Effect of Bacillus methylotrophicus on Tomato Plug Seedling
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Measurement Items and Methods
2.3.1. Plant Morphology and Biomass
2.3.2. Chlorophyll Content
2.3.3. Seedling Growth Function and Seedling Strength Index (SI)
2.3.4. Root Development Strategy
| Specific root length (SRL): | SRL = RL/RDW | (4) |
| Specific root surface area (SRA): | SRA = RA/RDW | (5) |
| Root tip density (RTD): | RTD = RT/RL | (6) |
| Root branch density (RBI): | RBI = RF/RL | (7) |
| Root tissue density (RTID): | RTID = RDW/RV | (8) |
| Root fineness (RFN): | RFN = RL/RV | (9) |
2.3.5. Physical and Chemical Properties of Substrates
2.3.6. Determination of Elemental Content of Plants
2.4. Data Processing and Statistical Analysis
3. Results Analysis
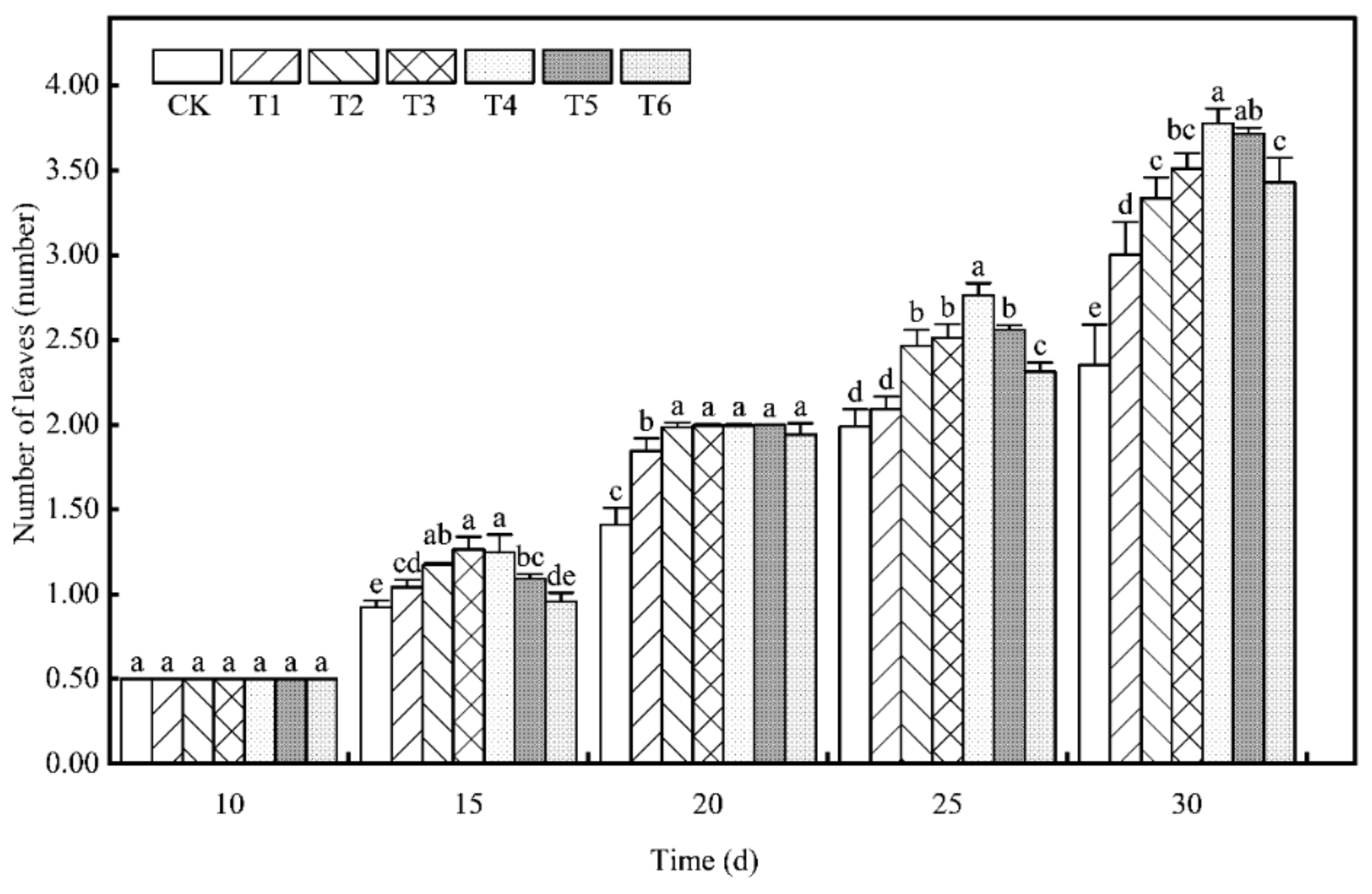
3.1. Effect of Bacterial Agentbacterial Agent Dosage on the Number of Leaves of Tomato Seedlings
3.2. Effect of Bacterial Agent Dosage on Above-Ground Morphological Indices of Tomato Seedlings
3.3. Effect of Bacterial Agent Dosage on the Biomass of Tomato Seedlings
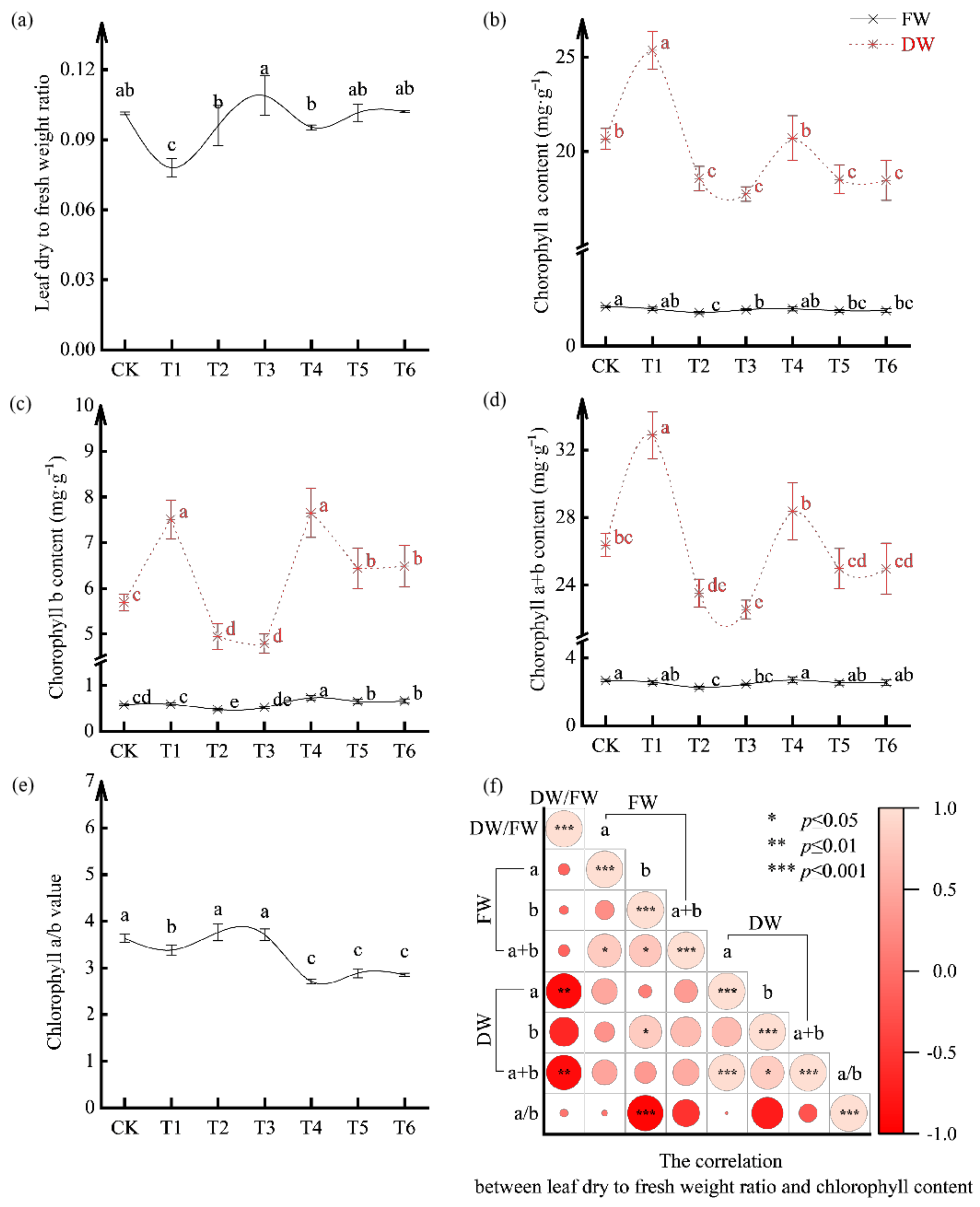
3.4. Effect of Bacterial Agent Dosage on Chlorophyll Content of Tomato Seedlings
3.5. Effect of Bacterial Agent Dosage on the G Value and Seedling Strength Index of Tomato Seedlings
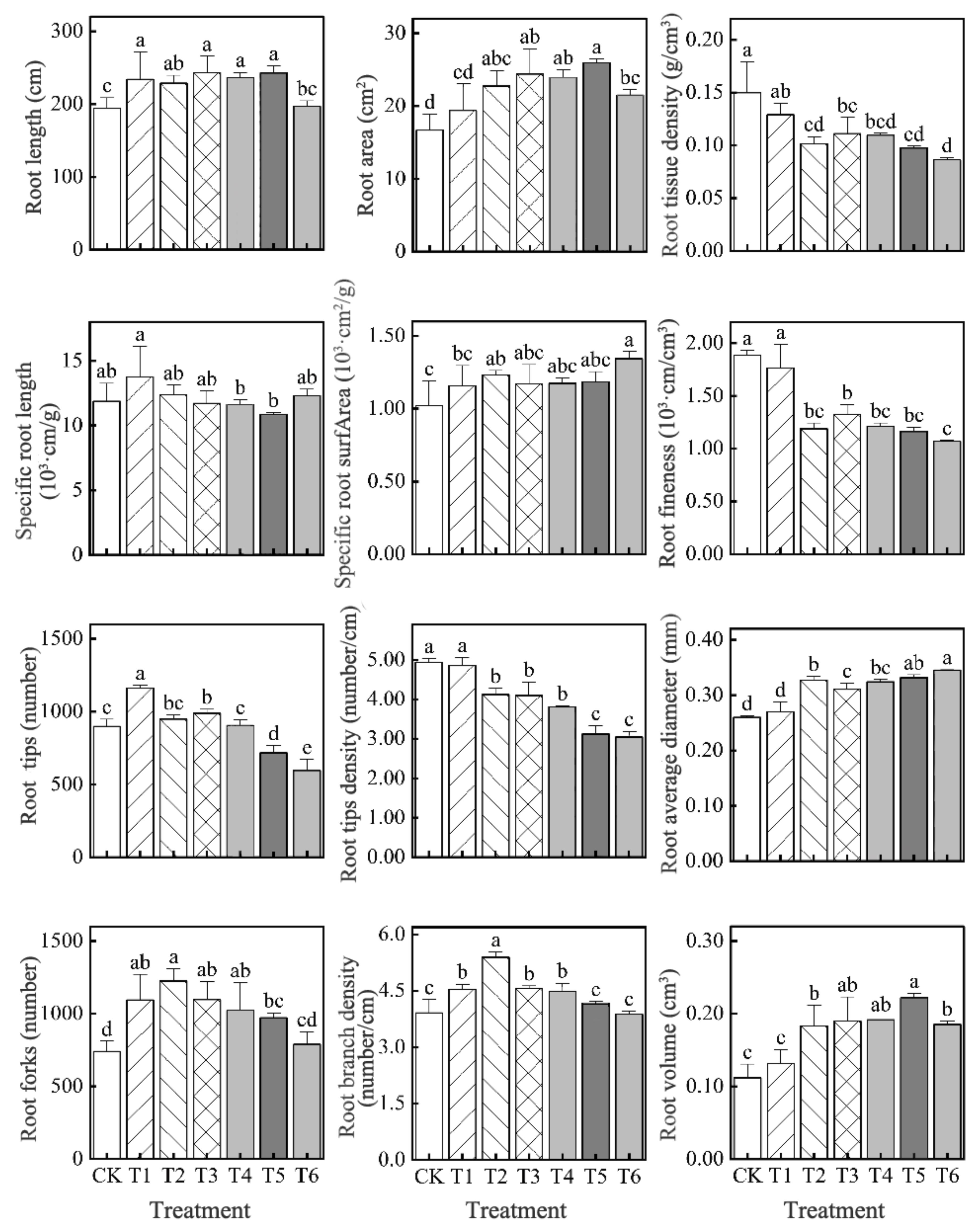
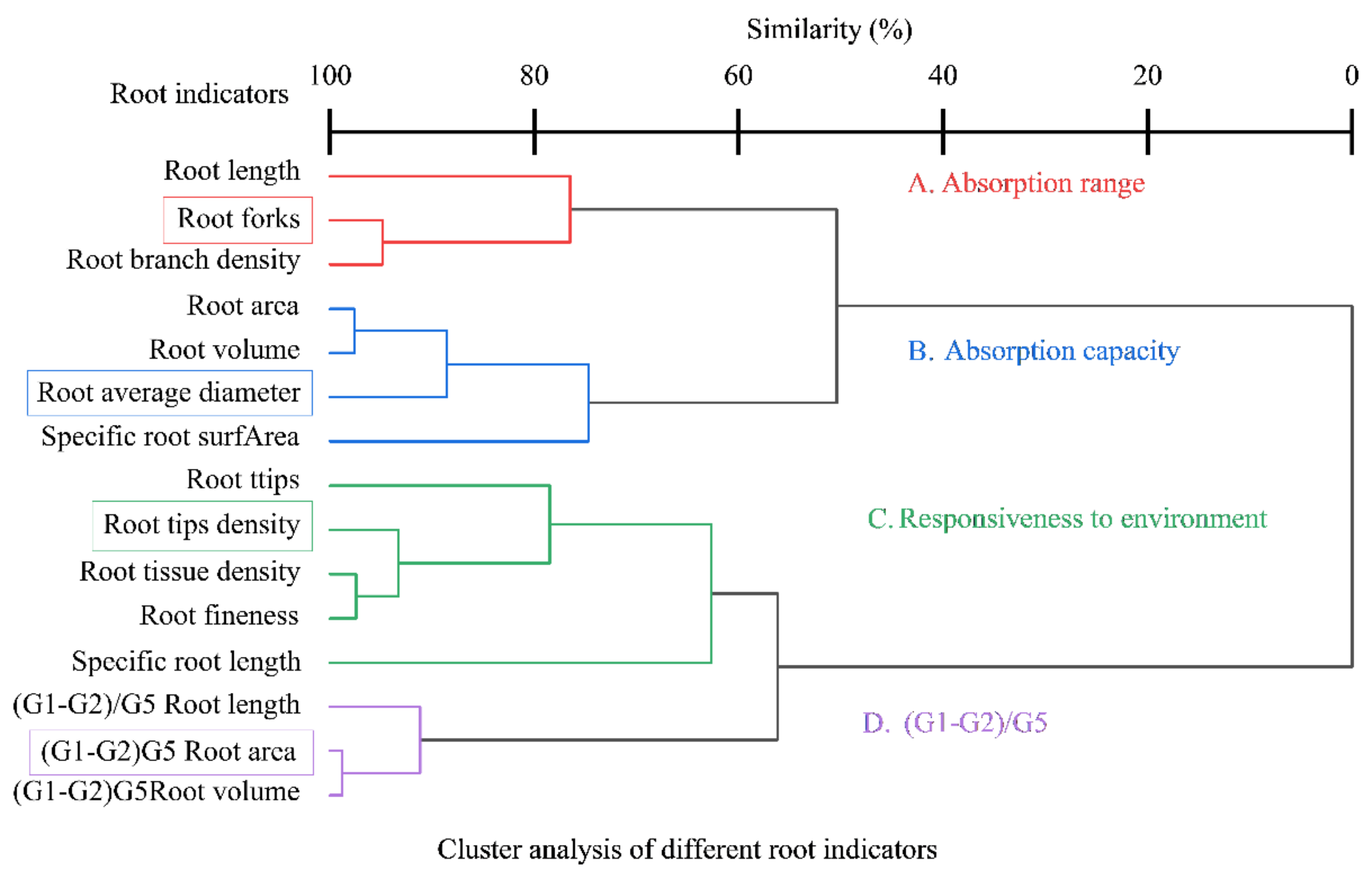
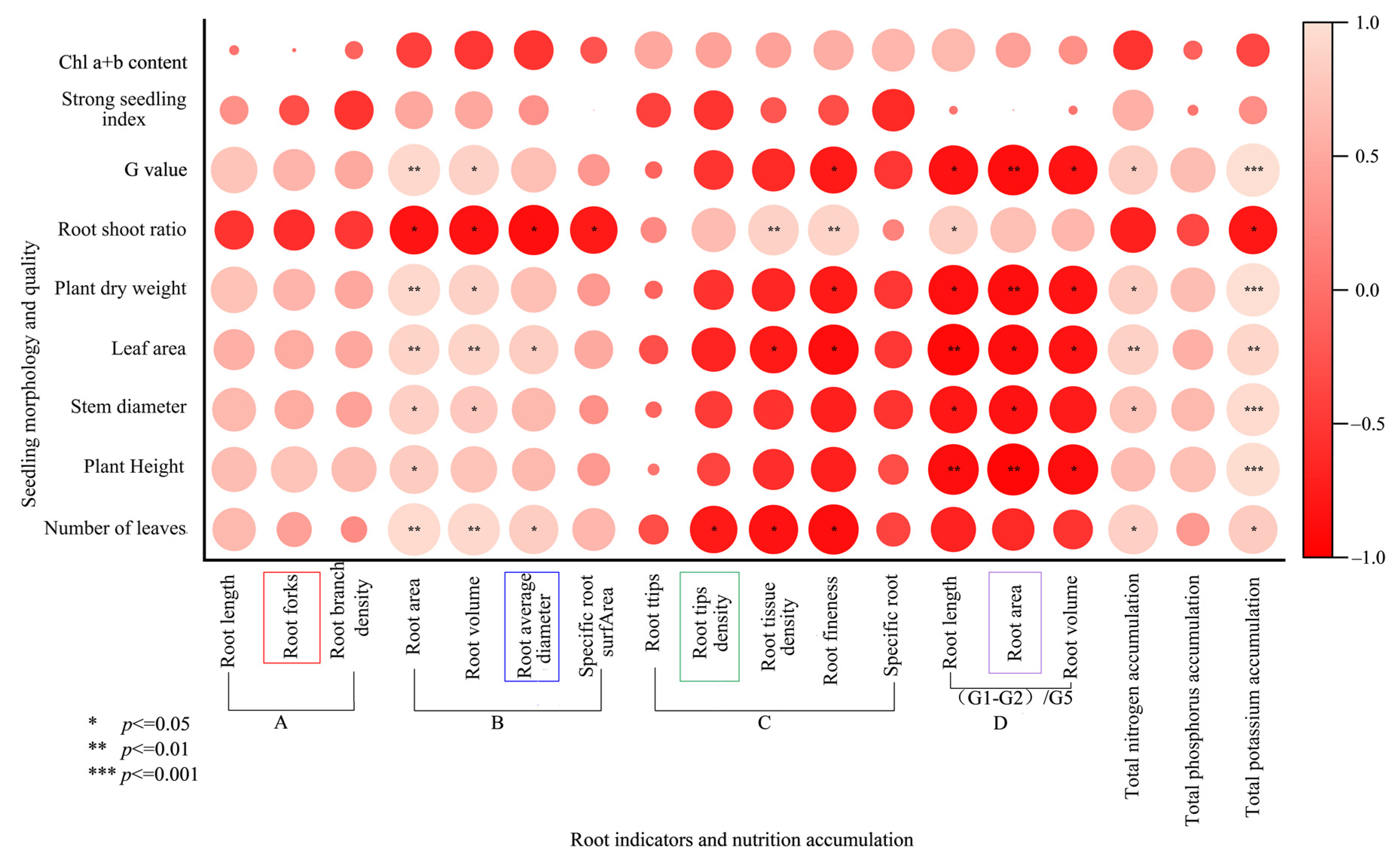
3.6. Effect of Bacterial Agent Dosage on the Root Morphology of Tomato Seedlings
3.7. Plasticity of Fine Root Structure of Tomato Seedlings at Different Diameter Levels in Response to Bacterial Agent Dosage
3.8. Effect of Bacterial Agent Dosage on the Accumulation and Distribution of Nitrogen, Phosphorus and Potassium in Tomato Seedlings
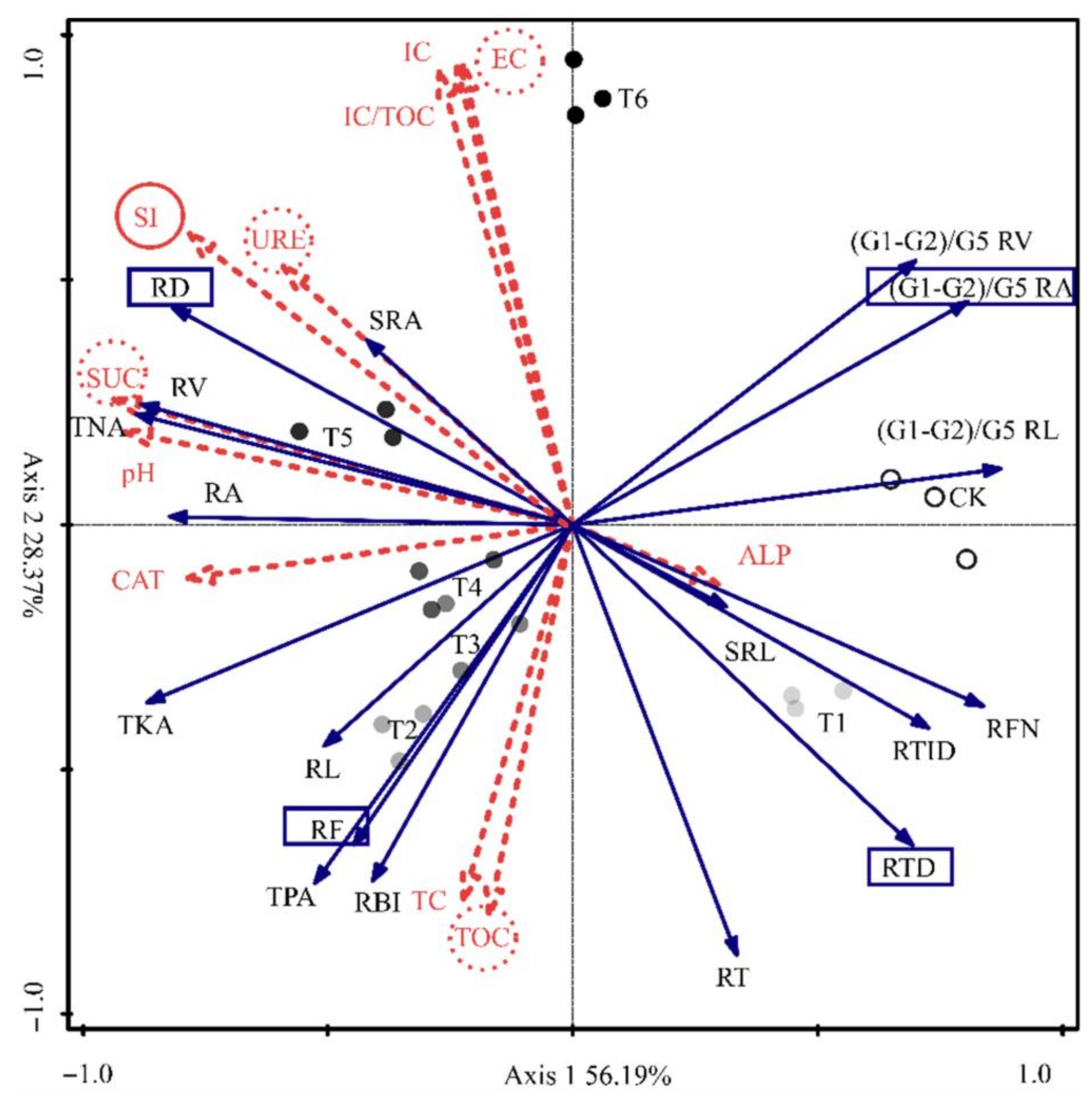
3.9. Effect of Bacterial Agent on the Physicochemical Properties of the Substrate
3.10. Effect of Substrate Physicochemical Properties on Root Morphological Characteristics and Element Uptake of Tomato Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2013, 374, 689–700. [Google Scholar] [CrossRef]
- Crowley, D.E. Microbial siderophores in the plant rhizosphere. In lron Nutrition in Plants and Rhizospheric Microorganisms; Springer: Dordrecht, The Netherlands, 2006; Volume 8, pp. 169–198. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Kucharova, Z.; Davranov, K.; Berg, G.; Makarova, N.; Azarova, T.; Lugtenberg, B. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fertil. Soils 2010, 47, 197–205. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Pineda, A.; Zheng, S.J.; Van Loon, J.J.; Pieterse, C.M.J.; Dicke, M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Guo, Q.; Li, Y.Z.; Sun, Y.F.; Xue, Q.H.; Lai, H.X. Streptomyces pactum Act12 controls tomato yellow leaf curl virus disease and alters rhizosphere microbial communities. Biol. Fertil. Soils 2019, 55, 149–169. [Google Scholar] [CrossRef]
