Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Biostimulant Treatment
2.4. Growth Parameters
2.5. Physiological Parameters
2.5.1. Chlorophyll Index (SPAD Value)
2.5.2. Leaf Gas Exchange Parameters
2.6. Yield Parameters
2.7. Quality Parameters
2.7.1. Total Soluble Solids (TSS)
2.7.2. Ascorbic Acid Content
2.7.3. Titratable Acidity
2.7.4. Lycopene Content
2.7.5. Total Sugars
2.8. Statistical Analysis
3. Results
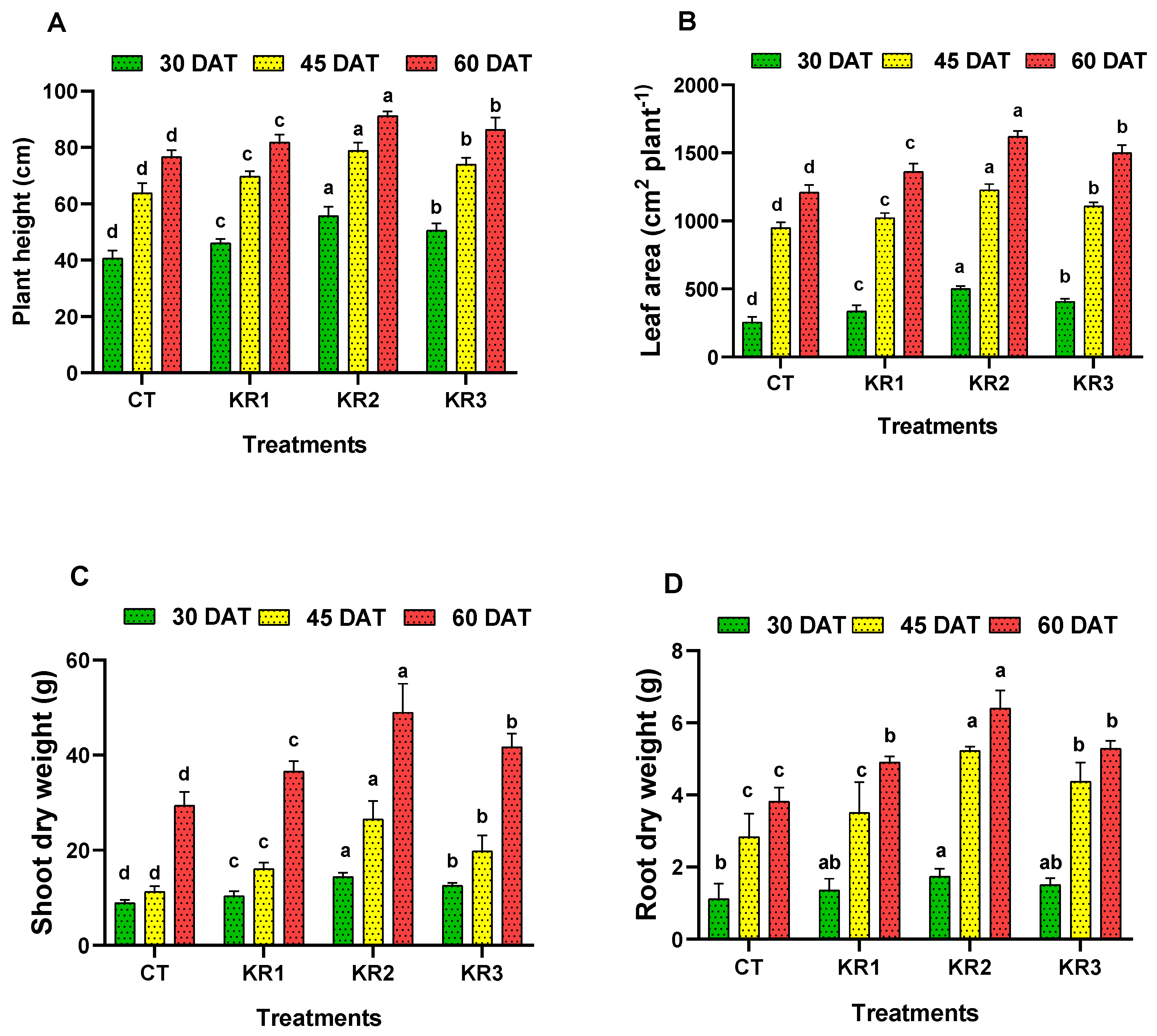
3.1. Growth Parameters
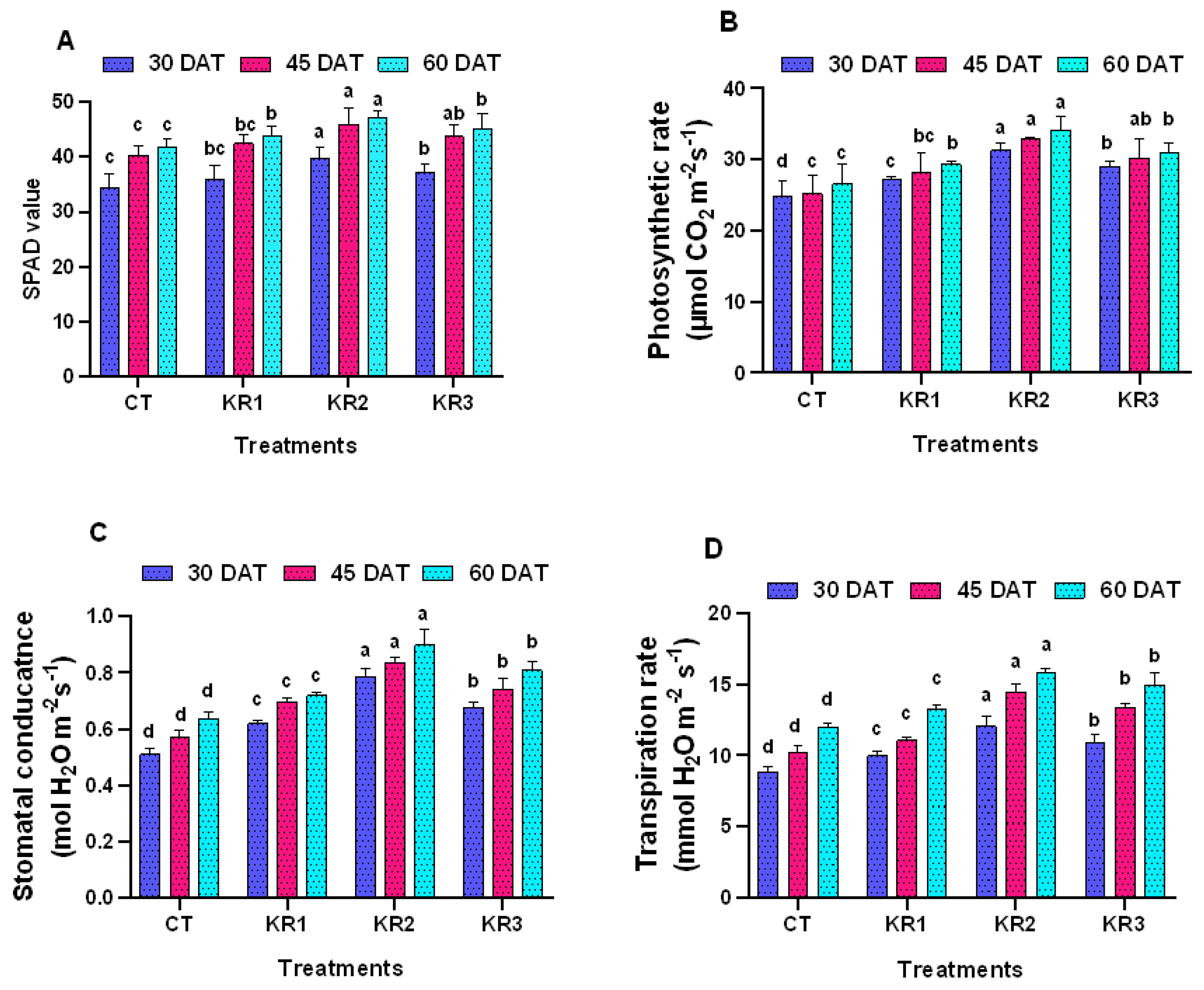
3.2. Physiological Parameters
3.2.1. SPAD Value (Chlorophyll Index)
3.2.2. Gas Exchange Parameters
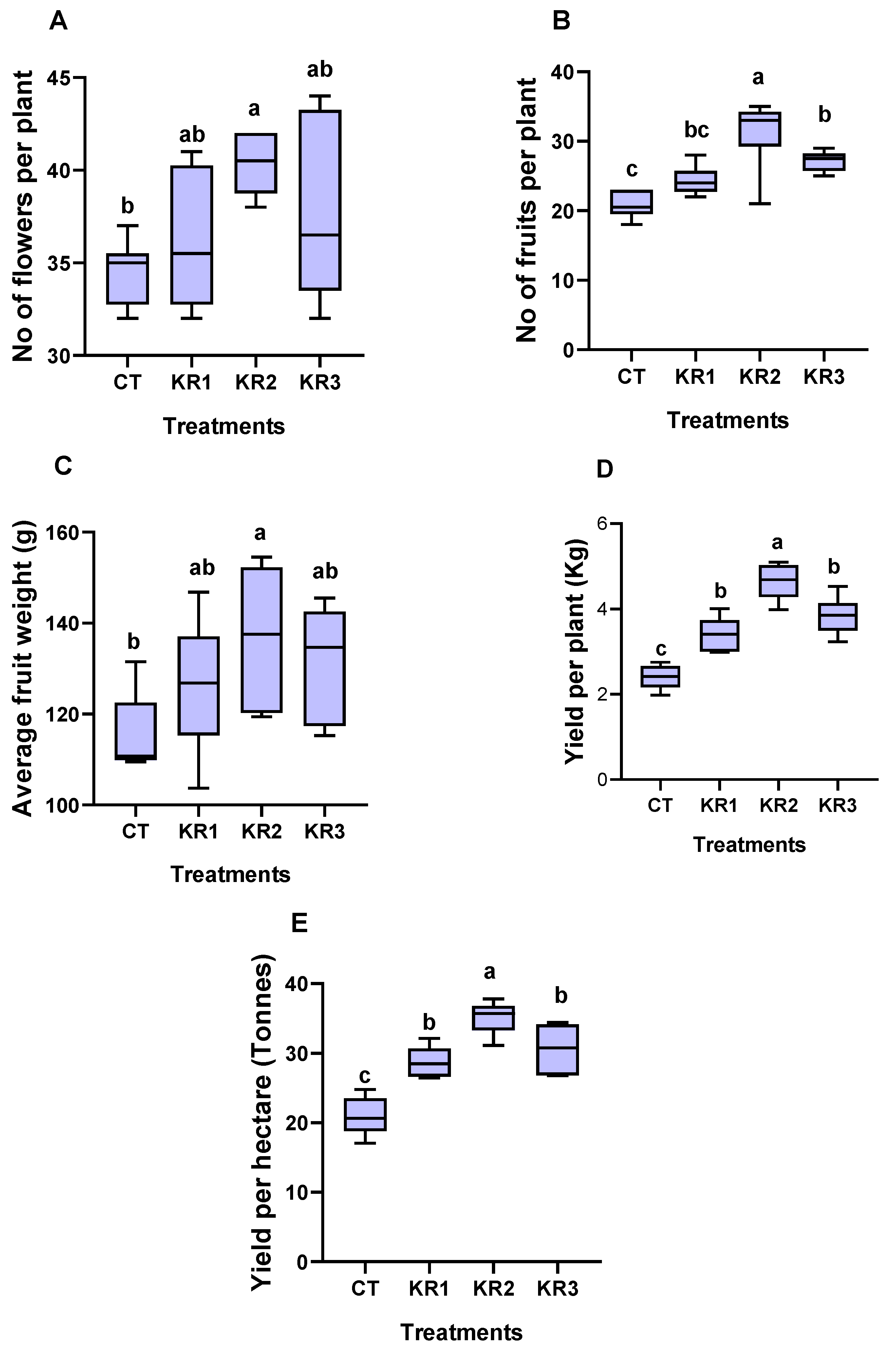
3.3. Yield Parameters
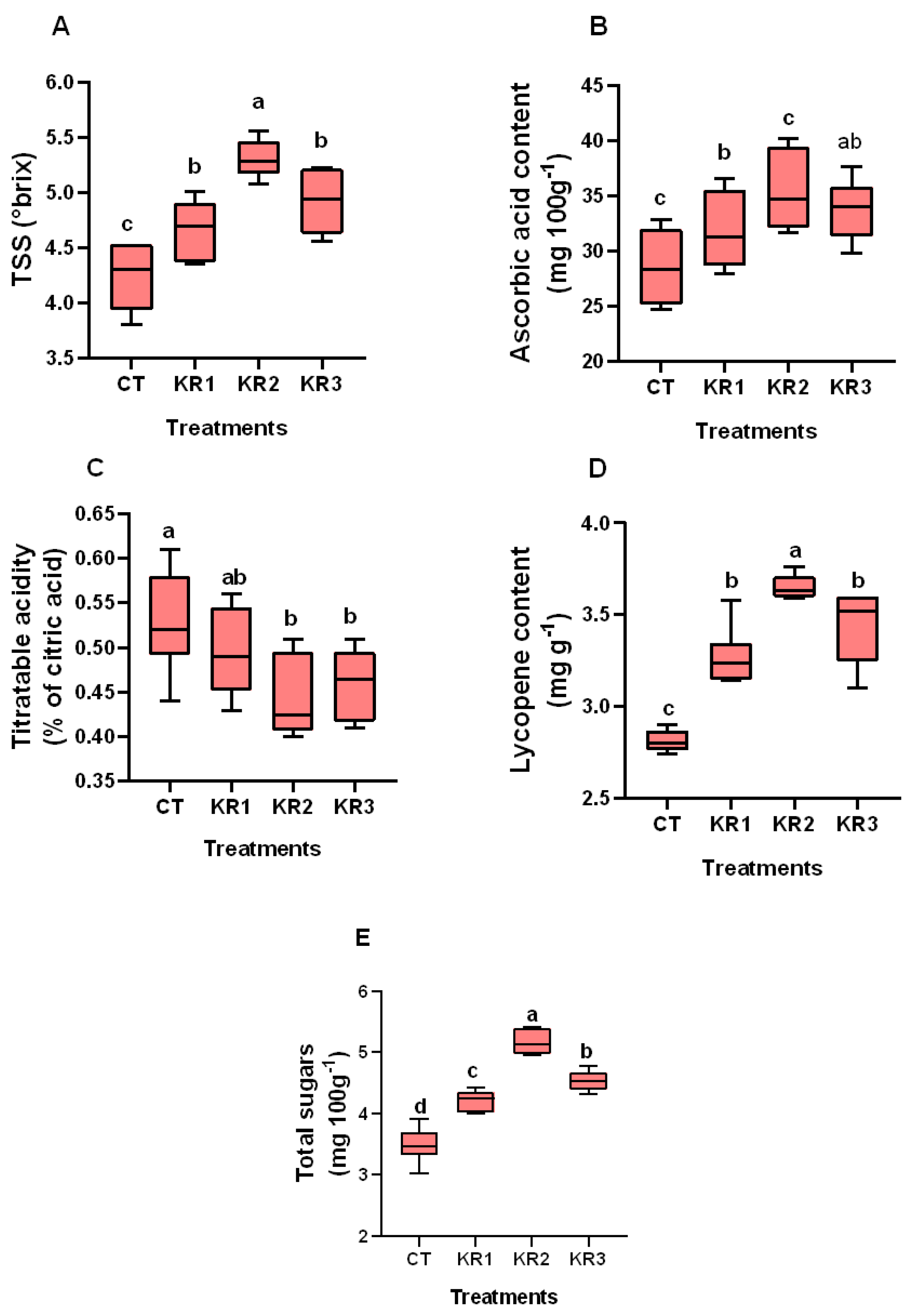
3.4. Fruit Quality Parameters
3.5. Correlation
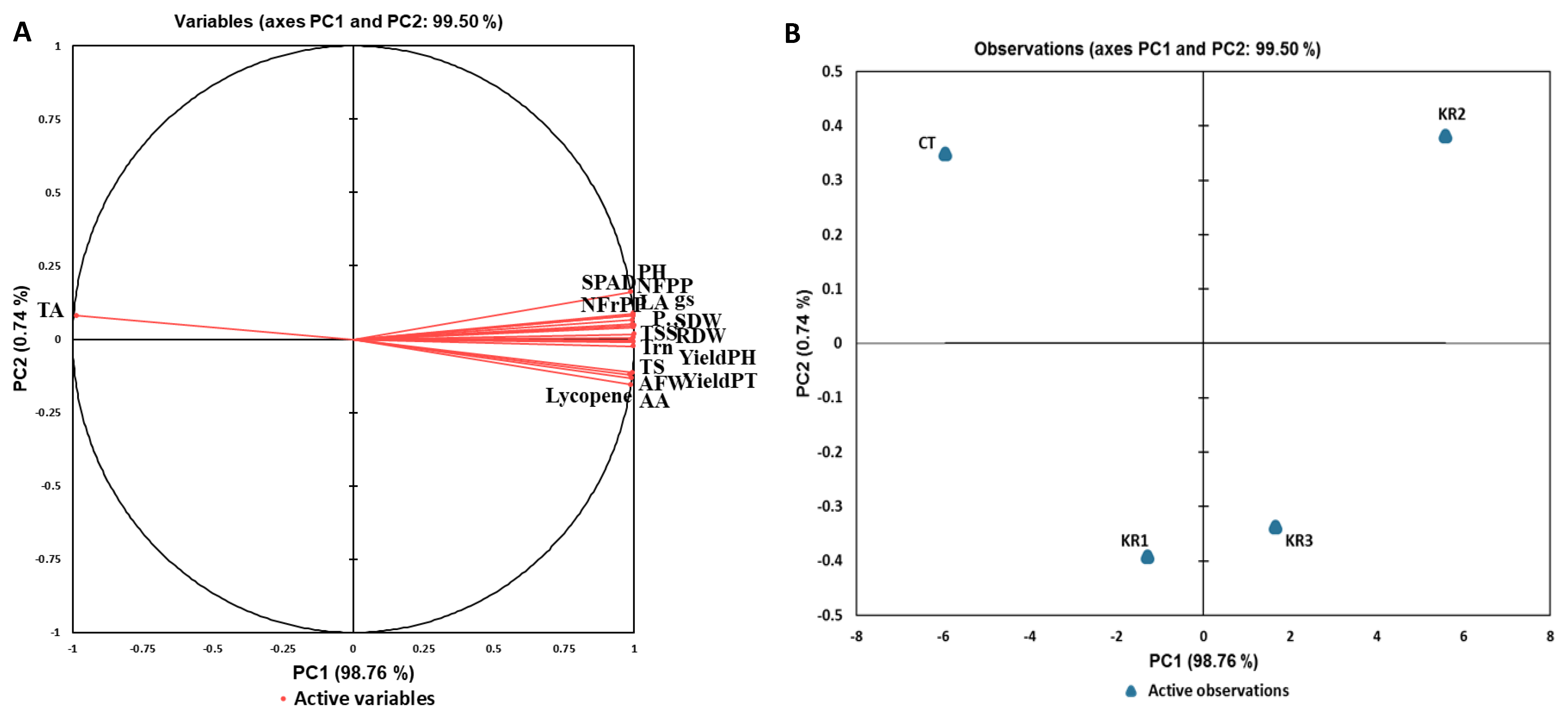
3.6. Principal Component Analysis
4. Discussion
4.1. Growth Parameters of Tomato Influenced by Kendal Root
4.2. Kendal Root Application on Physiological Parameters of Tomato
4.3. Yield Traits of Tomato Influenced by Kendal Root
4.4. Kendal Root Application on Quality Parameters of Tomato
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farooq, S.; Rather, A.; Gull, S.; Ahmad Ganai, A.; Masoodi, S.; Mohd Wani, F.; Ganaie, T.A. Physicochemical and nutraceutical properties of tomato powder as affected by pre-treatments, drying methods, and storage period. Int. J. Food Prop. 2020, 23, 797–808. [Google Scholar] [CrossRef]
- Rodriguez, A.; Sanders, I.R. The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J. 2015, 9, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Farneti, B. Tomato Quality: From the Field to the Consumer: Interactions between Genotype, Cultivation and Postharvest Conditions. Ph.D. Dissertation, Wageningen University and Research, Wageningen, The Netherlands, 2014. [Google Scholar]
