Abstract
Nitrate is a major source of the inorganic nitrogen taken up by the roots of plants. Nitrate sources are generally derived from inorganic minerals by an energy-consuming chemical process; as a result, the price of chemical fertilizers is gradually increasing year by year. NO3-N, generated from N2 using the plasma technique, is an alternative method of producing nitrate from the air. Therefore, in this research, we aimed to determine the efficiency of generating NO3-N using plasma-activated water (PAW) to replace nitrates from chemical fertilizer in a nutrient solution. Green oak lettuce (Lactuca sativa L.) was grown in a hydroponics system using the double-pot technique. The plants were supplied with three different nutrient solutions (based on Hoagland’s solution), i.e., T1, no nitrate in the nutrient solution (NO3− = 0); T2, using nitrate sourced from a commercial chemical fertilizer (normal nitrate); and T3, using a nitrate source generated using the pinhole plasma jet technique (plasma nitrate). The other macronutrients and micronutrients in each treatment were equally supplied. The results show that, at the harvested stage (21 days after the plants received treatment), the no-nitrate (T1) treatment provided lower growth and yields. Moreover, compared with the normal nitrate (T2) and plasma nitrate (T3), the results indicate that most growth and yields showed no statistical differences. In terms of nitrate accumulation within plants, it was found that the normal nitrate treatment (T2) had the highest levels of nitrate accumulation, in both the underground and aboveground parts of green oak lettuce. These results confirmed that plasma nitrate could be an alternative source of nitrate N which provided a safer way for the environment and human health in terms of nitrate accumulation. In addition, data related to the chemical analysis of free amino acid concentrations in each treatment are discussed in this research.
1. Introduction
Globally, the production levels of lettuces such as Batavia, butterhead, red oak, red coral, and green oak have risen due to population increases and growing health concerns [1]. According to the Food and Agricultural Organization of the United Nations [2], the global production of lettuce and chicory increased from 2015 to 2020 from approximately 26 to 28 million tons. Green oak (Lactuca sativa L.) is a leafy vegetable that is rich in nutrients such as fiber, folate, carotenoids, phenolic and antioxidant compounds, minerals, and various vitamins [3]. Although soil-grown lettuce is consumed worldwide, the risk of exposure to inorganic fertilizers and pesticides, which cause pollution and contamination, is high [4]. Hydroponics, an alternative method of growing lettuce, is an innovative agricultural technology that has been adopted in several countries. Due to the absence of soil, water is responsible for providing nutrients, hydration, and oxygen to plants or vegetables. Therefore, the risk of disease occurring due to organisms in the soil is low in such systems [5]. Furthermore, hydroponic systems are a highly popular technique for cultivating plants because they lead to improvements in growth, yields, income, profit, and the number of crops produced per year [6]. The fertilizer solution is an important factor in the functioning of hydroponics systems, and NO3-N is one of the main mineral elements that is usually derived from chemical fertilizers.
In agriculture, nitrogen is an essential macronutrient for plant growth and development. It is generally used to increase crop productivity, and the level of nitrogen available to plants is one of the factors that most strongly limit crop productivity. Plants normally absorb nitrogen in the form of NO3− and NH4+. Nitrate, as a signal molecule involved in plant development and metabolism, is the nutrient that leads to the production of amino acids and nitrogen compounds [7]. Therefore, NO3− accumulation in plant tissues can be influenced by many preharvest factors, including plant species, the photoperiod, temperature, light intensity, and the type of fertilizer applied; this can occur when the nitrate uptake exceeds plant assimilation [8].
Ammonia, nitrate, and urea are the three main nitrogen-based fertilizers. The production of nitrogen-based fertilizers has always been a challenge for horticulturists due to the increasing demand for such products [9]. Traditionally, ammonia was produced through the Haber–Bosch (HB) ammonia synthesis process, which consumes large amounts of energy and thus has a substantial carbon footprint [10]. The annual global energy consumption and CO2 emissions of ammonia production accounted for 1–2% and 300 million tons per year, respectively [11]. Consequently, it is imperative to ensure the availability of fertilizers that are safe for human health, sustainable, and environmentally friendly. Moreover, Russia is the second-largest exporter of fertilizers worldwide but terminated fertilizer exports during the 2022 Russia–Ukraine war, causing shortages around the world, in countries including Thailand [12,13]. Therefore, the cost of fertilizer is undergoing a continuous increase.
Plasma, which is the fourth state of matter, is a source of electrons, positively charged ions, radicals, gas atoms, molecules (in excited or basic states), and photons with a range of energies [14]. Plasma can occur in both natural phenomena on Earth (e.g., the aurora borealis and fire) and under laboratory conditions, by providing sufficient energy to excite gaseous molecules [15]. Plasma falls into the two categories of nonthermal plasma and thermal plasma. Nonthermal plasma has received considerable attention for applications in the agricultural sector [16]. Reactive oxygen and nitrogen species (RONS) generated from plasma are important factors that can enhance plant physiology and growth [17]. However, the nonthermal plasma technology in the form of gas is not sufficiently flexible for use in the agricultural sector due to the risk of plant surface damage [18]. Therefore, another form of plasma, which is more flexible than the gas form, is generated by exposing plasma to water; this is called plasma-activated water (PAW). Several researchers have proven the potential of PAW in various applications, including microbial inactivation [19,20], enhancement of seed germination [21,22], and plant growth improvement [23,24]. In addition to these applications in the agricultural sector, some research has used plasma-activated water as a source of nitrogen species. Graves et al. [25] indicated that using air plasma to activate liquid solutions of organic material resulted in a reduction in pH and the addition of NO2−, NO3−, and H2O2. Therefore, the generated NO3− provides plants with accessible nitrate, and the nitrate increases linearly with the increase in power usage under all tested flow rates (i.e., 0.5, 1.25, and 2.0 L/min) [26]. In addition, Carmassi et al. [27] investigated the effect of nonthermal plasma (NTP) technologies on hydroponic nutrient solutions and determined their effects on the growth and quality of baby-leaf lettuce (Lactuca sativa var. acephala Alef.). Their results prove that NTP treatment of the nutrient solution could improve the production and quality of baby-leaf lettuce grown in a hydroponic growing system (HGS). In hydroponics systems, the occurrence of algae leads to major problems, including intensive odors, a reduction in dissolved oxygen, nutrient deprivation, and a pH reduction in the system [28,29]. However, Date et al. [30] showed that plasma-activated nutrient solutions could reduce algae growth to a greater degree than standard nutrient solutions.