- Li, H.Y.; Qiu, Y.Z.; Yao, T.; Ma, Y.C.; Zhang, H.R.; Yang, X.L. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Zhu, T.H.; Liu, G.H.; Mao, C. Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biol. Fertil. Soils 2011, 47, 437–446. [Google Scholar] [CrossRef]
- Krishna, K.; Kuttum, M.; Vel, M.; Krishnan, S.; Anbalagan, A.; Awnindra, K.S.; Rai, K.G.; Sibnarayan, D.R. Growth enhancement in vegetable crops by multifunctional resident plant growth promoting rhizobacteria under tropical Island Ecosystem. Afr. J. Microbiol. Res. 2014, 8, 2436–2448. [Google Scholar] [CrossRef][Green Version]
- Ma, K.; Yang, F.; Duan, Y.K.; Tang, Y.L.; Cai, S.X.; Shi, X.J.; Yuan, Y.X. Research advances on the application technology of plant growth promoting rhizosphere in melon and vegetable industrial seedling production. China Cucurbits Veg. 2019, 32, 1–5. [Google Scholar] [CrossRef]
- Ansaril, A.F.; Ahmad, I.; Pichtel, J. Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Tomes, M.; Llamas, I.; Tomes, B.; Toral, L.; Sampedro, I.; Bejar, V. Growth promotion on horticultural crops and antifungal activity of Bacillus velezensis XT1. Appl. Soil Ecol. 2020, 150, 103453. [Google Scholar] [CrossRef]
- Shahid, M.; Akram, M.S.; Khan, M.A.; Zubair, M.; Shah, S.M.; Ismail, M.; Shabir, G.; Basheer, S.; Adam, K.; Tariq, M. A phytobeneficial strain Planomicrobium sp. MSSA-10 triggered oxidative stress responsive mechanisms and regulated the growth of pea plants under induced saline environment. J. Appl. Microbiol. 2018, 124, 1566–1579. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Kwon, S.W.; Sa, T.M. Bacillus methylotrophicus sp. nov.; a methanol-utilizing, plant-growth-promoting bacterium isolated from rice rhizosphere soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 2490–2495. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Babalola, O.O. Tackling maize fusariosis: In search of Fusarium graminearum biosuppressors. Arch. Microbiol. 2018, 200, 1239–1255. [Google Scholar] [CrossRef]
- Wang, A.; Hua, J.; Wang, Y.; Zhang, G.; Luo, S. Stereoisomers of nonvolatile acetylbutanediol metabolites produced by Bacillus velezensis WRN031 improved root elongation of maize and rice. J. Agric. Food Chem. 2020, 68, 6308–6315. [Google Scholar] [CrossRef]
- Hu, X.H.; Wang, J.Z.; Peng, T.L.; Yuan, L.Q. Effect of Bacillus methylotrophicus on cucumber acupoint plate seedling in summer. Trans. Chin. Soc. Agric. Mach. 2020, 51, 284–293. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhang, Q.; Gao, Z.X.; Ma, X.Q.; Qu, F.; Hu, X.H. Effects of two microbial agents on yield, quality and rhizosphere environment of autumn cucumber cultured in organic substrate. Sci. Agric. Sin. 2021, 54, 3077–3087. [Google Scholar] [CrossRef]
