Marker-Assisted Selection of Male-Sterile and Maintainer Line in Chili Improvement by Backcross Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. DNA Extraction and Polymerase Chain Reaction
2.3. Pollen Viability Test
3. Results
3.1. Analysis of the Rf-Linked DNA Marker and S-Linked DNA Marker
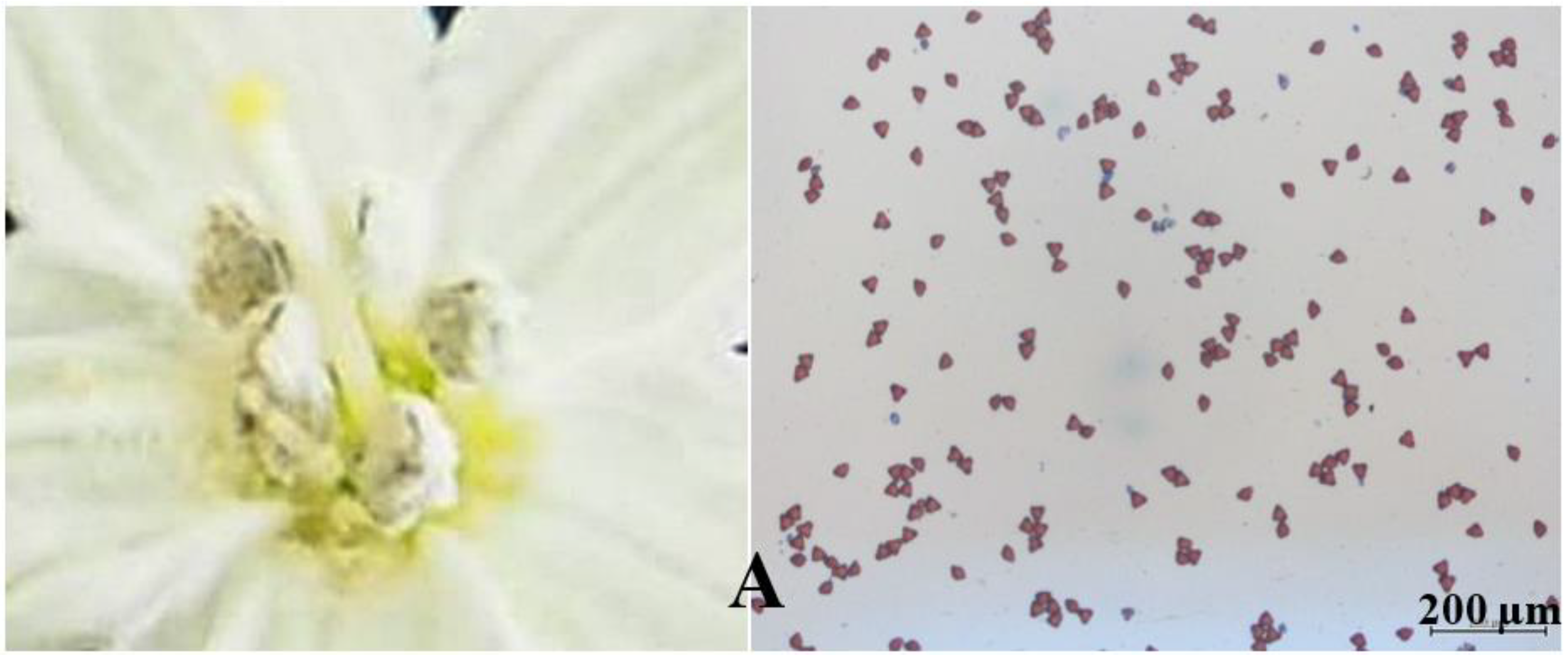
3.2. Pollen Viability Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozada, D.N.; Bosland, P.W.; Barchenger, D.W.; Haghshenas-Jaryani, M.; Sanogo, S.; Walker, S. Chile pepper (Capsicum) breeding and improvement in the “Multi-Omics” Era. Front. Plant Sci. 2022, 13, 879182. [Google Scholar] [CrossRef]
- Techawongstien, S. Chili: Production Management and Breeding; Press Media Co.: Bangkok, Thailand, 2006; pp. 2–6. (In Thai) [Google Scholar]
- Milerue, N.; Nikornpun, M. Studies on heterosis of chili (Capsicum annuum L.). Kasetsart J. (Nat. Sci.) 2000, 34, 90–196. [Google Scholar]
- Jeeatid, N.; Suriharn, B.; Techawongstien, S.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Evaluation of the effect of genotype-by-environment interaction on capsaicinoid production in hot pepper hybrids (Capsicum chinense Jacq.) under controlled environment. Sci. Hortic. 2018, 235, 334–339. [Google Scholar] [CrossRef]
- Kim, M.K.; Jang, J.H.; Potchanasin, P.; Chae, W.B.; Yoo, E.H.; Taek-Ryoun, K. Current status and breeding perspectives of major vegetable crops in Thailand. J. Korean Soc. Int. Agric. 2019, 31, 67–75. [Google Scholar] [CrossRef]
- Buchholz, K. The Biggest Producers of Chilis. Available online: https://www.statista.com/chart/28756/countries-producing-the-biggest-amount-of-dry-chilis-and-peppers/ (accessed on 28 February 2022).
