Regulatory Effect of Exogenous γ-Aminobutyric Acid on Respiratory Rate through the γ-Aminobutyric Acid Shunt in Malus baccata (L.) Borkh. Roots under Suboptimal Low Root-Zone Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Treatments
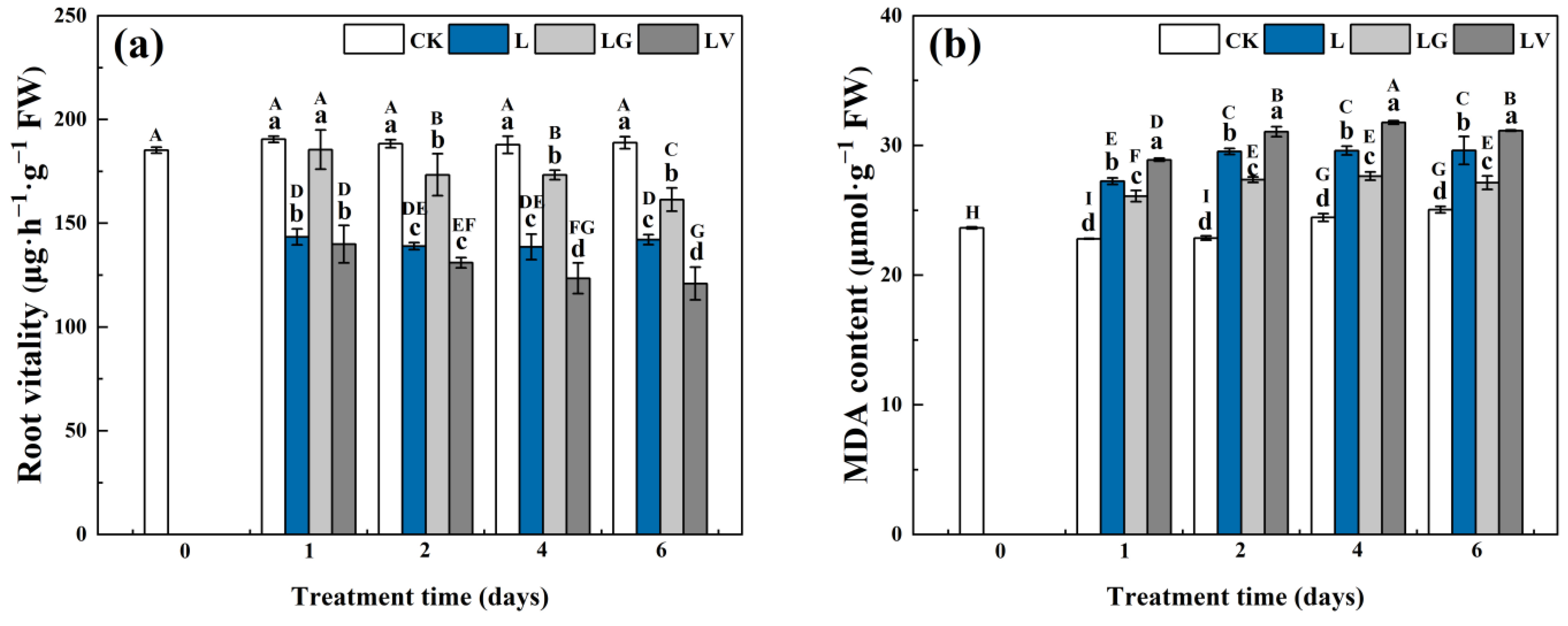
2.2. Determination of Root Vitality and Malondialdehyde (MDA) Content
2.3. Extraction and Determination of Glutamic Acid (Glu) and γ-Aminobutyric Acid (GABA) Content
2.4. Extraction and Determination of Succinate (Suc) Content
2.5. Extraction and Determination of Enzyme Activities in GABA Shunt
2.6. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
2.7. Respiratory Rate Determination
2.8. Statistical Analysis
3. Results
3.1. Root Vitality and Malondialdehyde (MDA) Content
3.2. Effect on the GABA Shunt
3.2.1. Metabolite Contents in the GABA Shunt
3.2.2. Key Enzyme Activities of GABA Shunt
3.2.3. Gene Expressions of Key Enzymes in the GABA Shunt
3.3. Effect on the Respiratory Rate in the Roots
3.3.1. Total Respiration Rate (VTotal)
3.3.2. Respiration Rate of Basic Biochemical Pathways
3.3.3. Respiratory Rate of Electron Transport Pathways in Mitochondria
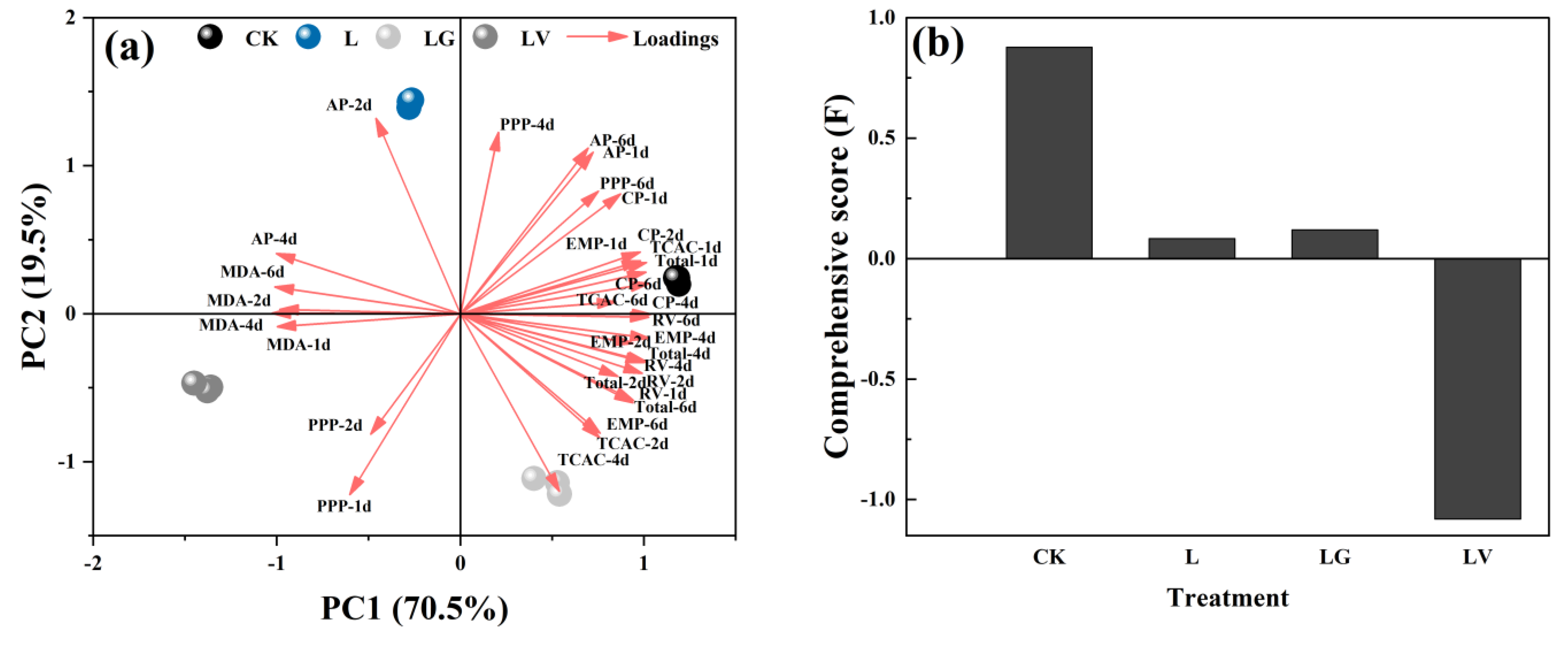
3.4. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [PubMed]
- Heidarvand, L.; Millar, A.H.; Taylor, N.L. Responses of the mitochondrial respiratory system to low temperature in plants. Crit. Rev. Plant Sci. 2017, 36, 217–240. [Google Scholar]
- Kerbler, S.M.; Taylor, N.L.; Millar, A.H. Cold sensitivity of mitochondrial ATP synthase restricts oxidative phosphorylation in Arabidopsis thaliana. New Phytol. 2019, 221, 1776–1788. [Google Scholar]
- Laube, J.; Sparks, T.H.; Estrella, N.; Hofler, J.; Ankerst, D.P.; Menzel, A. Chilling outweighs photoperiod in preventing precocious spring development. Glob. Chang. Biol. 2014, 20, 170–182. [Google Scholar]
- Tipov, A.F.; Shibaeva, T.G.; Ikkonena, E.N.; Sherudiloa, E.G. Plant responses to a daily short-term temperature drop: Phenomenology and mechanisms. Russ. J. Plant Physiol. 2020, 67, 1003–1017. [Google Scholar]
- Ma, H.Y.; Liu, G.C.; Lyu, D.G.; Qing, S.J. Responsive characteristics of respiratory metabolism pathway activity in ‘Hanfu’ apple flower buds under cold stress. J. Fruit Sci. 2012, 29, 317–321. [Google Scholar]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.; Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar]
- Li, L.J.; Lu, X.C.; Ma, H.Y.; Lyu, D.G. Jasmonic acid regulates the ascorbate–glutathione cycle in Malus baccata Borkh. roots under low root-zone temperature. Acta. Physiol. Plant. 2017, 39, 174. [Google Scholar]