- Mesa, J.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Piedras, J.M.B.; Caviedes, M.A.; Redondo-Gómez, S.; Mateos-Naranjo, E. Moving closer towards restoration of contaminated estuaries: Bioaugmentation with autochthonous rhizobacteria improves metal rhizoaccumulation in native Spartina maritima. J. Hazard. Mater. 2015, 300, 263–271. [Google Scholar] [CrossRef]
- FAOSTAT. Production/Yield Quantities of Tomatoes in World. 2022. Available online: https://www.fao.org/faostat/zh/#data/QCL/visualize (accessed on 8 August 2022).
- Guo, M.; Yang, M.; Liu, B.; Niu, P.; Yang, L. Status and development trend of vegetable seedling industry in china. J. Agric. Mech. Res. 2015, 37, 250–253. [Google Scholar] [CrossRef]
- Cui, Z.C.; Guan, C.S.; Yang, Y.T.; Gao, Q.S.; Chen, Y.S.; Xiao, T.Q. Research status of vegetable mechanical transplanting technology and equipment. J. Chin. Agric. Mech. 2020, 41, 85–92. [Google Scholar] [CrossRef]
- Shi, X.F.; Liang, H.; Zhu, J.H.; Wang, D.H.; Ge, M.H.; Huang, Y.; Zhou, M.B. The status, problems and countermeasures of industrialized vegetable seeding in Wuhan. China Cucurbits Veg. 2021, 34, 88–91. [Google Scholar] [CrossRef]
- El-Hady, N.A.A.A.; ElSayed, A.I.; El-saadany, S.S.; Deligios, P.A.; Ledda, L. Exogenous application of foliar salicylic acid and propolis enhances antioxidant defenses and growth parameters in tomato plants. Plants 2021, 10, 74. [Google Scholar] [CrossRef]
- Chen, F.M.; Chen, S.W. 1984. Study on the determination of chlorophyll content by mixed liquid method. For. Sci. Technol. 1984, 2, 4–8. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Jia, Y.B.; Yang, X.E.; Islam, E.; Feng, Y. Effects of potassium deficiency on chloroplast ultrastructure and chlorophyll fluo-rescence in inefficient and efficient genotype of rice. J. Plant Nutr. 2008, 31, 2105–2118. [Google Scholar] [CrossRef]
- Moore, B.C. Principal component analysis in linear systems:Controllability, observability, and modelreduction. IEEE Trans. Autom. Control 1981, 26, 17–32. [Google Scholar] [CrossRef]
- Gong, B.B.; Wang, N.; Zhang, T.J.; Wu, X.L.; Lu, G.Y.; Chu, X.P.; Gao, H.B. Selection of tomato seedling index based on comprehensive morphology and leaf chlorophyll content. Trans. Chin. Soc. Agric. Eng. 2019, 35, 237–244. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhou, M.; Li, P.; Sun, G.P.; Shi, S.L.; Xu, C.Y. Root morphological plasticity determing the adaptive strategies of Cotinus coggygria seedlings in barren soil environment. J. Beijing For. Univ. 2017, 39, 60–69. [Google Scholar] [CrossRef]
- Arima, S.; Saisho, K.; Harada, J. Morphological analysis of the rice root system based on root diameter. Jpn. J. Crop Sci. 2001, 70, 408–417. [Google Scholar] [CrossRef][Green Version]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Wei, J.; Wu, X.; Cui, R.; Li, G.; Zheng, F.; Tan, D. Humic acid fertilizer improved soil properties and soil microbial diversity of continuous cropping peanut: A three-year experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef]
