Synergistic Impact of Melatonin and Putrescine Interaction in Mitigating Salinity Stress in Snap Bean Seedlings: Reduction of Oxidative Damage and Inhibition of Polyamine Catabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
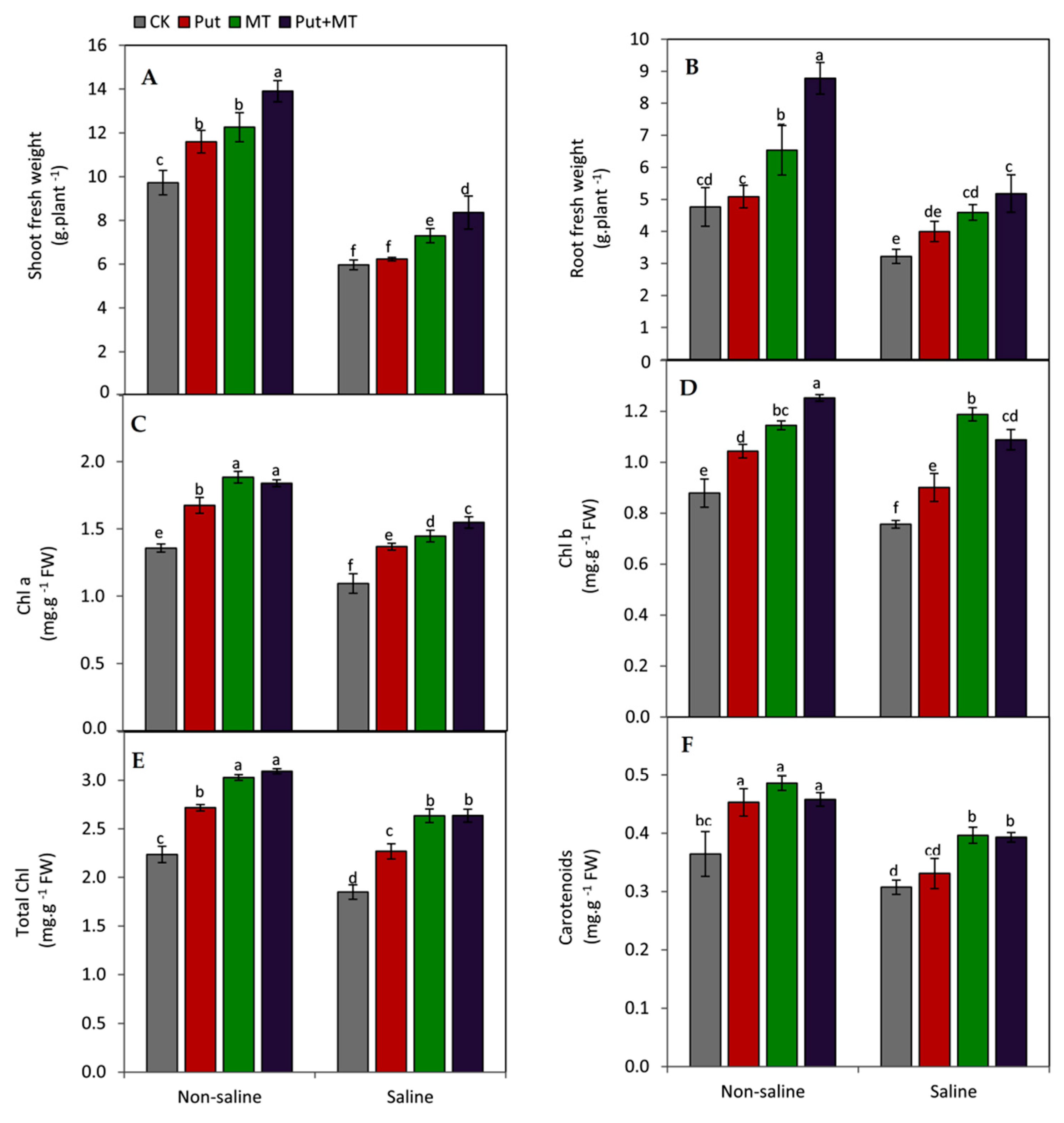
2.2. Plant Growth and Leaf Pigments
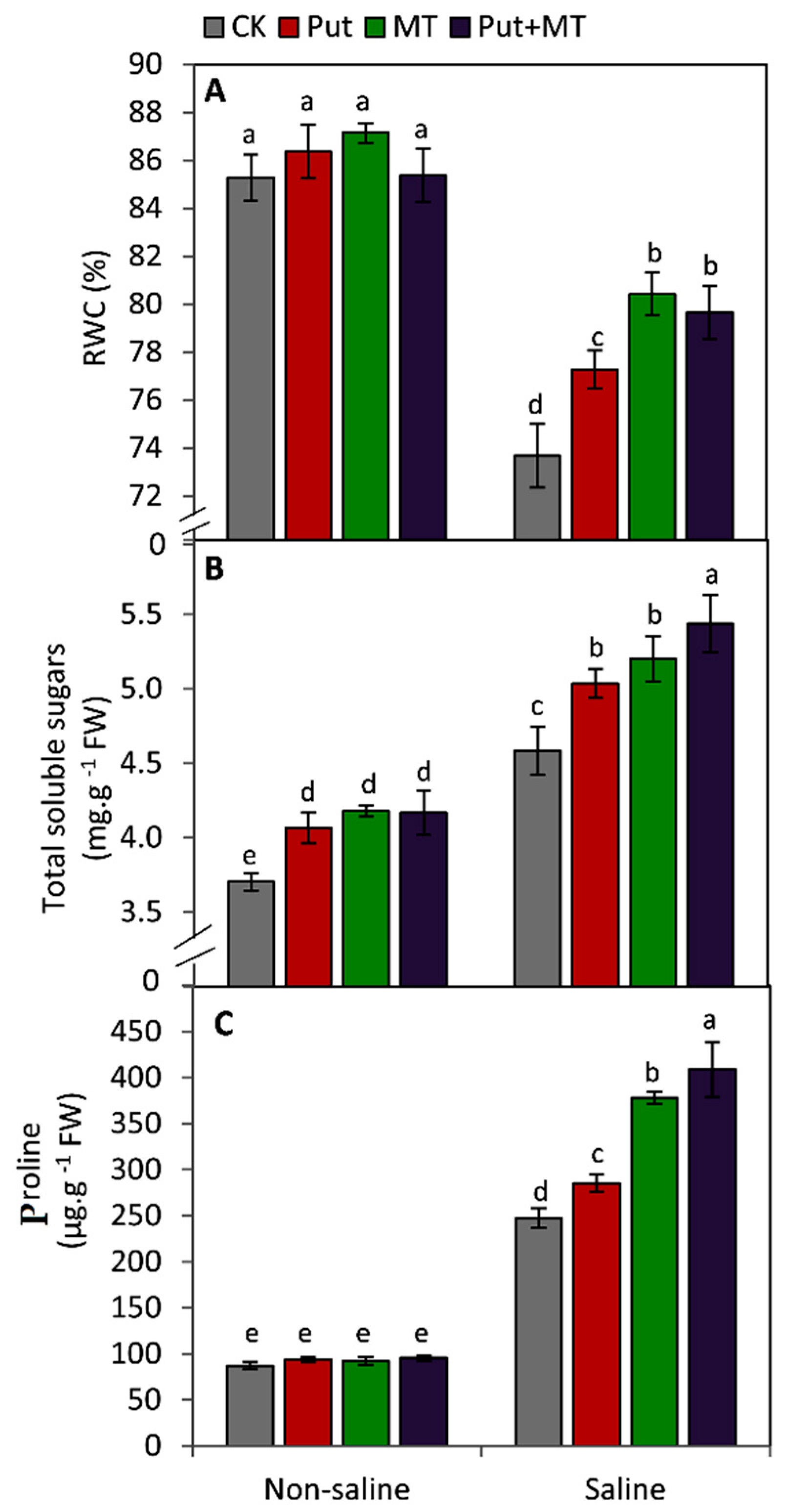
2.3. Leaf Relative Water Content and Osmolytes
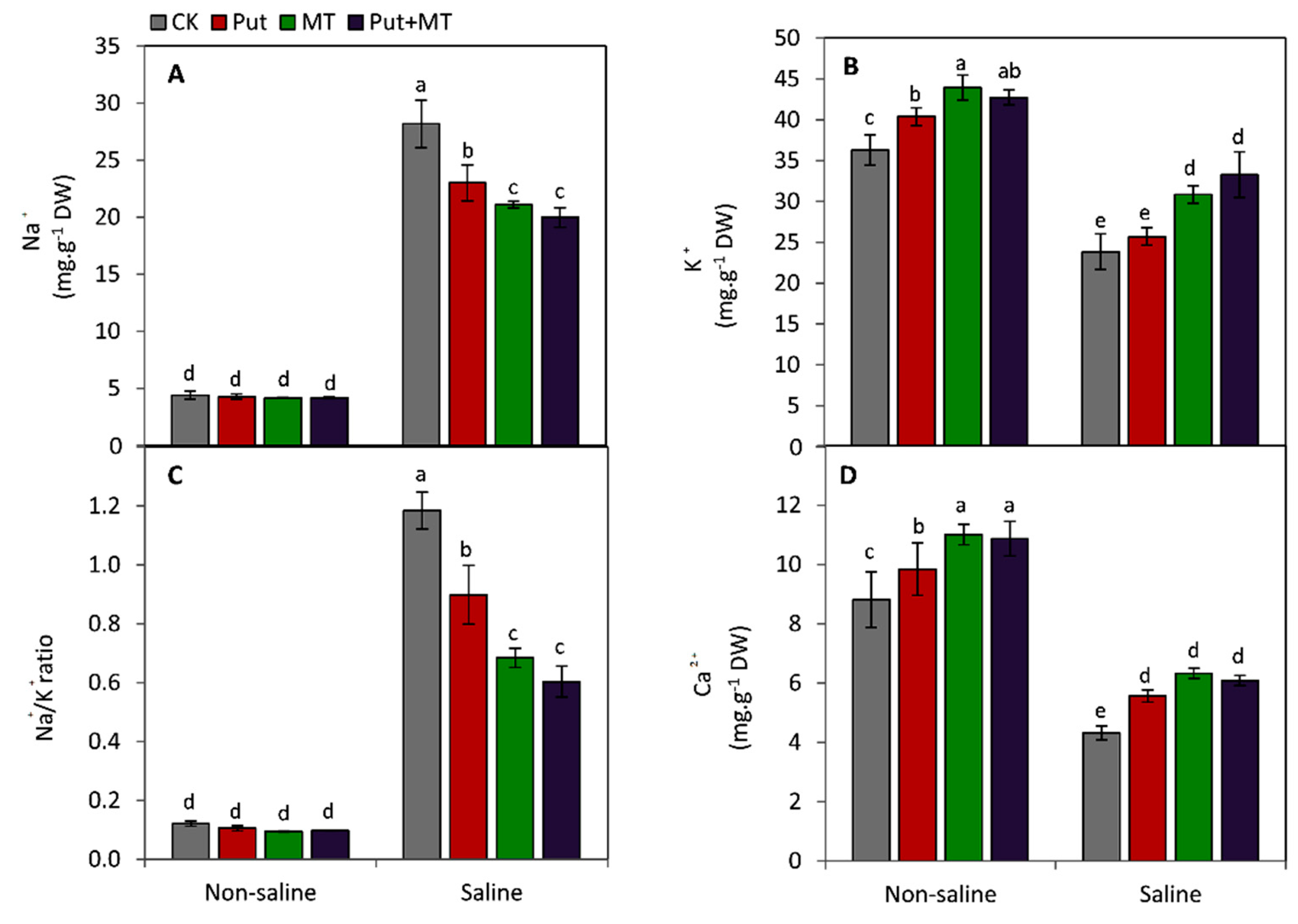
2.4. Determination of Na, K, and Ca
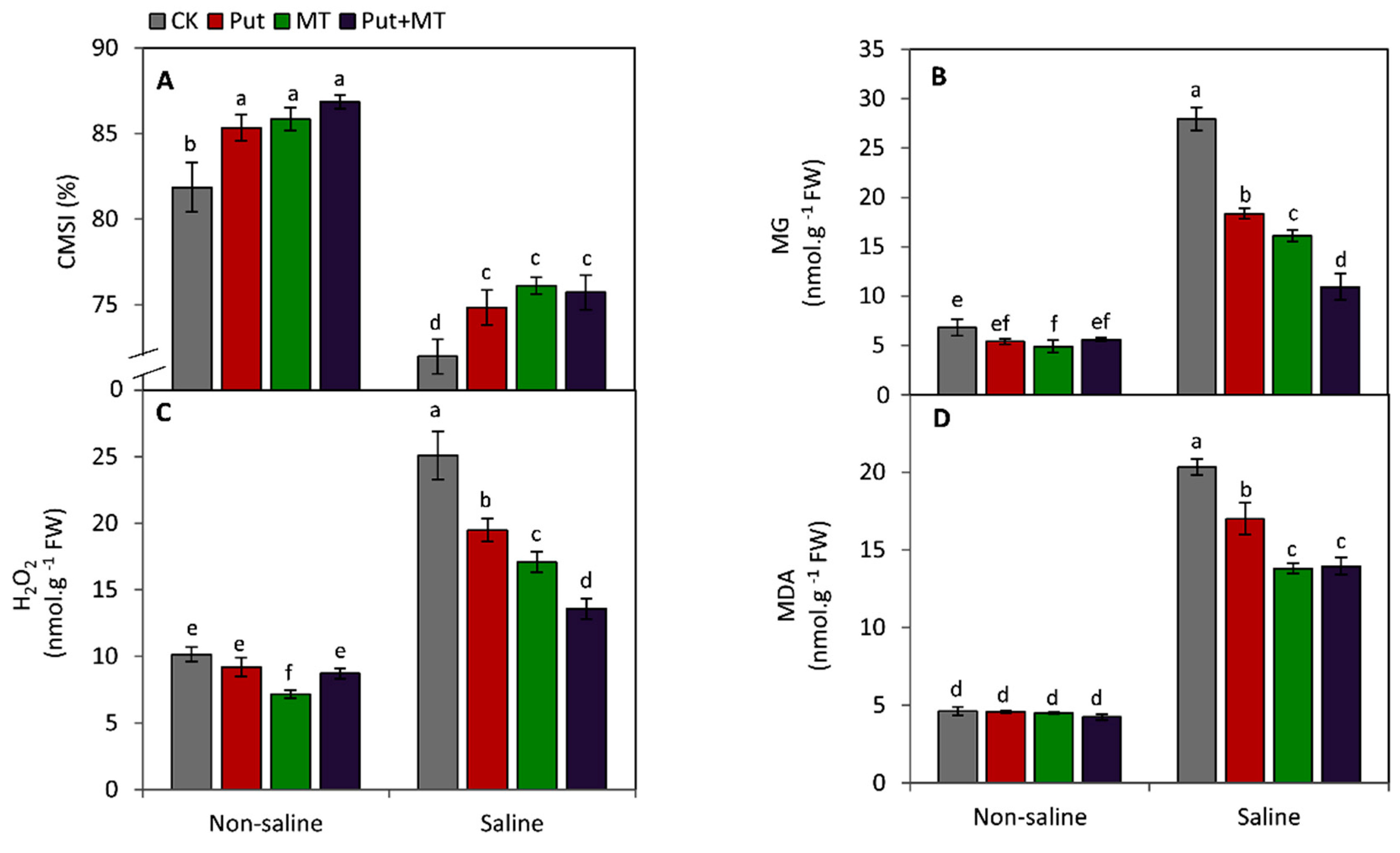
2.5. Determination of Cell Membrane Stability Index and Oxidative Damage
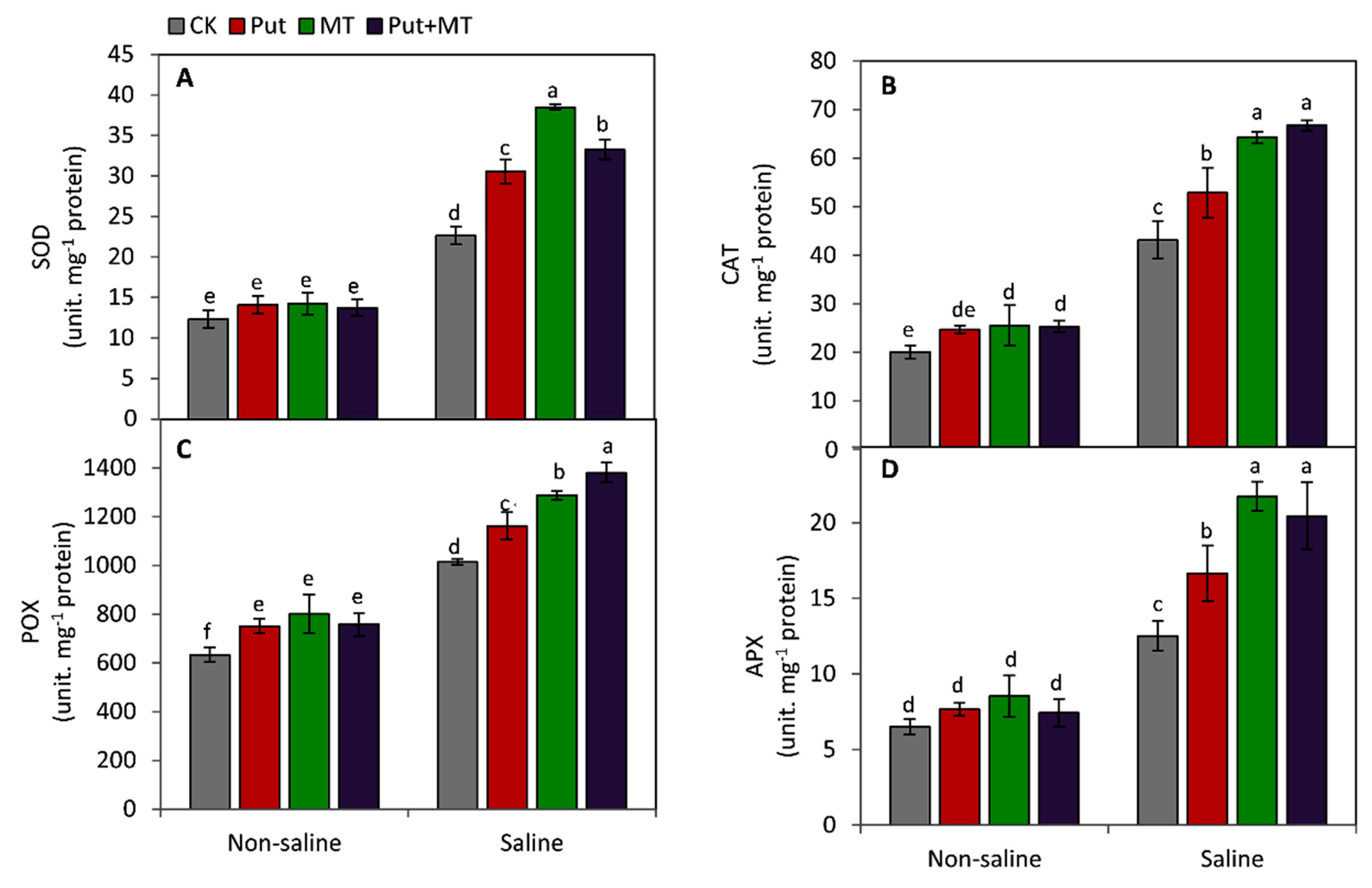
2.6. Measurement of the Activities of Antioxidant Enzymes
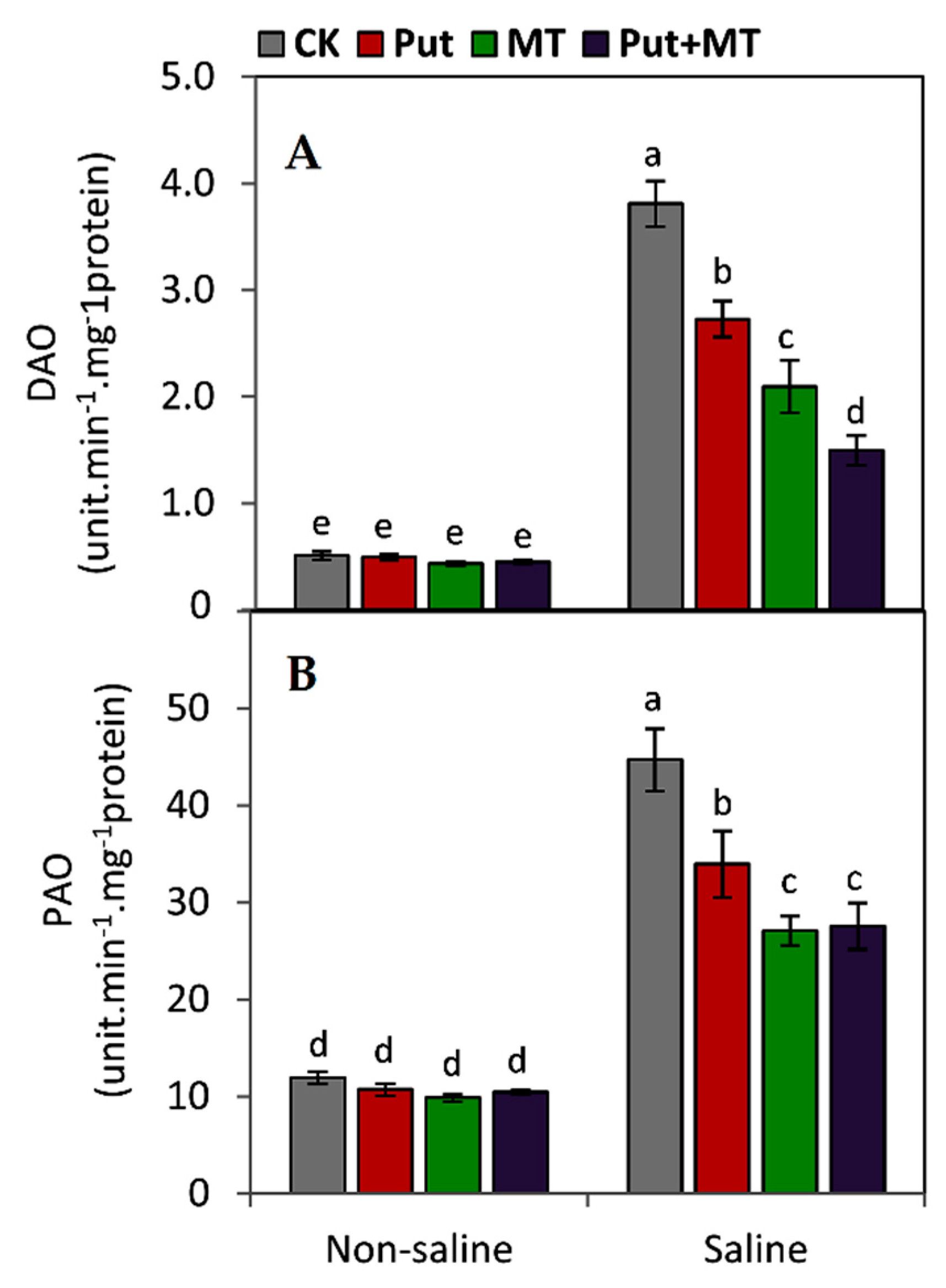
2.7. Determination the Activities of Polyamines Catabolism Enzymes
2.8. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ke, Q.; Ye, J.; Wang, B.; Ren, J.; Yin, L.; Deng, X.; Wang, S. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front. Plant Sci. 2018, 9, 914. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Shehata, W.F.; Hassan, U.; Shah, S.T.; Haleema, B.; Jalal, A.; Amin, R.; Khalid, M.A. Ascorbic acid enhances growth and yield of sweet peppers (Capsicum annum) by mitigating salinity stress. Gesunde Pflanz. 2022, 74, 423–433. [Google Scholar] [CrossRef]
- Mansour, M.M.F. The plasma membrane transport systems and adaptation to salinity. J. Plant Physiol. 2014, 171, 1787–1800. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; Alharbi, M.M.; Alenzi, A.M.; El-Beltagi, H.S.; Darwish, D.B.; Aldaej, M.I.; Shalaby, T.A.; Mansour, A.T.; El-Gabry, Y.A.; Ibrahim, M.F.M. Alpha Lipoic Acid as a Protective Mediator for Regulating the Defensive Responses of Wheat Plants against Sodic Alkaline Stress: Physiological, Biochemical and Molecular Aspects. Plants 2022, 11, 787. [Google Scholar] [CrossRef]
- Youssef, M.H.M.; Raafat, A.; El-Yazied, A.A.; Selim, S.; Azab, E.; Khojah, E.; El Nahhas, N.; Ibrahim, M.F.M. Exogenous Application of Alpha-Lipoic Acid Mitigates Salt-Induced Oxidative Damage in Sorghum Plants through Regulation Growth, Leaf Pigments, Ionic Homeostasis, Antioxidant Enzymes, and Expression of Salt Stress Responsive Genes. Plants 2021, 10, 2519. [Google Scholar] [CrossRef]