- Sebilo, M.; Mayer, B.; Nicolardot, B.; Pinay, G.; Mariotti, A. Long-term fate of nitrate fertilizer in agricultural soils. Proc. Natl. Acad. Sci. USA 2013, 110, 18185–18189. [Google Scholar] [CrossRef]
- Shubha, K.; Mukherjee, A.; Kumari, M.; Tiwari, K.; Meena, V.S. Bio-stimulants: An approach towards the sustainable vegetable production. In Agriculturally Important Microbes for Sustainable Agriculture: Volume I: Plant-Soil-Microbe Nexus; Meena, V., Mishra, P., Bisht, J., Pattanayak, A., Eds.; Springer: Singapore, 2017; Volume 1, pp. 259–277. [Google Scholar]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Koleska, I.; Hasanagic, D.; Todorovic, V.; Murtic, S.; Klokic, I.; Paradikovic, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Goni, O.; Quille, P.; Oconnell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Fleurence, J. Biostimulant Potential of Seaweed Extracts Derived from Laminaria and Ascophyllum nodosum Biostimulants. In Exploring Sources and Applications; Ramawat, N., Bhardwaj, V., Eds.; Springer: Singapore, 2022; pp. 31–49. [Google Scholar]
- Bantis, F.; Koukounaras, A. Ascophyllum nodosum and Silicon-Based Biostimulants differentially affect the physiology and growth of watermelon transplants under abiotic stress factors: The case of drought. Horticulturae 2022, 8, 1177. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A. Ascophyllum nodosum and silicon-based biostimulants differentially affect the physiology and growth of watermelon transplants under abiotic stress factors: The case of salinity. Plants 2023, 12, 433. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, H.; Piromsri, K.; Tisarum, R.; Cha-um, S.; Datta, A. Effects of an Ascophyllum nodosum seaweed extract application dose and method on growth, fruit yield, quality, and water productivity of tomato under water-deficit stress. S. Afr. J. Bot. 2022, 151, 95–107. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Ramnarine, S.D.B., Jr.; Jayaraman, J. Transcriptomic changes induced by applications of a commercial extract of Ascophyllum nodosum on tomato plants. Sci. Rep. 2022, 12, 8042. [Google Scholar] [CrossRef]