- Office of Permanent Secretary Ministry of Commerce, Exports Group Structure. Thailand Trading Report. 2019. Available online: http://www.ops3.moc.go.th/infor/menucomth/stru1export/exportre/report.asp (accessed on 17 September 2019).
- Hundal, J.S.; Dhall, R.K.; Tripathi, S.K. Breeding for hybrid hot pepper. In Hybrid Vegetable Development; Singh, P.K., Dasgupta, S.K., Tripathi, S.K., Eds.; Food Products Press: New York, NY, USA, 2004; pp. 40–44. [Google Scholar]
- Colombo, N.; Galmarini, C.R. The use of genetic, manual and chemical methods to control pollination in vegetable hybrid seed production: A review. Plant Breed. 2017, 136, 287–299. [Google Scholar] [CrossRef]
- Hussain, S.M.; Khursheed, H.; Farwah, S.; Rizvi, S.; Rashid, M.; Saleem, S.; Andrabi, N.; Rashid, H. Male sterility in vegetable crops. J. Pharmacogn. Phytochem. 2018, 7, 3390–3393. [Google Scholar]
- Chen, L.; Liu, Y.G. Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhang, D. Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci. 2018, 23, 53–65. [Google Scholar] [CrossRef]
- Ren, F.S.; Yang, H.F.; Jiao, Y.S.; Zhang, R.P.; Guo, Z.W.; Liu, H.J.; Sun, Q.; Li, X.J.; Tan, X.F.; Zhang, B.; et al. Fertility conversion between cytoplasmic maintainer lines and restorer lines through molecular marker-assisted selection in pepper (Capsicum annuum L.). Biologia 2022, 77, 2351–2358. [Google Scholar] [CrossRef]
- Wu, L.; Wang, P.; Wang, Y.; Cheng, Q.; Lu, Q.; Liu, J.; Li, T.; Ai, Y.; Yang, W.; Sun, L.; et al. Genome-wide correlation of 36 agronomic traits in the 287 pepper (Capsicum) accessions obtained from the SLAF-seq-based GWAS. Inter. J. Molecul. Sci. 2019, 20, 5675. [Google Scholar] [CrossRef]
