Abstract
The genus Diaporthe encompasses important plant pathogens, endophytes, and saprobes that are widely distributed in tropical and temperate regions. An accurate detection and identification of plant pathogens not only allows correct disease diagnosis but also increases the accuracy of taxonomic ambiguities for fungal-plant interactions purposes. Multi-omics approaches applied to this genus may represent valuable tools to unravel molecular mechanisms involved in the infection processes. Additionally, omics can provide adaptation patterns that make pathogens thrive under changing environmental conditions, and insights into the dual pathogen-endophyte lifestyle. Therefore, all published data covered in this literature review represents an important contribution to deepen the knowledge on the importance of omics in fungal-plant interactions. This accumulating evidence will speed up the research on formulating new strategies to control plant pathologies, to assist in the exploitation of endophytes for their function in plant hosts, and to underline molecular factors of fungal pathogenicity and endophytism in the genus Diaporthe.
1. Introduction
The genus Diaporthe is a species-rich phylogenetic group [1] that comprises several plant pathogens, endophytes, and saprobes [2]. As species of Diaporthe harbors phenotypic plasticity, their identification has relied mostly on the application of multilocus sequence analysis [3,4]. In this regard, the use of DNA sequences from type material provides the authentic biological material to investigate species boundaries in this genus [5,6]. However, the use of phylogenies to assess Diaporthe species boundaries remains a challenge for researchers [7], mainly due to the presence of high intraspecific and interspecific variability [3,8,9,10]. Moreover, species of this genus can be found co-occurring with other fungi (e.g., Neofusicoccum, Pestalotiopsis) on the same host under different lifestyles (e.g., endophyte, latent or harmful pathogen) [3,11], thus explaining the high number of taxa. Currently, the genus includes over 285 species supported by ex-type cultures and supplemented with DNA barcodes [1].
The early and accurate detection and identification of fungal plant pathogens is crucial to mitigate or prevent disease outbreaks, that may result in economic losses [12]. Therefore, the use of adequate phylogenetic methods is of paramount importance given that it increases the accuracy of taxonomic ambiguities [7] and provides insights for the discovery of natural products as well as additional information concerning the ecology and omics approaches among fungi [13]. For this reason, some authors have shown that besides phylogenetic analyses [1,3,9,14,15,16], the application of a cohesive approach comprising phylogenetic networks, studies on population genetic diversity, the detection of recombination, and the Genealogical Concordance Phylogenetic Species Recognition principle (GCPSR) are fundamental approaches to circumscribe the boundaries of species in the genus Diaporthe [8,10,17].
Genome analysis has allowed the identification of pathogenicity traits in different fungal plant pathogens [18,19,20]. In the genus Diaporthe, genome sequencing of pathogens has revealed genes encoding for plant cell wall degrading enzymes and enzymes involved in toxin production [21,22,23,24]. However, the genomic resources of species of Diaporthe are still limited due to the low number of available genomes in public databases. This impairs transcriptomic, proteomic, and metabolomic profiling of Diaporthe during host infection. Additionally, multi-omics not only gives insights into the molecular basis of the pathogenicity strategies used by Diaporthe but also molecular traits underlying the dual lifestyle switching (endophytic-pathogenic). Accumulating evidence suggests that species of Diaporthe have the ability to shift between endophytic and pathogenic behavior, depending on the host species and environmental stresses [3,9,21]. Moreover, it is also recognized that effector proteins could underpin the Diaporthe pathogenic lifestyle [24] and shape the fungal lifestyle on a given plant [25,26,27,28]. Nevertheless, our understanding of the molecular determinants of fungal pathogenicity and endophytism remains scarcely explored [29]. Thus, this review aims to elucidate how omics can deepen the knowledge of the mechanisms involved in pathogenicity and lifestyle switching in species of Diaporthe.
2. Criteria Used for Selection of Studies and Search Strategy
This study was carried out aiming to be an up-to-date review to summarize current scientific data regarding the pathogenicity mechanisms of species of Diaporthe, well known as important plant pathogens and endophytes. Moreover, the importance of using omics approaches to provide insights into the dual lifestyle of Diaporthe species was also discussed in this review. Considering this, the literature review was organized to deepen the knowledge on the importance to identify putative effectors and their functions, other pathogenicity-related genes, secondary metabolites and metabolic pathways, and proteins in species of Diaporthe. This knowledge will be crucial to identify mechanisms that may be involved in plant-pathogen cross-talks and in the transition from the endophytic to the pathogenic lifestyle. All scientific literature selected was mainly focused on the last decade (2012 to 2022), representing more than 83% of the references listed (146 out of 176).
3. Species of Diaporthe: Pathogens or Endophytes?
Species of the genus Diaporthe are cosmopolitan and well known as pathogens on several agricultural crops, forest trees, and ornamental plants (Table 1), causing diseases including root and fruit rots, dieback, stem cankers, leaf spots, wilting, pod blights, and seed decay [15,30,31,32]. For example, cankers caused by D. limonicola and D. melitensis were reported on lemon trees [11], and D. kongii, D. masirevicii, and D. ueckerae were found causing stem and peg dieback on peanut trees (Arachis hypogaea) [33]. Diaporthe ambigua, D. malorum, D. foeniculina, D. eres, and D. actinidiae are associated with cankers, shoot blight, and fruit rot on apple tree (Malus domestica) [30,34,35].
Recent studies using molecular data have shown that while a few species are host-specific, many others have an extensive host range [36,37]. For example, Diaporthe ampelina and D. citri are well-known pathogens associated only with Phomopsis cane and cankers of grapevines (Vitis vinifera) and melanose and gummosis on Citrus sp., respectively [27,38,39]. Similarly, D. helianthi is exclusively pathogenic to sunflower (Helianthus annuus), causing stem canker and twig blight [40] on crops in Europe, the USA, and Australia [41]. Other Diaporthe species are non-specific and infect a wide range of hosts [37,42]. For instance, D. novem has been reported as a pathogen on Aspalathus linearis, Citrus spp., Glycine max, H. annuus, and Hydrangea macrophylla [43]. Sunflower stem blight is also caused by D. gulyae, D. kochmanii, D. kongii, D. stewartii, D. phaseolorum, and D. novem [40,41]. Moreover, several Diaporthe species have been found associated with Phomopsis cane and leaf spot disease as well as cankers of grapevine [4,30,44].


Table 1.
Synopsis of some plant hosts reported for Diaporthe species.
Table 1.
Synopsis of some plant hosts reported for Diaporthe species.
| Plant Hosts | Species | References |
|---|---|---|
| Agricultural Crops | ||
| Prunus dulcis | D. amygdali, D. novem, D. foeniculina | [39,45,46,47] |
| Malus domestica | D. malorum, D. leucospermi, D. eres, D. ambigua, D. foeniculina | [34,35,48] |
| Vaccinium corymbosum | D. foeniculina, D. rudis, D. leucospermi, D. eres, D. ambigua, D. crousii, D. amygdali, D. oxe, D. passiflorae, D. malorum, D. hybrida | [49,50,51,52,53,54,55,56] |
| Citrus spp. | D. foeniculina, D. citri, D. citrishinensis, D. limonicola, D. masirevicii, D. passifloricola | [11,21,57,58] |
| Vitis vinifera | D. amygdali, D. celeris, D. rudis, D. ampelina, D. eres, D. gulyae, D. hungariae, D. sojae, D. guangxiensis, D. novem | [4,9,39,44,59] |
| Corylus avellana | D. eres, D. amygdali, D. sojae, D. cercidis, D. hungariae | [32,60] |
| Prunus persica | D. amygdali, D. eres, D. caryae, D. cercidis, D. hongkongensis, D. unshiuensis | [15,45,61] |
| Pyrus communis | D. infecunda, D. eres, D. terebinthifolii, D. phaseolorum, D. oxe, D. sojae, D. amygdali, D. hongkongensis | [14,35] |
| Gycine max | D. caulivora, D. longicolla, D. sojae, D. novem | [16,43,62,63] |
| Helianthus annus | D. helianthi, D. gulyae, D. novem, D. caulivora, D. kochmanii, D. kongii, D. stewartii | [40,41,43,64] |
| Juglans regia | D. capcisi, D. eres, D. amygdali | [32,65,66] |
| Forest Trees | ||
| Acacia spp. | D. accacigena, D. acaciarum, D. heterophyllae, D. fraxini-angustifoliae | [67,68,69] |
| Acer spp. | D. acericola, D. leucospermi | [37,70] |
| Eucalyptus spp. | D. crousii, D. malorum, D. eucalyptorum | [71,72] |
| Fraxinus spp. | D. silvicola, D. fraxinicola | [42,73] |
| Pinus spp. | D. eres, D. rudis | [71] |
| Quercus spp. | D. eres, D. foeniculina, D. rudis | [60,73] |
| Ornamental Plants | ||
| Camellia sinensis | D. amygdali, D. eres, D. hongkongensis, D. tulliensis, D. passiflorae | [74,75,76] |
| Foeniculum vulgare | D. foeniculina, D. angelicae | [77] |
| Hydrangea macrophylla | D. foeniculina, D. leucospermi, D. novem | [43,70] |
| Lithocarpus glabra | D. amygdali, D. longicicolla, D. lithocarpus | [78] |
| Rosa spp. | D. rosae, D. rosiphthora, D. rudis, D. eres, D. foeniculina | [3,79] |
Although species of Diaporthe are known as important plant pathogens and saprobes [3,7,9], they are also a major group of endophytes in stems and leaves of gymnosperms and angiosperms in tropical and temperate ecosystems [43,80]. Due to intercontinental trade of plant material, species of Diaporthe may behave as hitchhiking organisms [80] and are introduced into new areas as endophytes or latent pathogens acting as biotrophic at this stage. When the host is under stress conditions, the pathogen may switch to a necrotrophic stage inducing a phase of infection, and thus are called hemibiotrophs [6,81]. For example, it is assumed that D. rudis was imported to Chile via asymptomatic avocado fruit from California, causing then stem-end rot in avocados in Chile [82]. Moreover, some endophytes have been shown to act as pathogens, depending on the host and its health status [3]. Diaporthe sojae, previously found on Citrus as endophyte [83], was considered as the main causal agent of stem canker of soybean [16]. Moreover, D. caulivora is pathogenic to soybean [43] but endophytic in mangroves (Laguncularia racemosa) [84].