- Adebooye, O.C.; Schmitz-Eiberger, M.; Lankes, C.; Noga, G.J. Inhibitory effects of sub-optimal root zone temperature on leaf bioactive components, photosystem II (PS II) and minerals uptake in Trichosanthes cucumerina L. Cucurbitaceae. Acta Physiol. Plant. 2009, 32, 67–73. [Google Scholar] [CrossRef]
- Schmidt, J.; Messmer, M.; Wilbois, K.P. Beneficial microorganisms for soybean (Glycine max (L.) Merr), with a focus on low root-zone temperatures. Plant Soil. 2015, 397, 411–445. [Google Scholar]
- Anwar, A.; Di, Q.H.; Yan, Y.; He, C.X.; Li, Y.S.; Yu, X.C. Exogenous 24-epibrassinolide alleviates the detrimental effects of suboptimal root zone temperature in cucumber seedlings. Arch. Agro. Soil Sci. 2019, 65, 1927–1940. [Google Scholar]
- Zhen, A.; Zhang, Z.; Jin, X.Q.; Liu, T.; Ren, W.Q.; Hu, X.H. Exogenous GABA application improves the NO3−-N absorption and assimilation in Ca(NO3)2-treated muskmelon seedlings. Sci. Hortic. 2018, 227, 117–123. [Google Scholar]
- Nejad-Alimoradi, F.; Nasibi, F.; Manoochehri Kalantari, K.; Torkzadeh-Mahani, M. Spermine pre-treatment improves some physiochemical parameters and sodium transporter gene expression of pumpkin seedlings under salt stress. Russ. J. Plant Physiol. 2018, 65, 222–228. [Google Scholar]
- Shu, S.; Yuan, R.N.; Shen, J.L.; Chen, J.; Wang, L.J.; Wu, J.Q.; Sun, J.; Wang, Y.; Guo, S.R. The positive regulation of putrescine on light-harvesting complex II and excitation energy dissipation in salt-stressed cucumber seedlings. Environ. Exp. Bot. 2019, 162, 283–294. [Google Scholar]
- Shelp, B.J.; Bown, A.W.; Mclean, M.D. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999, 4, 446–452. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar]
- Kinnersley, A.M.; Turano, F.J. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2010, 19, 479–509. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Chiu, G.; Bajwa, V.S. Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. I. Pathway structure. Botany 2012, 90, 651–668. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signaling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not just a metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef]
- Long, Y.; Tyerman, S.D.; Gilliham, M. Cytosolic GABA inhibits anion transport by wheat ALMT1. New phytol. 2020, 225, 671–678. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Does the GABA shunt regulate cytosolic GABA? Trends Plant Sci. 2020, 25, 422–424. [Google Scholar] [PubMed]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar]