- Zhang, Z.; An, D.; Cao, Y.; Yu, H.; Zhu, Y.; Mei, Y.; Zhang, B.; Wang, L. Development and application of KASP markers associated with Restorer-of-fertility gene in Capsicum annuum L. Physiol. Mol. Biol. Plants 2021, 27, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Valde’s, M.H. A model for marker-based selection in gene introgression breeding programs. Crop Sci. 2000, 40, 91–98. [Google Scholar] [CrossRef]
- Wang, Q.S.; Zhang, X.; Li, C.Y.; Liu, Z.; Feng, Y.H. Directional transfer of a multiple-allele male sterile line in Brassica campestris L. ssp. chinensis (L.) Makino var. rosularis Tsen et Lee. Breed. Sci. 2014, 64, 149–155. [Google Scholar] [CrossRef]
- Kaushik, S.; Djiwanti, S.R. Genetic improvements of traits for enhancing NPK acquisition and utilization efficiency in plants. In Plant Macronutrient Use Efficiency: Molecular and Genomic Perspectives in Crop Plants; Hossain, M.A., Kamiya, T., Burritt, D., Tran, L.S.P., Fujiwara, T., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 267–283. [Google Scholar]
- Varshney, R.K.; Roorkiwal, M.; Sorrells, M.E. Genomic selection for crop improvement: An introduction. In Genomic Selection for Crop Improvement; Varshney, R.K., Roorkiwal, M., Sorrells, M.E., Eds.; Springer: New York, NY, USA, 2017; pp. 1–6. [Google Scholar]
- Lee, J.; Yoon, J.B.; Han, J.H.; Lee, W.P.; Do, J.W.; Ryu, H.S.; Kim, H.; Park, H.G. A codominant SCAR marker linked to the genic male sterility gene (ms1) in chili pepper (Capsicum annuum). Plant Breed. 2010, 129, 35–38. [Google Scholar] [CrossRef]
- Ji, J.J.; Huang, W.; Yin, Y.X.; Li, Z.; Gong, Z.H. Development of a SCAR marker for early identification of S-cytoplasm based on mitochondrial SRAP analysis in pepper (Capsicum annuum L.). Mol. Breed. 2014, 33, 679–690. [Google Scholar] [CrossRef]
- Yeh, T.; Lin, S.; Shieh, H.; Teoh, Y.; Kumar, S. Markers for cytoplasmic male sterility (CMS) traits in chili peppers (Capsicum annuum L.). I: Multiplex PCR and validation. SABRAO J. Breed. Genet. 2016, 48, 465–473. [Google Scholar]