According to the literature, species of Diaporthe can be found with different lifestyles in nature. Nevertheless, it is still unknown whether environmental changes or the reduction in host’s defense drive endophytes into pathogenicity [83]. Some authors stated that fungal lifestyle switches are a dynamic process [85]. Mishra et al. [86] concluded that the balance between nutrient requirements of a microorganism and plant defense is important in determining whether an organism turns endophytic or pathogenic. Several factors such as nutrition exchange, the genotype of host and the microbial organism, the microbial number, environmental changes, and microbial interactions may alter this balance [85,87,88,89]. Thus, an imbalance may result in the microorganism success by surpassing plant immunity, leading to disease development [25,86]. For instance, Hilário et al. [90] showed that a water deficit regime imposed on blueberry plants, enhanced the transition from a latent to a pathogenic stage of D. amygdali. Moreover, it has been reported that high light intensity induces the pathogenicity of Diplodia mutila, an endophyte from the tropical tree Iriartia deltoidei [91]. Hoffman and Arnold [92] reported that cupressaceous trees in non-native areas exhibited lower diversity of fungal endophytes than the native species. Moreover, the richness and diversity of endophytes in olive trees were also higher during the autumn season, as demonstrated by Materatski et al. [93], suggesting that the number of endophytes present in a given host may also define lifestyle switching.
Brader et al. [94] pointed out that endophyte strains from a certain host may not show symptoms on these plants but may be pathogenic on other hosts, suggesting that these strains are not true endophytes (those never causing disease) [25]. A recent investigation was carried out to distinguish between true endophytes and latent pathogens in diverse hosts [35]. Pathogenicity tests performed by the authors revealed that some species of Diaporthe did not cause disease and thus were considered as true endophytes. Nevertheless, other Diaporthe isolates were capable of causing disease, and therefore were characterized as latent pathogens [35]. In this regard, the presence of dual lifestyles in the genus Diaporthe still raises many doubts about their dynamics and behavior in plants. To better understand the dynamics of endophytism, it would be important to carry out comparative studies regarding gene expression and regulatory mechanisms in both plants and endophytes to understand how the same fungal species behaves as endophyte or pathogen [95].
4. Omics to Study Fungal Plant Pathogens
Recent advances in omics approaches such as transcriptomics and proteomics offer new opportunities to understand molecular mechanisms and to search for biomarkers expression for early diseases diagnosis (e.g., cancer, autoimmune diseases) [96,97]. Also in plant pathology, multi-omics (genomics, transcriptomics, proteomics, and metabolomics) can help mainly in the prevention and management of diseases [98]. The omics have been applied to elucidate the function of genes and the structure of the genome to provide insights into gene and protein expression and to understand the metabolic profiling of both the host and the pathogen during an infection process [99] (Figure 1). The application of omics in the genus Diaporthe is still poorly explored, although metabolomics has been widely applied to explore endophytic Diaporthe natural products for their potential applications in pharmacology [100]. Although the genus Diaporthe comprises important plant pathogens and endophytes, these species also have the ability to switch lifestyles [3,6,9]. Accordingly, multi-omics approaches could be crucial tools to unravel.
- (1)
- Adaptation patterns of pathogens under changing environmental conditions.
- (2)
- Molecular traits underlying the infection processes.
- (3)
- Patterns of endophytic fungal community and their implications for disease development.
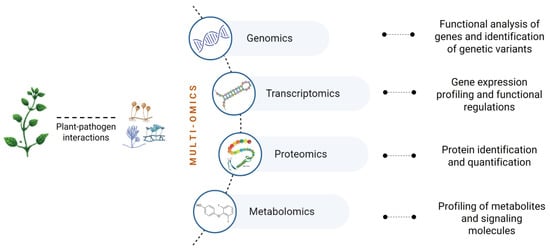
Figure 1.
Multi-omics approaches and obtained data for a thorough understanding of diverse aspects of plant pathogens and their cross-talks with plants.
4.1. Genomics
Since the sequencing of the first fungal genome, the yeast Saccharomyces cerevisiae in 1996 [101], advances in next-generation sequencing (NGS) technology have led to an increase in genomes [102], specifically from fungal pathogens that affect agriculture and forestry [103]. NGS is a rapid and high-throughput approach, and it is represented by different sequencing platforms such as AB SOLiD, Illumina HiSeq System, PacBio RS, and Oxford Nanopore Technology PromethION [104,105]. In 2011, the 1000 Fungal Genomes Project started with plans to sequence at least two reference genomes from each fungal family (http://1000.fungalgenomes.org; acceded on 5 February 2023). A search at the NCBI database (https://www.ncbi.nlm.nih.gov/; accessed on 10 February 2023) and the Genome Portal (https://genome.jgi.doe.gov/portal/; accessed on 10 February 2023) retrieved more than 12,200 and 2600 fungal genomes, respectively. Among these, over 11,550 genomes belong to the phylum Ascomycota that comprises the highest number of sequenced fungal genomes [106]. Despite the increasing number of fungal genomes over the last years, there are only a few genomes available in the genus Diaporthe. Table 2 sums up all species of Diaporthe with sequenced genomes deposited in NCBI and JGI databases.

Table 2.
Synopsis of all Diaporthe strains with genomes sequenced. (Note: NA stands for ‘not applicable’ meaning that the genome is available at the JGI Portal but has no Project ID).
The low number of genomes and annotations available impairs researchers to unveil key genes involved in the infection process of Diaporthe, as well as mechanisms involved in the dual lifestyle (pathogen-endophyte). To bridge this, studies on the genome sequencing of Diaporthe species have focused on genomic signatures that allow them to successfully invade and colonize the host plant through the presence of:
- (1)
- Hydrolytic enzymes to degrade plant cell wall polysaccharides (e.g., pectins, celluloses, and lignins) to ensure a successful entry into the host [21,22,23,24].
- (2)
- Biosynthetic gene clusters encoding for toxic metabolites that injure plant cells and enhance disease progression. (e.g., fusicoccin A, fusarin, and ACT-toxin II) [22,23].
- (3)
- Cellular transporters of ions (e.g., zinc, sulfur, copper), molecules that enhance pathogenicity (e.g., peroxiredoxin, tetraspanin), and sugars from plant polysaccharides degradation (e.g., xylose, inositol, and glycerol) [22].
- (4)
- Pathogenicity-related genes (e.g., acid aspartate and aminopeptidase) and candidate effectors (e.g., carboxylesterases, CFEM-domain, and laccases) that facilitate the host to be infected and manipulate the host immune defense [22,24].
Moreover, genome-wide association studies could be implemented to identify the genomic regions potentially associated with aggressiveness, through the analysis of single nucleotide polymorphism (SNP) data [118]. The analysis of SNPs between pathogenic and non-pathogenic fungi/endophytes is also a promising tool for the identification of candidate effectors underlying the pathogenicity of species of Diaporthe, as well as to understand the ecological and evolutionary dynamics of plant pathogens [119].
The integration of omics approaches can also speed up the identification of putative effectors in the genus Diaporthe and the characterization of their virulence functions in their host plants. Effectors are secreted proteins by fungal pathogens that modulate and interfere with plant defense responses [120]. Recently, Mena et al. [24] defined a set of proteins considered within the secretome of six Diaporthe species through a comparative analysis of available genomes. Moreover, Hilário et al. [22] have also identified candidate effectors from two Diaporthe species, through sequencing and analysis of their genomes (Table 3). This suggests that the genomes of species of Diaporthe have a large array of candidate effectors involved in pathogenicity, and some of them are common to other Diaporthe pathogens while others are Diaporthe-specific [24]. Nevertheless, future studies should be undertaken aiming to reveal effector functions during the infection process and to understand how effectors alter plant physiology, thus underpinning Diaporthe lifestyles [121]. Overall, genomic studies on Diaporthe intend to deepen the knowledge on:
- (1)
- Ecological selection and adaptation of species of Diaporthe to degrade the available biomass as carbon source [22,23,24].
- (2)
- Gene functions related to pathogenicity [22,24].
- (3)
- Phylogenomic studies to offer insights into phylogenetic inference of Diaporthe [21,102].
- (4)
- Genetic basis for multi-omics analyses to provide a thorough overview on plant-pathogen interactions [19,24,122,123,124].

Table 3.
Overview of some effector proteins identified in the genomes of species of Diaporthe.
Table 3.
Overview of some effector proteins identified in the genomes of species of Diaporthe.
