Ozone-Ultrafine Bubbles for Reducing Concentration of Citric Acid and Sodium Chloride for Trimmed Young Coconut Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemical Treatment
2.2. Disease Score
2.3. Mesocarp Whiteness (W) and Browning Index (BI)
2.4. Coconut Water and Coconut Meat Qualities
2.5. Total Phenolic Content of Coconut Mesocarp
2.6. Polyphenol Oxidase (PPO) Activity of Coconut Mesocarp
2.7. pH of Coconut Mesocarp
2.8. Microbiological Analysis
2.9. Statistical Analysis
3. Results
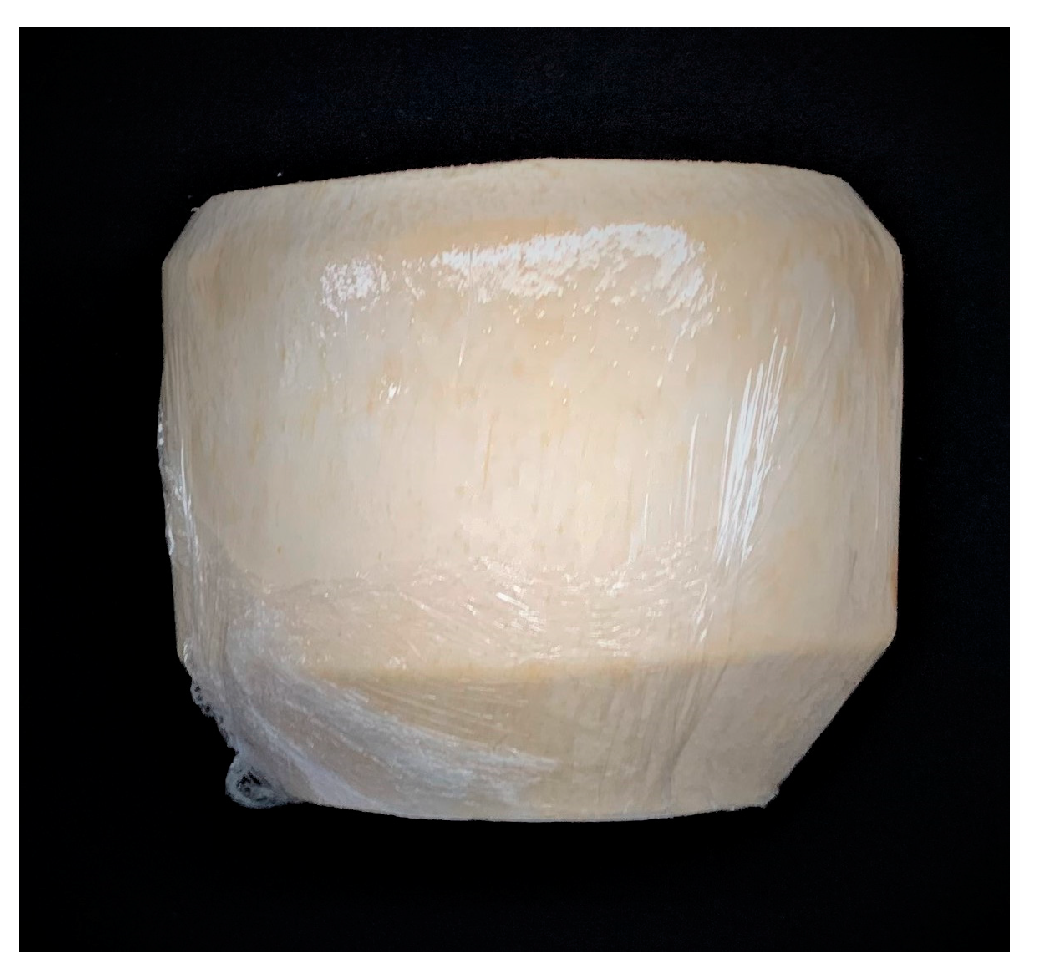
3.1. Coconut Fruit Appearance
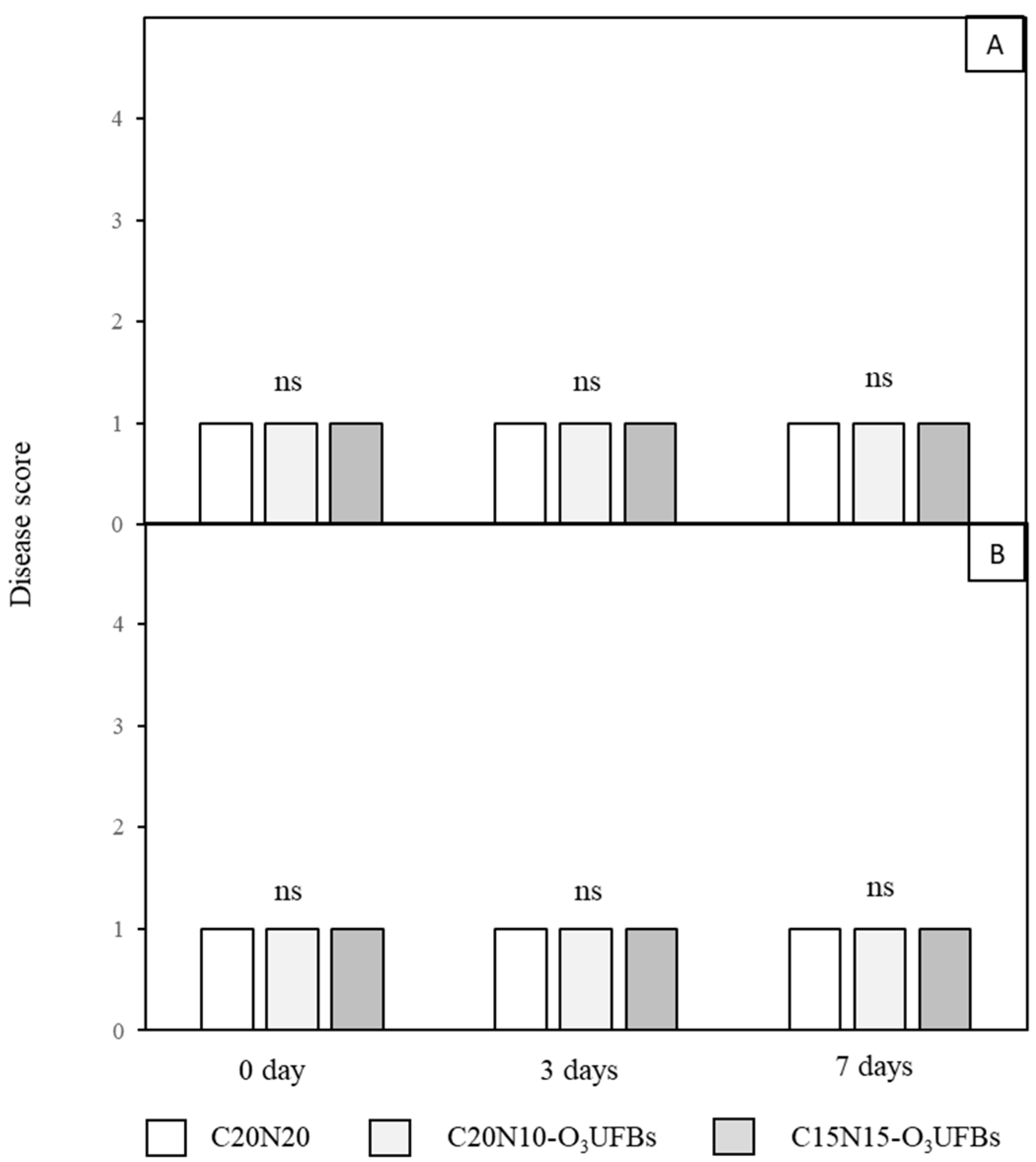
3.2. Disease Score
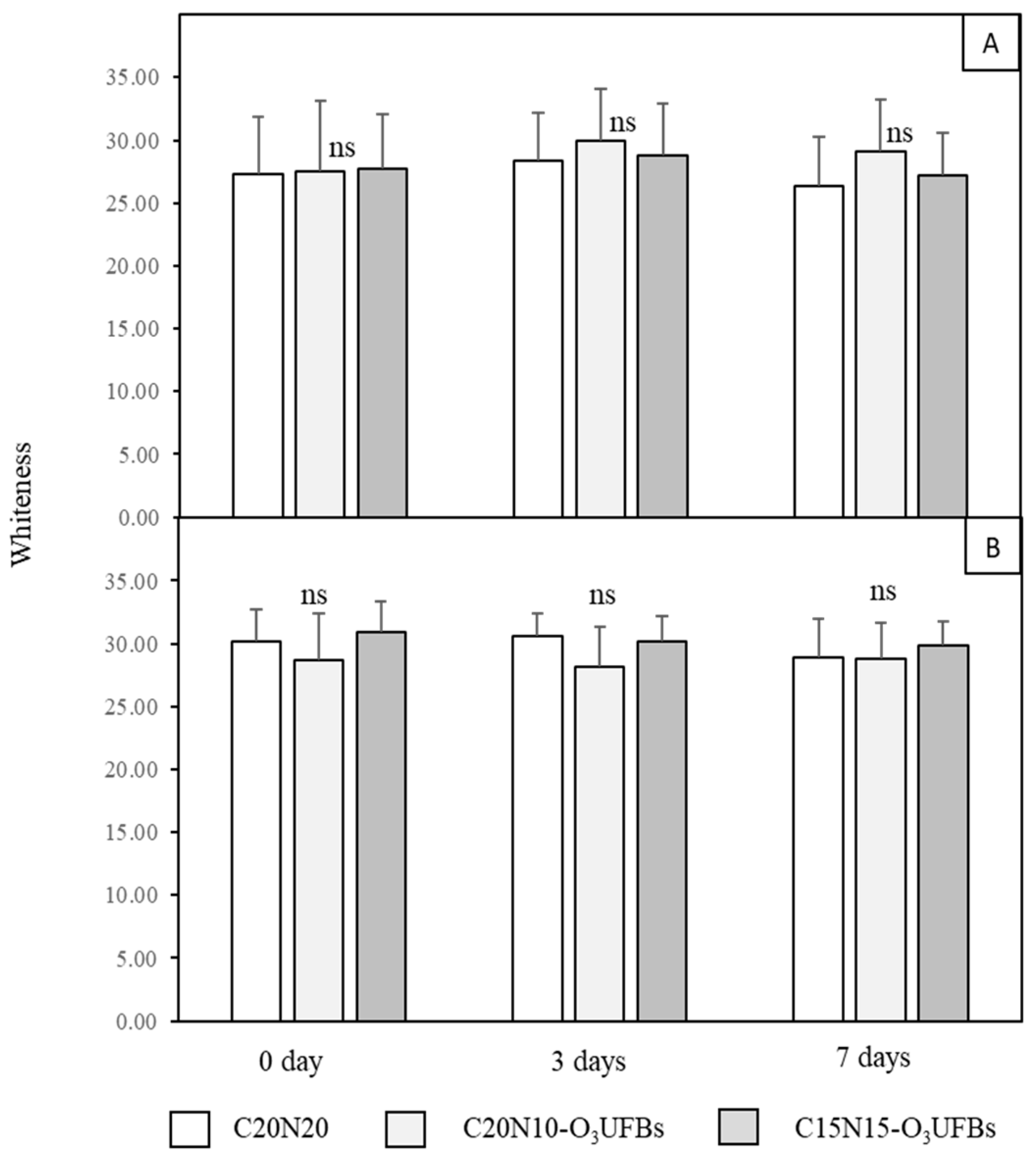
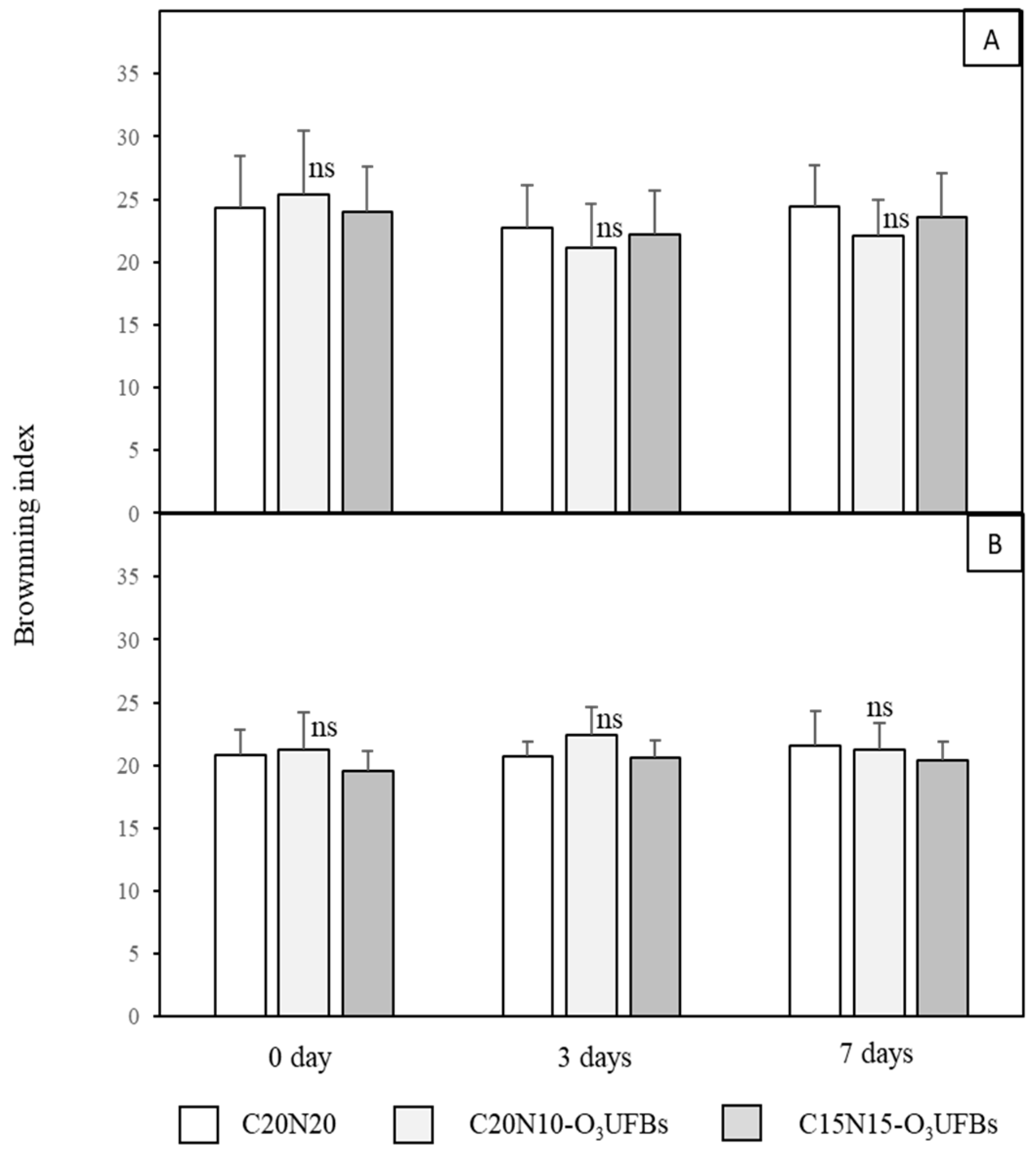
3.3. Mesocarp Whiteness (W) and Browning Index (BI)