- Ikuyinminu, E.; Goni, O.; Oconnell, S. Enhancing irrigation salinity stress tolerance and increasing yield in tomato using a precision engineered protein hydrolysate and Ascophyllum nodosum-derived biostimulant. Agronomy 2022, 12, 809. [Google Scholar] [CrossRef]
- Langowski, L.; Goni, O.; Ikuyinminu, E.; Feeney, E.; Oconnell, S. Investigation of the direct effect of a precision Ascophyllum nodosum biostimulant on nitrogen use efficiency in wheat seedlings. Plant Physiol. Biochem. 2022, 179, 44–57. [Google Scholar] [CrossRef]
- Repke, R.A.; Silva, D.M.R.; Dos Santos, J.C.C.; De Almeida Silva, M. Increased soybean tolerance to high-temperature through biostimulant based on Ascophyllum nodosum (L.) seaweed extract. J. Appl. Phycol. 2022, 34, 3205–3218. [Google Scholar] [CrossRef]
- Rajendran, R.; Jagmohan, S.; Jayaraj, P.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Effects of Ascophyllum nodosum extract on sweet pepper plants as an organic biostimulant in grow box home garden conditions. J. Appl. Phycol. 2022, 34, 647–657. [Google Scholar] [CrossRef]
- Rashad, Y.M.; El-Sharkawy, H.H.; Elazab, N.T. Ascophyllum nodosum extract and mycorrhizal colonization synergistically trigger immune responses in pea plants against Rhizoctonia root rot, and enhance plant growth and productivity. J. Fungi. 2022, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Prithiviraj, B. Ascophyllum nodosum biostimulant improves the growth of Zea mays grown under phosphorus impoverished conditions. Front. Plant Sci. 2021, 11, 601843. [Google Scholar] [CrossRef]