- Sun, G.S.; Dai, Z.L.; Bosland, P.W.; Wang, Q.; Sun, C.Q.; Zhang, Z.C.; Ma, Z.H. Characterizing and marker-assisting a novel chili pepper (Capsicum annuum L.) yellow bud mutant with cytoplasmic male sterility. Genet. Mol. Res. 2017, 16, gmr16019459. [Google Scholar] [CrossRef]
- Jo, Y.D.; Ha, Y.; Lee, J.H.; Park, M.; Bergsma, A.C.; Choi, H.I.; Goritschnig, S.; Kloosterman, B.; Van Dijk, P.J.; Choi, D.; et al. Fine mapping of restorer-of-fertility in pepper (Capsicum annuum L.) identified a candidate gene encoding a pentatricopeptide repeat (PPR)-containing protein. Theor. Appl. Genet. 2016, 129, 2003–2017. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Focus 1987, 12, 3–15. [Google Scholar]
- Winnepenninckx, B.; Backeljau, T.; de Wachter, R. Extraction of high molecular weight DNA from molluscs. Trends Genet. 1993, 9, 407. [Google Scholar]
- Kumchai, J.; Wei, Y.C.; Lee, C.Y.; Chen, F.C.; Chin, S.W. Production of interspecific hybrids between commercial cultivars of the eggplant (Solanum melongena L.) and its wild relative. S. Torvum. Genet. Mol. Res. 2013, 12, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Peterson, P.A. Cytoplasmically inherited male sterility in Capsicum. Am. Nat. 1958, 92, 111–119. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, P.; Liu, J.Q.; Wu, L.; Zhang, Z.P.; Li, T.T.; Gao, W.J.; Yang, W.C.; Sun, L.; Shen, H.L. Identification of candidate genes under lying genic male–sterile msc-1 locus via genome resequencing in Capsicum annuum L. Theor. App. Genet. 2018, 131, 1861–1872. [Google Scholar] [CrossRef]
- Gulyas, G.; Pakozdi, K.; Lee, J.S.; Hirata, Y. Analysis of fertility restoration by using cytoplasmic male-steriles red pepper (Capsicum annuum L.). Breed. Sci. 2006, 56, 331–334. [Google Scholar] [CrossRef]
