The Morpho-Anatomy of Nectaries and Chemical Composition of Nectar in Pear Cultivars with Different Susceptibility to Erwinia amlylovora
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Light Microscopy (LM)
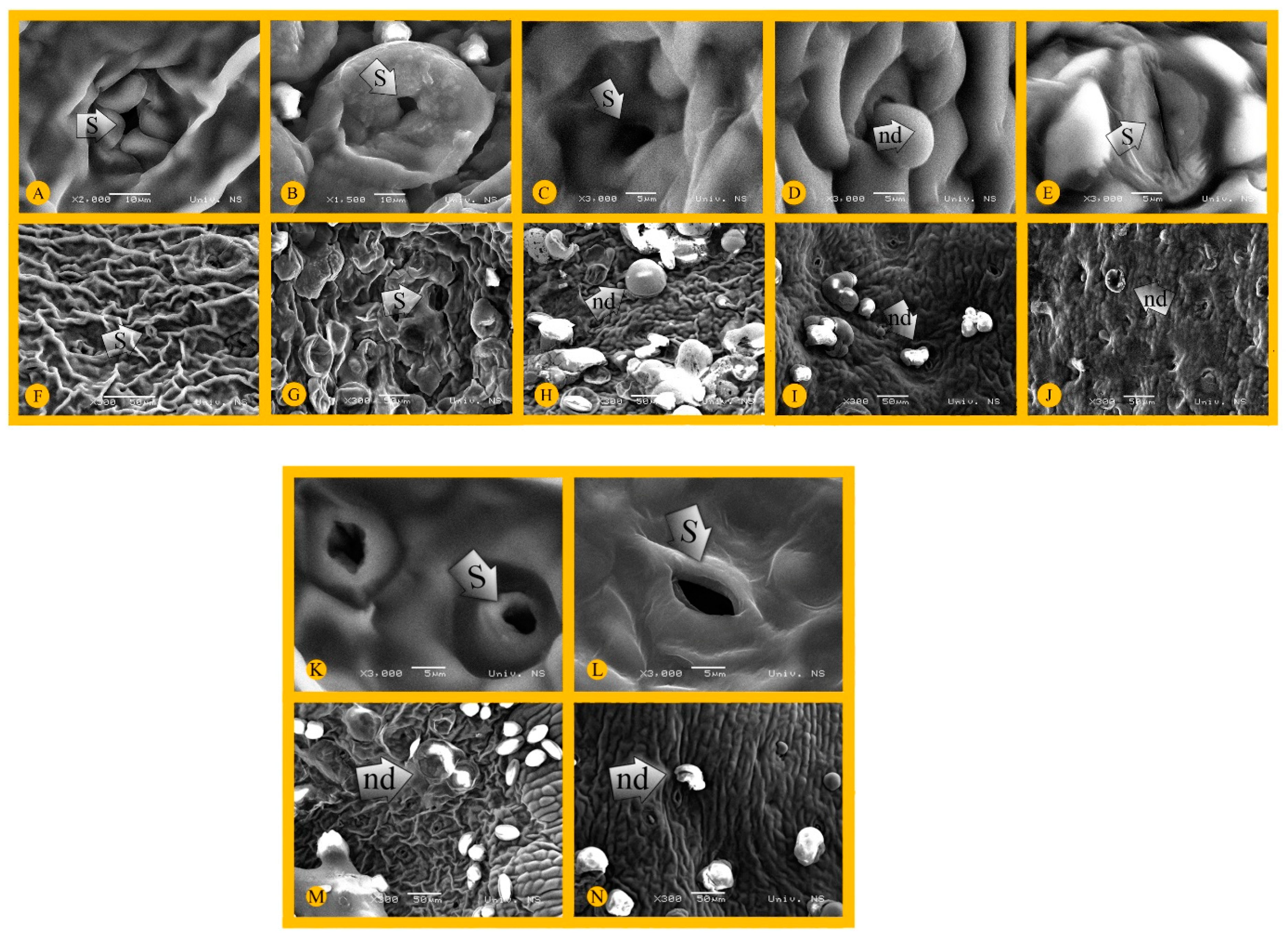
2.3. Scanning Electron Microscopy (SEM)
2.4. Chemical Analysis of Nectar
2.4.1. Reagents and Standards
2.4.2. Determination of Sugars and Sugar Alcohols by High-Performance Anion-Exchange Chromatography/Pulsed Amperometric Detection (HPAEC/PAD)
2.4.3. UHPLC–Orbitrap MS Analysis of Polyphenolic Compounds
2.5. Statistical Analysis
3. Results and Discussion
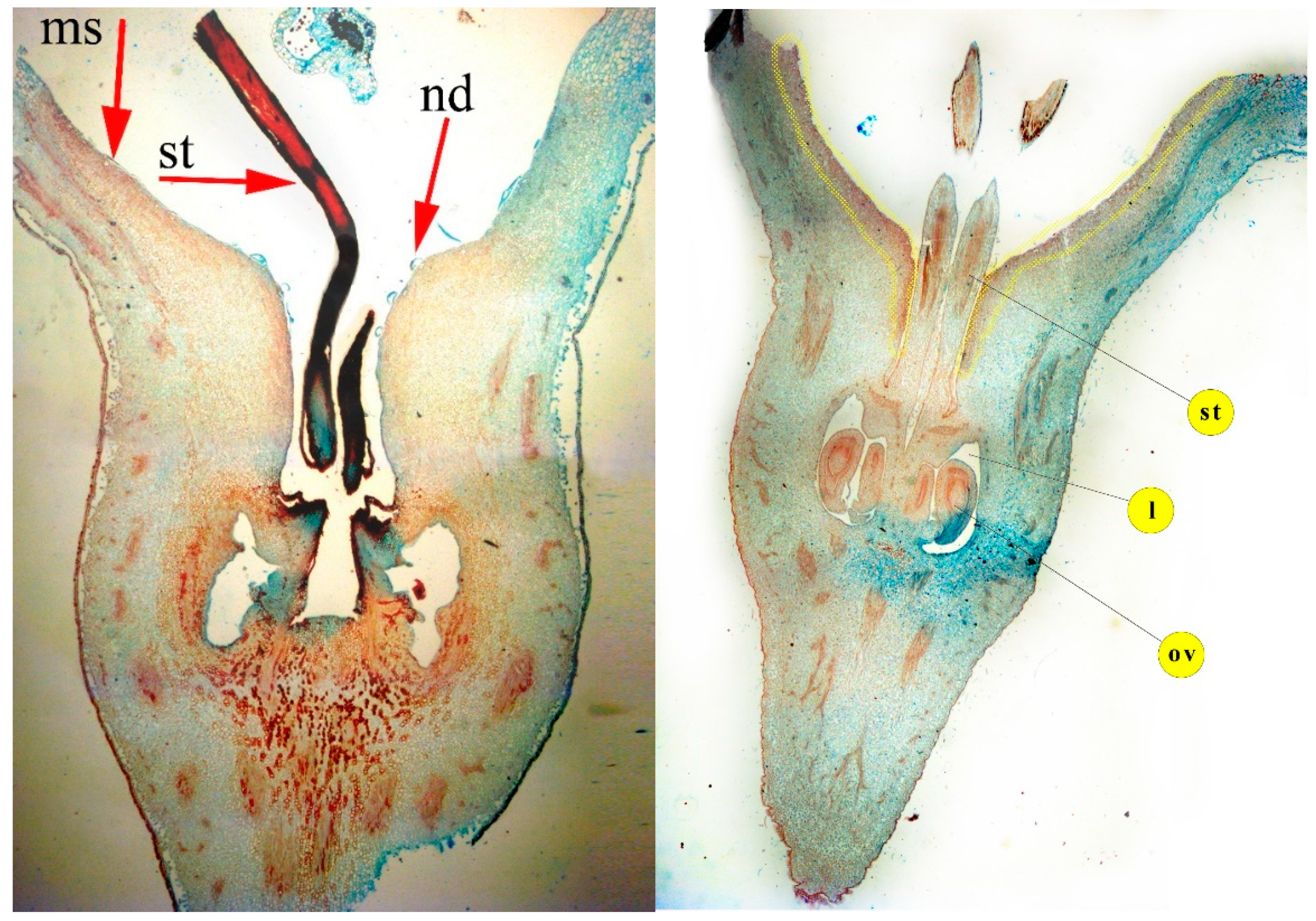
3.1. Topography and Micromorphology of Pear Nectary