- Al-Quraan, N.A. GABA shunt deficiencies and accumulation of reactive oxygen species under UV treatments: Insight from Arabidopsis thaliana calmodulin mutants. Acta Physiol. Plant. 2015, 37, 86. [Google Scholar]
- Chen, H.; Liu, T.; Xiang, L.; Hu, L.; Hu, X. GABA enhances muskmelon chloroplast antioxidants to defense salinity-alkalinity stress. Russ. J. Plant Physiol. 2018, 65, 674–679. [Google Scholar]
- Cheng, B.Z.; Li, Z.; Liang, L.L.; Cao, Y.Q.; Zeng, W.H.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Nie, G.; Liu, W.; et al. The gamma-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Hu, W.; Tian, S.B.; Di, Q.; Duan, S.H.; Dai, K. Effects of exogenous calcium on mesophyll cell ultrastructure, gas exchange, and photosystem II in tobacco (Nicotiana tabacum Linn.) under drought stress. Photosynthetica 2018, 56, 1204–1211. [Google Scholar] [CrossRef]
- Salvatierra, A.; Pimentel, P.; Almada, R.; Hinrichsen, P. Exogenous GABA application transiently improves the tolerance to root hypoxia on a sensitive genotype of Prunus rootstock. Environ. Exp. Bot. 2016, 125, 52–66. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, B.Y.; Peng, Y.X.; Liu, C.L.; Zhang, X.Z.; Zhang, Z.J.; Liang, W.; Ma, F.W.; Li, C.Y. Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids. Sci. Hortic. 2020, 261, 108982. [Google Scholar]
- Lu, X.C.; Li, L.J.; Hu, X.L.; Ma, H.Y.; Lyu, D.G. Effects of exogenous GABA on antioxidant function of Malus baccata Borkh. roots under suboptimal root-zone temperature. Acta Bot. Boreali-Occident. Sin. 2019, 39, 285–293. [Google Scholar]
- Su, H.; Li, L.J.; Ma, H.Y.; Lyu, D.G.; Sun, J. Calcium alleviates temperature stress by regulating nitrogen and respiratory metabolism in Malus baccata roots. Int. J. Agric. Biol. 2016, 18, 286–292. [Google Scholar] [CrossRef]
- Baum, G.; Lev-Yadun, S.; Fridmann, Y.; Arazi, T.; Katsnelson, H.; Zik, M.; Fromm, H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996, 15, 2988–2996. [Google Scholar]
- Liu, T.B.; Li, B.B.; Zhou, Y.F.; Chen, J.; Tu, H.Y. HPLC determination of γ-aminobutyric acid in Chinese rice wine using pre-column derivatization. J. Inst. Brewing. 2015, 121, 163–166. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Dong, C.X.; Lu, Y.L.; Miao, C.; Shen, Q.R. Study on the methods of extraction organic acids from different plant tissue by HPLC. J. Nanjing Agric. Univ. 2005, 28, 140–143. [Google Scholar]
- Arnetoll, M.; Montegrossi, G.; Buccianti, A.; Gonnelli, C. Determination of organic acids in plants of Silene paradoxa L. by HPLC. J. Agri. Food Chem. 2008, 56, 789–795. [Google Scholar] [CrossRef]