- Ali, J.; Jan, I.; Ullah, H.; Ahmed, N.; Alam, M.; Ullah, R.; Sayed, S. Influence of Ascophyllum nodosum extract foliar spray on the physiological and biochemical attributes of okra under drought stress. Plants 2022, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Monje, O.A.; Bugbee, B. Inherent limitations of nondestructive chlorophyll meters: A comparison of two types of meters. HortScience 1992, 27, 69–71. [Google Scholar] [CrossRef]
- Tigist, M.; Workneh, T.S.; Woldetsadik, K. Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 2013, 50, 477–486. [Google Scholar] [CrossRef]
- Ikewuchi, C.; Ikewuchi, C. Iodometric determination of the ascorbic acid (vitamin C) content of some fruits consumed in a university community in Nigeria. GJPAAS 2011, 17, 47–49. [Google Scholar]
- Ranganna, S. Handbook of analysis and quality control for fruit and vegetable products: Tata McGraw-Hill Education. J. Environ. Hortic. 1986, 514, 14–20. [Google Scholar]
- Hedge, J.; Hofreiter, B. Estimation of carbohydrate. In Methods in Carbohydrate Chemistry; Academic Press: New York, NY, USA, 1962; pp. 17–22. [Google Scholar]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of biostimulants in tomato plants (Solanum lycopersicum) to enhance plant growth and salt stress tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the biostimulant effects of a novel plant-based formulation on tomato crop. Sustainability 2022, 12, 8432. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Khouloud, M.; Farrie, Y.; Boulif, R.; Kadmiri, I.M.; Douira, A. Improving Growth, Yield, and Quality of Tomato Plants (Solanum lycopersicum L.) by the application of moroccan Seaweed-Based biostimulants under greenhouse conditions. Agronomy 2021, 11, 1373. [Google Scholar] [CrossRef]
- Ahmed, S.; Fahmy, A. Applications of natural polysaccharide polymers to overcome water scarcity on the yield and quality of tomato fruits. J. Soil Sci. Agric. Eng. 2019, 10, 199–208. [Google Scholar] [CrossRef]