3.2. Nectary Anatomy (Histological Analysis)
3.3. Sugars Analysis
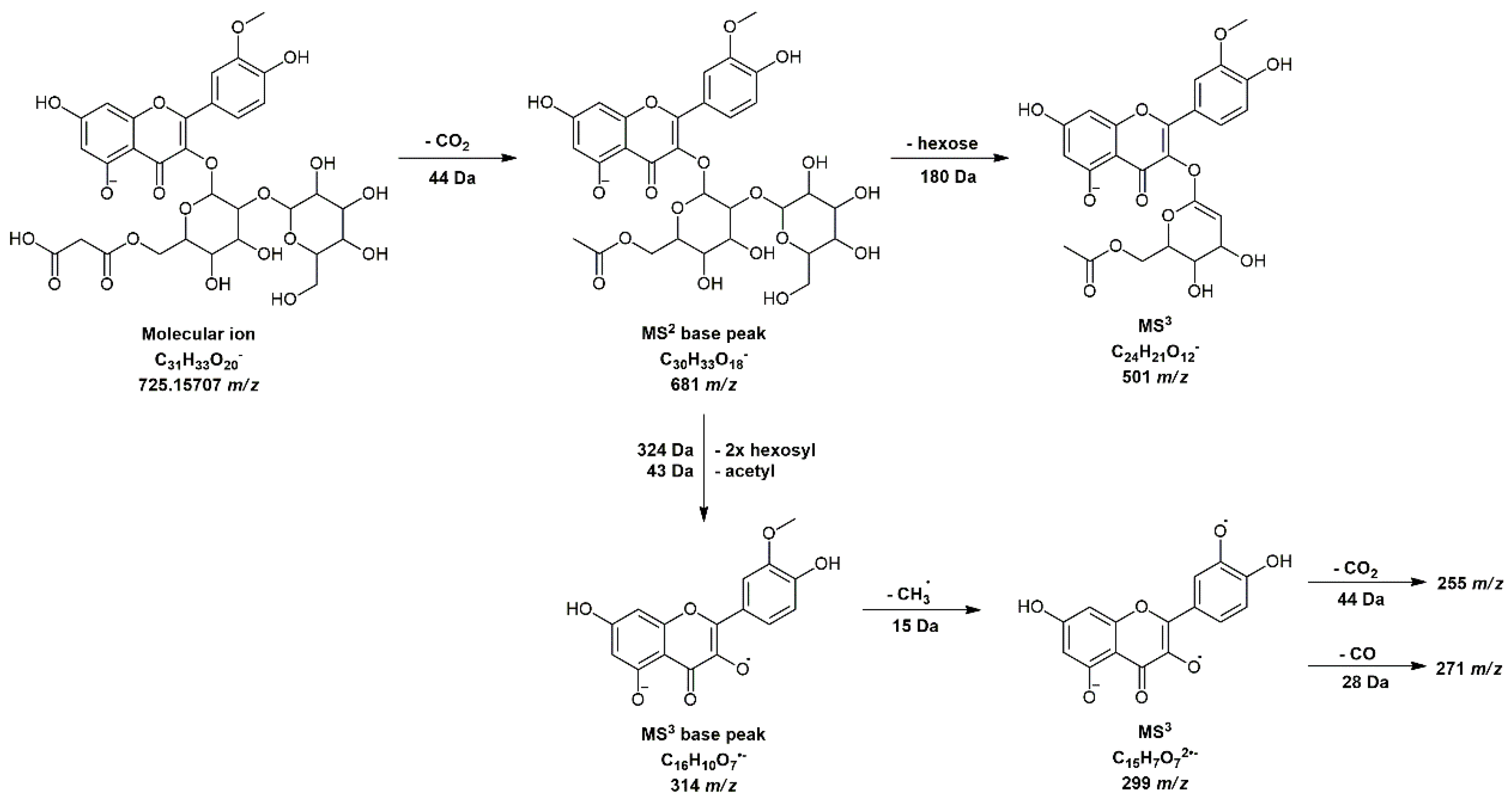
3.4. Identification of Pear Nectar Polyphenolics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hancock, J.F.; Lobos, G.A. Pears. In Temperate Fruit Crop Breeding: Germplasm to Genomics; Hancock, J.F., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 299–335. [Google Scholar] [CrossRef]
- Bao, L.; Chen, K.; Zhang, D.; Cao, Y.; Yamamoto, T.; Teng, Y. Genetic diversity and similarity of pear cultivars native to East Asia revealed by SSR (simple sequence repeat) markers. Genet. Resour. Crop Evol. 2007, 54, 959–971. [Google Scholar] [CrossRef]
- FAOStat. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 22 January 2023).