- Gallardo, M.; Cuartero, J.; Torre, L.; Padilla, F.M.; Segura, M.L.; Thompson, R.B. Modelling nitrogen, phosphorus, potassium, calcium and magnesium uptake, and uptake concentration, of greenhouse tomato with the VegSyst model. Sci. Hortic. 2021, 279, 109862. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Wang, N.; He, H.; Tan, Q.; Wen, B.; Zhang, R.; Sun, M.; Zhao, X.; Fu, X.; et al. Prunus persica terpene synthase PpTPS1 interacts with PpABI5 to enhance salt resistance in transgenic tomatoes. Front. Plant Sci. 2022, 13, 807342. [Google Scholar] [CrossRef]
- Breedt, G.; Labuschagne, N.; Coutinho, T.A. Seed treatment with selected plant growth-promoting rhizobacteria increases maize yield in the field. Ann. Appl. Biol. 2017, 171, 229–236. [Google Scholar] [CrossRef]
- Di Salvo, L.P.; Cellucci, G.C.; Carlino, M.E.; García de Salamone, I.E. Plant growth-promoting rhizobacteria inoculation and nitrogen fertilization increase maize (Zea mays L.) grain yield and modified rhizosphere microbial communities. Appl. Soil Ecol. 2018, 126, 113–120. [Google Scholar] [CrossRef]
- Santos, R.M.; Kandasamy, S.; Rigobelo, E.C. Sugarcane growth and nutrition levels are differentially affected by the application of PGPR and cane waste. Microbiol. Open 2018, 7, e00617. [Google Scholar] [CrossRef]
- Antunes, J.E.L.; Lyra, M.C.C.P.; Ollero, F.J.; Freitas, A.D.S.; Oliveira, L.M.S.; Araújo, A.S.F.; Figueiredo, M.V.B. Diversity of plant growth-promoting bacteria associated with sugarcane. Genet. Mol. Res. 2017, 16, gmr16029662. [Google Scholar] [CrossRef]
- Aquino, J.P.A.; Antunes, J.E.L.; Bonifácio, A.; Rocha, S.M.B.; Amorim, M.R.; Alcântara Neto, F.; Araujo, A.S.F. Plant growth-promoting bacteria improve growth and nitrogen metabolism in maize and sorghum. Theor. Exp. Plant Physiol. 2021, 33, 249–260. [Google Scholar] [CrossRef]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Da Silva, J.R.; Netto, A.T.; De Medeiros, B.P.; De Deus, B.C.S.; Silva, M.V.S.; Ferraz, T.M.; Campostrini, E.; Olivares, F.L. Endophytic diazotrophic bacteria mitigate water deprivation effects in pineapple explants during acclimatization. Theor. Exp. Plant Physiol. 2020, 32, 63–77. [Google Scholar] [CrossRef]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Agren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Collins, D.; Li, B.; Zhu, J.; Tavakkoli, E. Nutrient supply enhanced wheat residue-carbon mineralization, microbial growth, and microbial carbon-use efficiency when residues were supplied at high rate in contrasting soils. Soil Biol. Biochem. 2018, 126, 168–179. [Google Scholar] [CrossRef]