| Species | Effector Candidate | Effector Location | References |
|---|---|---|---|
| D. amygdali | glycosyl hydrolase family 61 | Apoplastic | [22] |
| aldehyde reductase 1 | Apoplastic | ||
| putative cfem domain-containing protein | Cytoplasmic | ||
| putative metalloprotease | Apoplastic | ||
| murein transglycosylase | Apoplastic | ||
| acetyl xylan esterase | Apoplastic | ||
| putative cerato-ulmin | Apoplastic | ||
| putative gas1-like protein | Apoplastic | ||
| putative secreted aspartic proteinase precursor | Apoplastic | ||
| Pectate lyase H | Apoplastic | ||
| glycosyl hydrolase family 61 | Apoplastic | ||
| D. capsici | sterigmatocystin biosynthesis peroxidase stcC | Apoplastic | [24] |
| pectate lyase F | Apoplastic | ||
| putative 1,4-beta-D-glucan cellobiohydrolase A | Apoplastic | ||
| putative proline-rich antigen | Apoplastic | ||
| chitin deacetylase | Apoplastic | ||
| xylanase G1 | Apoplastic | ||
| putative chitin binding protein | Apoplastic | ||
| putative mannose binding | Apoplastic | ||
| putative gas1-like protein | Apoplastic | ||
| glycoside hydrolase family 11 protein | Apoplastic | ||
| Cell wall glyco protein | Cytoplasmic | ||
| Poly(rC)-binding protein 4 | Cytoplasmic | ||
| D. caulivora | putative sterigmatocystin biosynthesis peroxidase stcC | Apoplastic | [24] |
| putative proline-rich antigen | Apoplastic | ||
| putative cytochrome p450 | Apoplastic | ||
| xylanase G1 | Apoplastic | ||
| glycoside hydrolase | Apoplastic | ||
| pectate lyase | Apoplastic | ||
| peptidase S41 family protein | Apoplastic | ||
| chitin deacetylase | Apoplastic | ||
| putative aldehyde dehydrogenase | Apoplastic | ||
| pectate lyase F | Apoplastic | ||
| putative 1,4-beta-D-glucan cellobiohydrolase A | Apoplastic | ||
| D. citri | chitin deacetylase | Apoplastic | [24] |
| Glucan endo-1,3-beta-glucosidase | Apoplastic | ||
| putative 1,4-beta-D-glucan cellobiohydrolase A | Apoplastic | ||
| putative sterigmatocystin biosynthesis peroxidase stcC | Apoplastic | ||
| cholera enterotoxin subunit A2 | Apoplastic | ||
| pectate lyase | Apoplastic | ||
| polysaccharide lyase family 3 protein | Apoplastic | ||
| Chitin binding protein | Apoplastic | ||
| Acetylxylan esterase-like protein | Apoplastic | ||
| pectate lyase F | Apoplastic | ||
| xylanase G1 | Apoplastic | ||
| putative riboflavin-aldehyde forming enzyme protein | Apoplastic | ||
| D. destruens | pectate lyase | Apoplastic | [24] |
| NPP1 domain-containing protein | Apoplastic | ||
| xylanase G1 | Apoplastic | ||
| cellulose binding CEL1 | Apoplastic | ||
| putative pectate lyase F | Apoplastic | ||
| Poly(rC)-binding protein 4 | Apoplastic | ||
| chitin deacetylase | Apoplastic | ||
| ribosomal protein s17 | Cytoplasmic | ||
| Protein CAP22 | Apoplastic | ||
| fungal cellulose binding domain-containing protein | Apoplastic | ||
| D. eres (syn. D. phragmitis) | pectate lyase | Apoplastic | [24] |
| Acetylxylan esterase 2 | Apoplastic | ||
| putative glutamine-serine-proline rich | Apoplastic | ||
| putative rhamnogalacturonan acetylesterase | Apoplastic | ||
| xylanase G1 | Apoplastic | ||
| Protein CAP22 | Apoplastic | ||
| lytic polysaccharide monooxygenase | Apoplastic | ||
| pectate lyase F | Apoplastic | ||
| putative proline-rich antigen | Apoplastic | ||
| chitin deacetylase | Apoplastic | ||
| D. eres (syn. D. vaccinii) | putative metalloprotease | Apoplastic | [22] |
| carbohydrate-binding module family 50 protein | Apoplastic | ||
| putative glycoside hydrolase family 61 protein | Apoplastic | ||
| acetylxylan esterase | Apoplastic | ||
| putative ricin b lectin | Apoplastic | ||
| putative pectate lyase b | Apoplastic | ||
| aldehyde reductase 1 | Apoplastic | ||
| putative npp1 domain | Cytoplasmic | ||
| putative pectinesterase | Cytoplasmic | ||
| putative pectate lyase | Apoplastic | ||
| disulfide-isomerase erp38 | Cytoplasmic | ||
| D. longicolla | polysaccharide lyase family 3 protein | Apoplastic | [24] |
| putative carbohydrate-binding module family 1 protein | Apoplastic | ||
| carbohydrate esterase family 5 protein | Apoplastic | ||
| starch binding domain-containing protein | Apoplastic | ||
| putative pectate lyase F | Apoplastic | ||
| Acetylxylan esterase 2 | Apoplastic | ||
| pectate lyase | Apoplastic | ||
| cell wall protein PhiA | Apoplastic | ||
| xylanase G1 | Apoplastic | ||
| cellulose binding CEL1 | Apoplastic | ||
| fungal cellulose binding domain-containing protein | Apoplastic | ||
| Protein CAP22 | Apoplastic |
4.2. Transcriptomics
The RNA-Seq technique has revolutionized the way in which transcriptomes are analyzed [125]. It promotes the understanding of gene expression under different conditions and allows for the discovery of new genes and transcription patterns, which helps to understand cell function and metabolic mechanisms [126]. As a result, it has been considered one of the most important applications of NGS technology, and one of the most important tools in plant pathology [125] since it allows to investigate the transcriptomic profiles of plant pathogens during infection [127,128]. As the interaction between plants and their pathogens is a dynamic process, these interactions should be analyzed as a dual process [127]. Hence, dual RNA sequencing allows to study host and pathogen transcriptomes simultaneously, detecting pathogen-specific transcripts as well as provides a more complete insight into the host defense mechanisms [129]. This approach has already been applied in studies of plant-pathogen interactions in crops such as grapevines [124,128]; peach [130] and potato [131]; medicinal plants [132]; and forest trees such as Eucalyptus sp. [133] and Pinus sp. [134].
However, the utilization of transcriptomics data is often hampered by the lack of annotations and genomes available, which is reflected in the scarce transcriptome studies, for example, in the genus Diaporthe. The few studies regarding the transcripts characterization in this genus are mainly based on quantitative PCR (qPCR). For example, Książkiewicz et al. [135] have used this technique to target genes on Lupinus angustifolius that confer resistance to D. toxica, the causal agent of lupinosis. Moreover, Elverson et al. [136] developed two qPCR assays to detect and quantify D. helianthi and D. gulyae on sunflower, the causing agents of Phomopsis stem canker. Hosseini et al. [137] have also established a multiplex qPCR to distinguish D. longicolla, D. caulivora, D. eres, and D. novem on soybean, which are responsible for seed decay, pod, and stem canker on this host. In another study, Fujiwara et al. [138] demonstrated that the qPCR assay they developed is useful to diagnose and quantify D. batatas and D. destruens in sweet potato, as they are the main causal agents of foot rot disease.
To our knowledge, Mena et al. [24] applied for the first time the dual RNA-Seq approach to the genus Diaporthe to evaluate how D. caulivora may affect soybean plants. The authors stated that the infected soybean with D. caulivora induces the reinforcement of cell walls, evidenced by the incorporation of phenolic compounds. Moreover, several defense genes were also upregulated, including those encoding a pathogenesis-related (PR) protein-1 (PR-1), a PR-10, a β-1,3-glucanase, two chitinases, two lipoxygenases, a phenylalanine-ammonia lyase, and a chalcone synthase [24,62]. Given the cosmopolitan behavior of species of Diaporthe, their ability to infect a wide range of hosts and their different lifestyles (e.g., endophytes and pathogens), transcriptome analyses of both the host and the pathogen, and the validation of the differentially expressed genes (DEGs) should be considered to understand the regulatory networks and mechanisms involved in infection processes. Such an approach would thus contribute to unravelling host-pathogen interactions to provide helpful information for the development of disease control strategies [132,133,134,139].
4.3. Proteomics
Profiling the protein expression can unravel the functions of different proteins by assessing the plant responses to environmental stresses, such as pathogen attack [99]. After the plant is stimulated by external stresses, their defensive response is rapidly generated, followed by changes in some physiological and biochemical characters (e.g., decrease in chlorophyl A and photosynthesis) [140]. For example, studies have demonstrated that Arabidopsis infected by Fusarium [141], rice infected by Magnaporthe oryzae [142], and strawberry leaves inoculated with Colletotrichum [143] showed an overexpression of peroxidase levels after pathogens infection, to scavenged reactive oxygen species (ROS). Additionally, PR proteins such as chitinases and plant β-1,3-glucanases are considered important components of plant defense mechanisms under a pathogen attack [144]. For instance, the above-mentioned proteins were upregulated in Triticum aestivum inoculated with F. equiseti [145] and in Populus trichocarpa after infection with Botryosphaeria dothidea [146].
When the fungus infects host plants, a series of effector proteins (e.g., cell wall degrading enzymes) are secreted into the host tissue to destroy intracellular components, interfering with their defense response [141,144]. The analysis of the proteome has been successfully made for some fungal plant pathogens. For instance, some studies have shown that cell wall degrading enzymes such as pectin, esterases, xylanases, pectate lyases, or galacturonases are upregulated in L. theobromae [143], M. oryzae [142], and F. graminearum [147], suggesting their pathogenicity on grapevines, rice, and barley, respectively. Moreover, the hydrolase glucan-β-glucosidase was found to be involved in the virulence of C. higginsianum [148] and Alternaria alternata [149]. Some effector proteins secreted by fungal pathogens, such as avirulence proteins (Avr), are delivered into the host plant, which have the potential to suppress pathogen-associated molecular patterns (PAMPs)-triggered immunity [150]. However, these pathogen-derived avirulence proteins are recognized by plant receptor proteins encoded by R genes, resulting in effector-triggered immunity that leads to fast responses [151].
As mentioned above, proteomics has been applied to unveil key proteins of several plant pathogens as well as those involved in plant defense under a pathogen attack. Nevertheless, no proteomic studies have been performed with members of the genus Diaporthe nor for their interaction with plants. As the proteome profiling during infection can identify specific proteins involved in plant disease resistance and pathogenicity processes [152], in-depth studies and comparative proteomics should be undertaken to reveal molecular mechanisms of Diaporthe—plant interactions as well as the susceptibility or resistance in plants. These studies will assist in the discovery of novel proteins that might be potential candidates for the enhancement of tolerance to fungal diseases.
4.4. Metabolomics
Currently, mass spectrometry (MS) has become a highly sensitive tool used for the identification and quantification of metabolites. Nuclear magnetic resonance (NMR) and types of mass analyzers are commonly used for metabolomic studies, such as capillary electrophoresis mass spectrometry (CE-MS), gas chromatography mass spectrometry (GC–MS), liquid chromatography mass spectrometry (LC–MS), and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) [153].
The increasing number of sequenced genomes and the scalable metabolomics approaches have largely expanded the access to the metabolite repertoire of fungi [154]. The awareness that fungi are a source of beneficial compounds came up after Sir Alexander Fleming in 1928 discovered penicillin [155]. This first broad-spectrum antibiotic was produced by the fungus Penicillium notatum (syn. P. rubens) and was considered as the ‘wonder drug’ of World War II [156]. After this event, the study of microorganisms as sources for antibiotics gave rise to the golden era for the discovery of natural products from fungi [157]. Species of the genus Diaporthe, for instance, are well known as producers of several compounds (e.g., polyketides, indoles, and terpenes) with potential applications in pharmacology and biomedicine [100].
Besides that, metabolomics research of plant pathogenic fungi has gained attention, since it allows the identification of metabolites, their functions, and metabolic pathways involved in pathogenicity [158,159,160]. Moreover, metabolomics profiling of host plants has also been performed to elucidate plant responses to abiotic and biotic stresses and to evaluate plant adaptations to such conditions [161]. For instance, Dickinson et al. [162] applied the LC–MS method to investigate metabolite changes of Medicago tranuclata under drought stress and infection with F. oxysporum. The authors stated that under pathogen infection, an increase in flavonoids, sucrose relocation from leaves to roots, and a decrease in organic acids were observed. Also, Jones et al. [163] used a meta-analytical method based on GC–MS/MS, LC–MS/MS, and NMR to evaluate rice plants at different time points after infection by M. grisea. These authors proposed that the production of a large amount of alanine caused by fungi may lead to cell death and thereby promoting M. grisea infection, suggesting that metabolomics may help evaluating the overall effects of pathogen infection on plant hosts [164].