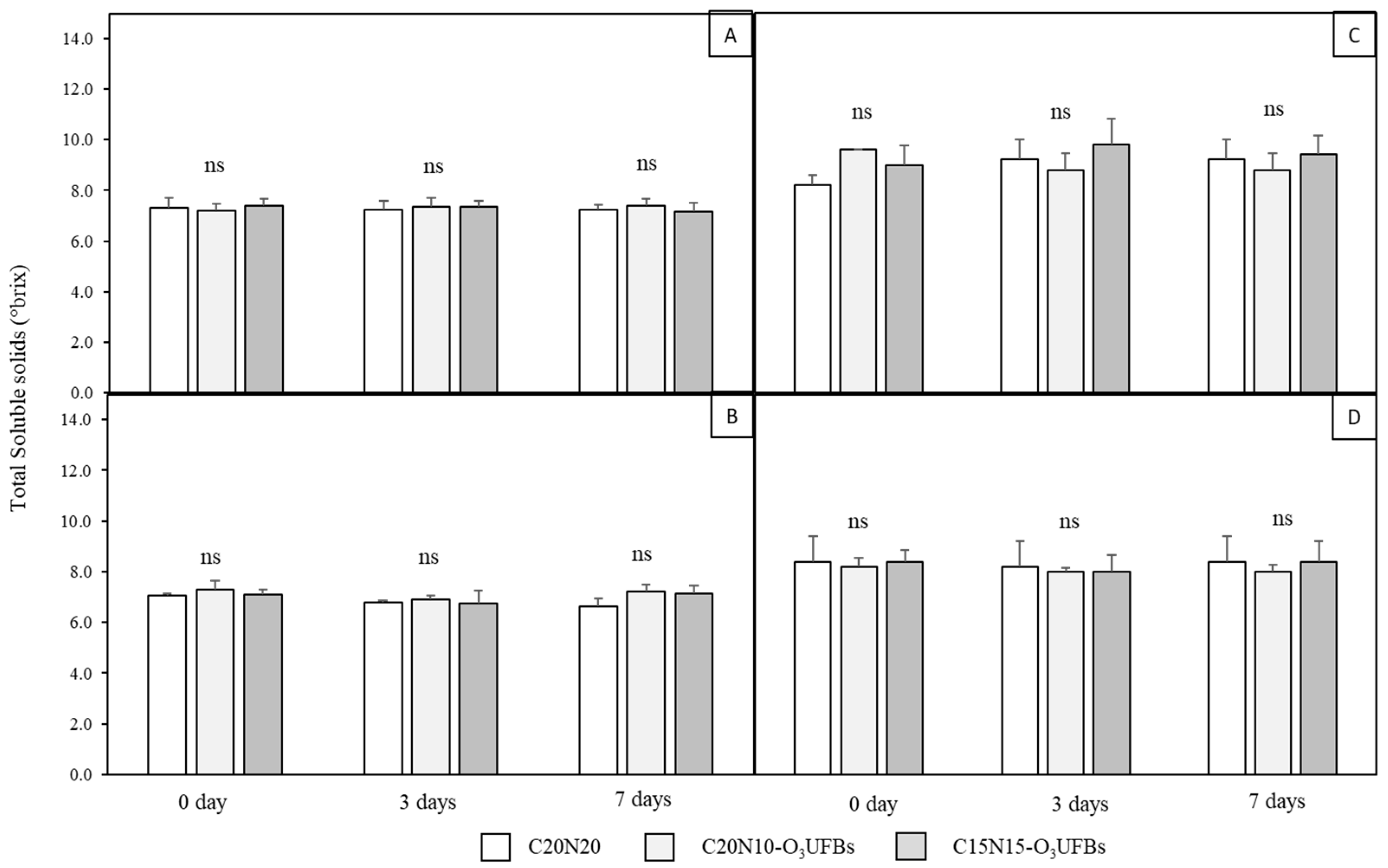
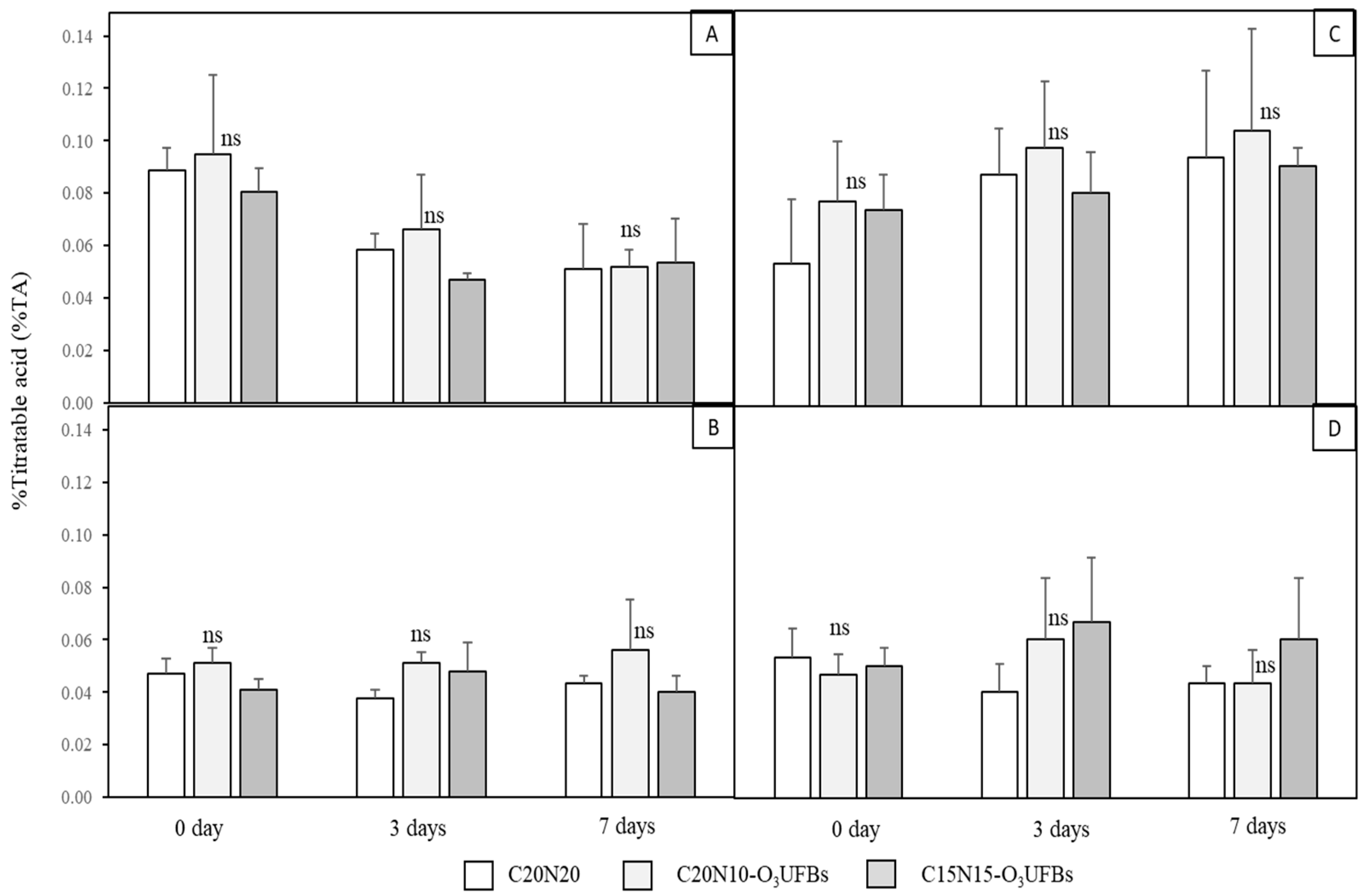
3.4. Coconut Water and Coconut Meat Qualities
3.4.1. Coconut Water
3.4.2. Coconut Meat
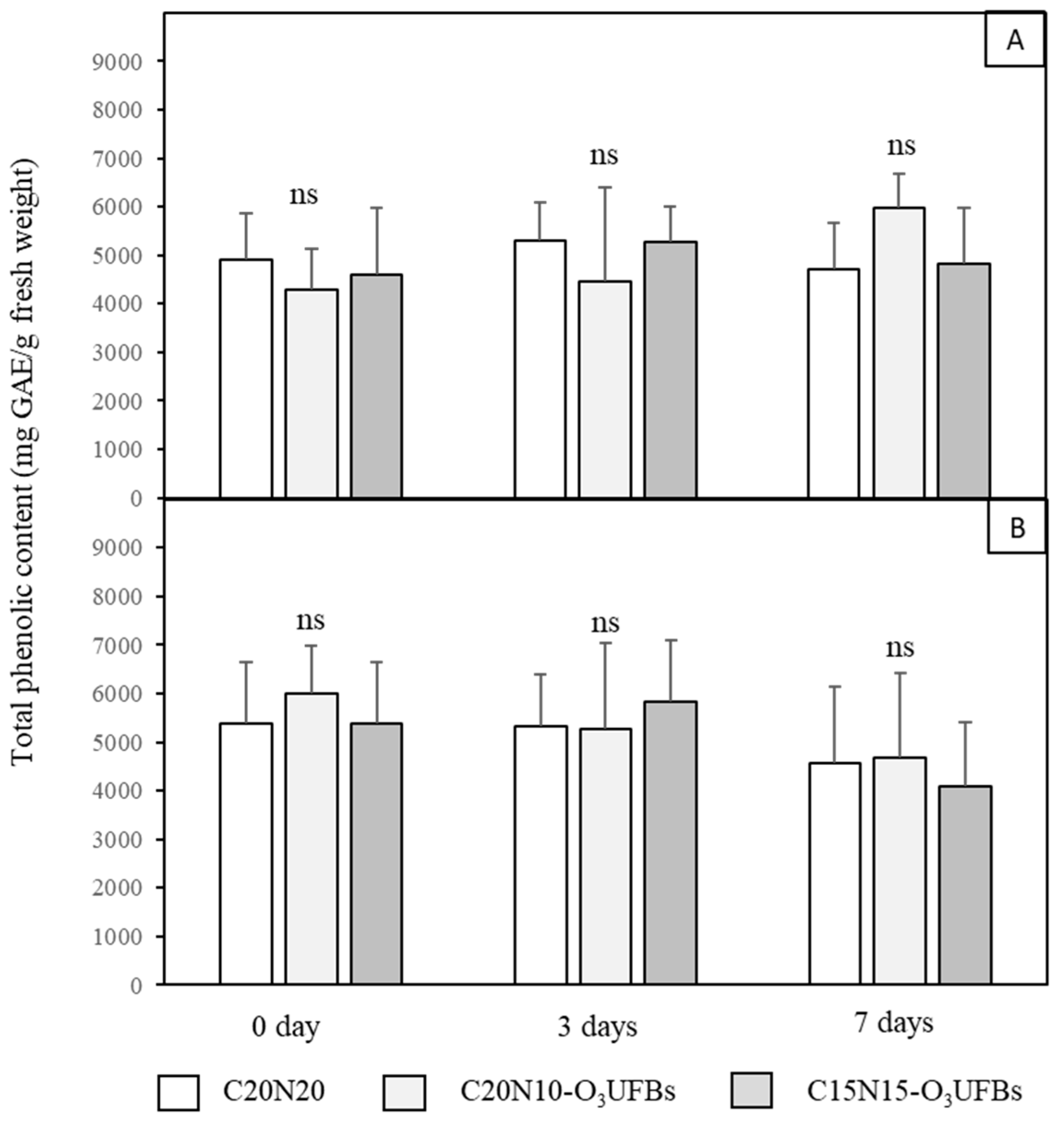
3.5. Total Phenolic Content of Coconut Mesocarp