- Mustroph, A.; Stock, J.; Hess, N.; Aldous, S.; Dreilich, A.; Grimm, B. Characterization of the phosphofructokinase gene family in rice and its expression under oxygen deficiency stress. Front. Plant Sci. 2013, 4, 125. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bouma, T.; Yanai, R.; Elkin, A.; Hartmond, U.; Flores-Alva, D.; Eissenstat, D. Estimating age-dependent costs and benefits of roots with contrasting life span: Comparing apples and oranges. New Phytol. 2001, 150, 685–695. [Google Scholar] [CrossRef]
- Yu, R.; Pan, R. Effect of blue light on the respiration of Rice (Oryza sativa) seeding. Chin. J. Rice Sci. 1996, 10, 159–162. [Google Scholar]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef]
- Sanchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Chang. Biol. 2014, 20, 408–417. [Google Scholar] [PubMed]
- Wang, Y.C.; Gu, W.R.; Meng, Y.; Xie, T.L.; Li, L.J.; Li, J.; Wei, S. γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Jia, Q.Y.; Ji, S.X.; Gong, B.B.; Li, J.R.; Lu, G.Y.; Gao, H.B. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar]
- Wang, D.M.; Chacher, B.; Liu, H.Y.; Wang, J.K.; Lin, J.; Liu, J.X. Effects of gamma-aminobutyric acid on feed intake, growth performance and expression of related genes in growing lambs. Animal 2015, 9, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) regulated plant defense: Mechanisms and opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef] [PubMed]
- Mazzucotelli, E.; Tartari, A.; Cattivelli, L.; Forlani, G. Metabolism of gamma-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J. Exp. Bot. 2006, 57, 3755–3766. [Google Scholar] [CrossRef]
- Mekonnen, D.W.; Flugge, U.I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar]
- Yang, R.Q.; Hui, Q.R.; Gu, Z.X. Effects of ABA and CaCl2 on GABA accumulation in fava bean germinating under hypoxia-NaCl stress. Biosci. Biotechnol. Biochem. 2016, 80, 540–546. [Google Scholar] [CrossRef]
- Xu, B.; Sai, N.; Gilliham, M. The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol. 2021, 187, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, F.; Killiny, N. The use of deuterium-labeled gamma-aminobutyric (D6-GABA) to study uptake, translocation, and metabolism of exogenous GABA in plants. Plant Methods 2020, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Yong, B.; Xie, H.; Li, Z.; Li, Y.P.; Zhang, Y.; Nie, G.; Zhang, X.Q.; Ma, X.; Huang, L.K.; Yan, Y.H.; et al. Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-Shunt, polyamines, and proline metabolism in white clover. Front. Physiol. 2017, 8, 1107. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Chiu, G.; Bajwa, V.S. Strategies and tools for studying the metabolismand function of γ-aminobutyrate in plants.II. Integrated analysis. Botany 2012, 90, 781–793. [Google Scholar] [CrossRef]