Some previous studies have indicated that nitrate in plasma-activated water effectively enhances plant growth, especially leafy plants, and little is known about the potential of nitrate from different nutrient solutions. Additionally, a pinhole plasma jet has not been applied to nitrate fertilizer generation before. Therefore, in the present study, we aimed to investigate the effects of nitrate synthesized from plasma-activated water on the growth performance, yields, and nutritional value of green oak lettuce in a hydroponics system. Plant morphological characteristics, including plant height, root length, canopy, leaf width, number of leaves, and leaf area were compared between three treatment groups (i.e., no nitrate, normal nitrate, and plasma nitrate). Finally, the chemical compositions of the three treatment groups of green oak were investigated and compared.
2. Materials and Methods
2.1. Plasma Fertilizer Preparation
A pinhole plasma jet was developed to generate plasma-activated water (PAW) at the Agriculture and Bio Plasma Technology Laboratory, Science and Technology Park, Chiang Mai University, Thailand. The schematic of the pinhole plasma jet discharge system is shown in Figure 1. This system consists of a pinhole plasma jet discharge system with a 125-watt neon transformer, a high-voltage power supply, a gas transport system to provide controlled flows of air and oxygen gas, control devices, and a tank containing tap water. Tap water (40 L) was used as a solution because it is convenient and cost-effective. The working gases were mixed from air and oxygen with flow rates of 1.5 and 1.5 L/min, respectively. After discharging the plasma for 16 h the nitrate and nitrite concentrations were set to approximately 800 mg/L, which is comparable with Hoagland fertilizer. The chemical composition of the PAW (i.e., NO3− and NO2−) was determined using a commercial kit containing Griess reagents (Cayman Chemicals, Ann Arbor, MI, USA), and a pink and brown azo-product was formed. The NO3− and NO2− concentrations were measured using established UV/Vis spectrophotometric methods (Shimadzu UV–1800, Kyoto, Japan) with maximum absorbance peaks at 372 and 507 nm, respectively. The concentration of H2O2 was determined using the iodometric titration method. Then, 1 mL of 2% potassium iodide solution and 2 M HCl was added to the PAW. The solution was kept in the dark for 15 min, resulting in the solution turning yellow in color. Then, 0.1 M sodium thiosulfate solution was added until the solution turned a lighter yellow. Dropping a starch indicator into the solution turned the solution blue in color. Then, the blue solution was titrated with a 0.1 M sodium thiosulfate solution until the solution turned clear. The concentrations of H2O2, NO3−, and NO2− in the PAW were measured immediately after plasma activation, and their values were 102.99, 883.59, and 31.56 mg/L, respectively.
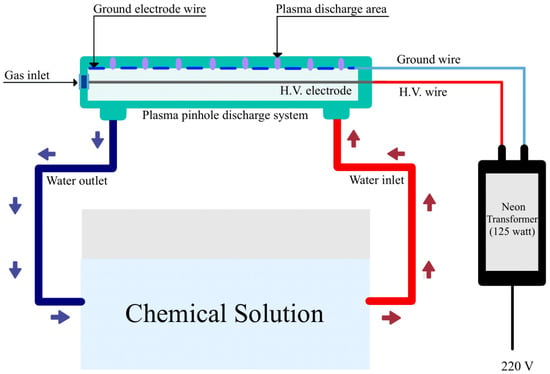
Figure 1.
Pinhole discharge system for generating plasma-activated water.
2.2. Plant Materials and Growth Conditions
The experiment was conducted in an evaporative greenhouse at H.M. The King Initiative Centre for Flower and Fruit Propagation, Chiang Mai University, Thailand. The temperature and relative humidity (RH) inside the evaporative greenhouse were 27.8 °C and 89.28%, respectively. The green oak lettuce (Lactuca sativa) seeds were germinated on a sponge in a plastic tray floating on a half-strength Hoagland fertilizer formula (Electrical conductivity (EC) = 1.4 µS/cm pH = 5.5) for 21 days. Then, the plants were transferred to the hydroponics system and supplied with three different nutrient solutions (based on Hoagland’s solution), i.e., T1 = no nitrate in the nutrient solution (NO3-N-free solution), T2 = using a nitrate source from a commercial chemical fertilizer (chemical fertilizer NO3-N), and T3 = using a nitrate source generated using the pinhole plasma jet technique (PAW-NO3-N-). The double-pot system used in this research is illustrated in Figure 2. The other macronutrients and micronutrients in each nutrient solution were equally supplied, as shown in Table 1.