It has also been suggested that metabolomic profiling in fungal-plant interactions provide important information for the early diagnosis of several fungal plant pathogens [161,164,165]. Hu et al. [164] used the GC–MS method to analyze the metabolic profiling of strawberry infected with Botrytis cinerea and identified biomarkers in the early stage of disease development. Moreover, Zeiss et al. [165] analyzed the metabolic profiling of tomato plants infected with Ralstonia solanacearum using the LC–MS method and detected metabolites that may be used as biomarkers for an early infection diagnosis (e.g., phenylpropanoids, phenolic acids, and flavonoids). Additionally, plant metabolomics can also help identify and link genes associated with resistance to fungal pathogens. For instance, Kage et al. [166] have also reported an increase in the metabolite coumaroylagmatine in a tolerant wheat variety to Fusarium head blight. The analysis of these compounds and their metabolic pathways paved the way for the detection of a gene (agmatine coumaroyl transferase) that confers resistance against F. graminearum [166].
Several studies have been focused to identify a wide range of metabolites produced by species of Diaporthe with biotechnological applications [100]. Nevertheless, there is still a lack of metabolomic studies on the interaction between species of Diaporthe and their hosts. Therefore, metabolomic approaches should be performed in Diaporthe-infected plants to elucidate the metabolic pathways involved in pathogenicity, as well as secreted metabolites as potential biomarkers for early disease diagnosis [158]. Moreover, unveiling metabolic features responsible for plant survival under stress conditions (e.g., pathogen attack) could facilitate crop improvement for biotic-stress tolerance, through the application of unique metabolites in formulations [99,161].
5. Omics to Underline Fungal Lifestyle Switches
Studies to understand the role of effector candidates during the infection process among species of Diaporthe could also reveal pathogenic lifestyles. Several studies have addressed the role of effectors to predict whether there are molecular differences between endophytes and pathogens [25]. For instance, Constantin et al. [26] found that strains of the endophytic F. oxysporum possessed fewer effectors than their pathogenic strains. On the contrary, Queiroz and Santana [167] revealed that both endophytic and pathogenic fungi share many of the proteins of their secretomes, and thus the difference between lifestyles were small. Genome analysis and the application of phylogenomics have revealed genes representing putative virulence factors involved in toxin production and wood degradation in members of the family Botryosphaeriaceae [168]. Moreover, genomics can also help understand the interactions between pathogens and endophytes as revealed for Verticillium dahliae [169]. Therefore, it is reasonable to state that using genome data could be an important approach to unveil virulence factors of endophytes in the genus Diaporthe.
Transcriptomics can also provide a deep understanding of the molecular traits that underline fungal lifestyle switching from endophytic to pathogenic state [166]. For instance, O’Connel et al. [28] have performed a comparative genomics and transcriptomics and showed that genes encoding secreted effectors, pectin-degrading enzymes, secondary metabolism enzymes, transporters, and peptidases are expanded in C. higginsianum. The authors stated that these genes are linked to pathogenic transitions: effectors and secondary metabolism enzymes are induced during the biotrophy (endophytic stage), whereas hydrolases and transporters are upregulated at the switch to necrotrophy (pathogenic stage) [28].
On the other hand, Zhou et al. [170] showed that the gene expression on both endophytic and saprophytic lifestyles of D. liquidambaris is strongly influenced by host environment. Additionally, it was suggested that mitogen-activated protein kinase (MAPK) signaling pathway may be involved in fungal lifestyle transition [170]. For instance, Becker et al. [171] found that the cell wall integrity (CWI) MAPK is a crucial signaling pathway for maintaining the mutualistic symbiotic interaction of Epichloë festucae with perennial ryegrass. On the other hand, the stress-activated MAPK pathway signaling and the production of ROS by the fungal NADPH oxidase (Nox) complex are key mechanisms that maintain a stable mutualistic association between E. festucae and ryegrass [172]. In this regard, when plants are infected by endophytic fungi, lacking functional Nox complexes or stress-activated MAPK signaling, the host can exhibit a slower growth while the fungus has a proliferative growth leading to a lifestyle switching from endophyte to pathogenic [173,174]. Such an outcome has been observed in M. grisea [175], B. cinerea [172], and D. liquidambaris [170].
Apart from studying the secondary metabolites involved in the pathogenesis, these compounds can also be potential determinants of endophytism [170]. For example, proteins involved in the biosynthesis of phenylalanine, tyrosine, tryptophan, sesquiterpenoids, and triterpenoids were upregulated in D. liquidambaris in the endophytic stage. Therefore, this suggests that endophytes may induce host plant synthesis of tryptophan and other alkaloid synthesis precursors, enhancing stress resistance of plants and limiting proliferation of endophytic fungi to a pathogenic stage [176]. Considering the dual lifestyle in the genus Diaporthe, omics technologies could be implemented in the future to gain insights into the candidate genes required to maintain the beneficial associations with plants and to understand the mechanisms underlying lifestyle switches.
6. Conclusions and Future Perspectives
Diaporthe is a species-rich genus that comprises endophytes and plant pathogens, causing disease on economically important crops. Omics technologies, such as genomics, transcriptomics, proteomics, and metabolomics, have become essential resources to understand plant—pathogen interactions for a sustainable agriculture. However, the limited number of available genomes of Diaporthe hampers further research on key transcripts, proteins, and metabolites involved in species pathogenicity. Therefore, to speed up the identification of pathogenicity determinants and their functions for a better knowledge of plant-Diaporthe cross-talks, this review summarized the need to adopt omics technologies of both the host and the pathogen to
- (1)
- Elucidate the broad spectrum of putative effectors underlying infection processes as well as to understand the ecological and evolutionary dynamics of plant pathogens.
- (2)
- Identify differentially expressed genes to understand regulatory networks involved in infection processes, which can provide helpful information for the development of disease control strategies.
- (3)
- Unravel novel proteins that might be potential candidates for the enhancement of tolerance to fungal diseases.
- (4)
- Identify secreted metabolites and metabolic pathways to identify candidate biomarkers in the early stage of disease development.
- (5)
- Provide evidence on the role of secreted effectors, pectin-degrading enzymes, secondary metabolism enzymes, MAPK signaling pathways and metabolites involved in pathogenicity as determinants of endophytism.
As stated in this review, the future application of omics techniques will surely enable new perspectives in deciphering Diaporthe—plant interactions. Incorporating omics data will improve our understanding of key molecular traits involved in host susceptibility/ resistant traits and in plant defense to offer novel crop protection opportunities. Moreover, multi-omics will also provide valuable information on pathogen virulence factors and the development of biomarkers and diagnostic kits, which will be fundamental for proper disease control and management. Despite the recognition of Diaporthe as the most common genera of endophytic fungi, up-to-date research should also be considered in the future. These should be focused on understanding which mechanisms and molecular traits underline the transition from an endophytic to a pathogenic lifestyle by addressing the following unanswered questions:
- (1)
- Are endophytic isolates location-specific and host-adapted?
- (2)
- Do isolates express multiple lifestyle?
- (3)
- Are endophytic and pathogenic fungi genetically differentiated?
- (4)
- What are the molecular differences between endophytes and pathogens?
- (5)
- Does an imbalance in the phyllosphere microbial community trigger life modes switching?
Therefore, future studies focused on the plant microbiome, for instance, would be an important approach to address the above-mentioned questions. This will offer insights into the endophytic community to unravel adaptation patterns in response to environmental stimuli, and to understand whether a dysbiosis may or may not affect the ability of fungi to switch life modes.
Author Contributions
Conceptualization: S.H.; Data curation: S.H.; Investigation and Methodology: M.F.M.G. and S.H.; Writing—original draft: S.H.; Writing—review and editing: M.F.M.G. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhunjun, C.S.; Niskanen, T.; Suwannarach, N.; Wannathes, N.; Chen, Y.J.; McKenzie, E.H.; Maharachchikumbura, S.S.; Buyck, B.; Zhao, C.L.; Fan, Y.G.; et al. The numbers of fungi: Are the most speciose genera truly diverse? Fungal Divers. 2022, 114, 387–462. [Google Scholar] [CrossRef]