- Itai, A. Pear. In Fruits and Nuts. Genome Mapping and Molecular Breeding in Plants; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4, pp. 157–171. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, F. Assessment of genetic diversity of Chinese sand pear landraces (Pyrus pyrifolia Nakai) using simple sequence repeat markers. Hortic. Sci. 2009, 44, 619–626. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Cerović, R.; Radošević, R.; Oparnica, Č.; Meland, M. Morphological and anatomical leaf characteristics of some European and Asian pear cultivars. Acta Hortic. 2021, 1303, 63–70. [Google Scholar] [CrossRef]
- Reiland, H.; Slavin, J. Systematic review of pears and health. Nutr. Today 2015, 50, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Meland, M.; Fotirić Akšić, M.; Cerović, R.; Frøynes, O.; Kaiser, C.; Maas, F. Evaluation of New Promising Norwegian Pear Cultivars in a Nordic Climate. J. Am. Pom. Soc. 2021, 75, 149–156. [Google Scholar]
- Przybyla, A.A.; Bokszczanin, K.L.; Schollenberger, M.; Gozdowski, D.; Madry, W.; Odziemkowski, S. Fire blight resistance of pear genotypes from different European countries. Trees 2012, 26, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Hevesi, M.; Göndör, M.; Kása, K.; Honty, K.; Tóth, M.G. Traditional and commercial apple and pear cultivars as sources of resistance to fire blight. EPPO Bull. 2004, 34, 377–380. [Google Scholar] [CrossRef]
- Thibault, B.; Paulin, J.P. Pear breeding and selection for fire blight resistance. Acta Hortic. 1984, 161, 141–146. [Google Scholar] [CrossRef]
- Malnoy, M.; Martens, S.; Norelli, J.L.; Barny, M.; Sundin, G.W.; Smits, T.H.M.; Duffy, B. Fire blight: Applied genomic insights of thepathogen and host. Annu. Rev. Phytopathol. 2012, 50, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Griffith, C.S.; Sutton, T.B.; Peterson, P.D. Fire Blight: The Foundation of Phytobacteriology; American Phytopathological Society: St. Paul, MN, USA, 2003; pp. 1–158. [Google Scholar]
- Piqué, N.; Miñana-Galbis, D.; Merino, S.; Tomás, J.M. Virulence factors of Erwinia amylovora: A review. Int. J. Mol. Sci. 2015, 16, 12836–12854. [Google Scholar] [CrossRef]
- Bubán, T.; Orosz-Kovács, Z. The nectary as the primary site of infection by Erwinia amylovora (Burr.) Winslow et al.: A mini review. Plant Syst. Evol. 2003, 238, 183–194. [Google Scholar] [CrossRef]
- Seneta, W.; Dolatowski, J. Dendrologia. Wyd; Naukowe PWN: Warszawa, Poland, 2009; pp. 1–544. [Google Scholar]
- Déri, H.; Orosz-Kovács, Z.; Farkas, Á. Morphological characterization of the floral nectary in some apple-shaped and pear-shaped quince cultivars. Acta Bot. Hung. 2007, 49, 171–187. [Google Scholar] [CrossRef]
- Farkas, Á.; Orosz-Kovács, Z.; Déri, H.; Chauhan, S.V.S. Floral nectaries in some apple and pear cultivars with special reference to bacterial fire blight. Curr. Sci. 2007, 92, 1286–1289. [Google Scholar]
- Farkas, A. Morphology and histology of the nectary in Hungarian local pear cultivars. Acta Hortic. 2005, 671, 127–135. [Google Scholar] [CrossRef]
- Radice, S.; Galati, B. Floral nectary ultrastructure of Prunus persica (L.) Batch cv. Forastero (Newcomer), an Argentine peach. Plant Syst. Evol. 2003, 238, 23–32. [Google Scholar] [CrossRef]
- Farkas, Á.; Zajácz, E. Nectar production for the Hungarian honey industry. Eur. J. Plant Sci. Biotechnol. 2007, 1, 125–151. [Google Scholar]
- Bernardello, G. A systematic survey of floral nectaries. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 19–128. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.M. Ultrastructural study on apricot floral nectary. J. Fruit Sci. 2011, 5, 792–797. [Google Scholar]
- Guffa, B.; Nedić, N.; Dabić Zagorac, D.; Tosti, T.; Gašić, U.; Natić, M.; Fotirić Akšić, M. Characterization of Sugar and Polyphenolic Diversity in Floral Nectar of Different ‘Oblačinska’ Sour Cherry Clones. Chem. Biodivers. 2017, 14, e1700061. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Masierowska, M.; Konarska, A. Characteristics of floral nectaries and nectar in two species of Crataegus (Rosaceae). Plant Syst. Evol. 2003, 238, 33–41. [Google Scholar] [CrossRef]
- Weryszko-Chmielewska, E.; Sulborska-Różycka, A.; Sawidis, T. Structure of the nectary in Chaenomeles japonica (Thunb.) Lindl. ex Spach. in different stages of flowering with focus on nectar secretion. Protoplasma 2022, 259, 1467–1476. [Google Scholar] [CrossRef]
- Kostryco, M.; Chwil, M. Nectar Secretion, Morphology, Anatomy and Ultrastructure of Floral Nectary in Selected Rubus idaeus L. Varieties. Agriculture 2022, 12, 1017. [Google Scholar] [CrossRef]
- Rubtsova, O.L.; Vakulenko, T.B.; Chyzhankova, V.I. Morphological features of nectaries of some species of the genus Rosa (Rosaceae). Ukr. Bot. J. 2022, 79, 103–113. (In Ukrainian) [Google Scholar] [CrossRef]
- Konarska, A.; Masierowska, M.; Weryszko-Chmielewska, E. The structure of nectaries and nectar secretion in common pear (Pyrus communis L.). J. Apic. Sci. 2005, 49, 85–92. [Google Scholar]
- Farkas, A.; Orosz-Kovács, Z.S. Primary and secondary attractants of flowers in pear Pyrus betulifolia. Acta Hortic. 2004, 636, 317–324. [Google Scholar] [CrossRef]