- Vogel, K.E. Backcross Breeding. Transgenic Maize Methods and Protocols Springer Protocals Methods in Molecular Biology; Humana Press: New York, NY, USA, 2009; pp. 161–169. [Google Scholar]
- Briggs, F.N.; Knowles, P.F. Introduction to Plant Breeding; Reinhold Publishing Coorperation: New York, NY, USA, 1967; pp. 162–174. [Google Scholar]
- Bellundagi, A.; Ramya, K.T.; Krishna, H.; Jain, N.; Shashikumara, P.; Singh, P.K.; Singh, G.P.; Prabhu, K.V. Marker-assisted backcross breeding for heat tolerance in bread wheat (Triticum aestivum L.). Front. Genet. 2022, 13, 1056783. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.G.; Rafii, M.Y.; Yusuff, O.; Martini, M.Y.; Ismail, M.R.; Ridzuan, R. Molecular confirmation of candidate Hsp70 gene associated with heat tolerancein BC3F2 advanced backcross lines and their phenotypic resemblance with recurrent chilli Kulai. Acta Agric. Scand. B 2020, 70, 252–264. [Google Scholar]
- Lee, J.; Yoon, J.B.; Park, H.G. A CAPS marker associated with the partial restoration of cytoplasmic male sterility in chili pepper (Capsicum annuum L.). Mol. Breed. 2008, 21, 95–104. [Google Scholar] [CrossRef]
- Jo, Y.D.; Kim, Y.M.; Park, M.N.; Yoo, J.H.; Park, M.; Kim, B.D.; Kang, B.C. Development and evaluation of broadly applicable markers for restorer-of-fertility in pepper. Mol. Breed. 2010, 25, 187–201. [Google Scholar] [CrossRef]
- Min, W.K.; Kim, S.; Sung, S.K.; Kim, B.D.; Lee, S. Allelic discrimination of the restorer of fertility gene and its inheritance in peppers (Capsicum annuum L.). Theor. Appl. Genet. 2009, 119, 1289–1299. [Google Scholar] [CrossRef]
- Nei, Z.; Song, Y.; Wang, H.; Chen, J.; Niu, Q.; Zhu, W. Fine mapping and gene analysis of restorer-of -fertility gene CaRfHZ in pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2022, 23, 7633. [Google Scholar]