- Chepkirui, C.; Stadler, M. The genus Diaporthe: A rich source of diverse and bioactive metabolites. Mycol. Prog. 2017, 16, 477–494. [Google Scholar] [CrossRef]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 2013, 31, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Mostert, L.; Crous, P.W.; Kang, J.C.; Phillips, A.J.L. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: Morphological, cultural, molecular and pathological characterization. Mycologia 2001, 93, 146–167. [Google Scholar] [CrossRef]
- Luo, M.; Guo, W.; Zhao, M.; Manawasinghe, I.S.; Guarnaccia, V.; Liu, J.; Hyde, K.D.; Dong, Z.; You, C. Endophytic Diaporthe Associated with Morinda officinalis in China. J. Fungi 2022, 8, 806. [Google Scholar] [CrossRef]
- Udayanga, D.; Liu, X.; McKenzie, E.H.C.; Chukeatirote, E.; Bahkali, A.H.A.; Hyde, K.D. The genus Phomopsis: Biology, applications, species concepts and names of common phytopathogens. Fungal Divers. 2011, 50, 189–225. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, F.; Duan, W.; Crous, P.W.; Cai, L. Diaporthe is paraphyletic. IMA Fungus 2017, 8, 163–187. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.; Lin, Y.; Fu, Y.; Zhu, F.; Luo, C. Phylogenetic and haplotype network analyses of Diaporthe eres species in China based on sequences of multiple loci. Biology 2021, 10, 179. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. Insights into the genus Diaporthe: Phylogenetic species delimitation in the D. eres species complex. Fungal Divers. 2014, 67, 203–229. [Google Scholar] [CrossRef]
- Hilário, S.; Gonçalves, M.F.M.; Alves, A. Using genealogical concordance and coalescent-based species delimitation to assess species boundaries in the Diaporthe eres complex. J. Fungi 2021, 7, 507. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Crous, P.W. Emerging citrus diseases in Europe caused by Diaporthe spp. IMA Fungus 2017, 8, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, G.; Prasannath, K. Recent advances in molecular diagnostics of fungal plant pathogens: A mini review. Front. Cell. Infect. Microbiol. 2021, 10, 600234. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.S.; Crous, P.W.; Bai, Q.; Fu, M.; Yang, M.M.; Wang, X.H.; Du, Y.M.; Hong, N.; Xu, W.X.; Wang, G.P. High diversity of Diaporthe species associated with pear shoot canker in China. Persoonia 2020, 45, 132–162. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Du, Y.; Yang, Z.; Huang, X.; Hong, N.; Xu, W.; Wang, G. Characterization of Diaporthe species associated with peach constriction canker, with two novel species from China. MycoKeys 2021, 80, 77. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Chukeatirote, E.; Hyde, K.D. The Diaporthe sojae species complex: Phylogenetic reassessment of pathogens associated with soybean, cucurbits and other field crops. Fungal Biol. 2015, 119, 383–407. [Google Scholar] [CrossRef]
- Hilário, S.; Santos, L.; Alves, A. Diaporthe amygdali, a species complex or a complex species? Fungal Biol. 2021, 125, 505–518. [Google Scholar] [CrossRef]
- Nagel, J.H.; Wingfield, M.J.; Slippers, B. Increased abundance of secreted hydrolytic enzymes and secondary metabolite gene clusters define the genomes of latent plant pathogens in the Botryosphaeriaceae. BMC Genom. 2021, 22, 589. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Yan, J.; Guo, M.; Xu, L.; Hou, L.; Zou, Q. Comparative genome analysis of plant ascomycete fungal pathogens with different lifestyles reveals distinctive virulence strategies. BMC Genom. 2022, 23, 34. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Wang, C.; Xu, J.R. Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genom. 2013, 14, 274. [Google Scholar] [CrossRef]
- Gai, Y.; Xiong, T.; Xiao, X.; Li, P.; Zeng, Y.; Li, L.; Riely, B.K.; Li, H. The Genome Sequence of the Citrus Melanose Pathogen Diaporthe citri and Two Citrus-Related Diaporthe Species. Phytopathology 2021, 111, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Hilário, S.; Gonçalves, M.F.M.; Fidalgo, C.; Tacão, M.; Alves, A. Genome Analyses of Two Blueberry Pathogens: Diaporthe amygdali CAA958 and Diaporthe eres CBS 160.32. J. Fungi 2022, 8, 804. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Darwish, O.; Alkharouf, N.W.; Musungu, B.; Matthews, B.F. Analysis of the genome sequence of Phomopsis longicolla: A fungal pathogen causing Phomopsis seed decay in soybean. BMC Genom. 2017, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Garaycochea, S.; Stewart, S.; Montesano, M.; Ponce De León, I. Comparative genomics of plant pathogenic Diaporthe species and transcriptomics of Diaporthe caulivora during host infection reveal insights into pathogenic strategies of the genus. BMC Genom. 2022, 23, 175. [Google Scholar] [CrossRef] [PubMed]
- Collinge, D.B.; Jensen, B.; Jørgensen, H.J. Fungal endophytes in plants and their relationship to plant disease. Curr. Opin. Microbiol. 2022, 69, 102177. [Google Scholar] [CrossRef]
- Constantin, M.E.; Fokkens, L.; De Sain, M.; Takken, F.L.; Rep, M. Number of candidate effector genes in accessory genomes differentiates pathogenic from endophytic Fusarium oxysporum strains. Front. Plant Sci. 2021, 12, 761740. [Google Scholar] [CrossRef]
- Lowe, R.G.; Howlett, B.J. Indifferent, affectionate, or deceitful: Lifestyles and secretomes of fungi. PLoS Pathog. 2012, 8, e1002515. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- Redkar, A.; Sabale, M.; Zuccaro, A.; Di Pietro, A. Determinants of endophytic and pathogenic lifestyle in root colonizing fungi. Curr. Opin. Plant Biol. 2022, 67, 102226. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, V.; Groenewald, J.Z.; Woodhall, J.; Armengol, J.; Cinelli, T.; Eichmeier, A.; Ezra, D.; Fontaine, F.; Gramaje, D.; Gutierrez-Aguirregabiria, A.; et al. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia 2018, 40, 135–153. [Google Scholar] [CrossRef]
- Fan, X.; Yang, Q.; Bezerra, J.D.; Alvarez, L.V.; Tian, C. Diaporthe from walnut tree (Juglans regia) in China, with insight of the Diaporthe eres complex. Mycol. Prog. 2018, 17, 841–853. [Google Scholar] [CrossRef]
- Arciuolo, R.; Santos, C.; Soares, C.; Castello, G.; Spigolon, N.; Chiusa, G.; Lima, N.; Battilani, P. Molecular characterization of Diaporthe species associated with hazelnut defects. Front. Plant Sci. 2020, 11, 611655. [Google Scholar] [CrossRef]
- Thompson, S.M.; Grams, R.A.; Neate, S.M.; Shivas, R.G.; Ryley, M.J.; Tan, Y.P.; Aitken, E.A.B.; Wright, G.C.; O’Connor, D.J. First reports of Diaporthe kongii, D. masirevicii, and D. ueckerae associated with stem and peg dieback on peanut in Australia. Plant Dis. 2018, 102, 1459. [Google Scholar] [CrossRef]
- Santos, L.; Phillips, A.J.L.; Crous, P.W.; Alves, A. Diaporthe species on Rosaceae with descriptions of D. pyracanthae sp. Mycosphere 2017, 8, 485–511. [Google Scholar] [CrossRef]
- Sessa, L.; Abreo, E.; Lupo, S. Diversity of fungal latent pathogens and true endophytes associated with fruit trees in Uruguay. J. Phytopathol. 2018, 166, 633–647. [Google Scholar] [CrossRef]
- Murali, T.; Suryanarayanan, T.; Geeta, R. Endophytic Phomopsis species: Host range and implications for diversity estimates. Can. J. Microbiol. 2006, 52, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, A.J.; Phillips, A.J.L. Advances in understanding Diaporthe (Editorial). Mycosphere 2017, 8, 7019. [Google Scholar]
- Cinelli, T.; Mondello, V.; Marchi, G.; Burruano, S.; Alves, A.; Mugnai, L. First report of Diaporthe eres associated with cane blight of grapevine (Vitis vinifera) in Italy. Plant Dis. 2016, 100, 532. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Baumgartner, K. Diversity of Diaporthe species associated with wood cankers of fruit and nut crops in northern California of fruit and nut crops in northern California. Mycologia 2015, 107, 926–940. [Google Scholar] [CrossRef]
- Mathew, F.M.; Olson, T.R.; Science, P.; Dakota, S. Identification of Sunflower (Helianthus annuus) accessions resistant to Diaporthe helianthi and Diaporthe gulyae. Plant Health Prog. 2018, 19, 97–102. [Google Scholar] [CrossRef]
- Thompson, S.M.; Tan, Y.P.; Young, A.J.; Neate, S.M.; Aitken, E.A.B.; Shivas, R.G. Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic Diaporthe (Phomopsis) species. Persoonia 2011, 27, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Fan, X.L.; Guarnaccia, V.; Tian, C.M. High diversity of Diaporthe species associated with dieback diseases in China, with twelve new species described. MycoKeys 2018, 39, 97–149. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Vrandecic, K.; Cosic, J.; Duvnjak, T.; Phillips, A.J.L. Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia 2011, 27, 9–19. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, J.M.; Groenewald, J.Z.; Farr, D.F.; Fourie, P.H.; Haleen, F.; Crous, P.W. Reassessment of Phomopsis on grapevine. Australas. Plant Pathol. 2005, 34, 27–39. [Google Scholar] [CrossRef]
- Diogo, E.L.; Santos, J.M.; Phillips, A.J.L. Phylogeny, morphology and pathogenicity of Diaporthe and Phomopsis species on almond in Portugal. Fungal Divers. 2010, 44, 107–115. [Google Scholar] [CrossRef]
- León, M.; Berbegal, M.; Rodríguez-Reina, J.M.; Elena, G.; Abad-Campos, P.; Ramón-Albalat, A.; Olmo, D.; Vicent, A.; Luque, J.; Miarnau, X.; et al. Identification and characterization of Diaporthe spp. associated with twig cankers and shoot blight of almonds in Spain. Agronomy 2020, 10, 1062. [Google Scholar] [CrossRef]
- Beluzán, F.; Miarnau, X.; Torguet, L.; Zazurca, L.; Abad-Campos, P.; Luque, J.; Armengol, J. Susceptibility of almond (Prunus dulcis) cultivars to twig canker and shoot blight caused by Diaporthe amygdali. Plant Dis. 2022, 106, 1890–1897. [Google Scholar] [CrossRef]
- Ali, S.; Renderos, W.; Bevis, E.; Hebb, J.; Abbasi, P.A. Diaporthe eres causes stem cankers and death of young apple rootstocks in Canada. Can. J. Plant Pathol. 2020, 42, 218–227. [Google Scholar] [CrossRef]
