | |||
| (a*) | 25.7 ± 1.0 a | 26.2 ± 0.9 a | |
| (b*) | 27.3 ± 1.1 a | 28.1 ± 0.8 a | |
| (a*/b*) | 0.94 ± 0.03 a | 0.93 ± 0.03 a | |
| Year (Y) Variety (V) Y × V | ns ** ns | ns ** ns | |
| Varieties | |||
|---|---|---|---|
| Characteristics | OSTGL | Rio Grande | |
| Lycopene (mg/kg fw) | 82.5 ± 4.2 a | 79.3 ± 5.1 a | |
| β-Carotene (mg/kg fw) | 5.2 ± 0.4 a | 4.9 ± 0.3 a | |
| Total phenols (mg GAE/kg fw) | 168.5 ± 6.3 a | 149.8 ± 7.1 b | |
| Flavonoids (mg RE/kg fw) | 118.7 ± 6.2 a | 105.8 ± 5.5 b | |
| Vitamin C (mg/kg fw) | 168.9 ± 7.2 a | 153.8 ± 6.8 b | |
| Vitamin E (mg/kg fw) | |||
| α-tocopherol | 21.03 ± 0.63 a | 18.03 ± 0.54 b | |
| β-tocopherol | 0 | 0 | |
| γ-tocopherol | 0.28 ± 0.01 | 0 | |
| Year (Y) | ns | ns | |
| Variety (V) | ** | ** | |
| Y × V | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romdhane, A.; Riahi, A.; Piro, G.; Lenucci, M.S.; Hdider, C. Agronomic Performance and Nutraceutical Quality of a Tomato Germplasm Line Selected under Organic Production System. Horticulturae 2023, 9, 490. https://doi.org/10.3390/horticulturae9040490
Romdhane A, Riahi A, Piro G, Lenucci MS, Hdider C. Agronomic Performance and Nutraceutical Quality of a Tomato Germplasm Line Selected under Organic Production System. Horticulturae. 2023; 9(4):490. https://doi.org/10.3390/horticulturae9040490
Chicago/Turabian StyleRomdhane, Amani, Anissa Riahi, Gabriella Piro, Marcello Salvatore Lenucci, and Chafik Hdider. 2023. "Agronomic Performance and Nutraceutical Quality of a Tomato Germplasm Line Selected under Organic Production System" Horticulturae 9, no. 4: 490. https://doi.org/10.3390/horticulturae9040490
APA StyleRomdhane, A., Riahi, A., Piro, G., Lenucci, M. S., & Hdider, C. (2023). Agronomic Performance and Nutraceutical Quality of a Tomato Germplasm Line Selected under Organic Production System. Horticulturae, 9(4), 490. https://doi.org/10.3390/horticulturae9040490