- Adeeba, S.; Naeem, M.; Shafia, N.; Mohd, I.; Tariq, A.; Nadeem, H.; Lalit, V. An evaluation of the effects of irradiated sodium alginate on the growth, physiological activities and essential oil production of fennel (Foeniculum vulgare Mill.). J. Med. Plant Res. 2011, 5, 15–21. [Google Scholar]
- Vera, J.; Castro, J.; Contreras, R.A.; Gonzalez, A.; Moenne, A. Oligo-carrageenans induce a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiol. Mol. Plant Pathol. 2012, 79, 31–39. [Google Scholar] [CrossRef]
- Gonzalez-Gonzalez, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sanchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Hernandez-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Murtic, S.; Oljaca, R.; Murtic, M.S.; Vrana, A.; Koleska, I.; Karic, L. Effects of seaweed extract on the growth, yield and quality of cherry tomato under different growth conditions. Acta Agric. Slov. 2018, 111, 315–325. [Google Scholar] [CrossRef]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Souri, M.K.; Bakhtiarizade, M. Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci. Hortic. 2019, 243, 472–476. [Google Scholar] [CrossRef]
- Della Lucia, M.C.; Baghdadi, A.; Mangione, F.; Borella, M.; Zegada-Lizarazu, W.; Ravi, S.; Monti, A. Transcriptional and physiological analyses to assess the effects of a novel biostimulant in tomato. Front. Plant Sci. 2022, 12, 781993. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, G.; Du, M.; Niu, C.; Zhang, P.; Zhang, X.; Bao, Z. Biostimulants promote plant vigor of tomato and strawberry after transplanting. Sci. Hortic. 2020, 267, 109355. [Google Scholar] [CrossRef]