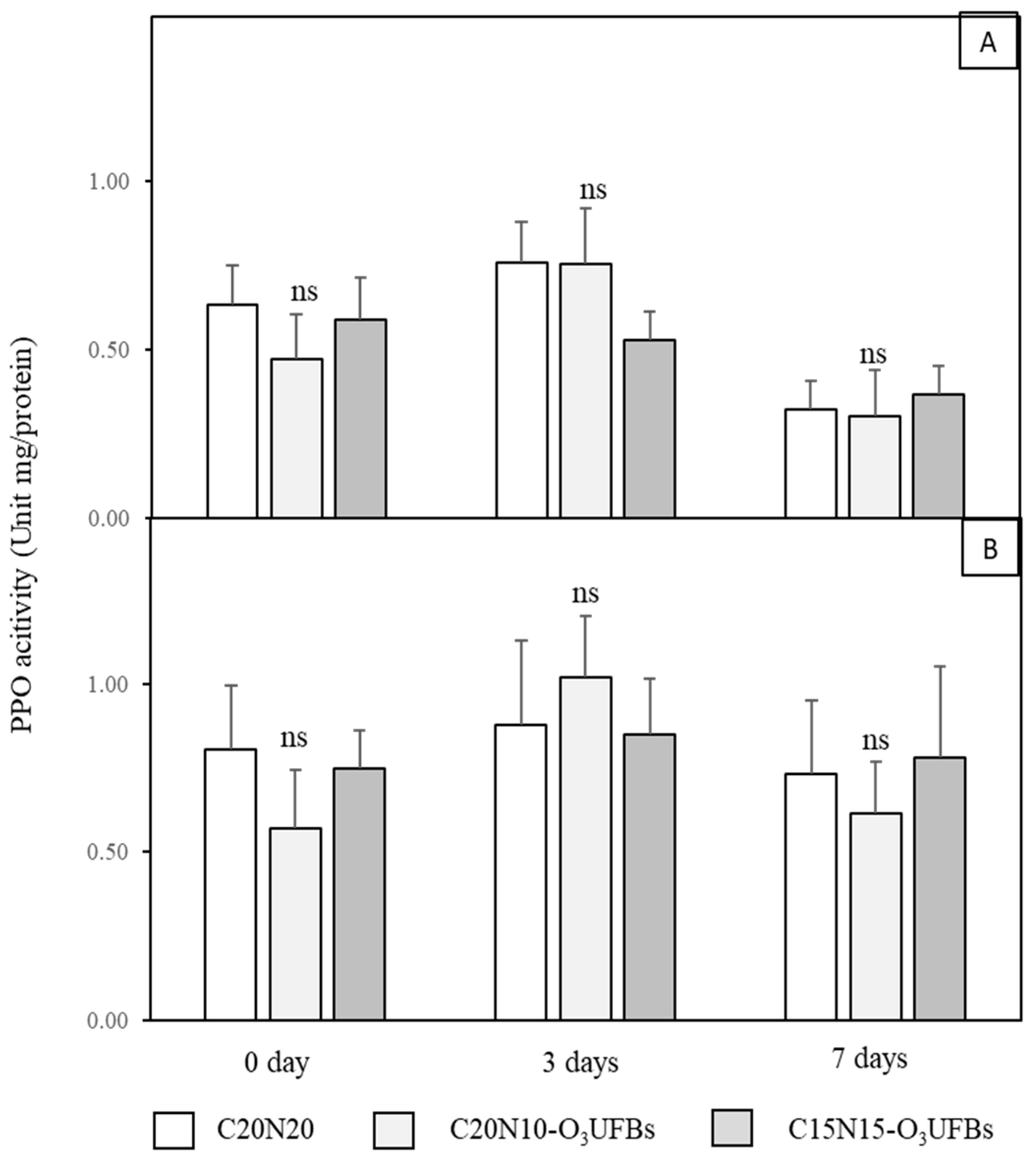
3.6. PPO Activity of Coconut Mesocarp
3.7. pH of Coconut Mesocarp
3.8. Microbial Analysis (Preliminary Result)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Office of Agricultural Economics. Statistics of Thai agricultural products trade with foreign countries, Agricultural Information Center, Office of Agricultural Economics, Ministry of Agriculture and Cooperatives, Bangkok; 2022. Available online: https://impexpth.oae.go.th/ (accessed on 15 May 2022).