- Bahadori, M.; Chen, C.; Lewis, S.; Boyd, S.; Kuzyakov, Y. Soil organic matter formation is controlled by the chemistry and bioavailability of organic carbon inputs across different land uses. Sci. Total Environ. 2021, 770, 145307. [Google Scholar] [CrossRef]
- Liu, S.H. Research progress on the effect of biochar on soil organic carbon. Agric. Biotechnol. 2020, 9, 79–81. [Google Scholar] [CrossRef]
- Ghorbani-Nasrabadi, R.; Greiner, R.; Alikhani, H.A.; Hamedi, J.; Yakhchali, B. Distribution of actinomycetes in different soil ecosystems and effect of media composition on extracellular phosphatase activity. J. Soil Sci. Plant Nutr. 2013, 13, 223–236. [Google Scholar] [CrossRef][Green Version]
- Rietz, D.; Haynes, R. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Karlen, D.L.; Tomer, M.D.; Neppel, J.; Cambardella, C.A. A preliminary watershed scale soil quality assessment in north central Iowa, USA. Soil Tillage Res. 2008, 99, 291–299. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Wang, X.; Shao, H.; Yang, J.; Wang, X. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar] [CrossRef]
- Urgenson, L.S.; Reichard, S.H.; Halpern, C.B. Multiple competitive mechanisms underlie the effects of a strong invader on early- to late-seral tree seedlings. J. Ecol. 2012, 100, 1204–1215. [Google Scholar] [CrossRef]
- Lobet, G.; Paez-Garcia, A.; Schneider, H.; Junker, A.; Atkinson, J.; Tracy, S. Demystifying roots: A need for clarification and extended concepts in root phenotyping. Plant Sci. 2018, 282, 11–13. [Google Scholar] [CrossRef]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Fujimura, S.; Suzuki, K.; Nagao, M.; Okada, M. Acclimation to root chilling increases sugar concentrations in tomato (Solanum lycopersicum L.) fruits. Sci. Hortic. 2012, 147, 34–41. [Google Scholar] [CrossRef]
- Henke, M.; Sarlikioti, V.; Kurth, W.; Buck-Sorlin, G.H.; Pagès, L. Exploring root developmental plasticity to nitrogen with a three-dimensional architectural model. Plant Soil 2014, 385, 49–62. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Hummel, I.; Vile, D.; Violle, C.; Devaux, J.; Ricci, B.; Blanchard, A.; Garnier, E.; Roumet, C. Relating root structure and anatomy to whole-plant functioning in 14 herbaceous Mediterranean species. New Phytol. 2007, 173, 313–321. [Google Scholar] [CrossRef]
- McCormack, M.L.; Guo, D. Impacts of environmental factors on fine root lifespan. Front. Plant Sci. 2014, 5, 205. [Google Scholar] [CrossRef]



| Treatment | Fresh Weight (g/strain) | Fresh Weight Proportion (%) | Root–Shoot Ratio (Fresh) | |||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Total | Root | Stem | Leaf | ||
| CK | 0.28 ± 0.01 cd | 0.92 ± 0.25 e | 0.73 ± 0.17 d | 1.93 ± 0.41 e | 15.16 ± 3.74 a | 47.30 ± 3.06 d | 37.54 ± 0.68 b | 0.18 ± 0.05 a |
| T1 | 0.29 ± 0.02 cd | 0.81 ± 0.04 e | 1.14 ± 0.09 b | 2.24 ± 0.14 e | 12.95 ± 0.03 a | 36.03 ± 0.67 e | 51.03 ± 0.64 a | 0.15 ± 0.00 ab |