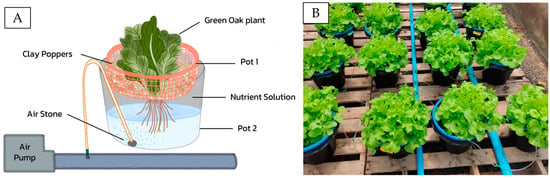
Figure 2.
(A) Diagram and (B) actual experimental setup of the double-pot system used in this experiment.

Table 1.
Macronutrient and micronutrient concentration (mg/L) in each nutrient solution.
The pH of each nutrient solution was adjusted to 5.5–6 by the pH-down adjuster and pH-up adjuster (KOH and diluted HNO3, respectively). The EC of plasma-activated water was 1.5 µS/cm. The pH, EC, and temperature were monitored using a SevenCompactTM Duo pH/Conductivity meter S213 (Mettler Toledo International Inc., Greifenseem, Switzerland). The solution was completely renewed every 7 days.
2.3. Measurement of Plant Morphology and Growth Characteristics
Four plant samples were randomly chosen from each replicate. Plant morphological characteristics, including plant height, root length, canopy width, leaf width, number of leaves, leaf area (LI-3100C, LI-COR Biosciences, Lincoln, OR, USA), and leaf color intensity (SPAD-502 Plus, Spectrum Technologies Inc., Aurora, IL, USA) were measured for plants from each treatment every seven days. The roots were rinsed with double-deionized water to remove visible hydroponic planting material (sponge) from the surface. At the same time, fresh and dry weights were also determined. To record the plants’ dry weights, the aboveground part and the underground part were washed with tap water, dried, and placed in an oven (UN55, MEMMERT, Büchenbach, Germany) at 60 °C for 1 week. The photosynthesis rate and stomatal conductance were measured at the harvest stage (when the plants had been cultivated for 21 days after treatment (DAT)) with a portable LCpro-SD (ADC BioScientific Ltd., Hoddesdon, England). The leaves’ greenness was evaluated using a SPAD-502 Plus chlorophyll meter (Spectrum Technologies Inc., Aurora, IL, USA).
2.4. Determination of Nitrate Contents in the Plants
After the lettuce samples were dried (~7 days in the oven), 10 mg of lettuce was immersed in 10 mL of distilled water in a centrifuge tube and incubated at room temperature for 1 night. The mixture was centrifuged at 15,000 rpm for 20 min. The supernatant was collected at 0.1 mL and added to 0.4 mL 5% (w/v) salicylic acid–sulfuric acid (5 g salicylic acid in 100 mL sulfuric acid) in each tube; the sample was mixed well, and then the reactants were incubated for 20 min at room temperature until they had cooled down. Then, 9.5 mL of 8% (w/v) NaOH solution was added to each tube and the tubes were cooled to room temperature (about 20–30 min). The absorbance measurement was made at 410 nm using established UV/Vis spectrophotometric methods (Shimadzu UV–1800, Kyoto, Japan). To generate the standard curve, the concentrations of the series of standard solutions were 0, 62.5, 125, 250, 500, and 1,000 mg/L KNO3. Additionally, the concentration of the KNO3 standard solution was plotted, and the R2 value was determined [31].
2.5. Determination of Total Phenolic Compounds
The total phenolic compounds were determined using a modified Folin–Ciolcateu method [32]. In brief, 1.0 g of fresh lettuce was extracted in 10 mL of 80% (v/v) methanol, sonicated for 5 min at 37 °C, and centrifuged at 4000 rpm for 10 min, followed by the collection of supernatants. An aliquot (500 µL) of supernatant was reacted with 2.5 mL 10% Folin–Ciolcalteu reagent, followed by the addition of 2.0 mL 7.5% Na2CO3 solution, and vortexed. The mixture was incubated at room temperature for 30 min. Absorbance was read at 765 nm using established UV/Vis spectrophotometric methods (Shimadzu UV–1800, Kyoto, Japan). Total phenolic content was calculated using the standard curves of 0, 5, 10, 20, 40, and 80 mg/L solutions of gallic acid (mg GAE/g FW) following the formula T = CV/M, where T = the total phenolic content in mg GAE/g FW, C = the concentration of gallic acid established from the calibration curve in mg/mL, V = the volume of the extract in mL, and M = the fresh weight of the plant extract in g.
2.6. Determination of Phosphorus, Potassium, Calcium, and Magnesium
The determination of exchangeable potassium and phosphorus in the fertilizer was conducted using an atomic emission spectrophotometer (AES) [33]; an atomic absorption spectrophotometer (AAS) was used to analyze calcium and magnesium [34].
2.7. Determination of Free Amino Acids
The amino acids in green oak leaves were identified using a high-speed amino acid analyzer (L-8900, Hitachi High-Technologies Corporation, Japan) equipped with a Hitachi custom ion-exchange column (4.6 × 60 mm) (Hitachi High-Technologies Corporation, Tokyo, Japan). For the analysis, a dry green oak powder was prepared using the methods described by Wang et al. [35].
2.8. Statistical Analysis
The data are presented as mean values ± standard deviation and were obtained using SPSS software (IBM Corp., Armonk, NY, USA). The one-way analysis of variance (ANOVA) and subsequent multiple range test using the least significant difference method (LSD) were performed to judge the differences between the groups. The different lowercase letters represent statistically significant differences at probability p < 0.05.