- Jangchud, K.; Puchakawimol, P.; Jangchud, A. Quality changes of burnt aromatic coconut during 28-day storage in different packages. LWT-Food Sci. Technol. 2007, 40, 1232–1239. [Google Scholar] [CrossRef]
- Mohpraman, K.; Siriphanich, J. Safe use of sodium metabisulfite in young coconuts. Postharvest Biol. Technol. 2012, 65, 76–78. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Alegre, I.; Usall, J.; Torres, R.; Viñas, I. Evaluation of alternative sanitizers to chlorine disinfection for reducing foodborne pathogens in fresh-cut apple. Postharvest Biol. Technol. 2011, 59, 289–297. [Google Scholar] [CrossRef]
- Davidson, P.; Taylor, T. Chemical preservatives and natural antimicrobial compounds. Food Microbiol. Fundam. Front. 2007, 3, 713–745. [Google Scholar]
- Nguyen, D.T.N.; Tongkhao, K.; Tongchitpakdee, S. Application of citric acid, sodium chloride and peroxyacetic acid as alternative chemical treatment for organic trimmed aromatic coconut. Chiang Mai Univ. J. Nat. Sci. 2019, 18, 444–460. [Google Scholar] [CrossRef]
- Saijai, S.; Thonglek, V.; Yoshikawa, K. Sterilization effects of ozone fine (micro/nano) bubble water. Int. J. Plasma Environ. Sci. Technol. 2019, 12, 55–58. [Google Scholar]
- Batagoda, J.W.; Hewage, S.D.A.; Meegoda, J.N. Nano-ozone bubbles for drinking water treatment. J. Environ. Eng. Sci. 2018, 14, 57–66. [Google Scholar] [CrossRef]
- Yuk, H.; Yoo, M.; Yoon, J.; Moon, K.; Marshall, D.L.; Oh, D. Effect of Combined Ozone and Organic Acid Treatment for Control of Escherichia coli O157:H7 and Listeria monocytogenes on Lettuce. J. Food Sci. 2006, 71, M83–M87. [Google Scholar] [CrossRef]
- Hunter, R.S.; Harold, R.W. The Measurement of Appearance; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- Campos, C.F.; Souza, P.E.A.; Coelho, J.V.; Gloria, M.B.A. Chemical composition, enzyme activity and effect of enzyme inactivation on flavor quality of green coconut water. J. Food Process. Preserv. 1996, 20, 487–500. [Google Scholar] [CrossRef]
- Bradford, M.N. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Anderton, A. Microbiological quality of products used in enteral feeds. J. Hosp. Infect. 1986, 7, 68–73. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, Q.; Dong, X.; Pan, H.; Chen, J.; Zheng, Z.P. Oxyresveratrol and ascorbic acid O/W microemulsion: Preparation, characterization, anti-isomerization and potential application as antibrowning agent on fresh-cut lotus root slices. Food Chem. 2017, 214, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.; da Silva, A.J.R.; Lopes, M.L.M.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale, L.) after processing. Food Chem. 2011, 125, 128–132. [Google Scholar] [CrossRef]
- Janovitz-Klapp, A.H.; Richard, F.C.; Goupy, P.M.; Nicolas, J.J. Inhibition studies on apple polyphenol oxidase. J. Agric. Food Chem. 1990, 38, 926–931. [Google Scholar] [CrossRef]
- Laurenti, E.; Suriano, G.; Ghibaudi, E.M.; Ferrari, R.P. Ionic strength and pH effect on the Fe(III)-imidazolate bond in the heme pocket of horseradish peroxidase: An EPR and UV–visible combined approach. J. Inorg. Biochem. 2000, 81, 259–266. [Google Scholar] [CrossRef]
- Liao, T.; Liu, J.; Sun, Y.; Zou, L.; Zhou, L.; Liu, C.; Liu, W. Differential inhibitory effects of organic acids on pear polyphenol oxidase in model systems and pear puree. J. Food Sci. Technol. 2020, 118, 108–704. [Google Scholar] [CrossRef]
- Farungsang, U.; Farungsang, N.; Kunprom, C. The relation between husk trimming process and fungal colonization onpartial husk-trimmed Nam-Hom coconut. In Proceedings of the 10th National Postharvest Technology Conference, Khon Kaen, Thailand, 23–24 August 2012; Volume 43, pp. 519–522. [Google Scholar]