- Kałuzewicz, A.; Krzesinski, W.; Spizewski, T.; Zaworska, A. Effect of biostimulants on several physiological characteristics and chlorophyll content in broccoli under drought stress and re-watering. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 197–202. [Google Scholar] [CrossRef]
- Rashid, N.; Khan, S.; Wahid, A.; Ibrar, D.; Hasnain, Z.; Irshad, S.; Kamran, M. Exogenous application of biostimulants and synthetic growth promoters improved the productivity and grain quality of quinoa linked with enhanced photosynthetic pigments and metabolomics. Agronomy 2021, 11, 2302. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Dell Aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Carillo, P. Ascophyllum nodosum based extracts counteract salinity stress in tomato by remodelling leaf nitrogen metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Rouphael, Y.; Corrado, G.; Colla, G.; De Pascale, S.; Dell Aversana, E.; Damelia, L.I.; Carillo, P. Biostimulation as a means for optimizing fruit phytochemical content and functional quality of tomato landraces of the San Marzano area. Foods 2021, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-priming with seaweed extracts enhances yield of salt-stressed tomato plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Islam, M.N.; Uddain, J.; Chowdhury, M.S.N.; Subramaniam, S. Synergistic effect of microbial and nonmicrobial biostimulants on growth, yield, and nutritional quality of organic tomato. Crop Sci. 2020, 60, 2102–2114. [Google Scholar] [CrossRef]
- Mannino, G.; Campobenedetto, C.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. The application of a plant biostimulant based on seaweed and yeast extract improved tomato fruit development and quality. Biomolecules 2020, 10, 1662. [Google Scholar] [CrossRef] [PubMed]

| Soil Properties | Range |
|---|---|
| Texture | Clay loam |
| pH | 7.57 |
| Electrical conductivity (dS m−1) | 0.66 |
| Soil organic carbon (%) | 0.69 |