3. Results and Discussions
3.1. Changes in the NO3 Concentration in the Solution
According to several studies, reactive nitrogen species including NO2− and NO3− are the main long-living species produced during the plasma–water interaction [36]. Various studies have examined the chemical reaction for the generation of nitrate. Al-Sharify et al. [37] proposed one of the possible nitrate-generation paths, as presented in Equation (1). In the present study, the pinhole plasma jet was directly discharged into water, in which the obtained concentrations of nitrate and nitrite were higher than when the jet was discharged above the water’s surface [38].
NO2− + H2O2 + H+ → NO3− + H2O + H+
Nitrate is a general form of nitrogen taken up and assimilated by plants. Andrews and Raven [39] explained that nitrate that is taken up by plants is reduced to nitrite and ammonium (NH4+) by the enzymes nitrate reductase (NR) and nitrite reductase (NiR), respectively. Table 2 shows the remaining nitrate concentration in each nutrient solution (i.e., T1, T2, and T3) at each sampling time (i.e., 7, 14, and 21 DAT). Note that each nutrient solution was mixed and renewed every seven days. Therefore, the initial concentration of nitrate was the same as that on day 1. As shown in Table 2, the nitrate concentration in T3 decreased by approximately 33.75%, 35.88%, and 49.09% after 7 DAT, 14 DAT, and 21 DAT, respectively. The reduction of nitrate in T3 was more than that in T1 and T2 at every sampling time. The concentration of nitrate generated in PAW gradually decreased with the increased storage time [38]. This indicates that plants can easily take in NO3-N generated from PAW.

Table 2.
Nitrate content remaining in each treatment at each sampling time.
3.2. Growth Characteristics and Yields
Visualizations from above and from the side of the plants supplied with the nutrient solutions without nitrate (T1), with normal nitrate (T2), and with plasma nitrate (T3) are shown in Figure 3. T1 plants exhibited smaller heads and longer roots than T2 and T3. Jia et al. [40] indicated that the root foraging response prompted by increasing root length results from a lack of nitrogen. They explained that variation in root elongation under low N results from variation in the brassinosteroid (BR) biosynthesis gene DWARF1 and from the overexpression of DWF1. The average morphological features including plant height, root length, canopy width, leaf width, number of leaves, and leaf area after the plants were harvested at 21 days after treatment (DAT) are presented in Table 3. Plant height, leaf width, canopy width, and the number of leaves in the T2 and T3 groups were not statistically different (p > 0.05). Similar results were obtained by Noh et al. [41], which indicates that growth parameters are not significantly different between lettuces grown with and without plasma-activated water. In contrast, lettuce cultivated under T3 had a leaf area larger than T1 and T2 by 75.41% and 27.77%, respectively. Even though the number of leaves was not significantly different between T2 and T3, this difference can lead to a significantly larger leaf area. A larger leaf area could result in a better interception of light and a higher photosynthetic rate [42]. RONS generated in plasma-activated water play a key role in stimulating plant growth and responses to stress [27].
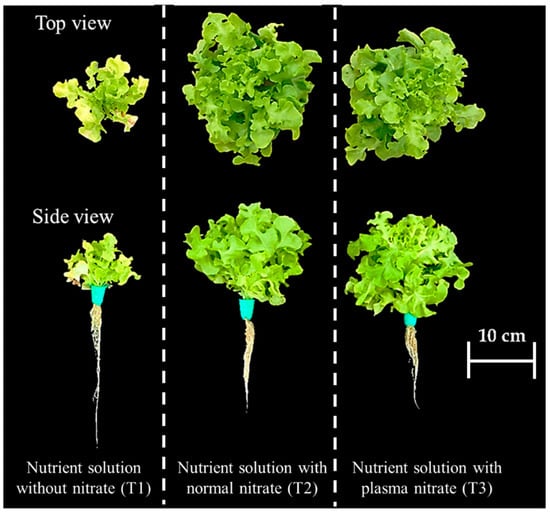
Figure 3.
Growth of green oak lettuce grown in a hydroponics system and supplied with different NO3− sources at harvest stage (21 days after treatment).

Table 3.
The morphologies of hydroponically grown lettuce cultivated in nutrient solutions without nitrate (T1), normal nitrate (T2), and plasma nitrate (T3) at harvest stage (21 DAT).
Chlorophyll, which gives plants their green color, has nitrogen as a part of its molecular makeup. The samples cultivated in the T3 group had higher levels of nitrate uptake than the T2 group, as presented in Table 2. In addition, Table 4 shows that plants cultivated with a nutrient solution containing plasma nitrate (T3) had significantly higher (p < 0.05) greenness of leaves than T1 and T2, by 5.5 and 3.45 SPAD units, respectively. This indicates that plasma nitrate could be assimilated into chlorophyll molecules to a greater extent than in the other treatments. Nitrogen deficiency leads to a reduction in chlorophyll in leaves, which means that the leaves are less green [43,44], as shown in T1. Chlorophyll is an important photosynthetic pigment and the leaves’ appearance represents the rate of photosynthesis. Furthermore, the chlorophyll content directly relates to the leaves’ area. A larger leaf area represents a higher photosynthetic rate as there is a larger area for absorbing light [44]. The T2 and T3 groups presented significantly higher photosynthetic rates than the T1 group, and a similar trend was seen for the leaf area. Kučerová et al. [45] demonstrated that both nitrate concentration and hydrogen peroxide are responsible for higher photosynthesis rates. Nitrogen is a fundamental constituent of chlorophyll, which correlates with the photosynthetic rate, carboxylation enzymes, and protein membranes [46]. Correspondingly, the lowest photosynthesis rate was observed in the T1 group. The stomatal conductance of the green oak cultivated in the T2 and T3 groups was significantly higher than in the T1 group. Many studies have indicated that stomatal conductance is a physiological plant mechanism that plays an important role in photosynthetic processes [47,48]. Saberi et al. [49] also showed the positive effects of cold plasma technology under haze conditions on the photosynthetic rate, chlorophyll content, and stomatal conductance of wheat, which increased by 34%, 32%, and 93%, respectively. As a result, nitrate content from any source (e.g., normal nitrate nutrient solutions and plasma nitrate) is essential to most growth parameters, including plant height, root length, leaf area, photosynthetic rate, and dry fresh weight.