- Celmeli, T.; Sari, H.; Canci, H.; Sari, D.; Adak, A.; Eker, T.; Toker, C. The nutritional content of common bean (Phaseolus vulgaris L.) landraces in comparison to modern varieties. Agronomy 2018, 8, 166. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ibrahim, H.A.; Abd El-Gawad, H. Folic acid as a protective agent in snap bean plants under water deficit conditions. J. Hortic. Sci. Biotechnol. 2021, 96, 94–109. [Google Scholar] [CrossRef]
- Câmara, C.R.; Urrea, C.A.; Schlegel, V. Pinto beans (Phaseolus vulgaris L.) as a functional food: Implications on human health. Agriculture 2013, 3, 90–111. [Google Scholar] [CrossRef]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch. Intern. Med. 2001, 161, 2573–2578. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D.; Hollman, P.; Katan, M. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Didinger, C.; Foster, M.T.; Bunning, M.; Thompson, H.J. Nutrition and Human Health Benefits of Dry Beans and Other Pulses. In Dry Beans and Pulses: Production, Processing, and Nutrition; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 481–504. [Google Scholar] [CrossRef]
- Sangaramoorthy, M.; Koo, J.; John, E.M. Intake of bean fiber, beans, and grains and reduced risk of hormone receptor-negative breast cancer: The San Francisco Bay Area Breast Cancer Study. Cancer Med. 2018, 7, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Darmadi-Blackberry, I.; Wahlqvist, M.L.; Kouris-Blazos, A.; Steen, B.; Lukito, W.; Horie, Y.; Horie, K. Legumes: The most important dietary predictor of survival in older people of different ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220. [Google Scholar]
- Alsamadany, H.; Mansour, H.; Elkelish, A.; Ibrahim, M.F. Folic Acid Confers Tolerance against Salt Stress-Induced Oxidative Damages in Snap Beans through Regulation Growth, Metabolites, Antioxidant Machinery and Gene Expression. Plants 2022, 11, 1459. [Google Scholar] [CrossRef]
- Farag, H.A.; Ibrahim, M.F.; El-Yazied, A.A.; El-Beltagi, H.S.; El-Gawad, H.G.A.; Alqurashi, M.; Shalaby, T.A.; Mansour, A.T.; Alkhateeb, A.A.; Farag, R. Applied Selenium as a Powerful Antioxidant to Mitigate the Harmful Effects of Salinity Stress in Snap Bean Seedlings. Agronomy 2022, 12, 3215. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, C.; Xu, L.; Niu, H.; Liu, Q.; Huang, Y.; Lv, G.; Yang, H.; Li, M. Melatonin and indole-3-acetic acid synergistically regulate plant growth and stress resistance. Cells 2022, 11, 3250. [Google Scholar] [CrossRef]