| Available nitrogen (kg ha−1) | 275 |
| Available phosphorous (kg ha−1) | 38 |
| Available potassium (kg ha−1) | 975 |
| Variables | BS | PH | LA | SDW | RDW | SPAD | Pn | gs | Trn | NFPP | NFrPP | AFW | YieldPP | YieldPH | TSS | AA | TA | Lycopene | TS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS | 1 | ||||||||||||||||||
| PH | 0.608 ** | 1 | |||||||||||||||||
| LA | 0.678 ** | 0.864 ** | 1 | ||||||||||||||||
| SDW | 0.579 ** | 0.782 ** | 0.870 ** | 1 | |||||||||||||||
| RDW | 0.542 ** | 0.839 ** | 0.898 ** | 0.881 ** | 1 | ||||||||||||||
| SPAD | 0.474 * | 0.732 ** | 0.661 ** | 0.649 ** | 0.741 ** | 1 | |||||||||||||
| Pn | 0.519 ** | 0.817 ** | 0.839 ** | 0.790 ** | 0.822 ** | 0.648 ** | 1 | ||||||||||||
| Gs | 0.640 ** | 0.822 ** | 0.900 ** | 0.894 ** | 0.890 ** | 0.682 ** | 0.852 ** | 1 | |||||||||||
| Trn | 0.717 ** | 0.852 ** | 0.910 ** | 0.853 ** | 0.887 ** | 0.701 ** | 0.787 ** | 0.915 ** | 1 | ||||||||||
| NFPP | 0.338 | 0.550 ** | 0.549 ** | 0.452 * | 0.581 ** | 0.745 ** | 0.462 * | 0.491 * | 0.463 * | 1 | |||||||||
| NFrPP | 0.508 * | 0.824 ** | 0.731 ** | 0.673 ** | 0.721 ** | 0.538 ** | 0.809 ** | 0.785 ** | 0.749 ** | 0.424 * | 1 | ||||||||
| AFW | 0.389 | 0.554 ** | 0.537 ** | 0.398 | 0.614 ** | 0.314 | 0.426 * | 0.439 * | 0.576 ** | 0.379 | 0.588 ** | 1 | |||||||
| YieldPP | 0.584 ** | 0.830 ** | 0.881 ** | 0.785 ** | 0.894 ** | 0.613 ** | 0.797 ** | 0.857 ** | 0.880 ** | 0.452 * | 0.799 ** | 0.752 ** | 1 | ||||||
| YieldPH | 0.576 ** | 0.824 ** | 0.853 ** | 0.839 ** | 0.906 ** | 0.752 ** | 0.813 ** | 0.838 ** | 0.849 ** | 0.526 ** | 0.657 ** | 0.475 * | 0.757 ** | 1 | |||||
| TSS | 0.545 ** | 0.808 ** | 0.786 ** | 0.809 ** | 0.848 ** | 0.707 ** | 0.763 ** | 0.783 ** | 0.760 ** | 0.607 ** | 0.725 ** | 0.449 * | 0.778 ** | 0.753 ** | 1 | ||||