Table 4.
Leaves color intensity, photosynthesis rate, and stomatal conductance of hydroponically grown lettuce nutrient solution without nitrate (T1), normal nitrate (T2), and plasma nitrate (T3) at 42 days.
Non-significant (p > 0.05) differences in the fresh- and dry-weight yields were observed between T2 and T3 (Table 5). However, plants cultivated with a nutrient solution without nitrate had significantly lower fresh and dry weights compared to the T2 and T3 groups (p < 0.05). Nitrogen is known to be the mineral element that is most necessary for plant growth, and nitrate (NO3−) is the major form in which plants absorb nitrogen. Several studies indicate that plasma-activated water can be used as a nitrogen fertilizer as it generates nitrogen species (e.g., NO2− and NO3−) [50,51]. The effects of plasma-activated water on the germination rate, growth characteristics, and total phenolic and flavonoid contents of mung bean sprouts were investigated by Fan et al. [52]. Their results show a remarkable increase in growth characteristics (i.e., stem length and average weight) with PAW15 (with a discharge time of 15 s). The positive effects of PAW treatment on mass accumulation could arise due to the stimulation of protein synthesis [53]. However, in our research, we found non-significant differences in the effects on plant morphology and growth characteristics between T2 and T3. The reason for this could be that the nitrate concentration is equivalent to the normal nitrate nutrient solution and the plasma nitrate.

Table 5.
Yields of hydroponically grown lettuce cultivated in nutrient solution without nitrate (T1), normal nitrate (T2), and plasma nitrate (T3) at 21 DAT.
3.3. Nutritional Quality Analysis
Leafy vegetables, especially lettuce, are considered to be a notable nitrate-accumulating species [54]. By analyzing the NO3− content in the aboveground and underground parts of green oak lettuce, the plants cultivated in normal nitrate nutrient solution (T2) were shown to exhibit the highest accumulation of NO3− content (p < 0.05), as shown in Table 6. However, high nitrate content poses a risk to human health and the surrounding environment [54]. Interestingly, lettuce cultivated in the T3 group showed significantly lower nitrate concentrations than those of the T2 group, even though the initial nitrate concentrations of the fertilizers were not significantly different. This result could indicate that NO3-N produced using cold plasma technology (T3) is rapidly assimilated into an organic form such as free amino acids, as shown in Table 7; therefore, the NO3-N accumulation in the vacuole was lower than in the T2 group. This result has implications that may be beneficial to the environment and to human health.

Table 6.
Nitrate concentration, total nitrogen, and total phenolic in grown lettuce at 42 days.

Table 7.
Concentration of amino acids (nmol/mg) in green oak lettuce after being cultivated in nutrient solution without nitrate (T1), normal nitrate (T2), and plasma nitrate (T3).
The results of the present study show that the lettuce cultivated with T2 had levels of total phenolic compounds 0.35 ± 0.05 mg GAE/g FW higher than that cultivated with T3 by 0.28 ± 0.05 mg GAE/g FW (p < 0.05), as shown in Table 6. Phenolic compounds, one of the most abundant groups of secondary metabolites found in vegetables, contain several thousand compounds. Several environmental factors, including temperature, light, and nutrient management, are responsible for increasing the biosynthesis of phenolic compounds in plants [55]. Nitrogen, an important nutrient in plants, plays a critical role in regulating phenolic accumulation in plants [56]. Previous research has studied the effect of PAW on the total phenolic compounds in apples [57], mung bean sprouts [58], and rocket leaves [59]. Most of this research indicates that phenolic accumulation depends on plasma exposure time, whereby longer treatment times lead to lower levels of phenolic compounds [50,57]. Since PAW has stronger oxidation abilities with increasing exposure time, the rapid oxidation of phenols causes an immediate reduction in the phenolic content levels [60]. In the present work, plasma nitrate did not induce phenolic compounds in plants to the same extent as commercial fertilizers do (i.e., normal nitrate nutrient solution); however, our results still indicate an effective growth enhancement. Therefore, further research should investigate the suitable conditions for generating plasma nitrate to enhance total phenolic accumulation in plants.