- Schmid, M.; Davison, T.S.; Henz, S.R.; Pape, U.J.; Demar, M.; Vingron, M.; Scholkopf, B.; Weigel, D.; Lohmann, J.U. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005, 37, 501–506. [Google Scholar] [CrossRef]
- Bouche, N.; Fait, A.; Zik, M.; Fromm, H. The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol. Biol. 2004, 55, 315–325. [Google Scholar] [CrossRef]
- Trobacher, C.P.; Zarei, A.; Liu, J.Y.; Clark, S.M.; Bozzo, G.G.; Shelp, B.J. Calmodulin-dependent and calmodulin-independent glutamate decarboxylases in apple fruit. BMC Plant Biol. 2013, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Shimajiri, Y.; Ozaki, K.; Kainou, K.; Akama, K. Differential subcellular localization, enzymatic properties and expression patterns of gamma-aminobutyric acid transaminases (GABA-Ts) in rice (Oryza sativa). J. Plant Physiol. 2013, 170, 196–201. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Naderi, R.; Jannatizadeh, A.; Babalar, M.; Sarcheshmeh, M.A.; Faradonbe, M.Z. Impact of exogenous GABA treatments on endogenous GABA metabolism in anthurium cut flowers in response to postharvest chilling temperature. Plant Physiol. Biochem. 2016, 106, 11–15. [Google Scholar] [CrossRef]
- Hu, X.H.; Xu, Z.R.; Xu, W.N.; Li, J.M.; Zhao, N.; Zhou, Y. Application of gamma-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol. Biochem. 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Chen, W.C.; Cui, P.J.; Sun, H.Y.; Guo, W.Q.; Yang, C.W.; Jin, H.; Fang, B.; Shi, D.C. Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.). Ind. Crop. Prod. 2009, 30, 351–358. [Google Scholar] [CrossRef]
- Lin, Q.; Qian, J.; Zhao, C.N.; Wang, D.L.; Liu, C.R.; Wang, Z.D.; Sun, C.D.; Chen, K.S. Low temperature induced changes in citrate metabolism in Ponkan (Citrus reticulata Blanco cv. Ponkan) fruit during maturation. PLoS ONE 2016, 11, e0156703. [Google Scholar] [CrossRef] [PubMed]
- Sil, P.; Das, P.; Biswas, A.K. Silicon induced mitigation of TCA cycle and GABA synthesis in arsenic stressed wheat (Triticum aestivum L.) seedlings. S. Afr. J. Bot. 2018, 119, 340–352. [Google Scholar] [CrossRef]
- Atkin, O.; Tjoelker, M. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Gropman, A. Vigabatrin and newer interventions in succinic semialdehyde dehydrogenase deficiency. Ann. Neurol. 2003, 54 (Suppl. S6), S66–S72. [Google Scholar] [CrossRef] [PubMed]