- Lombard, L.; Van Leeuwen, G.C.M.; Guarnaccia, V.; Polizii, G.; Van Rijswick, P.C.J.; Rosendahl, C.H.M.; Gabler, J.; Crous, P.W. Diaporthe species associated with Vaccinium, with specific reference to Europe. Phytopathol. Mediterr. 2014, 53, 287–299. [Google Scholar]
- Elfar, K.; Torres, R.; Díaz, G.A.; Latorre, B. Characterization of Diaporthe australafricana and Diaporthe spp. associated with stem canker of blueberry in Chile. Plant Dis. 2013, 97, 1042–1050. [Google Scholar] [CrossRef]
- Hilário, S.; Santos, L.; Alves, A. Diversity and Pathogenicity of Diaporthe Species Revealed from a Survey of Blueberry Orchards in Portugal. Agriculture 2021, 11, 1271. [Google Scholar] [CrossRef]
- Farr, D.F.; Castlebury, L.A.; Rossman, A.Y. Morphological and molecular characterization of Phomopsis vaccinii and additional isolates of Phomopsis from blueberry and cranberry in the eastern United States. Mycologia 2002, 94, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Cardinaals, J.; Wenneker, M.; Voogd, B.; Van Leeuwen, M. Pathogenicity of Diaporthe spp. on two blueberry cultivars (Vaccinium corymbosum). Bull. OEPP 2018, 48, 128–134. [Google Scholar] [CrossRef]
- Hilário, S.; Amaral, I.A.; Gonçalves, M.F.M.; Lopes, A.; Santos, L.; Alves, A. Diaporthe species associated with twig blight and dieback of Vaccinium corymbosum in Portugal, with description of four new species. Mycologia 2020, 112, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Nabetani, K.; Wood, B.; Sabaratnam, S. Role of pycnidia in twig and blossom blight and stem dieback of highbush blueberry caused by Phomopsis vaccinii in British Columbia. Can. J. Plant Pathol. 2017, 39, 405–421. [Google Scholar] [CrossRef]
- van Bruggen, A.H.C.; West, J.S.; van der Werf, W.; Potting, R.P.J.; Gardi, C.; Koufakis, I.; Zelenev, V.V.; Narouei-Khandan, H.; Shilder, A.; Harmon, P. Input data needed for a risk model for the entry, establishment and spread of a pathogen (Phomopsis vaccinii) of blueberries and cranberries in the EU. Ann. Appl. Biol. 2018, 172, 126–147. [Google Scholar] [CrossRef]
- Chaisiri, C.; Liu, X.Y.; Yin, W.X.; Luo, C.X.; Lin, Y. Morphology characterization, molecular phylogeny, and pathogenicity of Diaporthe passifloricola on Citrus reticulata cv. Nanfengmiju in Jiangxi Province, China. Plants 2021, 10, 218. [Google Scholar] [CrossRef]
- Dong, Z.; Manawasinghe, I.S.; Huang, Y.; Shu, Y.; Phillips, A.J.L.; Dissanayake, A.J.; Hyde, K.D.; Xiang, M.; Luo, M. Endophytic Diaporthe associated with Citrus grandis cv. Tomentosa in China. Front. Microbiol. 2021, 11, 3621. [Google Scholar] [CrossRef] [PubMed]
- Manawasinghe, I.S.; Dissanayake, A.; Li, X.; Liu, M.; Wanasinghe, D.; Xu, J.; Zhao, W.; Zhang, W.; Zhou, Y.; Hyde, K.; et al. High genetic diversity and species complexity of Diaporthe associated with grapevine dieback in China. Front. Microbiol. 2019, 10, 1936. [Google Scholar] [CrossRef]
- Wiman, N.G.; Webber, J.B., III; Wiseman, M.; Merlet, L. Identity and pathogenicity of some fungi associated with hazelnut (Corylus avellana L.) trunk cankers in Oregon. PLoS ONE 2019, 14, e0223500. [Google Scholar] [CrossRef]
- Thomidis, T.; Michailides, T.J. Studies on Diaporthe eres as a new pathogen of peach trees in Greece. Plant Dis. 2009, 93, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Stewart, S.; Montesano, M.; Ponce de León, I. Soybean stem canker caused by Diaporthe caulivora; pathogen diversity, colonization process, and plant defense activation. Front. Plant Sci. 2020, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wang, T.C.; Seo, M.J. First report of soybean pod and stem blight caused by Diaporthe phaseolorum var. sojae in Taiwan. Plant Dis. 2009, 93, 202. [Google Scholar] [CrossRef]
- Olson, T.R.; Kontz, B.; Gulya, T.J.; Markell, S.G.; Mathew, F.M. First report of Diaporthe stewartii causing Phomopsis stem canker of sunflower (Helianthus annuus) in Minnesota. Plant Dis. 2017, 101, 382. [Google Scholar] [CrossRef]
- Fang, X.; Qin, K.; Li, S.; Han, S.; Zhu, T. Whole genome sequence of Diaporthe capsici, a new pathogen of walnut blight. Genomics 2020, 112, 3751–3761. [Google Scholar] [CrossRef]
- Meng, L.; Yu, C.; Wang, C.; Li, G. First report of Diaporthe amygdali causing walnut twig canker in Shandong province of China. Plant Dis. 2018, 102, 1859. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Wingfield, M.J.; Akulov, A.; Carnegie, A.J.; Cheewangkoon, R.; Gramaje, D.; Groenewald, J.Z.; Guarnaccia, V.; Halleen, F.; et al. Genera of phytopathogenic fungi: GOPHY 2. Stud. Mycol. 2019, 92, 47–133. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Shivas, R.G.; Edwards, J.; Seifert, K.A.; Alfenas, A.C.; Burgess, T.I.; Carnegie, A.J.; Hardy, G.E.S.J.; Hiscock, N.; et al. Fungal Planet description sheets: 69–91. Persoonia 2011, 26, 108–156. [Google Scholar] [CrossRef]
- Tan, Y.P.; Edwards, J.; Grice, K.R.E.; Shivas, R.G. Molecular phylogenetic analysis reveals six new species of Diaporthe from Australia. Fungal Divers. 2013, 61, 251–260. [Google Scholar] [CrossRef]
- Santos, J.M.; Correia, V.G.; Phillips, A.J.L. Primers for mating-type diagnosis in Diaporthe and Phomopsis: Their use in teleomorph induction in vitro and biological species definition. Fungal Biol. 2010, 114, 255–270. [Google Scholar] [CrossRef]
- Lopes, A.F.; Batista, E.; Hilário, S.; Santos, L.; Alves, A. Occurrence of Diaporthe species in Eucalyptus globulus, Pinus pinaster and Quercus suber in Portugal. For. Pathol. 2021, 51, e12674. [Google Scholar] [CrossRef]
- Crous, P.W.; Summerell, B.A.; Shivas, R.G.; Burgess, T.I.; Decock, C.A.; Dreyer, L.L.; Granke, L.L.; Guest, D.I.; Hardy, G.; Hausbeck, M.K.; et al. Fungal Planet description sheets: 107–127. Persoonia 2012, 28, 138–182. [Google Scholar] [CrossRef]
- Jiang, N.; Voglmayr, H.; Piao, C.G.; Li, Y. Two new species of Diaporthe (Diaporthaceae, Diaporthales) associated with tree cankers in the Netherlands. MycoKeys 2021, 85, 31. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Tsai, I.; Wang, J.Y.; Withee, P.; Tanjira, M.; Lin, S.R.; Suwannarach, N.; Kumla, J.; Elgorban, A.M.; Cheewangkoon, R. Molecular phylogenetic diversity and biological characterization of Diaporthe species associated with leaf spots of Camellia sinensis in Taiwan. Plants 2021, 10, 1434. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, F.; Cai, L. Unravelling Diaporthe species associated with Camellia. Syst. Biodivers. 2016, 14, 102–117. [Google Scholar] [CrossRef]
- Li, Y.; Tan, P.; Zhao, D.G. Diaporthe nobilis, a new record on Camellia sinensis in Guizhou Province, China. Mycosphere 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Santos, J.M.; Phillips, A.J.L. Resolving the complex of Diaporthe (Phomopsis) species occurring on Foeniculum vulgare in Portugal. Fungal Divers. 2009, 34, 111–125. [Google Scholar]
- Gao, Y.; Su, Y.; Sun, W.; Cai, L. Diaporthe species occurring on Lithocarpus glabra in China, with descriptions of five new species. Fungal Biol. 2015, 119, 295–309. [Google Scholar] [CrossRef]
- Caio, P.; Bruno, F.; Carlos, A.P.; Robert, B. Diaporthe rosiphthora sp. nov.: Yet another rose dieback fungus. Crop Prot. 2021, 139, 105365. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Camporesi, E.; Hyde, K.D.; Zang, W.; Yan, J.Y.; Li, X.H. Molecular phylogenetic analysis reveals seven new Diaporthe species from Italy. Mycosphere 2017, 8, 853–877. [Google Scholar] [CrossRef]
- Torres, C.; Camps, R.; Aguirre, R.; Besoain, X.A. First report of Diaporthe rudis in Chile causing stem-end rot on ‘Hass’ avocado fruit imported from California, USA. Plant Dis. 2016, 100, 1951. [Google Scholar] [CrossRef]
- Huang, F.; Udayanga, D.; Wang, X.; Hou, X.; Mei, X.; Fu, Y.; Hyde, K.D.; Li, H. Endophytic Diaporthe associated with Citrus: A phylogenetic reassessment with seven new species from China. Fungal Biol. 2015, 119, 331–347. [Google Scholar] [CrossRef]
- Sebastianes, F.L.; Lacava, P.T.; Fávaro, L.C.; Rodrigues, M.B.; Araújo, W.L.; Azevedo, J.L.; Pizzirani-Kleiner, A.A. Genetic transformation of Diaporthe phaseolorum, an endophytic fungus found in mangrove forests, mediated by Agrobacterium tumefaciens. Curr. Genet. 2012, 58, 21–33. [Google Scholar] [CrossRef]
- Kuo, H.C.; Hui, S.; Choi, J.; Asiegbu, F.O.; Valkonen, J.; Lee, Y.H. Secret lifestyles of Neurospora crassa. Sci. Rep. 2014, 4, 5135. [Google Scholar] [CrossRef]
- Mishra, S.; Bhattacharjee, A.; Sharma, S. An ecological insight into the multifaceted world of plant-endophyte association. Crit. Rev. Plant Sci. 2021, 40, 127–146. [Google Scholar] [CrossRef]
- Abramczyk, B.; Marzec-Grządziel, A.; Grządziel, J.; Król, E.; Gałązka, A.; Oleszek, W. Biocontrol Potential and Catabolic Profile of Endophytic Diaporthe eres Strain 1420S from Prunus domestica L. in Poland—A Preliminary Study. Agronomy 2022, 12, 165. [Google Scholar] [CrossRef]
- Ghanbary, E.; Fathizadeh, O.; Pazhouhan, I.; Zarafshar, M.; Tabari, M.; Jafarnia, S.; Parad, G.A.; Bader, M.K.F. Drought and pathogen effects on survival, leaf physiology, oxidative damage, and defense in two middle eastern oak species. Forests 2021, 12, 247. [Google Scholar] [CrossRef]
- Kogel, K.H.; Franken, P.; Hückelhoven, R. Endophyte or parasite—What decides? Curr. Opin. Plant Biol. 2006, 9, 358–363. [Google Scholar] [CrossRef]
- Hilário, S.; Pinto, G.; Monteiro, P.; Santos, L.; Alves, A. The impact of two Diaporthe spp. on Vaccinium corymbosum physiological fitness under different water availability scenarios. Eur. J. Plant Pathol. 2023, 1–17. [Google Scholar] [CrossRef]
- Álvarez-Loayza, P.; White, J.F., Jr.; Torres, M.S.; Balslev, H.; Kristiansen, T.; Svenning, J.C.; Gil, N. Light converts endosymbiotic fungus to pathogen, influencing seedling survival and niche-space filling of a common tropical tree, Iriartea deltoidea. PLoS ONE 2011, 6, e16386. [Google Scholar] [CrossRef]