- Arnao, M.B.; Giraldo-Acosta, M.; Castejón-Castillejo, A.; Losada-Lorán, M.; Sánchez-Herrerías, P.; El Mihyaoui, A.; Cano, A.; Hernández-Ruiz, J. Melatonin from Microorganisms, Algae, and Plants as Possible Alternatives to Synthetic Melatonin. Metabolites 2023, 13, 72. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, e0216575. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Zhang, Z.-W.; Chen, Y.-E.; Ding, C.-B.; Yuan, S.; Reiter, R.J.; Yuan, M. Melatonin: A potential agent in delaying leaf senescence. Crit. Rev. Plant Sci. 2021, 40, 1–22. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 2019, 10, 54. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.-D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin Suppressed the Heat Stress-Induced Damage in Wheat Seedlings by Modulating the Antioxidant Machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ding, F. Melatonin Mitigates Chilling-Induced Oxidative Stress and Photosynthesis Inhibition in Tomato Plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- El-Yazied, A.A.; Ibrahim, M.F.; Ibrahim, M.A.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M. Melatonin Mitigates Drought Induced Oxidative Stress in Potato Plants through Modulation of Osmolytes, Sugar Metabolism, ABA Homeostasis and Antioxidant Enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.F.; Elbar, O.H.A.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; El-Gawad, H.G.A. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef] [PubMed]
- Gohari, G.; Farhadi, H.; Panahirad, S.; Zareei, E.; Labib, P.; Jafari, H.; Mahdavinia, G.; Hassanpouraghdam, M.B.; Ioannou, A.; Kulak, M. Mitigation of salinity impact in spearmint plants through the application of engineered chitosan-melatonin nanoparticles. Int. J. Biol. Macromol. 2023, 224, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.; Khan, M.N.; Al-Amri, A.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of Avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Cao, K.; Hu, L.; Du, T.; Baluška, F.; Zou, Z. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress. Front. Plant Sci. 2016, 7, 1823. [Google Scholar] [CrossRef]