- Dhaliwal, M.S.; Jindal, S.K. Induction and exploitation of nuclear and cytoplasmic male sterility in pepper (Capsicum spp.): A review. J. Hortic. Sci. Biotechnol. 2014, 89, 471–479. [Google Scholar] [CrossRef]
- Usman, M.; Ziaf, K.; Ye, Z. Breeding and crop improvement. In Breeding of Horticultural Crops; Khan, A.S., Ziaf, K., Eds.; Horticulture Science and Technology, University of Agriculture: Faisalabad, Pakistan, 2017; pp. 25–65. [Google Scholar]
- Jugulam, M.; Ziauddin, A.; So, K.K.Y.; Chen, S.; Hall, J.C. Transfer of Dicamba tolerance from Sinapis arvensis to Brassica napus via embryo rescue and recurrent backcross breeding. PLoS ONE 2015, 10, e0141418. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Havey, M.J. Variable penetrance among different sources of the male fertility restoration allele of onion. Hortscience 2020, 55, 543–546. [Google Scholar] [CrossRef]
- Rattenbury, J.A. Specific staining of nucleolar substance with aceto-carmine. Stain Technol. 1952, 27, 113–120. [Google Scholar] [CrossRef]
- Dapson, R.W. The history, chemistry, and modes of action of carmine and related dyes. Biotech. Histochem. 2007, 82, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Ting, L.; Yixin, A.; Qiaohua, L.; Yihao, W.; Lang, W.; Jinqiu, L.; Liang, S.; Huolin, S. Phenotypic, genetic, and molecular function of msc-2, a genic male sterile mutant in pepper (Capsicum annuum L.). Theor. Appl. Genet. 2019, 133, 843–855. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.N.; Hsu, Y.C.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Luo, X.D.; Dai, L.F.; Wang, S.B.; Wolukau, J.; Jáhn, M.; Chen, J.F. Male gamete development and early tapetal degeneration in cytoplasmic male-sterile pepper investigated by meiotic, anatomical and ultrastructural analyse. Plant Breed. 2006, 125, 395–399. [Google Scholar] [CrossRef]