- Hoffman, M.T.; Arnold, A.E. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol. Res. 2008, 112, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Materatski, P.; Varanda, C.; Carvalho, T.; Dias, A.B.; Campos, M.D.; Rei, F.; do Rosário Félix, M. Spatial and temporal variation of fungal endophytic richness and diversity associated to the phyllosphere of olive cultivars. Fungal Biol. 2019, 123, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Brader, G.; Corretto, E.; Sessitsch, A. Metagenomics of Plant Microbiomes. In Functional Metagenomics: Tools and Applications; Charles, T., Liles, M., Sessitsch, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 179–200. [Google Scholar]
- Akram, S.; Ahmed, A.; He, P.; He, P.; Liu, Y.; Wu, Y.; Munir, S.; He, Y. Uniting the Role of Endophytic Fungi against Plant Pathogens and Their Interaction. J. Fungi 2023, 9, 72. [Google Scholar] [CrossRef]
- Husseini, A.A.; Derakhshandeh, M.; Tatlisu, N.B. Comprehensive review of transcriptomics (RNAs) workflows from blood specimens. Sep. Purif. Rev. 2022, 51, 57–77. [Google Scholar] [CrossRef]
- Alexovič, M.; Lindner, J.R.; Bober, P.; Longuespée, R.; Sabo, J.; Davalieva, K. Human peripheral blood mononuclear cells: A review of recent proteomic applications. Proteomics 2022, 22, 2200026. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gold, K.M.; Jiménez-Gasco, M.D.M.; Filgueiras, C.C.; Willett, D.S. A multi-omics approach to solving problems in plant disease ecology. PLoS ONE 2020, 15, e0237975. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Mangat, H.K.; Pathak, R.K.; Yadav, I.S. Harnessing the potential of omics for prevention and management of the complex crop plant’s diseases. J. Proteins Proteom. 2021, 12, 227–245. [Google Scholar] [CrossRef]
- Xu, T.-C.; Lu, Y.-H.; Wang, J.-F.; Song, Z.-Q.; Hou, Y.-G.; Liu, S.-S.; Liu, C.-S.; Wu, S.-H. Bioactive secondary metabolites of the genus Diaporthe and anamorph Phomopsis from terrestrial and marine habitats and endophytes: 2010–2019. Microorganisms 2021, 9, 217. [Google Scholar] [CrossRef]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M.; et al. Life with 6000 genes. Science 1996, 274, 546–567. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, J.; Bhattacharya, D. Advances in fungal phylogenomics and their impact on fungal systematics. Adv. Genet. 2017, 100, 309–328. [Google Scholar] [PubMed]
- Aylward, J.; Steenkamp, E.T.; Dreyer, L.L.; Roets, F.; Wingfield, B.D.; Wingfield, M.J. A plant pathology perspective of fungal genome sequencing. IMA Fungus 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, X.; Xhang, X.; Zou, H.; Chen, J.; Fang, B.; Huang, L. Draft genome sequence of Diaporthe batatatis causing dry rot disease in sweet potato. Plant Dis. 2022, 106, 737–740. [Google Scholar] [CrossRef]
- Hill, R.; Leitch, I.J.; Gaya, E. Targeting Ascomycota genomes: What and how big? Fungal Biol. Rev. 2021, 36, 52–59. [Google Scholar] [CrossRef]
- Morales-Cruz, A.; Amrine, K.C.; Blanco-Ulate, B.; Lawrence, D.P.; Travadon, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Distinctive expansion of gene families associated with plant cell wall degradation, secondary metabolism, and nutrient uptake in the genomes of grapevine trunk pathogens. BMC Genom. 2015, 16, 469. [Google Scholar] [CrossRef]
- Savitha, J.; Bhargavi, S.D.; Praveen, V.K. Complete genome sequence of the endophytic fungus Diaporthe (Phomopsis) ampelina. Genome Announc. 2016, 4, e00477. [Google Scholar] [CrossRef]
- Li, S.; Song, Q.; Martins, A.M.; Cregan, P. Draft genome sequence of Diaporthe aspalathi isolate MS-SSC91, a fungus causing stem canker in soybean. Genom. Data 2016, 7, 262–263. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Yang, Y.; Zou, H.; Fang, B.; Liu, W. High-Quality genome resource of Diaporthe destruens causing foot rot disease of sweet potato. Plant Dis. 2021, 105, 3279–3281. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chaisiri, C.; Lin, Y.; Yin, W.X.; Luo, C.X. Whole-Genome sequence of Diaporthe citri Isolate NFHF-8-4, the causal Agent of Citrus melanose. Mol. Plant-Microbe Interact. 2021, 34, 845–847. [Google Scholar] [CrossRef]
- Wang, X.; Dong, H.; Lan, J.; Liu, Y.; Liang, K.; Lu, Q.; Fang, Z.; Liu, P. High-quality genome resource of the pathogen of Diaporthe (Phomopsis) phragmitis causing kiwifruit soft rot. Mol. Plant-Microbe Interact. 2021, 34, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, R.; Scala, F.; Vergara, M.; Thon, M.R.; Ruocco, M. Draft whole-genome sequence of the Diaporthe helianthi 7/96 strain, causal agent of sunflower stem canker. Genom. Data 2016, 10, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, I.B.; Konkel, Z.M.; Scott, K.L.; Valero David, G.E.; Slot, J.C.; Peduto Hand, F. Whole-Genome Sequence Data for the Holotype Strain of Diaporthe ilicicola, a Fungus Associated with Latent Fruit Rot in Deciduous Holly. Microbiol. Resour. Announc. 2022, 11, e00631-22. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Song, Q.; Ji, P.; Cregan, P. Draft genome sequence of Phomopsis longicolla type strain TWH P74, a fungus causing Phomopsis seed decay in soybean. Genome Announc. 2015, 3, e00010-15. [Google Scholar] [CrossRef] [PubMed]
- Heng, Z.; You, Q.; Li, Z.; Sun, B.; Xu, X.; Li, Y.; Li, Z.; Wang, H.; Gong, C.; Xu, X.; et al. The first genome sequence of Phomopsis vexans: A fungal pathogen causing Phomopsis blight in eggplant. Biologia 2023, 78, 543–548. [Google Scholar] [CrossRef]
- Tulsook, K.; Isarangkul, D.; Sriubolmas, N.; Kittakoop, P.; Wiyakrutta, S. Draft genome sequence of Diaporthe sp. strain HANT25, an endophytic fungus producing mycoepoxydiene. Microbiol. Resour. Announc. 2020, 9, e00805-20. [Google Scholar] [CrossRef]
- Vieira, A.; Silva, D.N.; Várzea, V.; Paulo, O.S.; Batista, D. Genome-wide signatures of selection in Colletotrichum kahawae reveal candidate genes potentially involved in pathogenicity and aggressiveness. Front. Microbiol. 2019, 10, 1374. [Google Scholar] [CrossRef]
- Hartmann, F.E. Using structural variants to understand the ecological and evolutionary dynamics of fungal plant pathogens. New Phytol. 2022, 234, 43–49. [Google Scholar] [CrossRef]
- Boufleur, T.R.; Massola Júnior, N.S.; Tikami, Í.; Sukno, S.A.; Thon, M.R.; Baroncelli, R. Identification and comparison of Colletotrichum secreted effector candidates reveal two independent lineages pathogenic to soybean. Pathogens 2021, 10, 1520. [Google Scholar] [CrossRef]
- Selin, C.; De Kievit, T.R.; Belmonte, M.F.; Fernando, W.D. Elucidating the role of effectors in plant-fungal interactions: Progress and challenges. Front. Microbiol. 2016, 7, 600. [Google Scholar] [CrossRef]
- Ball, B.; Langille, M.; Geddes-McAlister, J. Fun(gi)omics: Advanced and diverse technologies to explore emerging fungal pathogens and define mechanisms of antifungal resistance. MBio 2020, 11, e01020-20. [Google Scholar] [CrossRef]
- Félix, C.; Meneses, R.; Gonçalves, M.F.M.; Tilleman, L.; Duarte, A.S.; Jorrín-Novo, J.V.; Van de Peer, Y.; Deforce, D.; Van Nieuwerburgh, F.; Esteves, A.C.; et al. A multi-omics analysis of the grapevine pathogen Lasiodiplodia theobromae reveals that temperature affects the expression of virulence-and pathogenicity-related genes. Sci. Rep. 2019, 9, 13144. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Nunes, R.B.; Tilleman, L.; Van de Peer, Y.; Deforce, D.; Van Nieuwerburgh, F.; Esteves, A.C.; Alves, A. Dual RNA sequencing of Vitis vinifera during Lasiodiplodia theobromae infection unveils host-pathogen interactions. Int. J. Mol. Sci. 2019, 20, 6083. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, S.; Visser, E.A.; Zwart, L.; Du Toit, Y.; Bhadauria, V.; Shuey, L.S. Dual RNA-seq to elucidate the plant-pathogen duel. Curr. Issues Mol. Biol. 2017, 27, 127–142. [Google Scholar] [PubMed]
- Zhang, W.; Yan, J.; Li, X.; Xing, Q.; Chethana, K.T.; Zhao, W. Transcriptional response of grapevine to infection with the fungal pathogen Lasiodiplodia theobromae. Sci. Rep. 2019, 9, 5387. [Google Scholar] [CrossRef]
- Westermann, A.J.; Gorski, S.A.; Vogel, J. Dual RNA-seq of pathogen and host. Nat. Rev. Microbiol. 2012, 10, 618. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y.; Li, Z.; Zhang, H.; Ye, J.; Li, G. Gene expression changes during the gummosis development of peach shoots in response to Lasiodiplodia theobromae infection using RNA-Seq. Front. Physiol. 2016, 7, 170. [Google Scholar] [CrossRef]
- Li, H.; Hu, R.; Fan, Z.; Chen, Q.; Jiang, Y.; Huang, W.; Tao, X. Dual RNA-Seq reveals the genome-wide expression profiles during the compatible and incompatible interactions between Solanum tuberosum and Phytophthora infestans. Front. Plant Sci. 2022, 13, 817199. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, M.; Ma, Y.; Du, Z.; Yuan, N.; Li, Y.; Xiao, J.; Zhang, Y. Whole-genome and time-course dual RNA-Seq analyses reveal chronic pathogenicity-related gene dynamics in the ginseng rusty root rot pathogen Ilyonectria robusta. Sci. Rep. 2020, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Shuey, L.S.; Naidoo, S.; Mamni, T.; Berger, D.K.; Myburg, A.A.; van den Berg, N.; Naidoo, S. Dual RNA-sequencing of Eucalyptus nitens during Phytophthora cinnamomi challenge reveals pathogen and host factors influencing compatibility. Front. Plant Sci. 2016, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ballesteros, C.; Pinto, G.; Amaral, J.; Valledor, L.; Alves, A.; Diez, J.J.; Martín-García, J. Dual RNA-sequencing analysis of resistant (Pinus pinea) and susceptible (Pinus radiata) hosts during Fusarium circinatum challenge. Int. J. Mol. Sci. 2021, 22, 5231. [Google Scholar] [CrossRef]