- Azizi, F.; Amiri, H.; Ismaili, A. Melatonin improves salinity stress tolerance of Phaseolus vulgaris L. cv. Pak by changing antioxidant enzymes and photosynthetic parameters. Acta Physiol. Plant. 2022, 44, 40. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.; Yu, X.; Kiprotich, F.; Han, H.; Guan, R.; Wang, R.; Shen, W. Nitric oxide is required for melatonin-enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanati, F.; Sajedi, R.H. Crosstalk between melatonin and Ca2+/CaM evokes systemic salt tolerance in Dracocephalum kotschyi. J. Plant Physiol. 2020, 252, 153237. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radic. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Egea-Cortines, M.; Mizrahi, Y. Polyamines in cell division, fruit set and development, and seed germination. In Slocum RD and Flores HE (eds) Biochemistry and Physiology of Polyamines in Plants; CRC Press: Boca Raton, FL, USA, 1991; pp. 143–158. [Google Scholar]
- Kuznetsov, V.V.; Shevyakova, N.I. Polyamines and stress tolerance of plants. Plant Stress 2007, 1, 50–71. [Google Scholar] [CrossRef]
- Kaur-Sawhney, R.; Tiburcio, A.F.; Altabella, T.; Galston, A.W. Polyamines in plants: An overview. J. Mol. Cell Biol. 2003, 2, 1–12. [Google Scholar]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Hamdani, S.; Gauthier, A.; Msilini, N.; Carpentier, R. Positive charges of polyamines protect PSII in isolated thylakoid membranes during photoinhibitory conditions. Plant Cell Physiol. 2011, 52, 866–873. [Google Scholar] [CrossRef]
- Pottosin, I.; Shabala, S. Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef]
- Todorova, D.; Katerova, Z.; Shopova, E.; Jodinskienė, M.; Jurkoniene, S.; Sergiev, I. Responses of pea plants to heat stress and spermine treatment. Zemdirb.-Agric. 2016, 103, 99–106. [Google Scholar] [CrossRef]
- Groppa, M.D.; Tomaro, M.L.; Benavides, M.P. Polyamines and heavy metal stress: The antioxidant behavior of spermine in cadmium-and copper-treated wheat leaves. Biometals 2007, 20, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Chattopadhayay, M.K.; Tiwari, B.S.; Chattopadhyay, G.; Bose, A.; Sengupta, D.N.; Ghosh, B. Protective role of exogenous polyamines on salinity-stressed rice (Oryza sativa) plants. Physiol. Plant. 2002, 116, 192–199. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Matkovics, B.; Kecskemeti, V.; Varga, S.I.; Novak, Z.; Kertesz, Z. Antioxidant properties of di-and polyamines. Comp. Biochem. Physiol.. B Comp. Biochem. 1993, 104, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Chakraborty, M.; Saha, J.; Gupta, B.; Gupta, K. Polyamines: Osmoprotectants in plant abiotic stress adaptation. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: New Delhi, India, 2016; pp. 97–127. [Google Scholar]