- Guo, J.; Wang, P.; Cheng, Q.; Sun, L.; Wang, H.; Wang, Y.; Kao, L.; Li, Y.; Qiu, T.; Yang, W.; et al. Proteomic analysis reveals strong mitochondrial involvement in cytoplasmic male sterility of pepper (Capsicum annuum L.). J. Proteom. 2017, 168, 15–27. [Google Scholar] [CrossRef]
- Ahmadikhah, A.; Mirarab, M.; Pahlevani, M.H.; Nayyeripasand, L. Marker-assisted backcrossing to develop an elite cytoplasmic male sterility line in rice. Plant Genome 2015, 8, plantgenome2014.07.0031. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B 2008, 363, 557–572. [Google Scholar] [CrossRef] [PubMed]
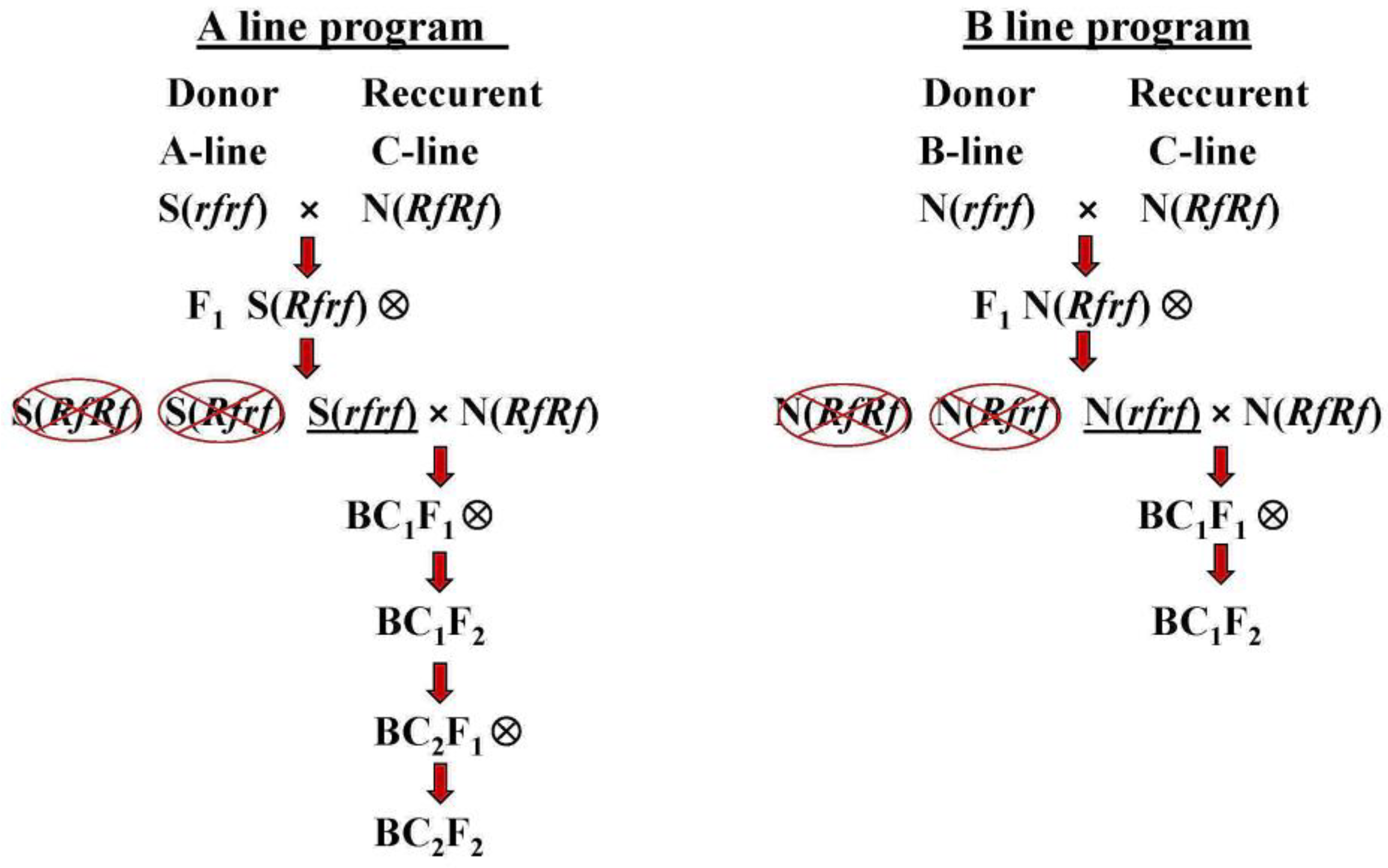



| Group | Code | Genotype | Phenotype |
|---|---|---|---|
| A-line | A1 | S rfrf | Sterile |
| B-line | B1 | N rfrf | Maintainer (Fertile) |
| C-line | C1 | N RfRf | Restorer (Fertile) |
| C3 | N RfRf | Restorer (Fertile) | |
| cross | |||
| BC2F2A1 × C1 | - | - | |
| BC2F2A1 × C3 | - | - | |
| BC1F2B1 × C1 | - | - | |
| BC1F2B1 × C3 | - | - |
| Marker Name | 5′ to 3′ Sequence | Annealing Temperature (°C) | Product Size (bp) | References |
|---|---|---|---|---|
| S or N cytoplasm | ||||
| CMS-SCAR130/140 | F: TTACGGCTCGTTACCGCAGCG R: CAATTGACCGACCCGCCAT | 57 | 130/140 | Ji et al. (2014) [21] |
| Rf locus | ||||
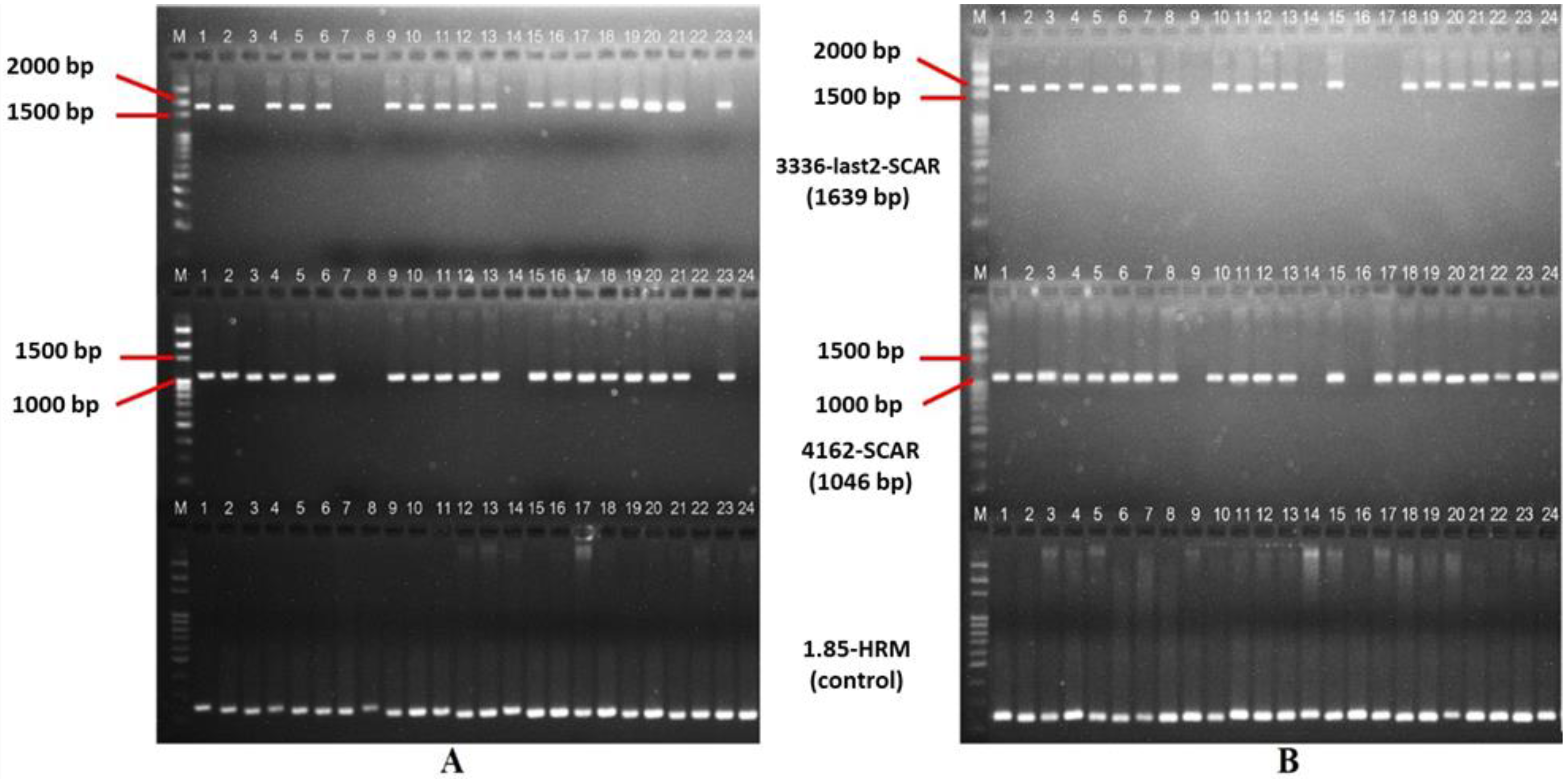
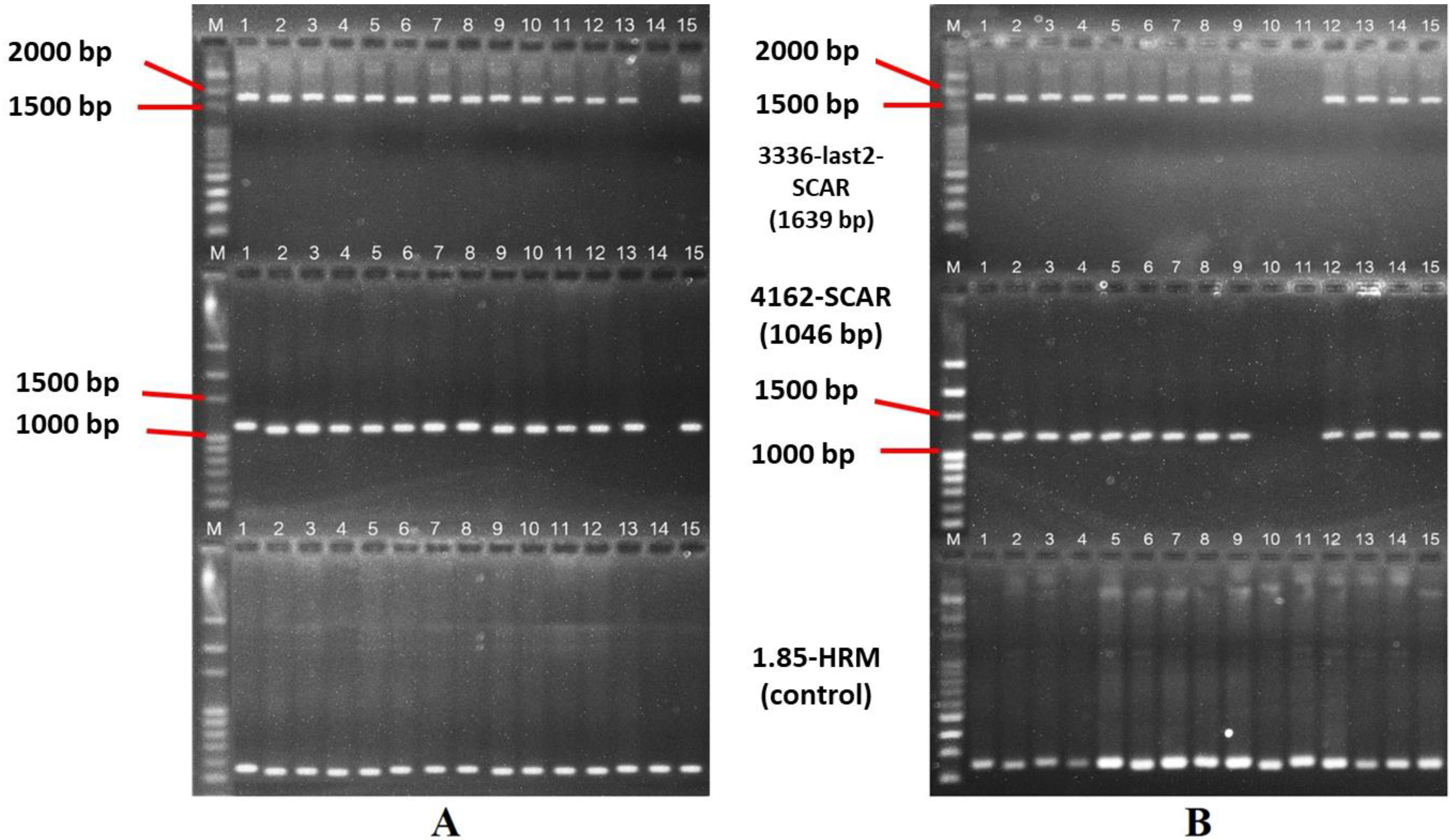
| 3336-last2-SCAR | F: CATCGAACTGATACGGAAGGAC R: TAACACTACTTGGGGAAAGCG | 52 | 1639 | Jo et al. (2016) [24] |
| 4162-SCAR | F: GCAGTTCGGTTTTACGGAGTTAC R: CCATTGGACAAAAGGGGATC | 51 | 1046 | Jo et al. (2016) [24] |
| 1.85-HRM | F: GACATGCAAGGTAAGGCTGC R: CACAAATTCTGGCTATCGGTC | 52 | 250 | Jo et al. (2016) [24] |
| BC2F2 | Plant No. | Markers | Genotype | Phenotype | |
|---|---|---|---|---|---|