| T2 | 0.31 ± 0.05 bcd | 2.10 ± 0.04 ab | 1.22 ± 0.04 ab | 3.62 ± 0.13 ab | 8.41 ± 1.04 b | 57.91 ± 0.95 a | 33.68 ± 0.09 d | 0.09 ± 0.01 c |
| T3 | 0.32 ± 0.03 abc | 1.81 ± 0.09 c | 1.10 ± 0.06 bc | 3.23 ± 0.17 c | 9.79 ± 0.72 b | 56.16 ± 0.94 abc | 34.05 ± 0.22 d | 0.11 ± 0.01 c |
| T4 | 0.36 ± 0.02 a | 2.24 ± 0.09 a | 1.35 ± 0.03 a | 3.96 ± 0.07 a | 9.04 ± 0.60 b | 56.71 ± 1.57 ab | 34.25 ± 1.04 d | 0.10 ± 0.01 c |
| T5 | 0.35 ± 0.02 ab | 1.90 ± 0.14 bc | 1.23 ± 0.06 ab | 3.48 ± 0.08 bc | 10.07 ± 0.65 b | 54.45 ± 3.01 bc | 35.48 ± 2.36 cd | 0.11 ± 0.01 bc |
| T6 | 0.26 ± 0.01 d | 1.41 ± 0.11 d | 0.98 ± 0.07 c | 2.65 ± 0.18 d | 9.85 ± 0.45 b | 53.03 ± 0.39 c | 37.12 ± 0.05 bc | 0.11 ± 0.01 c |
| Treatment | Dry Weight (10−1 g/strain) | Dry Weight Proportion (%) | Root–Shoot Ratio (Dry) | |||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Total | Root | Stem | Leaf | ||
| CK | 0.17 ± 0.01 cd | 0.44 ± 0.17 c | 0.74 ± 0.17 c | 1.34 ± 0.29 c | 12.94 ± 3.00 a | 31.88 ± 4.60 d | 55.18 ± 1.60 abc | 0.15 ± 0.04 a |
| T1 | 0.17 ± 0.01 cd | 0.53 ± 0.02 bc | 0.89 ± 0.02 b | 1.58 ± 0.04 bc | 10.73 ± 0.32 ab | 33.16 ± 0.03 cd | 56.11 ± 0.29 ab | 0.12 ± 0.00 b |
| T2 | 0.19 ± 0.02 bc | 0.84 ± 0.00 a | 1.17 ± 0.07 a | 2.20 ± 0.03 a | 8.44 ± 1.15 c | 38.28 ± 0.88 a | 53.28 ± 2.03 c | 0.09 ± 0.01 b |
| T3 | 0.21 ± 0.01 ab | 0.79 ± 0.03 a | 1.19 ± 0.05 a | 2.19 ± 0.04 a | 9.48 ± 0.34 bc | 36.01 ± 1.63 abc | 54.50 ± 1.37 bc | 0.10 ± 0.00 b |
| T4 | 0.20 ± 0.02 ab | 0.87 ± 0.03 a | 1.29 ± 0.04 a | 2.37 ± 0.04 a | 8.58 ± 0.43 bc | 36.92 ± 1.30 ab | 54.50 ± 1.30 bc | 0.09 ± 0.01 b |
| T5 | 0.21 ± 0.02 a | 0.76 ± 0.00 a | 1.25 ± 0.09 a | 2.22 ± 0.09 a | 9.44 ± 0.54 bc | 34.14 ± 1.75 bcd | 56.41 ± 1.28 ab | 0.10 ± 0.01 b |
| T6 | 0.16 ± 0.00 d | 0.59 ± 0.05 b | 1.00 ± 0.07 b | 1.75 ± 0.09 b | 9.15 ± 0.59 bc | 33.63 ± 0.49 bcd | 57.22 ± 0.10 a | 0.10 ± 0.01 b |
| Treatment | (G1-G2)/G5 | ||
|---|---|---|---|
| Root Length | Root Area | Root Volume | |
| CK | 373.17 ± 22.23 a | 29.02 ± 1.21 a | 3.27 ± 0.54 a |
| T1 | 373.70 ± 4.37 a | 27.36 ± 2.41 a | 2.95 ± 0.41 ab |
| T2 | 159.71 ± 2.48 c | 11.31 ± 0.82 c | 1.18 ± 0.07 d |
| T3 | 255.63 ± 18.52 b | 19.58 ± 1.35 b | 2.41 ± 0.09 bc |
| T4 | 251.43 ± 20.30 b | 16.90 ± 0.70 b | 1.85 ± 0.08 c |
| T5 | 236.03 ± 47.10 b | 17.94 ± 0.96 b | 2.01 ± 0.14 c |
| T6 | 284.85 ± 31.10 b | 28.17 ± 3.73 a | 3.48 ± 0.48 a |
| Nutrient | Treatment | Accumulation (mg/strain) | Total | Distribution Ratio (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaf | Root | Stem | Leaf | |||
| Total nitrogen | CK | 0.48 ± 0.01 b | 1.00 ± 0.12 c | 3.36 ± 0.46 c | 4.84 ± 0.57 c | 9.98 ± 0.21 a | 20.69 ± 2.45 bc | 69.32 ± 9.40 a |
| T1 | 0.40 ± 0.03 c | 0.94 ± 0.02 c | 3.34 ± 0.25 c | 4.68 ± 0.25 c | 8.56 ± 0.73 b | 20.15 ± 0.36 bc | 71.29 ± 5.36 a | |