- Sakamoto, A.; Terui, Y.; Uemura, T.; Igarashi, K.; Kashiwagi, K. Polyamines regulate gene expression by stimulating translation of histone acetyltransferase mRNAs. J. Biol. Chem. 2020, 295, 8736–8745. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Abd El-Gawad, H.G.; Mukherjee, S.; Farag, R.; Abd Elbar, O.H.; Hikal, M.; Abou El-Yazied, A.; Abd Elhady, S.A.; Helal, N.; ElKelish, A.; El Nahhas, N. Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L. Plant Signal. Behav. 2021, 16, 1853384. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Ieggli, C.; Bohrer, D.; Do Nascimento, P.; De Carvalho, L.; Garcia, S. Determination of sodium, potassium, calcium, magnesium, zinc, and iron in emulsified egg samples by flame atomic absorption spectrometry. Talanta 2010, 80, 1282–1286. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Elkelish, A.; Niedbała, G.; Farag, R.; Wojciechowski, T.; Mukherjee, S.; Abou-Hadid, A.F.; El-Hennawy, H.M.; Abou El-Yazied, A.; Abd El-Gawad, H.G. Protective Effect of γ-Aminobutyric Acid Against Chilling Stress during Reproductive Stage in Tomato Plants Through Modulation of Sugar Metabolism, Chloroplast Integrity, and Antioxidative Defense Systems. Front. Plant Sci. 2021, 12, 663750. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Hossain, M.Z.; Fujita, M. Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 2009, 3, 53. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Dias, M.A.; Costa, M.M. Effect of low salt concentrations on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Su, G.X.; Zhang, W.H.; Liu, Y.L. Involvement of hydrogen peroxide generated by polyamine oxidative degradation in the development of lateral roots in soybean. J. Integr. Plant Biol. 2006, 48, 426–432. [Google Scholar] [CrossRef]
- SAS. SAS/STAT User’s Guide, Release 6.03 ed.; SAS Inst. Inc.: Cary, NC, USA, 1988. [Google Scholar]
- Bayuelo-Jimenez, J.; Jasso-Plata, N.; Ochoa, I. Growth and physiological responses of Phaseolus species to salinity stress. Int. J. Agron. 2012, 2012, 527673. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Abdelaal, K.; Alsubeie, M.S.; Hafez, Y.; Emeran, A.; Moghanm, F.; Okasha, S.; Omara, R.; Basahi, M.A.; Darwish, D.B.E.; Ibrahim, M. Physiological and Biochemical Changes in Vegetable and Field Crops under Drought, Salinity and Weeds Stresses: Control Strategies and Management. Agriculture 2022, 12, 2084. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Al-Otaibi, H.H.; Parmar, A.; Ramadan, K.; Lobato, A.K.d.S.; El-Mogy, M.M. Application of Potassium Humate and Salicylic Acid to Mitigate Salinity Stress of Common Bean. Life 2023, 13, 448. [Google Scholar] [CrossRef]