- Książkiewicz, M.; Rychel-Bielska, S.; Plewiński, P.; Nuc, M.; Irzykowski, W.; Jędryczka, M.; Krajewski, P. The resistance of narrow-leafed lupin to Diaporthe toxica is based on the rapid activation of defense response genes. Int. J. Mol. Sci. 2021, 22, 574. [Google Scholar] [CrossRef]
- Elverson, T.R.; Kontz, B.J.; Markell, S.G.; Harveson, R.M.; Mathew, F.M. Quantitative PCR assays developed for Diaporthe helianthi and Diaporthe gulyae for phomopsis stem canker diagnosis and germplasm screening in sunflower (Helianthus annuus). Plant Dis. 2020, 104, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Voegele, R.T.; Link, T.I. Establishment of a quadruplex real-time PCR assay to distinguish the fungal pathogens Diaporthe longicolla, D. caulivora, D. eres, and D. novem on soybean. PLoS ONE 2020, 16, e0257225. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Kobayashi, Y.O.; Usui, M.; Nishioka, K.; Nakamura, M.; Kawano, S.; Okada, Y.; Kobayashi, A.; Miyasaka, A.; Hirayae, K.; et al. Real-Time PCR assay for the diagnosis and quantification of co-infections by Diaporthe batatas and Diaporthe destruens in sweet potato. Front. Plant Sci. 2020, 12, 694053. [Google Scholar] [CrossRef]
- Qi, H.; Jiang, Z.; Zhang, K.; Yang, S.; He, F.; Zhang, Z. PlaD: A transcriptomics database for plant defense responses to pathogens, providing new insights into plant immune system. Genom. Proteom. Bioinform. 2018, 16, 283–293. [Google Scholar] [CrossRef]
- Yang, H.; Luo, P. Changes in photosynthesis could provide important insight into the interaction between wheat and fungal pathogens. Int. J. Mol. Sci. 2021, 22, 8865. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Kim, S.T.; Kim, S.G.; Hwang, D.H.; Kang, S.Y.; Kim, H.J.; Lee, B.H.; Lee, J.J.; Kang, K.Y. Proteomic analysis of pathogen-responsive proteins from rice leaves induced by rice blast fungus, Magnaporthe grisea. Proteomics 2004, 4, 3569–3578. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, W.; Xin, Y.; Zhang, H.; Yan, C.; Yu, H.; Liu, H.; Xiao, W.; Wang, S.; Zheng, G.; et al. Proteomic analysis of strawberry leaves infected with Colletotrichum fragariae. J. Proteom. 2012, 75, 4074–4090. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Hussain, A.; Ali, Q.; Saleem, M.H.; Abualreesh, M.H.; Alatawi, A.; Ali, S.; Munis, M.F.H. Disease severity, resistance analysis, and expression profiling of pathogenesis-related protein genes after the inoculation of Fusarium equiseti in wheat. Agronomy 2021, 11, 2124. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Lü, Q.; Yan, D.; Liu, Z.; Zhang, X. Comparative proteomic analysis of plant-pathogen interactions in resistant and susceptible poplar ecotypes infected with Botryosphaeria dothidea. Phytopathology 2019, 109, 2009–2021. [Google Scholar] [CrossRef]
- Yang, F.E.N.; Jensen, J.D.; Svensson, B.; Jørgensen, H.J.; Collinge, D.B.; Finnie, C. Secretomics identifies Fusarium graminearum proteins involved in the interaction with barley and wheat. Mol. Plant Pathol. 2012, 13, 445–453. [Google Scholar] [CrossRef]
- Huser, A.; Takahara, H.; Schmalenbach, W.; O’Connell, R. Discovery of pathogenicity genes in the crucifer anthracnose fungus Colletotrichum higginsianum, using random insertional mutagenesis. Mol. Plant-Microbe Interact. 2009, 22, 143–156. [Google Scholar] [CrossRef]
- Reuveni, M.; Sheglov, N.; Eshel, D.; Prusky, D.; Ben-Arie, R. Virulence and the production of endo-1, 4-β-glucanase by Isolates of Alternaria alternata involved in the moldy-core disease of apples. J. Phytopathol. 2007, 155, 50–55. [Google Scholar] [CrossRef]
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351. [Google Scholar] [CrossRef]
- Silva, M.D.C.; Guerra-Guimarães, L.; Diniz, I.; Loureiro, A.; Azinheira, H.; Pereira, A.P.; Tavares, S.; Batista, D.; Várzea, V. An overview of the mechanisms involved in Coffee-Hemileia vastatrix interactions: Plant and pathogen perspectives. Agronomy 2022, 12, 326. [Google Scholar] [CrossRef]
- Balotf, S.; Wilson, R.; Tegg, R.S.; Nichols, D.S.; Wilson, C.R. Shotgun proteomics as a powerful tool for the study of the proteomes of plants, their pathogens, and plant-pathogen interactions. Proteomes 2022, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Spina, R.; Saliba, S.; Dupire, F.; Ptak, A.; Hehn, A.; Piutti, S.; Poinsignon, S.; Leclerc, S.; Bouguet-Bonnet, S.; Laurain-Mattar, D. Molecular identification of endophytic bacteria in Leucojum aestivum in vitro culture, NMR-based metabolomics study and LC-MS analysis leading to potential Amaryllidaceae alkaloid production. Int. J. Mol. Sci. 2021, 22, 1773. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2011, 2, 87–95. [Google Scholar] [CrossRef]
- Quinn, R. Rethinking antibiotic research and development: World War II and the penicillin collaborative. Am. J. Public Health 2013, 103, 426–434. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Esteves, A.C.; Alves, A. Marine Fungi: Opportunities and Challenges. Encyclopedia 2022, 2, 559–577. [Google Scholar] [CrossRef]
- Chen, H.; Singh, H.; Bhardwaj, N.; Bhardwaj, S.K.; Khatri, M.; Kim, K.H.; Peng, W. An exploration on the toxicity mechanisms of phytotoxins and their potential utilities. Crit. Rev. Environ. Sci. Technol. 2022, 52, 395–435. [Google Scholar] [CrossRef]
- Feussner, I.; Polle, A. What the transcriptome does not tell—Proteomics and metabolomics are closer to the plants’ patho-phenotype. Curr. Opin. Plant Biol. 2015, 26, 26–31. [Google Scholar] [CrossRef]
- Tan, K.C.; Ipcho, S.V.; Trengove, R.D.; Oliver, R.P.; Solomon, P.S. Assessing the impact of transcriptomics, proteomics and metabolomics on fungal phytopathology. Mol. Plant Pathol. 2010, 10, 703–771. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Masamba, P.; Kappo, A.P. Metabolomics and Chemoinformatics in Agricultural Biotechnology Research: Complementary Probes in Unravelling New Metabolites for Crop Improvement. Biology 2022, 11, 1156. [Google Scholar] [CrossRef]
- Dickinson, E.; Rusilowicz, M.J.; Dickinson, M.; Charlton, A.J.; Bechtold, U.; Mullineaux, P.M.; Wilson, J. Integrating transcriptomic techniques and k-means clustering in metabolomics to identify markers of abiotic and biotic stress in Medicago truncatula. Metabolomics 2018, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.A.H.; Griffin, J.L.; Jung, Y.H.; Shibato, J.; Rakwal, R.; Agrawal, G.K.; Jwa, N.S. Using metabolic profiling to assess plant-pathogen interactions: An example using rice (Oryza sativa) and the blast pathogen Magnaporthe grisea. Eur. J. Plant Pathol. 2011, 129, 539–554. [Google Scholar] [CrossRef]
- Hu, Z.; Chang, X.; Dai, T.; Li, L.; Liu, P.; Wang, G.; Liu, P.; Huang, Z.; Liu, X. Metabolic profiling to identify the latent infection of strawberry by Botrytis cinerea. Evol. Bioinform. 2019, 15, 1176934319838518. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Metabolomic profiling of the host response of tomato (Solanum lycopersicum) following infection by Ralstonia solanacearum. Int. J. Mol. Sci. 2019, 20, 3945. [Google Scholar] [CrossRef] [PubMed]
- Kage, U.; Karre, S.; Kushalappa, A.C.; McCartney, C. Identification and characterization of a fusarium head blight resistance gene TaACT in wheat QTL-2DL. Plant Biotechnol. J. 2017, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.B.D.; Santana, M.F. Prediction of the secretomes of endophytic and nonendophytic fungi reveals similarities in host plant infection and colonization strategies. Mycologia 2020, 112, 491–503. [Google Scholar] [CrossRef]
- Garcia, J.F.; Lawrence, D.P.; Morales-Cruz, A.; Travadon, R.; Minio, A.; Hernandez-Martinez, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Phylogenomics of plant associated Botryosphaeriaceae species. Front. Microbiol. 2021, 12, 587. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Dung, J.K.S.; Johnson, D.A. From pathogen to endophyte: An endophytic population of Verticillium dahliae evolved from a sympatric pathogenic population. New Phytol. 2019, 222, 497–510. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Huang, P.W.; Dai, C.C. Endophytism or saprophytism: Decoding the lifestyle transition of the generalist fungus Phomopsis liquidambari. Microbiol. Res. 2018, 206, 99–112. [Google Scholar] [CrossRef]
- Becker, Y.; Eaton, C.J.; Brasell, E.; May, K.J.; Becker, M.; Hassing, B.; Cartwright, G.M.; Reinhold, L.; Scott, B. The fungal cell-wall integrity MAPK cascade is crucial for hyphal network formation and maintenance of restrictive growth of Epichloë festucae in symbiosis with Lolium perenne. Mol. Plant Microbe Interact. 2015, 28, 69–85. [Google Scholar] [CrossRef]
- Segmüller, N.; Kokkelink, L.; Giesbert, S.; Odinius, D.; van Kan, J.; Tudzynski, P. NADPH oxidases are involved in differentiation and pathogenicity in Botrytis cinerea. Mol. Plant Microbe Interact. 2008, 21, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Eaton, C.J.; Cox, M.P.; Scott, B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 2011, 180, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.M.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A critical review on communication mechanism within plant-endophytic fungi interactions to cope with biotic and abiotic stresses. J. Fungi 2021, 7, 719. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.J.; Wang, Z.Y.; Jones, M.A.; Smirnoff, N.; Talbot, N.J. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. USA 2007, 104, 11772–11777. [Google Scholar] [CrossRef]
- Lahrmann, U.; Strehmel, N.; Langen, G.; Frerigmann, H.; Leson, L.; Ding, Y.; Scheel, D.; Herklotz, S.; Hilbert, M.; Zuccaro, A. Mutualistic root endophytism is not associated with the reduction of saprotrophic traits and requires a noncompromised plant innate immunity. New Phytol. 2015, 207, 841–857. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).