- Farkas, Á.; Orosz Kovács, Z.; Bubán, T. Floral biological studies on pear cultivars in relation to fire blight susceptibility. Int. J. Hortic. Sci. 2004, 10, 25–30. [Google Scholar] [CrossRef]
- Canto, A.; Herrera, C.M.; Medrano, M.; Perez, R.; Garcia, I.M. Pollinator foraging modifies nectar sugar composition in Helleborus foetidus (Ranunculaceae): An experimental test. Am. J. Bot. 2008, 95, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Nagy Tóth, E.; Szabó, L.G.; Botz, L.; Orosz- Kovács, Z. Effect of rootstocks on floral nectar composition in apple cultivars. Plant Syst. Evol. 2003, 283, 43–55. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Thornburg, R.W. Nectar chemistry. In Nectaries and Nectar; Nicolson, S.W., Nepi, M., Pacini, E., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 215–264. [Google Scholar] [CrossRef]
- Heil, M. Nectar: Generation, regulation and ecological functions. Trends Plant Sci. 2011, 16, 191–200. [Google Scholar] [CrossRef]
- Hevesi, M.; Farkas, A.; Kása, A.; Orosz-Kovács, Z. Carbohydrate utilization of Erwinia amylovora in vitro. In. J. Hortic. Sci. 2004, 10, 31–34. [Google Scholar] [CrossRef]
- Pontais, I.; Treutter, D.; Paulin, J.P.; Brisset, M.N. Erwinia amylovora modifies phenolic profiles of susceptible and resistant apple through its type III secretion system. Physiol. Plant 2008, 132, 262–271. [Google Scholar] [CrossRef]
- González-Teuber, M.; Eilmus, S.; Muck, A.; Svatos, A.; Heil, M. Pathogenesis-related proteins protect extrafloral nectar from microbial infestation. Plant J. 2009, 58, 464–473. [Google Scholar] [CrossRef]
- Nogueira, F.C.S.; Farias, A.R.B.; Teixeira, F.M.; Domont, G.B.; Campos, F.A.P. Common Features Between the Proteomes of Floral and Extrafloral Nectar From the Castor Plant (Ricinus communis) and the Proteomes of Exudates From Carnivorous Plants. Front. Plant Sci. 2018, 9, 549. [Google Scholar] [CrossRef]
- Kurilla, A.; Toth, T.; Dorgai, L.; Darula, A.; Lakatos, T.; Silhavy, D.; Kerenyi, Z.; Dallmann, G. Nectar and stigma exudate-specific expression of an acidic chitinase could partially protect certain apple cultivars against fire blight disease. Planta 2020, 251, 20. [Google Scholar] [CrossRef]
- Nepi, M. Beyond nectar sweetness: The hidden ecological role of non-protein amino acids in nectar. J. Ecol. 2014, 102, 108–115. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Skene, K.R.; Formanek, P. Non-proteinamino acids: Plant, soil and ecosystem interactions. Plant Soil 2011, 342, 31–48. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Dabić, D.; Gašić, U.; Zec, G.; Vulić, T.; Tešić, Ž.; Natić, M. Polyphenolic Profile of Pear Leaves with Different Resistance to Pear Psylla (Cacopsylla pyri). J. Agric. Food Chem. 2015, 63, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: Oxford, UK, 1999; pp. 1–322. [Google Scholar]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: Harlow, UK, 2010; pp. 1–278. [Google Scholar]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic profiles of leaves, grapes and wine of grapevine variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Nagy Tóth, E.; Bubán, T.; Hevesi, M.; Orosz-Kovács, Z.; Szabó, L.G. Morphological characteristics of the nectary and composition of nectar in flowers of selected apple cultivars. Acta Hortic. 2000, 538, 301–308. [Google Scholar] [CrossRef]
- Zeng, W.; Melotto, M.; He, S.Y. Plant stomata: A checkpoint of host immunity and pathogen virulence. Curr. Opin. Biotechnol. 2010, 21, 599–603. [Google Scholar] [CrossRef]
- Davis, A.R.; Gunning, B.E.S. The modified stomata of the floral nectary of Vicia faba L. Development, anatomy and ultrastructure. Protoplasma 1992, 166, 134–152. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; He, S.Y. Role of Stomata in Plant Innate Immunity and Foliar Bacterial. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Orosz-Kovács, Z. Nectary structures in cherry cultivars. Acta Agron. Hung. 1993, 42, 239–253. [Google Scholar]
- Wilson, M.; Sigee, D.C.; Epton, H.A.S. Erwinia amylovora Infection of hawthorn blossom, II The stigma. J. Phytopath. 1989, 127, 1–14. [Google Scholar] [CrossRef]
- Spinelli, F.; Ciampolini, F.; Cresti, M.; Geider, K.; Costa, G. Influence of stigmatic morphology on flower colonization by Erwinia amylovora and Pantoea agglomerans. Eur. J. Plant Pathol. 2005, 113, 395–405. [Google Scholar] [CrossRef]
- Wilson, M.; Sigee, D.C.; Epton, H.A.S. Erwinia amylovora infection of hawthorn blossom: III. The Nectary. J. Phytopath. 1990, 128, 62–74. [Google Scholar] [CrossRef]
- Davis, A.R. Searching and breeding for structural features of flowers correlated with high nectar-carbohydrate production. Acta Hortic. 2001, 561, 107–121. [Google Scholar] [CrossRef]
- Faoro, I.D.; Orth, A.I. Flower visiting insects during the bloom period of Japanese pear orchards in Brazil. Acta Hortic. 2015, 1094, 275–279. [Google Scholar] [CrossRef]
- Jacquemart, A.-L.; Michotte-van der, A.A.; Raspé, O. Compatibility and pollinator efficiency tests on Pyrus communis L. cv. Conférence. J. Hortic. Sci. Biotechnol. 2006, 81, 827–830. [Google Scholar] [CrossRef]