- Gao, W.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef]
- Tan, X.L.; Fan, Z.q.; Kuang, J.f.; Lu, W.j.; Reiter, R.J.; Lakshmanan, P.; Su, X.g.; Zhou, J.; Chen, J.Y.; Shan, W. Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J. Pineal Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef]
- Sigmaz, B.; Agar, G.; Arslan, E.; Aydin, M.; Taspinar, M.S. The role of putrescine against the long terminal repeat (LTR) retrotransposon polymorphisms induced by salinity stress in Triticum aestivum. Acta Physiol. Plant. 2015, 37, 251. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, B.; Li, W.; Song, H.; Yu, Y.; Chen, J. Polyamines enhance chilling tolerance of cucumber (Cucumis sativus L.) through modulating antioxidative system. Sci. Hortic. 2009, 122, 200–208. [Google Scholar] [CrossRef]
- Zhao, F.; Qin, P. Protective effect of exogenous polyamines on root tonoplast function against salt stress in barley seedlings. Plant Growth Regul. 2004, 42, 97–103. [Google Scholar] [CrossRef]
- Farsaraei, S.; Mehdizadeh, L.; Moghaddam, M. Seed priming with putrescine alleviated salinity stress during germination and seedling growth of medicinal pumpkin. J. Soil Sci. Plant Nutr. 2021, 21, 1782–1792. [Google Scholar] [CrossRef]
- Hamdy, A.E. Effect of GA3 and NAA on growth, yield and fruit quality of Washington navel orange. Egypt. J. Hortic. 2017, 44, 33–43. [Google Scholar] [CrossRef]
- Abd Elbar, O.H.; Farag, R.E.; Shehata, S.A. Effect of putrescine application on some growth, biochemical and anatomical characteristics of Thymus vulgaris L. under drought stress. Ann. Agric. Sci. 2019, 64, 129–137. [Google Scholar] [CrossRef]
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; Yu, J.Q.; Xu, M.X. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR 1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef]
- Palma, F.; Lluch, C.; Iribarne, C.; García-Garrido, J.M.; Tejera García, N.A. Combined effect of salicylic acid and salinity on some antioxidant activities, oxidative stress and metabolite accumulation in Phaseolus vulgaris. Plant Growth Regul. 2009, 58, 307–316. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Öztürk, L.; Demir, Y. Effects of putrescine and ethephon on some oxidative stress enzyme activities and proline content in salt stressed spinach leaves. Plant Growth Regul. 2003, 40, 89–95. [Google Scholar] [CrossRef]