The effects of plasma nitrate (T3), especially the role of NO3− species and reactive oxygen species, the nutrient solution without nitrate (T1), and normal nitrate (T2) on the concentration of 17 free amino acids were determined and are shown in Table 7. The results illustrate that threonine, serine, glutamic acid, alanine, tyrosine, phenylalanine, lysine, and arginine have significantly higher concentrations in T3 than in T2 (p < 0.05). Zhou et al. [61] showed that RNS generated from PAW, especially NO3−, was assimilated by plant enzymes, leading to the generation of organic nitrogen compounds, such as free amino acids, in accordance with the assimilation pathway of nitrate. Therefore, nitrate content generated from plasma nitrate might more easily transform into amino acids. However, green oak lettuces cultivated in the T3 group showed significantly lower levels of proline, glycine, methionine, isoleucine, leucine, and histidine than those in the T2 group (p < 0.05). Han et al. [62] explained that cold plasma treatment causes the chemical modification of amino acids through hydroxylation, dehydrogenation, nitration, and dimerization, leading to the degradation of sulfur-containing and aromatic amino acids. Interestingly, very small amounts of methionine were present in the green oak cultivated with T1 (Table 7). Methionine, a sulfur-containing amino acid, is an essential component for the synthesis of all proteins. Wilson et al. [63] also indicated that both nitrogen and sulfur affect the synthesis of amino acids.
4. Conclusions
Air and oxygen were applied using a pinhole plasma jet with a flow rate of 1.5 L/min and discharged for 16 h to generate a nitrogen fertilizer with a nitrate concentration of approximately 800 mg/L. Plant height, root length, canopy width, leaf width, number of leaves, photosynthesis rate, and stomatal conductance of green oak lettuce cultivated in plasma nitrate solution compared to those grown in the normal nitrate nutrient solution were not significantly different, except in respect of leaf area and leaf greenness. Similarly, the yields, including the fresh and dry weights of plants cultivated in plasma nitrate, were the same as those of the plants cultivated in commercial nitrate. Significantly lower levels of chemical compositions (including nitrate concentrations in the underground and aboveground parts and the total phenolic compounds) were found in green oak irrigated with plasma nitrate than in that grown with a normal nitrate nutrient solution (p < 0.05). The presence of fewer nitrate residues in the green oak samples indicates a reduced threat to human health and to the environment. Moreover, plasma nitrate was rapidly assimilated to free amino acids, resulting in significantly higher concentrations of amino acids including Thr, Ser, Glu, Ala, Tyr, Phe, Lys, and Arg than in the normal nitrate nutrient solution. These results could indicate that plasma-activated water can be used as an alternative nitrogen fertilizer, as well as an innovative environmentally friendly technology. However, the nitrogen assimilation pathway from plasma fertilizers should be further explored in order to understand why the nitrate levels of green oak cultivated in plasma nitrate are lower than those of the normal nitrate nutrient solution. Moreover, the economic effectiveness of this method should be determined to prepare for the adoption of this technology in the commercial sector.
Author Contributions
Conceptualization, S.R., C.I. and S.-n.T.; methodology, S.J., M.I. and S.-n.T.; software, P.S. and S.J.; validation, P.S., S.J. and S.-n.T.; formal analysis, P.S. and S.J.; investigation, P.S. and S.J.; resources, S.R., K.P. and C.S.; data curation, S.J. and M.I.; writing—original draft preparation, P.S., S.J. and M.I.; writing—review and editing, S.R., K.P. and C.I.; visualization, P.S., S.J. and M.I.; supervision, S.R., C.S. and S.-n.T.; project administration, W.S.; funding acquisition, C.S. and S.-n.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding from Fundamental Fund 2022, Chiang Mai University (FF65/117).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This work was supported by H.M. The King Initiative Centre for Flower and Fruit Propagation, Chiang Mai with the research location. The authors also thank all staff members at the Agriculture and Bio Plasma Technology Center (ABPlas), Science and Technology Park, Chiang Mai University, Thailand, (CMU SteP), for their efforts and research support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pornsuriya, C.; Ito, S.; Sunpapao, A. First report of leaf spot on lettuce caused by Curvularia aeria. J. Gen. Plant Pathol. 2018, 84, 296–299. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations (FAOSTAT). Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 15 November 2022).
- Camejo, D.; Frutos, A.; Mestre, T.C.; del Carmen Piñero, M.; Rivero, R.M.; Martínez, V. Artificial light impacts the physical and nutritional quality of lettuce plants. Hortic. Environ. Biotechnol. 2020, 61, 69–82. [Google Scholar] [CrossRef]
- Shohael, A.; Hrisha, A.A.; Ahamed, T.; Khatun, S. An easy and reproducible field to table technology for the production of hydroponics lettuce in Bangladesh International Journal of Agronomy and Agricultural Research (IJAAR). Int. J. Agron. Agric. Res. 2017, 10, 37–47. [Google Scholar]
- Market Analysis Report. Hydroponics Market Size, Share&Trends Analysis Report by Type (Aggregate Systems, Liquid Systems), by Crops (Tomatoes, Lettuce, Peppers, Cucumbers, Herbs), By Region, And Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/hydroponics-market (accessed on 15 November 2022).