- Deleu, C.; Faes, P.; Niogret, M.F.; Bouchereau, A. Effects of the inhibitor of the gamma-aminobutyrate-transaminase, vinyl-gamma-aminobutyrate, on development and nitrogen metabolism in Brassica napus seedlings. Plant Physiol. Biochem. 2013, 64, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bown, A.W.; Zarei, A. 4-Aminobutyrate (GABA): A metabolite and signal with practical significance. Botany 2017, 95, 1015–1032. [Google Scholar] [CrossRef]
- Glanemann, C.; Loos, A.; Gorret, N.; Willis, L.B.; O’Brien, X.M.; Lessard, P.A.; Sinskey, A.J. Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: Implications for DNA microarray analysis. Appl. Microbiol. Biotechnol. 2003, 61, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Shen, Z.J.; Zhang, Y.P.; Han, J.; Ma, R.J.; Korir, N.K.; Yu, M.L. Cloning and expression of genes related to the sucrose-metabolizing enzymes and carbohydrate changes in peach. Acta Physiol. Plant. 2012, 35, 589–602. [Google Scholar] [CrossRef]
- Atkin, O.; Edwards, E.; Loveys, B. Response of root respiration to changes in temperature and its relevance to global warming. New Phytol. 2000, 147, 141–154. [Google Scholar] [CrossRef]
- Zhou, W.J.; Qin, S.J.; Lyu, D.G.; Zhang, P. Soil sterilisation and plant growth-promoting rhizobacteria promote root respiration and growth of sweet cherry rootstocks. Arch. Agron. Soil Sci. 2014, 61, 361–370. [Google Scholar] [CrossRef]
- Shelp, B.J.; Mullen, R.T.; Waller, J.C. Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci. 2012, 17, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Chiu, G.Z.; Yu, G.H.; Trobacher, C.P.; Shelp, B.J. Salinity-regulated expression of genes involved in GABA metabolism and signaling. Botany 2017, 95, 621–627. [Google Scholar] [CrossRef]
- Chandel, N.S. Evolution of mitochondria as signaling organelles. Cell Metab. 2015, 22, 204–206. [Google Scholar] [CrossRef]
- Pu, X.J.; Lv, X.; Tan, T.H.; Fu, F.Q.; Qin, G.W.; Lin, H.H. Roles of mitochondrial energy dissipation systems in plant development and acclimation to stress. Ann. Bot. 2015, 116, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Whelan, J.; Soole, K.L.; Day, D.A. Organization and regulation of mitochondrial respiration in plants. Annu. Rev. Plant Biol. 2011, 62, 79–104. [Google Scholar] [CrossRef]
- Millar, A.H.; Day, D.A. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Lett. 1996, 398, 155–158. [Google Scholar] [CrossRef]
- Farre, G.; Blancquaert, D.; Capell, T.; Van Der Straeten, D.; Christou, P.; Zhu, C. Engineering complex metabolic pathways in plants. Annu. Rev. Plant Biol. 2014, 65, 187–223. [Google Scholar] [CrossRef]