| 3336-last2-SCAR | 4162-SCAR | ||||
| A1 × C1 | 1–2, 4–6, 9–13, 15–21, 23 | + | + | S Rf_ | Fertile |
| A1 × C1 | 3, 7–8, 14, 22, 24 | − | + | S rfrf | Sterile |
| A1 × C3 | 1–8, 10–13, 15, 18–24 | + | + | S Rf_ | Fertile |
| A1 × C3 | 9, 14, 16–17, | − | + | S rfrf | Sterile |
| BC1F2 | Plant No. | Markers | Genotype | Phenotype | |
|---|---|---|---|---|---|
| 3336-last2-SCAR | 4162-SCAR | ||||
| B1 × C1 | 1–13, 15 | + | + | N Rf_ | Fertile |
| B1 × C1 | 14 | − | − | N rfrf | Maintainer |
| B1 × C3 | 1–9, 12–15 | + | + | S Rf_ | Fertile |
| B1 × C3 | 10–11 | − | − | N rfrf | Maintainer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na Jinda, A.; Nikornpun, M.; Jeeatid, N.; Thumdee, S.; Thippachote, K.; Pusadee, T.; Kumchai, J. Marker-Assisted Selection of Male-Sterile and Maintainer Line in Chili Improvement by Backcross Breeding. Horticulturae 2023, 9, 357. https://doi.org/10.3390/horticulturae9030357
Na Jinda A, Nikornpun M, Jeeatid N, Thumdee S, Thippachote K, Pusadee T, Kumchai J. Marker-Assisted Selection of Male-Sterile and Maintainer Line in Chili Improvement by Backcross Breeding. Horticulturae. 2023; 9(3):357. https://doi.org/10.3390/horticulturae9030357
Chicago/Turabian StyleNa Jinda, Aatjima, Maneechat Nikornpun, Nakarin Jeeatid, Siwaporn Thumdee, Kamon Thippachote, Tonapha Pusadee, and Jutamas Kumchai. 2023. "Marker-Assisted Selection of Male-Sterile and Maintainer Line in Chili Improvement by Backcross Breeding" Horticulturae 9, no. 3: 357. https://doi.org/10.3390/horticulturae9030357
APA StyleNa Jinda, A., Nikornpun, M., Jeeatid, N., Thumdee, S., Thippachote, K., Pusadee, T., & Kumchai, J. (2023). Marker-Assisted Selection of Male-Sterile and Maintainer Line in Chili Improvement by Backcross Breeding. Horticulturae, 9(3), 357. https://doi.org/10.3390/horticulturae9030357