- Siringam, K.; Theerawipa, K.; Hlaihakhot, N. Effect of nutrient solution on growth of lettuce (Lactuca sativa L.) cultivated under hydroponic system. Thai Sci. Technol. J. 2014, 22, 828–836. [Google Scholar]
- Hou, C.-Y.; Kong, T.-K.; Lin, C.-M.; Chen, H.-L. The Effects of Plasma-Activated Water on Heavy Metals Accumulation in Water Spinach. Appl. Sci. 2021, 11, 5304. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- van der Hoeven, M.; Kobayashi, Y.; Diercks, R. Technology roadmap: Energy and GHG reductions in the chemical industry via catalytic processes. Int. Energy Agency 2013, 56, 12–16. [Google Scholar]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K. Beyond fossil fuel–driven nitrogen transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef]
- Tanabe, Y.; Nishibayashi, Y. Developing more sustainable processes for ammonia synthesis. Coord. Chem. Rev. 2013, 257, 2551–2564. [Google Scholar] [CrossRef]
- Chu, D.-T.; Vu Ngoc, S.-M.; Vu Thi, H.; Nguyen Thi, Y.-V.; Ho, T.-T.; Hoang, V.-T.; Singh, V.; Al-Tawfiq, J.A. COVID-19 in Southeast Asia: Current status and perspectives. Bioengineered 2022, 13, 3797–3809. [Google Scholar] [CrossRef]
- Sapbamrer, R.; Chittrakul, J.; Sirikul, W.; Kitro, A.; Chaiut, W.; Panya, P.; Amput, P.; Chaipin, E.; Sutalangka, C.; Sidthilaw, S. Impact of COVID-19 Pandemic on Daily Lives, Agricultural Working Lives, and Mental Health of Farmers in Northern Thai-land. Sustainability 2022, 14, 1189. [Google Scholar] [CrossRef]
- Randeniya, L.K.; de Groot, G.J. Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: A review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Perinban, S.; Orsat, V.; Raghavan, V. Nonthermal plasma–liquid interactions in food processing: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1985–2008. [Google Scholar] [CrossRef] [PubMed]
- Park, D.P.; Davis, K.; Gilani, S.; Alonzo, C.-A.; Dobrynin, D.; Friedman, G.; Fridman, A.; Rabinovich, A.; Fridman, G. Reactive nitrogen species produced in water by non-equilibrium plasma increase plant growth rate and nutritional yield. Curr. Appl. Phys. 2013, 13, S19–S29. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhou, L.; Xie, J.; He, C. Plasma-activated water production and its application in agriculture. J. Sci. Food Agric. 2021, 101, 4891–4899. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, R.; Tian, Y.; Su, B.; Wang, K.; Yu, S.; Zhang, J.; Fang, J. Sterilization Efficiency of a Novel Electrochemical Dis-infectant against Staphylococcus aureus. Environ. Sci. Technol. 2016, 50, 3184–3192. [Google Scholar] [CrossRef]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal Effects against S. aureus and Physicochemical Properties of Plasma Activated Water stored at different temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Lan, Q.; Pritchard, H.W.; Xue, H.; Wang, X. Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol. Biochem. 2016, 109, 406–415. [Google Scholar] [CrossRef]
- Mitra, A.; Li, Y.-F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of surface-borne microorgan-isms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Sinclair, A.J.; Cahill, D.M.; Wang, X.; Dai, X.J. Nitrate and hydrogen peroxide generated in water by electrical discharges stimulate wheat seedling growth. Plasma Chem. Plasma Process. 2017, 37, 1393–1404. [Google Scholar] [CrossRef]
- Sarinont, T.; Katayama, R.; Wada, Y.; Koga, K.; Shiratani, M. Plant growth enhancement of seeds immersed in plasma activated water. MRS Adv. 2017, 2, 995–1000. [Google Scholar] [CrossRef]
- Graves, D.B.; Bakken, L.B.; Jensen, M.B.; Ingels, R. Plasma Activated Organic Fertilizer. Plasma Chem. Plasma Process. 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Wu, S.; Thapa, B.; Rivera, C.; Yuan, Y. Nitrate and nitrite fertilizer production from air and water by continuous flow liq-uid-phase plasma discharge. J. Environ. Chem. Eng. 2021, 9, 104761. [Google Scholar] [CrossRef]
- Carmassi, G.; Cela, F.; Trivellini, A.; Gambineri, F.; Cursi, L.; Cecchi, A.; Pardossi, A.; Incrocci, L. Effects of Nonthermal Plasma (NTP) on the Growth and Quality of Baby Leaf Lettuce (Lactuca sativa var. acephala Alef.) Cultivated in an Indoor Hydroponic Growing System. Horticulturae 2022, 8, 251. [Google Scholar] [CrossRef]
- Jamie. Algae in Hydroponics: Types, Causes, Effects, Treating & More. Available online: https://whyfarmit.com/algae-in-hydroponics/ (accessed on 16 November 2022).
- Stephens, O. Algae in Hydroponics: How to Get Rid of and Prevent Its Growth. Available online: https://thehydroponicsplanet.com/how-to-get-rid-of-algae-in-hydroponics-for-good/ (accessed on 15 November 2022).