| AA | 0.469 * | 0.539 ** | 0.532 ** | 0.553 ** | 0.658 ** | 0.686 ** | 0.605 ** | 0.574 ** | 0.653 ** | 0.378 | 0.562 ** | 0.438 * | 0.616 ** | 0.652 ** | 0.586 ** | 1 | |||
| TA | −0.461 * | −0.602 ** | −0.621 ** | −0.387 | −0.480 * | −0.380 | −0.436 * | −0.480 * | −0.591 ** | −0.376 | −0.521 ** | −0.241 | −0.437 * | −0.531 ** | −0.451 * | −0.253 | 1 | ||
| Lycopene | 0.650 ** | 0.779 ** | 0.864 ** | 0.816 ** | 0.826 ** | 0.746 ** | 0.741 ** | 0.851 ** | 0.876 ** | 0.569 ** | 0.692 ** | 0.477 * | 0.811 ** | 0.830 ** | 0.689 ** | 0.574 ** | −0.544 ** | 1 | |
| TS | 0.593 ** | 0.860 ** | 0.892 ** | 0.820 ** | 0.910 ** | 0.782 ** | 0.751 ** | 0.879 ** | 0.899 ** | 0.587 ** | 0.768 ** | 0.534 ** | 0.848 ** | 0.859 ** | 0.751 ** | 0.707 ** | −0.604 ** | 0.886 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramaniyan, L.; Veerasamy, R.; Prabhakaran, J.; Selvaraj, A.; Algarswamy, S.; Karuppasami, K.M.; Thangavel, K.; Nalliappan, S. Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 348. https://doi.org/10.3390/horticulturae9030348
Subramaniyan L, Veerasamy R, Prabhakaran J, Selvaraj A, Algarswamy S, Karuppasami KM, Thangavel K, Nalliappan S. Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.). Horticulturae. 2023; 9(3):348. https://doi.org/10.3390/horticulturae9030348
Chicago/Turabian StyleSubramaniyan, Lakshmi, Ravichandran Veerasamy, Jeyakumar Prabhakaran, Anandakumar Selvaraj, Senthil Algarswamy, Kalarani M. Karuppasami, Kalaiselvi Thangavel, and Sakthivel Nalliappan. 2023. "Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.)" Horticulturae 9, no. 3: 348. https://doi.org/10.3390/horticulturae9030348
APA StyleSubramaniyan, L., Veerasamy, R., Prabhakaran, J., Selvaraj, A., Algarswamy, S., Karuppasami, K. M., Thangavel, K., & Nalliappan, S. (2023). Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.). Horticulturae, 9(3), 348. https://doi.org/10.3390/horticulturae9030348