- Braun, P.G.; Hildebrand, P.D.; Jamieson, A.R. Resistance of raspberry cultivars to fire blight. HortScience 2004, 39, 1189–1192. [Google Scholar]
- Seeburger, V.C.; D’Alvise, P.; Shaaban, B.; Schweikert, K.; Lohaus, G.; Schroeder, A.; Hasselmann, I.M. The trisaccharide melezitose impacts honey bees and their intestinal microbiota. PLoS ONE 2020, 15, e0230871. [Google Scholar] [CrossRef]
- Colaric, M.; Stampar, F.; Hudina, M. Changes in sugars and phenolics concentrations of Williams pear leaves during the growing season. Can. J. Plant Sci. 2006, 86, 1203–1208. [Google Scholar] [CrossRef]
- Baker, H.G.; Baker, I. Floral nectar sugar constituents in relation to pollinator type. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1983; pp. 117–141. [Google Scholar]
- Farkas, A.; Orosz-Kovács, Z.; Szabó, L.G. Insect attraction of flowers in pear cultivars. Acta Hortic. 2002, 596, 773–776. [Google Scholar] [CrossRef]
- Meheriuk, M.; Lane, W.D.; Hall, J.W. Influence of cultivar on nectar sugar content in several species of tree fruits. HortScience 1987, 22, 448–450. [Google Scholar] [CrossRef]
- Romeis, J.; Wäckers, F.L. Nutritional suitability of individual carbohydrates and amino acids for adult Pieris brassicae. Physiol. Entomol. 2002, 27, 148–156. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.Q.; Xue, B.; Liu, Z.D.; Zou, L. Chemical Composition and Functional Characteristics of Dietary Fibers from Pingguoli Pear (Pyrus bretschneideri Rehd. cv. Pingguoli Pear). Curr. Top. Nutraceutical Res. 2020, 18, 39. [Google Scholar]
- Fischer, M.K.; Shingleton, A.W. Host plant and ants influence the honeydew sugar composition of aphids. Funct. Ecol. 2001, 15, 544–550. [Google Scholar] [CrossRef]
- Lingner, U.; Steffen, M.; Sode, B.; Deising, H.B.; Sauer, N. Functional Characterization of a Eukaryotic Melibiose Transporter. Plant Physiol. 2011, 156, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Soto, V.; Silva, M.F.; Galmarini, C. Effect of Nectar Composition on Bee Attraction for Onion Seed Production. In Nectar—Production, Chemical Composition and Benefits to Animals and Plants, II. Series: Plant Science Research and Practices; Peck, R.L., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 41–58. [Google Scholar]
- Deachathai, S.; Mahabusarakam, W.; Phongpaichit, S.; Taylor, W.C.; Zhang, Y.J.; Yang, C.R. Phenolic compounds from the flowers of Garcinia dulcis. Phytochemistry 2006, 67, 464–469. [Google Scholar] [CrossRef]
- del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-Garcia, O.; del Río, J.A.; Ortuño, A.; Quirin, K.W.; Gerard, D. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, A.; Qian, M.; Li, J.; Li, Y.; Shen, J.; et al. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. 2020, 18, 1223–1240. [Google Scholar] [CrossRef]
- Ferreres, F.; Llorach, R.; Gil-Izquierdo, A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Şenkardes, I.; Dogan, A.; Sinan, K.I.; Uysal, S.; Aumeeruddy-Elalfi, Y.; et al. Modern and traditional extraction techniques affect chemical composition and bioactivity of Tanacetum parthenium (L.) Sch.Bip. Ind. Crops Prod. 2020, 146, 112202. [Google Scholar] [CrossRef]
- Andrade, P.B.; Carvalho, A.R.F.; Seabra, R.M.; Ferreira, M.A. A Previous Study of Phenolic Profiles of Quince, Pear, and Apple Purees by HPLC Diode Array Detection for the Evaluation of Quince Puree Genuineness. J. Agric. Food Chem. 1998, 46, 968–972. [Google Scholar] [CrossRef]
- Brahem, M.; Renard, C.M.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.-Y.; Gao, W.-Y.; Wang, Y.; Wang, H.-Y.; Cao, J.-G.; Huang, L.-Q. Chemical composition and anti-inflammatory and antioxidant activities of eight pear cultivars. J. Agric. Food Chem. 2012, 60, 8738–8744. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, J.Y.; Lee, H.J.; Park, K.Y.; Ma, Y.-K.; Lee, S.-H.; Cho, J.A.; Kim, W.-S.; Park, K.H.; Moon, J.-H. Isolation and identification of phenolic compounds from an Asian pear (Pyrus pyrifolia Nakai) fruit peel. Food Sci. Biotechnol. 2011, 20, 1539–1545. [Google Scholar] [CrossRef]
- Horváth, G.; Farkas, A.; Szabó, G.L. Flavonoids, chalcones and phenyl-propanoids in apple and pear flowers. Int. J. Hortic. Sci. 2004, 10, 35–38. [Google Scholar] [CrossRef]
- Cui, T.; Nakamura, K.; Ma, L.; Li, J.; Kayahara, H. Analysis of arbutin and chlorogenic acid the major phenolic constituents in Oriental pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- Gunen, Y.; Misirli, A.; Gulcan, R. Leaf phenolic content of pear cultivars resistant or susceptible to fire blight. Sci. Hortic. 2005, 105, 213–221. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.; Gašić, U.; Dragana, D.Z.; Sredojević, M.; Tosti, T.; Natić, M.; Meland, M. Chemical Fingerprint of ‘Oblačinska’ Sour Cherry (Prunus cerasus L.) Pollen. Biomolecules 2019, 9, 391. [Google Scholar] [CrossRef]
- Kostić, A.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT 2019, 112, 108244. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Streker, P.; Klaiber, I.; Carle, R. Detection of isorhamnetin glycosides in extracts of apples (Malus domestica cv. “Brettacher”) by HPLC-PDA and HPLC-APCI-MS/MS. Phytochem. Anal. 2002, 13, 87–94. [Google Scholar] [CrossRef]