- Sharma, A.; Slathia, S.; Choudhary, S.P.; Sharma, Y.P.; Langer, A. Role of 24-epibrassinolide, putrescine and spermine in salinity stressed Adiantum capillus-veneris leaves. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 183–192. [Google Scholar] [CrossRef]
- EL-Bauome, H.A.; Abdeldaym, E.A.; Abd El-Hady, M.A.; Darwish, D.B.E.; Alsubeie, M.S.; El-Mogy, M.M.; Basahi, M.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M. Exogenous Proline, Methionine, and Melatonin Stimulate Growth, Quality, and Drought Tolerance in Cauliflower Plants. Agriculture 2022, 12, 1301. [Google Scholar] [CrossRef]
- Sheokand, S.; Kumari, A.; Sawhney, V. Effect of nitric oxide and putrescine on antioxidative responses under NaCl stress in chickpea plants. Physiol. Mol. Biol. Plants 2008, 14, 355–362. [Google Scholar] [CrossRef]
- Vakharia, D.; Kukadia, A.; Parameswaran, M. Polyamines in response to artificial water stress in groundnut seedlings. Indian J. Plant Physiol. 2003, 8, 383–387. [Google Scholar]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Radyukina, N.; Shevyakova, N. Polyamines and stress: Biological role, metabolism, and regulation. Russ. J. Plant Physiol. 2006, 53, 583–604. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Rahman, A.; Alam, M.M.; Mahmud, J.-A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Shi, L.; Xu, L.; Shen, Z.; Chen, W.; Yang, Z. Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci. Rep. 2018, 8, 806. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Cui, Y.; Yin, Y. Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant Growth Regul. 2017, 83, 441–454. [Google Scholar] [CrossRef]
- Zamani, Z.; Amiri, H.; Ismaili, A. Improving drought stress tolerance in fenugreek (Trigonella foenum-graecum) by exogenous melatonin. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 154, 643–655. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (C ucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Yildirim, E.; Dursun, A.; Mohamedsrajaden, N. Putrescine, spermine and spermidine mitigated the salt stress damage on Pepper (Capsicum annum L.) seedling. Yüzüncü Yıl Üniversitesi Tarım Bilim. Derg. 2019, 29, 290–299. [Google Scholar] [CrossRef]