| T2 | 0.44 ± 0.04 bc | 1.39 ± 0.13 b | 4.55 ± 0.41 b | 6.38 ± 0.33 b | 6.96 ± 0.65 cde | 21.80 ± 2.07 b | 71.24 ± 6.43 a | |
| T3 | 0.50 ± 0.02 b | 1.39 ± 0.09 b | 4.49 ± 0.22 b | 6.37 ± 0.27 b | 7.80 ± 0.25 bc | 21.76 ± 1.36 b | 70.44 ± 3.46 a | |
| T4 | 0.44 ± 0.01 bc | 1.85 ± 0.29 a | 4.49 ± 0.92 b | 6.78 ± 0.67 b | 6.55 ± 0.14 de | 27.26 ± 4.26 a | 66.19 ± 13.55 a | |
| T5 | 0.59 ± 0.07 a | 1.68 ± 0.09 a | 5.56 ± 0.38 a | 7.83 ± 0.33 a | 7.53 ± 0.93 bcd | 21.43 ± 1.14 b | 71.04 ± 4.84 a | |
| T6 | 0.39 ± 0.06 c | 1.09 ± 0.16 c | 4.83 ± 0.17 ab | 6.31 ± 0.21 b | 6.22 ± 0.88 e | 17.26 ± 2.53 c | 76.52 ± 2.64 a | |
| Total phosphorus | CK | 0.23 ± 0.01 a | 0.51 ± 0.03 c | 0.92 ± 0.12 d | 1.66 ± 0.16 c | 13.72 ± 0.40 a | 30.66 ± 2.09 ab | 55.62 ± 7.11 b |
| T1 | 0.19 ± 0.02 b | 0.56 ± 0.01 bc | 1.04 ± 0.07 bcd | 1.79 ± 0.07 bc | 10.80 ± 0.89 b | 31.24 ± 0.51 ab | 57.96 ± 4.09 b | |
| T2 | 0.17 ± 0.02 bc | 0.66 ± 0.05 a | 1.22 ± 0.09 ab | 2.05 ± 0.05 a | 8.23 ± 0.74 cd | 32.32 ± 2.58 a | 59.45 ± 4.36 b | |
| T3 | 0.18 ± 0.00 b | 0.60 ± 0.04 ab | 1.18 ± 0.05 ab | 1.95 ± 0.07 ab | 9.31 ± 0.23 c | 30.47 ± 1.83 ab | 60.21 ± 2.37 b | |
| T4 | 0.16 ± 0.00 c | 0.68 ± 0.10 a | 1.12 ± 0.20 abc | 1.96 ± 0.13 a | 8.11 ± 0.12 d | 34.57 ± 4.86 a | 57.32 ± 10.22 b | |
| T5 | 0.18 ± 0.02 bc | 0.53 ± 0.03 bc | 1.26 ± 0.07 a | 1.97 ± 0.08 a | 9.08 ± 1.01 cd | 27.03 ± 1.40 b | 63.89 ± 3.69 ab | |
| T6 | 0.09 ± 0.01 d | 0.30 ± 0.04 d | 0.97 ± 0.03 cd | 1.36 ± 0.04 d | 6.71 ± 0.71 e | 21.75 ± 2.63 c | 71.54 ± 2.04 a | |
| Total potassium | CK | 0.71 ± 0.03 b | 3.02 ± 0.36 c | 2.87 ± 0.35 d | 6.60 ± 0.16 d | 10.71 ± 0.39 a | 45.75 ± 5.41 ab | 43.54 ± 5.27 bc |
| T1 | 0.68 ± 0.04 b | 3.60 ± 0.06 c | 3.56 ± 0.21 c | 7.84 ± 0.23 c | 8.65 ± 0.55 b | 45.92 ± 0.78 ab | 45.43 ± 2.62 bc | |
| T2 | 0.70 ± 0.05 b | 5.33 ± 0.42 ab | 4.96 ± 0.41 a | 10.99 ± 0.83 ab | 6.36 ± 0.49 d | 48.54 ± 3.78 a | 45.11 ± 3.76 bc | |
| T3 | 0.78 ± 0.03 a | 5.13 ± 0.29 b | 4.94 ± 0.25 a | 10.85 ± 0.43 ab | 7.19 ± 0.24 c | 47.30 ± 2.70 a | 45.51 ± 2.32 bc | |
| T4 | 0.66 ± 0.00 b | 6.14 ± 0.97 a | 4.61 ± 0.72 ab | 11.41 ± 0.43 a | 5.81 ± 0.00 d | 53.77 ± 8.46 a | 40.41 ± 6.27 c | |
| T5 | 0.69 ± 0.05 b | 4.88 ± 0.32 b | 4.97 ± 0.24 a | 10.54 ± 0.14 b | 6.56 ± 0.47 cd | 46.28 ± 3.06 ab | 47.16 ± 2.26 b | |
| T6 | 0.50 ± 0.05 c | 3.04 ± 0.35 c | 4.25 ± 0.16 b | 7.79 ± 0.24 c | 6.45 ± 0.63 cd | 39.04 ± 4.50 b | 54.52 ± 1.99 a | |
| Treatment | EC (10−1 mS/m) | pH | Substrate Carbon Content (%) | |||
|---|---|---|---|---|---|---|
| Total Carbon | Inorganic Carbon | Total Organic Carbon | Inorganic Carbon/Total Organic Carbon | |||
| Original substrate | 150.00 ± 3.00 f | 6.21 ± 0.03 d | 29.65 ± 1.13 b | 0.00 ± 0.00 d | 29.65 ± 1.13 b | 0.00 ± 0.00 e |
| CK | 449.33 ± 6.11 d | 5.18 ± 0.03 e | 28.33 ± 0.95 bc | 0.00 ± 0.00 d | 28.33 ± 0.95 bc | 0.00 ± 0.00 e |