- Dias, L.G.; Tolentino, G.; Pascoal, A.; Estevinho, L.M. Effect of processing conditions on the bioactive compounds and biological properties of bee pollen. J. Apic. Res. 2016, 55, 357–365. [Google Scholar] [CrossRef]
- Riveros, A.J.; Gronenberg, W. The flavonoid rutin protects the bumble bee Bombus impatiens against cognitive impairment by imidacloprid and fipronil. J. Exp. Biol. 2022, 225, jeb244526. [Google Scholar] [CrossRef]
- Skočajić, D.; Gašić, U.; Dabić Zagorac, D.; Nešić, M.; Tešić, Ž.; Meland, M.; Fotirić Akšić, M. Analysis of Phenolic Compounds for the Determination of Grafts (in) Compatibility Using In Vitro Callus Cultures of Sato-Zakura Cherries. Plants 2021, 10, 2822. [Google Scholar] [CrossRef] [PubMed]
- Genzel, F.; Dicke, M.D.; Junker-Frohn, L.V.; Neuwohner, A.; Thiele, B.; Putz, A.; Usadel, B.; Wormit, A.; Wiese-Klinkenberg, A. Impact of Moderate Cold and Salt Stress on the Accumulation of Antioxidant Flavonoids in the Leaves of Two Capsicum Cultivars. J. Agric. Food Chem. 2021, 69, 6431–6443. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, M.A.; Said, A.; Antje, H. NMR and radical scavenging activities of patuletin from Urtica urens against aflatoxin B. Pharm. Biol. 2005, 43, 515–525. [Google Scholar] [CrossRef]
- Bergman, M.; Varshavsky, L.; Gottlieb, H.E.; Grossman, S. The antioxidant activity of aqueous spinach extract: Chemical identification of active fractions. Phytochemistry 2001, 58, 143–152. [Google Scholar] [CrossRef]
- Burlec, A.F.; Pecio, Ł.; Kozachok, S.; Mircea, C.; Corciovă, A.; Vereștiuc, L.; Cioancă, O.; Oleszek, W.; Hăncianu, M. Phytochemical Profile, Antioxidant Activity, and Cytotoxicity Assessment of Tagetes erecta L. Flowers. Molecules 2021, 26, 1201. [Google Scholar] [CrossRef] [PubMed]
- Abrol, D.P. Pollination Biology: Biodiversity Conservation and Agricultural Production; Springer: New York, NY, USA, 2012; pp. 413–458. [Google Scholar]
- Faizi, S.; Siddiqi, H.; Bano, S.; Naz, A.; Lubna, A.; Mazhar, K.; Nasim, S.; Riaz, T.; Kamal, S.; Ahmad, A.; et al. Antibacterial and Antifungal Activities of Different Parts of Tagetes patula.: Preparation of Patuletin Derivatives. Pharm. Biol. 2008, 46, 309–320. [Google Scholar] [CrossRef]
- Mosić, M.; Trifković, J.; Vovk, I.; Gašić, U.; Tešić, Ž.; Šikoparija, B.; Milojković-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef] [PubMed]



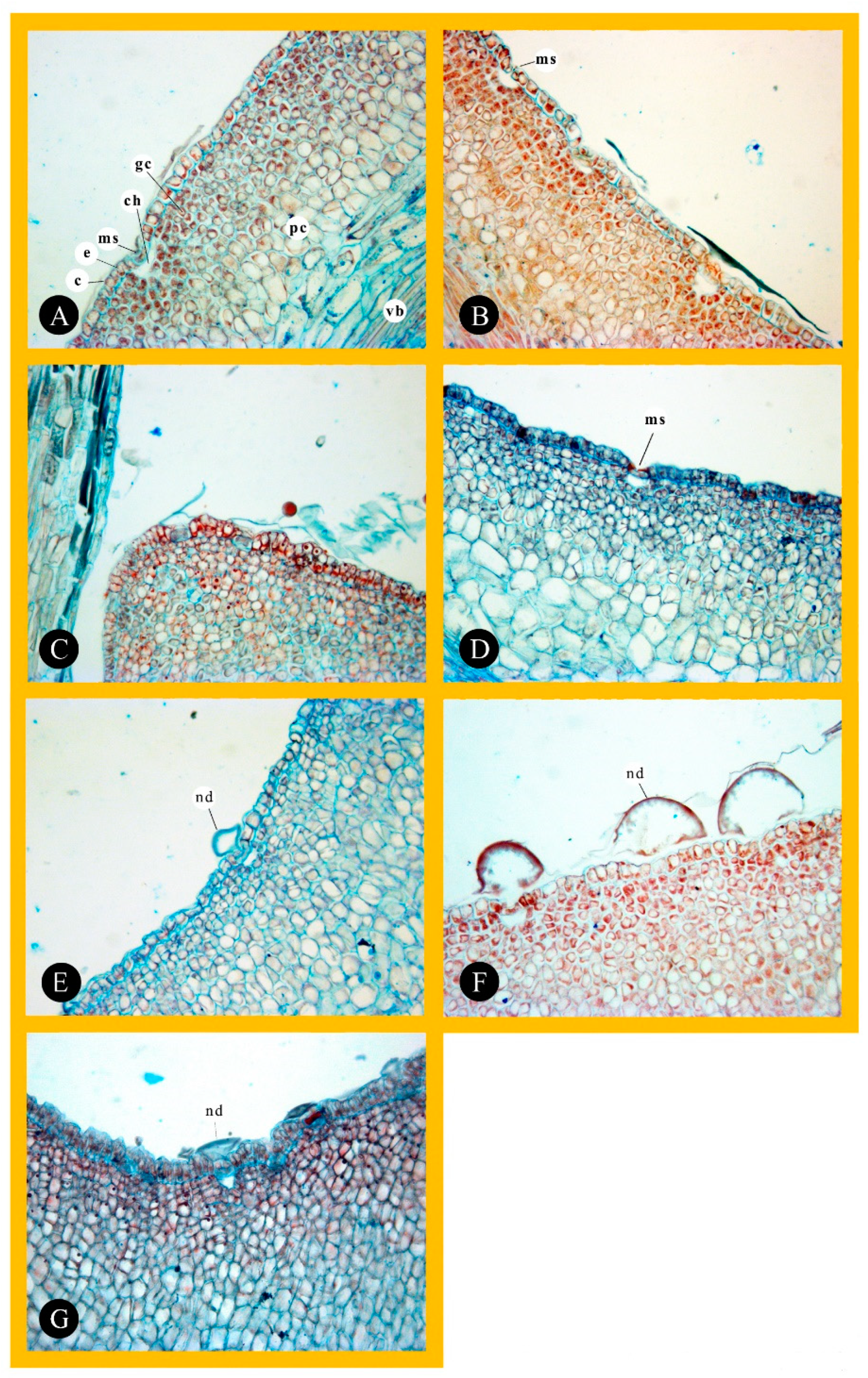
| Cultivar | Length of Epidermal Cell (µm) | Width of Epider- mal Cell (µm) | Length of Glandular Cell (µm) | Width of Glandular Cell (µm) | Length of Hypantium Cell (µm) | Width of Hypantium Cell (µm) | Radius of Substomatal Chamber (µm) | Stomatal Guard Cells Length (µm) |
|---|---|---|---|---|---|---|---|---|
| Williams (SUS) ** | 33.49 a * | 24.27 de | 23.79 bc | 20.48 de | 51.03 b | 30.71 a | 52.91 c | 19.00 bc |
| Bella di Guigno (MS) | 34.89 a | 25.40 e | 24.13 c | 19.29 cd | 46.31 a | 31.64 a | 56.75 d | 12.15 a |
| Poire de Cure (MS) | 37.30 b | 26.27 e | 23.84 bc | 22.94 e | 65.39 d | 33.62 a | 73.67 f | 20.93 bc |
| Alexand. Lucas (LS) | 33.40 a | 23.54 de | 22.77 b | 17.16 b | 59.61 c | 37.22 cd | 59.87 e | 19.01 bc |
| Chojuro (HR) | 33.19 a | 18.09 bc | 20.17 a | 17.16 b | 66.43 d | 36.33 b | 46.62 b | 20.03 bc |
| Nijisseiki (HR) | 37.47 b | 14.09 a | 22.31 b | 14.50 a | 77.44 e | 47.79 e | 24.16 a | 22.99 c |
| Kieffer (HR) | 32.99 a | 20.54 c | 24.69 c | 17.18 b | 66.02 d | 39.85 d | 56.20 de | 37.85 d |
| Sugar Components | Williams | Bella di Guigno | Poire de Cure | Alexander Lucas | Chojuro | Nijisseiki | Kieffer |
|---|---|---|---|---|---|---|---|
| Arabinose | 1.999 d | 3.190 g | 2.326 f | 1.657 c | 1.031 a | 1.256 b | 2.141 e |
| Erythritol | 0.965 e * | 0.271 a | 0.999 ef | 0.887 d | 0.659 c | 0.533 b | 1.023 f |
| Fructose | 184.544 a | 195.603 b | 204.022 c | 207.460 c | 214.435 d | 215.677 d | 204.619 c |
| Galactitol | 2.875 a | 3.345 a | 3.256 a | 3.487 a | 5.112 b | 4.652 b | 2.145 a |
| Galactose | 2.326 b | 1.595 a | 3.333 c | 3.541 c | 4.985 d | 5.633 e | 3.326 c |
| Gentiobiose | 0.085 c | 0.016 a | 0.054 b | 0.061 b | 0.156 e | 0.145 d | 0.065 b |
| Glucose | 166.078 a | 188.638 b | 207.215 c | 200.340 bc | 212.886 d | 210.621 d | 205.664 c |
| Isomaltose | 0.270 ab | 0.518 c | 0.299 b | 0.786 d | 0.246 a | 1.023 e | 0.526 c |
| Isomaltotriose | 0.256 a | 0.906 f | 0.336 b | 0.443 c | 0.453 c | 0.679 e | 0.553 d |
| Maltose | 0.785 b | 0.801 bc | 0.886 c | 0.934 d | 3.256 e | 4.179 f | 0.457 a |
| Maltotriose | 0.112 a | 0.545 d | 0.105 a | 0.253 b | 0.237 b | 0.333 c | 0.253 b |
| Manitol | 0.989 c | 0.690 a | 0.852 b | 0.954 c | 1.690 f | 1.556 e | 1.124 d |
| Melesitose | 0.052 b | 0.032 a | 0.065 b | 0.099 c | 0.885 c | 0.986 c | 0.124 d |
| Melibiose | 0.089 a | 0.079 a | 0.124 b | 0.211 c | 0.527 e | 0.411 d | 0.236 c |
| Panose | 0.265 b | 0.282 b | 0.279 b | 0.295 bc | 0.300 cd | 0.333 d | 0.152 a |
| Raffinose | 9.652 a | 13.271 b | 10.236 a | 11.067 ab | 17.653 c | 16.326 c | 8.652 a |