- Qu, M.; Zheng, G.; Hamdani, S.; Essemine, J.; Song, Q.; Wang, H.; Chu, C.; Sirault, X.; Zhu, X.-G. Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey. Plant Physiol. 2017, 175, 248–258. [Google Scholar] [CrossRef]
- Shi, K.; Huang, Y.; Xia, X.; Zhang, Y.; Zhou, Y.; Yu, J. Protective role of putrescine against salt stress is partially related to the improvement of water relation and nutritional imbalance in cucumber. J. Plant Nutr. 2008, 31, 1820–1831. [Google Scholar] [CrossRef]
- Yu, Z.; Jia, D.; Liu, T. Polyamine oxidases play various roles in plant development and abiotic stress tolerance. Plants 2019, 8, 184. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; El-Yazied, A.A.; El-Gawad, H.G.A.; Kandeel, M.; Shalaby, T.A.; Mansour, A.T.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Alkhateeb, A.A.; Ibrahim, M.F.M. Synergistic Impact of Melatonin and Putrescine Interaction in Mitigating Salinity Stress in Snap Bean Seedlings: Reduction of Oxidative Damage and Inhibition of Polyamine Catabolism. Horticulturae 2023, 9, 285. https://doi.org/10.3390/horticulturae9020285
El-Beltagi HS, El-Yazied AA, El-Gawad HGA, Kandeel M, Shalaby TA, Mansour AT, Al-Harbi NA, Al-Qahtani SM, Alkhateeb AA, Ibrahim MFM. Synergistic Impact of Melatonin and Putrescine Interaction in Mitigating Salinity Stress in Snap Bean Seedlings: Reduction of Oxidative Damage and Inhibition of Polyamine Catabolism. Horticulturae. 2023; 9(2):285. https://doi.org/10.3390/horticulturae9020285
Chicago/Turabian StyleEl-Beltagi, Hossam S., Ahmed Abou El-Yazied, Hany G. Abd El-Gawad, Mahmoud Kandeel, Tarek A. Shalaby, Abdallah Tageldein Mansour, Nadi Awad Al-Harbi, Salem Mesfir Al-Qahtani, Abdulmalik A. Alkhateeb, and Mohamed F. M. Ibrahim. 2023. "Synergistic Impact of Melatonin and Putrescine Interaction in Mitigating Salinity Stress in Snap Bean Seedlings: Reduction of Oxidative Damage and Inhibition of Polyamine Catabolism" Horticulturae 9, no. 2: 285. https://doi.org/10.3390/horticulturae9020285
APA StyleEl-Beltagi, H. S., El-Yazied, A. A., El-Gawad, H. G. A., Kandeel, M., Shalaby, T. A., Mansour, A. T., Al-Harbi, N. A., Al-Qahtani, S. M., Alkhateeb, A. A., & Ibrahim, M. F. M. (2023). Synergistic Impact of Melatonin and Putrescine Interaction in Mitigating Salinity Stress in Snap Bean Seedlings: Reduction of Oxidative Damage and Inhibition of Polyamine Catabolism. Horticulturae, 9(2), 285. https://doi.org/10.3390/horticulturae9020285