- Erdal, S.; Genisel, M.; Turk, H.; Dumlupinar, R.; Demir, Y. Modulation of alternative oxidase to enhance tolerance against cold stress of chickpea by chemical treatments. J. Plant Physiol. 2015, 175, 95–101. [Google Scholar] [CrossRef]
- Turk, H.; Genisel, M. Melatonin-related mitochondrial respiration responses are associated with growth promotion and cold tolerance in plants. Cryobiology 2020, 92, 76–85. [Google Scholar] [CrossRef]
- Wang, Y.C.; Luo, Z.S.; Khan, Z.U.; Mao, L.C.; Ying, T.J. Effect of nitric oxide on energy metabolism in postharvest banana fruit in response to chilling stress. Postharvest Biol. Technol. 2015, 108, 21–27. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef] [PubMed]




| Genes | Sequence ID | Primer Sequence (5′-3′) |
|---|---|---|
| Actin | - | F: GGCTGGATTTGCTGGTGATG R: TGCTCACTATGCCGTGCTCA |
| GAD1 | NM_001294057.1 | F: TCAGCCCACTTTCACCCT R: CATCCGAACTTCCTCAAACTAT |
| GAD2 | NM_001293835.1 | F: GGAGCCAATGTCCAGGTG R: GCCGAGGATAGCAGCAAC |
| GAD3 | NM_001294084.1 | F: CGGTGGGACAGACACAGAGA R: CACTCCGACTAGTAGCATTTTGCA |
| GABA-T1 | NM_001328897.1 | F: CTATTTATTGCCGACGAG R: ACAAGAACAGCACCGATT |
| GABA-T2 | NM_001293859.1 | F: ACACTGACTGCCCACATT R: TCACAAGAACAGCACCAA |
| SSADH1 | XM_008357890.3 | F: CAGTGGCACCCCTTTTGC R: GCAGCTAACCCTGCATTGGT |
| SSADH2 | XM_029110078.1 | F: TCATACTTTGATACCTCATCCTCCAT R: GAGCAGCAGGATAGAAATTTGAATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Dai, P.; Ma, H.; Lyu, D. Regulatory Effect of Exogenous γ-Aminobutyric Acid on Respiratory Rate through the γ-Aminobutyric Acid Shunt in Malus baccata (L.) Borkh. Roots under Suboptimal Low Root-Zone Temperature. Horticulturae 2023, 9, 268. https://doi.org/10.3390/horticulturae9020268
Lu X, Dai P, Ma H, Lyu D. Regulatory Effect of Exogenous γ-Aminobutyric Acid on Respiratory Rate through the γ-Aminobutyric Acid Shunt in Malus baccata (L.) Borkh. Roots under Suboptimal Low Root-Zone Temperature. Horticulturae. 2023; 9(2):268. https://doi.org/10.3390/horticulturae9020268
Chicago/Turabian StyleLu, Xiaochen, Ping Dai, Huaiyu Ma, and Deguo Lyu. 2023. "Regulatory Effect of Exogenous γ-Aminobutyric Acid on Respiratory Rate through the γ-Aminobutyric Acid Shunt in Malus baccata (L.) Borkh. Roots under Suboptimal Low Root-Zone Temperature" Horticulturae 9, no. 2: 268. https://doi.org/10.3390/horticulturae9020268
APA StyleLu, X., Dai, P., Ma, H., & Lyu, D. (2023). Regulatory Effect of Exogenous γ-Aminobutyric Acid on Respiratory Rate through the γ-Aminobutyric Acid Shunt in Malus baccata (L.) Borkh. Roots under Suboptimal Low Root-Zone Temperature. Horticulturae, 9(2), 268. https://doi.org/10.3390/horticulturae9020268