| Ribose | 0.885 d | 0.318 a | 0.899 d | 0.725 c | 0.599 b | 0.326 a | 0.562 b |
| Sorbitol | 84.294 a | 82.330 a | 117.365 b | 121.220 b | 134.365 c | 186.326 d | 185.326 d |
| Stachyose | 0.652 ab | 0.522 a | 0.771 bc | 0.877 c | 1.653 e | 1.025 d | 0.790 bc |
| Sucrose | 12.666 a | 10.604 a | 39.873 c | 17.812 b | 21.326 b | 19.425 a | 21.198 a |
| Trechalose | 1.856 f | 1.422 d | 0.652 b | 0.976 c | 1.986 g | 0.443 a | 1.543 e |
| Turanose | 0.074 a | 0.095 b | 0.088 ab | 0.100 b | 0.237 d | 0.252 d | 0.125 c |
| Xylose | 1.256 d | 0.853 b | 1.385 e | 1.123 c | 0.356 a | 0.445 a | 0.996 b |
| No | Compound Name | tR, min | Molecular Formula, [M–H]− | Calculated Mass, [M–H]− | Exact Mass, [M–H]− | Δ mDa | MS2 Fragments, (% Base Peak) | MS3 Fragments, (% Base Peak) |
|---|---|---|---|---|---|---|---|---|
| Phenolic acid derivatives | ||||||||
| 1 | 3-O-p-Coumaroylquinic acid | 5.00 | C16H17O8− | 337.09289 | 337.09012 | 2.77 | 119(9), 163(100), 173(3), 191(7) | 119(100) |
| 2 | 5-O-Caffeoylquinic acid a | 5.11 | C16H17O9− | 353.08781 | 353.08485 | 2.96 | 179(4), 191(100), 192(9), 215(4) | 85(68), 87(25), 93(44), 109(41), 111(24), 127(100), 173(59) |
| 3 | p-Hydroxybenzoic acid a | 5.22 | C7H5O3− | 137.02442 | 137.02359 | 0.83 | 93(100), 109(25) | − |
| 4 | Ferulic acid hexoside | 5.38 | C16H19O9− | 355.10346 | 355.10034 | 3.12 | 193(100), 194(15), 293(3) | 134(77), 149(92), 178(100) |
| 5 | 5-O-Caffeoylquinic acid isomer | 5.57 | C16H17O9− | 353.08781 | 353.08486 | 2.95 | 177(3), 179(3), 191(100), 192(8), 215(8), 307(3) | 85(97), 87(35), 93(84), 111(43), 127(100), 171(37), 173(78) |
| 6 | Aesculetin a | 5.60 | C9H5O4− | 177.01933 | 177.01877 | 0.56 | 131(41), 133(34), 135(100), 147(19) | 91(100), 107(6) |
| 7 | Caffeic acid a | 5.62 | C9H7O4− | 179.03498 | 179.03389 | 1.09 | 135(100) | 79(4), 91(49), 107(100), 117(39), 135(4) |
| 8 | 4-O-p-Coumaroylquinic acid | 5.75 | C16H17O8− | 337.09289 | 337.08989 | 3.00 | 163(13), 173(92), 174(8), 191(100), 192(8), 298(5), 299(7) | 85(100), 87(13), 93(27), 111(12), 127(11), 153(9), 173(68) |
| 9 | 5-O-p-Coumaroylquinic acid | 6.15 | C16H17O8− | 337.09289 | 337.09029 | 2.60 | 163(3), 164(3), 191(100), 192(3) | 71(22), 83(84), 85(37), 111(47), 115(16), 127(100), 171(37) |
| 10 | p-Coumaric acid a | 6.51 | C9H7O3− | 163.04007 | 163.03917 | 0.90 | 119(100) | 91(100) |
| 11 | Dicaffeoylquinic acid | 7.00 | C25H23O12− | 515.11950 | 515.11573 | 3.77 | 353(100), 354(17) | 135(8), 173(3), 179(39), 191(100) |
| 12 | Trimethylellagic acid hexoside | 8.31 | C23H21O13− | 505.09876 | 505.09496 | 3.80 | 342(37), 343(100), 344(28), 345(3), 425(3), 460(3), 463(3) | 300(5), 328(100) |
| Flavonoid glycosides | ||||||||
| 13 | Isorhamnetin 3,7-di-O-hexoside | 5.39 | C28H31O17− | 639.15667 | 639.15160 | 5.07 | 315(20), 316(11), 477(100), 478(17), 479(5), 519(20), 593(9) | 283(10), 299(34), 314(18), 315(49), 316(6), 342(7), 462(100) |
| 14 | Patuletin 3-O-(6″-hexosyl)-malonyl-hexoside | 5.95 | C31H33O21− | 741.15198 | 741.14688 | 5.10 | 697(100), 698(21) | 209(12), 315(35), 316(13), 330(100), 331(35), 535(14), 655(32) |
| 15 | Isorhamnetin 3-O-(2″-hexosyl)-hexoside | 6.01 | C28H31O17− | 639.15667 | 639.15126 | 5.41 | 271(25), 299(43), 300(71), 314(61), 315(72), 459(100), 477(25) | 138(11), 272(4), 351(4), 354(5), 369(5), 444(100), 445(13) |
| 16 | Isorhamnetin 3-O-(2″-hexosyl-6″-malonyl-hexoside | 6.27 | C31H33O20− | 725.15707 | 725.15239 | 4.68 | 681(100), 682(31) | 255(24), 271(38), 299(66), 300(33), 314(100), 315(87), 501(40) |
| 17 | Isorhamnetin 3-O-(2″-rhamnosyl)-hexoside | 6.27 | C28H31O16− | 623.16176 | 623.15741 | 4.35 | 271(19), 299(62), 300(34), 314(100), 315(66), 459(50), 503(16) | 271(3), 299(100) |
| 18 | Luteolin 7-O-(6″-rhamnosyl)-hexoside | 6.31 | C27H29O15− | 593.15119 | 593.14659 | 4.60 | 285(100), 286(16), 550(3) | 175(57), 197(58), 199(89), 217(100), 241(83), 243(79), 267(37) |
| 19 | Quercetin 3-O-(6″-rhamnosyl)-glucoside (Rutin) a | 6.31 | C27H29O16− | 609.14611 | 609.14343 | 2.68 | 255(4), 271(6), 300(33), 301(100), 343(5) | 151(79), 179(100), 257(16), 273(20) |
| 20 | Patuletin 3-O-(6″-malonyl)-hexoside | 6.70 | C25H23O16− | 579.09916 | 579.09506 | 4.10 | 535(100), 536(21) | 181(5), 315(19), 316(21), 330(48), 331(100), 493(25), 520(6) |
| 21 | Isorhamnetin 3-O-(6″-rhamnosyl)-hexoside | 6.81 | C28H31O16− | 623.16176 | 623.15741 | 4.35 | 299(7), 300(17), 315(100), 316(16) | 300(100), 271(3) |
| 22 | Isorhamnetin 3-O-hexoside | 6.89 | C22H21O12− | 477.10385 | 477.10023 | 3.62 | 299(18), 300(27), 314(23), 315(100), 316(21), 462(23), 463(8) | 300(100), 255(20), 271(14) |
| 23 | Syringetin 3-O-hexoside | 7.00 | C23H23O13− | 507.11441 | 507.11064 | 3.77 | 329(73), 330(22), 344(90), 345(43), 346(11), 492(100), 493(20) | 286(5), 301(20), 314(4), 329(100), 330(5) |
| 24 | Apigenin 7-O-glucoside (Apigetrin) a | 7.11 | C21H19O10− | 431.09837 | 431.09454 | 3.83 | 268(18), 269(100), 270(16), 311(4), 401(3) | 107(13), 117(12), 169(18), 197(13), 201(15), 225(100), 269(10) |