- Kobayashi, F.; Sugiura, M.; Ikeura, H.; Sato, K.; Odakea, S.; Hayata, Y. Inactivation of Fusarium oxysporum f. sp. melonis and Pectobacterium carotovorum subsp. carotovorum in hydroponic nutrient solution by low-pressure carbon dioxide microbubbles. Sci. Hortic. 2013, 164, 596–601. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Hashizume, K.; Nishimura, Y. Development of supply system of microbubble ozonated water in agriculture. Hortic. Environ. Biotechnol. 2010, 51, 21–27. [Google Scholar]
- Seridou, P.; Kalogerakis, N. Disinfection applications of ozone micro- and nanobubbles. Environ. Sci. Nano 2021, 8, 3493–3510. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Formation of hydroxyl radicals by collapsing ozone microbubbles under strong acid conditions. J. Phys. Chem. 2007, 111, 11443–11446. [Google Scholar] [CrossRef] [PubMed]
- Treesuwan, K.; Jirapakkul, W.; Tongchitpakdee, S.; Chonhenchob, V.; Mahakarnchanakul, W.; Moonmangmee, S.; Tongkhao, K. Effect of controlled atmospheric conditions combined with salt acid immersion on trimmed young coconut qualities during cold storage. Food Packag. Shelf Life 2022, 32, 100857. [Google Scholar] [CrossRef]
- Watada, A.E.; Ko, N.P.; Minott, D.A. Factors affecting quality of fresh-cut horticultural products. Postharvest Biol. Technol. 1996, 9, 115–125. [Google Scholar] [CrossRef]
- Hirahara, Y.; Iwata, K.; Nakamuro, K. Effect of citric acid on prolonging the half-life of dissolved ozone in water. Food Saf. 2019, 7, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulou, E.; Varzakas, T. Effect of storage conditions on the sensory quality, colour and texture of fresh-cut minimally processed cabbage with the addition of ascorbic acid, citric acid and calcium chloride. Food Nutr. Sci. 2011, 2, 956–963. [Google Scholar] [CrossRef]
- Eicken, C.; Krebs, B.; Sacchettini, J.C. Catechol oxidase-structure and activity. Curr. Opin. Struct. Biol. 1999, 9, 677–683. [Google Scholar] [CrossRef]
- Luengwilai, K.; Beckles, D.M.; Pluemjit, O.; Siriphanich, J. Postharvest quality and storage life of ‘Makapuno’ coconut (Cocos nucifera L.). Sci. Hortic. 2014, 175, 105–110. [Google Scholar] [CrossRef]
- Jirapong, C.; Changprasert, S.; Kanlayanarat, S.; Uthairatanakij, A.; Bodhipadma, K.; Noichinda, S.; Wongs-Aree, C. Characterization of the liquid endosperm attributes in young coconut fruit during storage. Int. Food Res. J. 2018, 25, 2650–2656. [Google Scholar]
- Kannangara, A.C.; Chandrajith, V.G.G.; Ranaweera, K.K.D.S. Comparative analysis of coconut water in four different maturity stages. J. Pharmacogn. Phytochem. 2018, 7, 1814–1817. [Google Scholar]


| Treatments | Total Plate Count (CFU/g) | Yeast and Mold (CFU/g) |
|---|---|---|
| C20N20 | 5.0 × 105 | 3.1 × 108 |
| C20N10-O3UFBs | 2.5 × 106 | 8.2 × 107 |
| C15N15-O3UFBs | 6.7 × 105 | 6.0 × 107 |
| F-test | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathomaim, S.; Jarussophon, S.; Arikit, S.; Imsabai, W. Ozone-Ultrafine Bubbles for Reducing Concentration of Citric Acid and Sodium Chloride for Trimmed Young Coconut Preservation. Horticulturae 2023, 9, 284. https://doi.org/10.3390/horticulturae9020284
Pathomaim S, Jarussophon S, Arikit S, Imsabai W. Ozone-Ultrafine Bubbles for Reducing Concentration of Citric Acid and Sodium Chloride for Trimmed Young Coconut Preservation. Horticulturae. 2023; 9(2):284. https://doi.org/10.3390/horticulturae9020284
Chicago/Turabian StylePathomaim, Supat, Suwatchai Jarussophon, Siwaret Arikit, and Wachiraya Imsabai. 2023. "Ozone-Ultrafine Bubbles for Reducing Concentration of Citric Acid and Sodium Chloride for Trimmed Young Coconut Preservation" Horticulturae 9, no. 2: 284. https://doi.org/10.3390/horticulturae9020284
APA StylePathomaim, S., Jarussophon, S., Arikit, S., & Imsabai, W. (2023). Ozone-Ultrafine Bubbles for Reducing Concentration of Citric Acid and Sodium Chloride for Trimmed Young Coconut Preservation. Horticulturae, 9(2), 284. https://doi.org/10.3390/horticulturae9020284