- Date, M.B.; Rivero, W.C.; Tan, J.; Specca, D.; Simon, J.; Salvi, D.; Karwe, M.V. Effect of Plasma-Activated Nutrient Solution (Pans) on Sweet Basil (O. basilicum L.) Grown Using an Ebb and Flow Hydroponic System. Soc. Sci. Res. Netw. 2022. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y. Nitrate Assay for Plant Tissues. Bio-Protoc. J. 2017, 7, e2029. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Miller, D.D.; Rutzke, M. Atomic absorption and emission spectroscopy. In Food Analysis, 3rd ed.; Kluwer: New York, NY, USA, 2003; pp. 401–421. [Google Scholar]
- Bisergaeva, R.A.; Sirieva, Y.N. Determination of calcium and magnesium by atomic absorption spectroscopy and flame pho-tometry. J. Phys. Conf. Ser. 2020, 1691, 012055. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Shuang, F.-F.; Yang, J.-X.; Jv, Y.-X.; Hu, R.-Z.; Chen, T.; Yao, X.-H.; Zhao, W.-G.; Liu, L.; Zhang, D.-Y. A rapid and efficient method of microwave-assisted extraction and hydrolysis and automatic amino acid analyzer determination of 17 amino acids from mulberry leaves. Ind. Crops Prod. 2022, 186, 115271. [Google Scholar] [CrossRef]
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive nitrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J. Phys. D Appl. Phys. 2020, 53, 223001. [Google Scholar] [CrossRef]
- Al-Sharify, Z.T.; Al-Sharify, T.A.; al-Obaidy, B.W.; al-Azawi, A.M. Investigative Study on the Interaction and Applications of Plasma Activated Water(PAW). IOP Conf. Ser. Mater. Sci. Eng. 2020, 870, 012042. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A. Root or shoot nitrate assimilation in terrestrial vascular plants—Does it matter? Plant Soil 2022, 476, 31–62. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wirén, N. The Root Foraging Response under Low Nitrogen Depends on DWARF1-Mediated Brassi-nosteroid Biosynthesis. Plant Physiol. 2020, 183, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.W.; Park, J.S.; Kim, S.J.; Kim, D.-W.; Kang, W.S. Effect of Plasma-activated Water Process on the Growth and Functional Substance Content of Lettuce during the Cultivation Period in a Deep Flow Technique System. Prot. Hortic. Plant Fact. 2020, 29, 464–472. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shen, M.; Hou, J.; Shao, H.; Dong, Y.; Jiang, J. Improving Seed Germination and Peanut Yields by Cold Plasma Treatment. Plasma Sci. Technol. 2016, 18, 1027–1033. [Google Scholar] [CrossRef]
- Ndiffo Yemeli, G.B.; Švubová, R.; Kostolani, D.; Kyzek, S.; Machala, Z. The effect of water activated by nonthermal air plasma on the growth of farm plants: Case of maize and barley. Plasma Process. Polym. 2021, 18, 2000205. [Google Scholar] [CrossRef]
- Than, H.A.Q.; Pham, T.H.; Nguyen, D.K.V.; Pham, T.H.; Khacef, A. Non-thermal Plasma Activated Water for Increasing Ger-mination and Plant Growth of Lactuca sativa L. Plasma Chem. Plasma Process. 2022, 42, 73–89. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Bačovčinová, M.; Hensel, K. Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters. Appl. Sci. 2021, 11, 1985. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2016, 68, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, S.; Balestra, G.M. The modulation of stomatal conductance and photosynthetic parameters is involved in Fusarium head blight resistance in wheat. PLoS ONE 2020, 15, e0235482. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef]
- Saberi, M.; Modarres Sanavy, M.A.; Zare, R.; Ghomi, H. Improvement of Photosynthesis and Photosynthetic Productivity of Winter Wheat by Cold Plasma Treatment under Haze Condition. J. Agric. Sci. Technol. 2019, 21, 1889–1904. [Google Scholar]
- Rahman, M.M.; Sajib, S.A.; Rahi, M.S.; Tahura, S.; Roy, N.C.; Parvez, S.; Reza, M.A.; Talukder, M.R.; Kabir, A.H. Mechanisms and Signaling Associated with LPDBD Plasma Mediated Growth Improvement in Wheat. Sci. Rep. 2018, 8, 10498. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, Z.; Tounekti, T.; Khemira, H. Cold plasma treatment to release dormancy and improve growth in grape buds: A promising alternative to natural chilling and rest breaking chemicals. Sci. Rep. 2020, 10, 2667. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of plasma-activated water treatment on seed germination and growth of mung bean sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Stoleru, V.; Burlica, R.; Mihalache, G.; Dirlau, D.; Padureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniuga, O.; Patras, A. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-W.; Sung, Y.; Chen, B.-C.; Lai, H.-Y. Effects of Nitrogen Fertilizers on the Growth and Nitrate Content of Lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public Health 2014, 11, 4427–4440. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; McGhie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV light regulates flavonoid metabolism in apple (Malus × domestica). Plant Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, X.; Zhang, Y.; Li, K.; Jin, B.; Lu, L.; Jin, C.; Lin, X. Reduced nitrogen supply enhances the cellular antioxidant potential of phenolic extracts through alteration of the phenolic composition in lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2019, 99, 4761–4771. [Google Scholar] [CrossRef] [PubMed]
- Tappi, S.; Ramazzina, I.; Rizzi, F.; Sacchetti, G.; Ragni, L.; Rocculi, P. Effect of Plasma Exposure Time on the Polyphenolic Profile and Antioxidant Activity of Fresh-Cut Apples. Appl. Sci. 2018, 8, 1939. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Laurita, R.; Gozzi, G.; Tappi, S.; Capelli, F.; Bisag, A.; Laghi, G.; Gherardi, M.; Cellini, B.; Abouelenein, D.; Vittori, S.; et al. Effect of plasma activated water (PAW) on rocket leaves decontamination and nutritional value. Innov. Food Sci. Emerg. Technol. 2021, 73, 102805. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. Technol. 2020, 59, 102256. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, J.-H.; Sun, D.-W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 794–811. [Google Scholar] [CrossRef]
- Wilson, T.L.; Guttieri, M.J.; Nelson, N.O.; Fritz, A.; Tilley, M. Nitrogen and sulfur effects on hard winter wheat quality and as-paragine concentration. J. Cereal Sci. 2020, 93, 102969. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).