| 25 | Isorhamnetin 3-O-(6″-malonyl)-hexoside | 7.19 | C25H23O15− | 563.10424 | 563.10005 | 4.19 | 519(100), 520(19) | 299(3), 300(39), 314(9), 315(100) |
| 26 | Isorhamnetin 3-O-(6″acetyl)-hexoside | 7.20 | C23H23O13− | 519.11441 | 519.11105 | 3.36 | 282(3), 299(4), 300(33), 315(100), 316(13), 317(7) | 255(15), 271(13), 272(20), 300(100) |
| 27 | Syringetin 3-O-(6″-malonyl)-hexoside | 7.34 | C26H25O16− | 593.11481 | 593.11022 | 4.59 | 549(100), 550(19) | 330(28), 344(6), 345(100) |
| 28 | Syringetin 3-O-(6″-acetyl)-hexoside | 7.34 | C25H25O11− | 549.12498 | 549.12087 | 4.11 | 329(37), 330(30), 344(25), 345(100), 346(12), 534(39), 535(14) | 285(4), 287(5), 301(6), 302(20), 315(3) 330(100) |
| 29 | Isorhamnetin 3-O-(6″-malonyl)-hexoside isomer | 7.38 | C25H23O15− | 563.10424 | 563.10016 | 4.08 | 519(100), 520(16) | 255(5), 300(48), 314(53), 315(100), 357(7), 459(22), 477(6) |
| No | Compound Name | Williams (SUS) ** | Bella di Guigno (MS) | Poire de Cure (MS) | Alexander Lucas (LS) | Chojuro (HR) | Nijisseiki (HR) | Kieffer (HR) |
|---|---|---|---|---|---|---|---|---|
| Phenolic acid derivatives | ||||||||
| 1 | 3-O-p-Coumaroylquinic acid | − | 0.038 a * | 0.102 b | − | 0.400 c | − | − |
| 2 | 5-O-Caffeoylquinic acid a | − | 0.051 a | 0.047 a | − | 0.073 b | 0.048 a | 0.199 c |
| 3 | p-Hydroxybenzoic acid a | 0.049 c | 0.004 a | 0.002 a | 0.028 b | 0.048 c | 0.005 a | 0.072 d |
| 4 | Ferulic acid hexoside | − | 0.172 c | 0.038 a | 0.155 b | 0.983 e | 0.199 d | 0.193 d |
| 5 | 5-O-Caffeoylquinic acid isomer | − | 0.068 a | 0.062 a | − | − | − | 1.266 b |
| 6 | Aesculetin a | − | − | 0.003 b | − | 0.002 ab | − | 0.001 a |
| 7 | Caffeic acid a | − | − | − | − | 0.086 | − | − |
| 8 | 4-O-p-Coumaroylquinic acid | − | − | 0.122 a | − | 0.465 b | − | − |
| 9 | 5-O-p-Coumaroylquinic acid | − | − | 0.060 a | − | 0.172 c | − | 0.105 b |
| 10 | p-Coumaric acid a | − | − | 0.001 a | − | 0.003 b | − | − |
| 11 | Dicaffeoylquinic acid | − | 0.051 a | 0.159 c | 0.125 b | 0.214 d | 0.541 e | 2.238 f |
| 12 | Trimethylellagic acid hexoside | − | − | − | − | 0.116 | − | − |
| Flavonoid glycosides | ||||||||
| 13 | Isorhamnetin 3,7-di-O-hexoside | − | − | 0.074 | − | − | − | − |
| 14 | Patuletin 3-O-(6″-hexosyl)-malonyl-hexoside | − | − | − | − | − | 0.107 | − |
| 15 | Isorhamnetin 3-O-(2″-hexosyl)-hexoside | − | 0.137 | − | 0.223 c | − | 0.423 d | 0.125 a |
| 16 | Isorhamnetin 3-O-(2″-hexosyl-6″-malonyl-hexoside | − | 0.152 b | 0.168 c | 0.279 d | − | 0.423 e | 0.144 a |
| 17 | Isorhamnetin 3-O-(2″-rhamnosyl)-hexoside | − | − | − | − | 0.115 a | 0.336 c | 0.145 b |
| 18 | Luteolin 7-O-(6″-rhamnosyl)-hexoside | − | − | − | − | 0.169 | − | − |
| 19 | Quercetin 3-O-(6″-rhamnosyl)-glucoside (Rutin) a | − | − | − | − | 0.024 a | − | 0.072 b |
| 20 | Patuletin 3-O-(6″-malonyl)-hexoside | − | − | − | − | − | 0.540 | − |
| 21 | Isorhamnetin 3-O-(6″-rhamnosyl)-hexoside | − | − | 0.037 a | − | 0.253 c | − | 0.102 b |
| 22 | Isorhamnetin 3-O-hexoside | 0.359 c | 0.760 e | 0.101 a | 0.647 d | 0.194 b | 1.565 f | 0.387 c |
| 23 | Syringetin 3-O-hexoside | 0.107 b | 0.155 c | − | 0.087 a | − | 0.362 d | − |
| 24 | Apigenin 7-O-glucoside (Apigetrin)a | − | − | − | − | 0.012 | − | − |
| 25 | Isorhamnetin 3-O-(6″-malonyl)-hexoside | 0.608 a | 1.845 d | 1.667 b | 3.128 e | 1.687 c | 5.065 g | 4.107 f |
| 26 | Isorhamnetin 3-O-(6″acetyl)-hexoside | − | 0.253 c | 0.152 a | 0.324 de | 0.184 b | 0.356 e | 0.318 d |
| 27 | Syringetin 3-O-(6″-malonyl)-hexoside | 0.150 a | 0.379 c | 0.209 b | 0.625 e | 0.436 d | 1.476 g | 0.927 f |
| 28 | Syringetin 3-O-(6″-acetyl)-hexoside | − | 0.286 b | 0.119 a | 0.412 d | 0.348 c | 0.836 f | 0.501 e |
| 29 | Isorhamnetin 3-O-(6″-malonyl)-hexoside isomer | − | 0.054 a | − | 0.111 b | − | 0.197 c | 0.266 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotirić Akšić, M.; Mačukanović-Jocić, M.; Radošević, R.; Nedić, N.; Gašić, U.; Tosti, T.; Tešić, Ž.; Meland, M. The Morpho-Anatomy of Nectaries and Chemical Composition of Nectar in Pear Cultivars with Different Susceptibility to Erwinia amlylovora. Horticulturae 2023, 9, 424. https://doi.org/10.3390/horticulturae9040424
Fotirić Akšić M, Mačukanović-Jocić M, Radošević R, Nedić N, Gašić U, Tosti T, Tešić Ž, Meland M. The Morpho-Anatomy of Nectaries and Chemical Composition of Nectar in Pear Cultivars with Different Susceptibility to Erwinia amlylovora. Horticulturae. 2023; 9(4):424. https://doi.org/10.3390/horticulturae9040424
Chicago/Turabian StyleFotirić Akšić, Milica, Marina Mačukanović-Jocić, Radenko Radošević, Nebojša Nedić, Uroš Gašić, Tomislav Tosti, Živoslav Tešić, and Mekjell Meland. 2023. "The Morpho-Anatomy of Nectaries and Chemical Composition of Nectar in Pear Cultivars with Different Susceptibility to Erwinia amlylovora" Horticulturae 9, no. 4: 424. https://doi.org/10.3390/horticulturae9040424
APA StyleFotirić Akšić, M., Mačukanović-Jocić, M., Radošević, R., Nedić, N., Gašić, U., Tosti, T., Tešić, Ž., & Meland, M. (2023). The Morpho-Anatomy of Nectaries and Chemical Composition of Nectar in Pear Cultivars with Different Susceptibility to Erwinia amlylovora. Horticulturae, 9(4), 424. https://doi.org/10.3390/horticulturae9040424