| T1 | 346.00 ± 3.61 e | 6.24 ± 0.15 d | 26.37 ± 0.22 e | 0.00 ± 0.00 d | 26.37 ± 0.22 de | 0.00 ± 0.00 e |
| T2 | 440.67 ± 6.11 d | 7.44 ± 0.03 ab | 31.50 ± 0.79 a | 0.00 ± 0.00 d | 31.50 ± 0.79 a | 0.00 ± 0.00 e |
| T3 | 476.33 ± 13.05 c | 7.13 ± 0.08 c | 29.31 ± 0.56 b | 0.05 ± 0.01 d | 29.28 ± 0.53 b | 0.15 ± 0.02 d |
| T4 | 490.33 ± 12.74 c | 7.53 ± 0.06 a | 27.83 ± 0.29 cd | 0.14 ± 0.01 c | 27.74 ± 0.22 cd | 0.49 ± 0.02 c |
| T5 | 553.00 ± 16.09 b | 7.54 ± 0.04 a | 26.84 ± 0.42 de | 0.57 ± 0.05 b | 26.28 ± 0.37 e | 2.15 ± 0.17 b |
| T6 | 868.00 ± 17.09 a | 7.39 ± 0.03 b | 23.77 ± 1.27 f | 0.93 ± 0.07 a | 23.14 ± 1.35 f | 4.10 ± 0.01 a |
| Treatment | URE (mg/g/d) | SUC (mg/g/d) | CAT (mg/g/d) | ALP (mg/g/d) |
|---|---|---|---|---|
| Original substrate | - | - | - | - |
| CK | 0.18 ± 0.03 d | 13.34 ± 1.53 b | 7.84 ± 0.77 d | 0.78 ± 0.03 a |
| T1 | 0.64 ± 0.06 c | 11.78 ± 1.67 b | 8.28 ± 0.33 cd | 0.71 ± 0.02 a |
| T2 | 0.73 ± 0.06 bc | 54.89 ± 2.41 a | 9.00 ± 0.12 ab | 0.73 ± 0.05 a |
| T3 | 0.72 ± 0.05 bc | 47.29 ± 5.73 a | 9.48 ± 0.29 a | 0.75 ± 0.01 a |
| T4 | 0.73 ± 0.02 bc | 51.74 ± 5.73 a | 9.06 ± 0.19 ab | 0.69 ± 0.09 a |
| T5 | 0.83 ± 0.04 b | 56.07 ± 8.44 a | 9.15 ± 0.37 ab | 0.69 ± 0.06 a |
| T6 | 1.18 ± 0.13 a | 52.06 ± 7.47 a | 8.54 ± 0.19 bc | 0.71 ± 0.11 a |
| Substrate Properties | Simple Effects | Conditional Effects | ||||
|---|---|---|---|---|---|---|
| Explanation % | Pseudo-F | p | Explanation % | Pseudo-F | p | |
| SUC | 43.50 | 14.60 | 0.002 | 43.50 | 14.60 | 0.002 |
| pH | 41.00 | 13.20 | 0.002 | 2.00 | 1.40 | 0.162 |
| CAT | 30.20 | 8.20 | 0.002 | 1.20 | 0.90 | 0.502 |
| URE | 24.90 | 6.30 | 0.002 | 4.80 | 3.00 | 0.014 |
| EC | 23.80 | 5.90 | 0.002 | 24.50 | 13.80 | 0.002 |
| IC/TOC | 23.90 | 6.00 | 0.004 | 1.40 | 1.00 | 0.344 |
| IC | 24.20 | 6.10 | 0.002 | 1.00 | 0.70 | 0.644 |
| TOC | 17.50 | 4.00 | 0.014 | 3.80 | 2.60 | 0.022 |
| TC | 17.40 | 4.00 | 0.022 | 0.60 | 0.40 | 0.886 |
| ALP | 5.00 | 1.00 | 0.41 | 1.80 | 1.30 | 0.248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Meng, X.; Peng, T.; Hu, X. Effect of Bacillus methylotrophicus on Tomato Plug Seedling. Horticulturae 2022, 8, 947. https://doi.org/10.3390/horticulturae8100947
Sun M, Meng X, Peng T, Hu X. Effect of Bacillus methylotrophicus on Tomato Plug Seedling. Horticulturae. 2022; 8(10):947. https://doi.org/10.3390/horticulturae8100947
Chicago/Turabian StyleSun, Min, Xiangguang Meng, Tieli Peng, and Xiaohui Hu. 2022. "Effect of Bacillus methylotrophicus on Tomato Plug Seedling" Horticulturae 8, no. 10: 947. https://doi.org/10.3390/horticulturae8100947
APA StyleSun, M., Meng, X., Peng, T., & Hu, X. (2022). Effect of Bacillus methylotrophicus on Tomato Plug Seedling. Horticulturae, 8(10), 947. https://doi.org/10.3390/horticulturae8100947






