Abstract
Heracleum sphondylium L., commonly known as hogweed, common hogweed, or cow parsnip, is an herbaceous plant of the Apiaceae family native to Europe and Asia. This wild edible plant is common in grasslands, herb-rich meadows, hedges, wooded areas, roadsides, and railway embankments and in both waste and cultivated grounds. This review presents both the characteristics and ethnobotany, as well as the findings, technical advances, and potential of hogweed research with the goal of improving and disseminating knowledge regarding the value and potential of this wild edible plant. Current knowledge suggests that H. sphondylium L. shows good potential as a new cash crop, being an interesting food ingredient and also a source of compounds with biological activities. Therefore, hogweed may be proposed as a new horticultural crop, although several aspects of cultivation must be examined before full domestication.
1. Introduction
In the past, the collecting of wild edible plants (WEP) was the only option for survival during famines and chronic poverty [1,2,3,4], and for this reason, they are also known as “phyto-alymurgic plants” [5]. Today, WEP can be considered a great historical and cultural heritage that can restore a link to agrobiodiversity [6] and old gastronomic traditions [7] and improve diets [8]. Several studies have been carried out with the aim of cultivating certain WEP such as Asparagus acutifolius L. [9], Borago officinalis L., Taraxacum officinalis L. [10], Muscari comosum (L.) Mill. [11], and Brassica fruticulosa Cyr. [12]. Apart from these examples, an estimated 30,000 plant species are considered edible; however, nowadays, very few of them are grown as crops or are cultivated on a commercially significant scale.
The genus Heracleum is one of the largest of the Apiaceae family, including about 125 species. Among them, Heracleum sphondylium L. (Figure 1) occurs in most of Europe and parts of Asia and North Africa [13]. This species, commonly known as hogweed, common hogweed, or cow parsnip, is a perennial or biennial herbaceous plant of the Apiaceae family native to Europe and Asia. It is also called “eltrot”; however, this is not a specific common name for this species [14]. The American species H. maximum (also called cow parsnip) is sometimes included as a subspecies of H. sphondylium L.

Figure 1.
Plants of hogweed (Heracleum sphondylium L.). Picture by Eleonora Matarrese.
The morphological similarity of the species within the genus and the difficulty of botanical identification have led to several synonyms and naming issues. For example, the classification of the species now widely known as H. maxima has been inconsistent. In literature, the scientific names H. lanatum, H. maximum, and others are used interchangeably. Before the 2000s, the previous name was more popular; today, the middle name is more popular.
In some parts of the world, H. sphondylium is used as an ingredient for several traditional recipes. For example, borscht derives from an ancient soup originally cooked with stems, leaves, and umbels of common hogweed, which resulted in its Slavic name [15]. The use of this species for a liqueur preparation in France and as food or a food additive in some Asian countries has also been reported [16]. Furthermore, this species is used in traditional medicine as an aphrodisiac, vasodilator, tonic, antihypertensive, and sedative and to treat dysentery, dyspepsia, digestive, and gynaecological problems [17].
Given the increasing scientific interest in H. sphondylium (Figure 2), in this review, its ethnobotany, characteristics, and potential will be presented with the aim of spreading knowledge and prospects for its cultivation as a new cash crop.
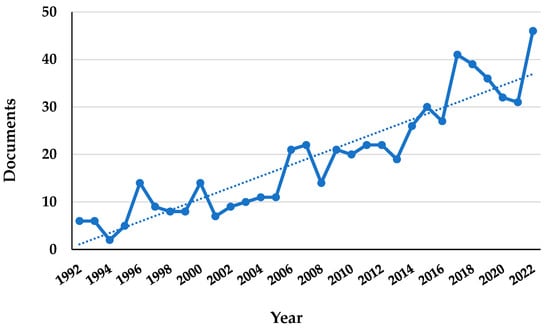
Figure 2.
Increasing number of articles on H. sphondylium published from 1992 to 2022 (data retrieved from Scopus® database). The dashed line indicates the growing trend of the published articles.
2. Methodology
We could not identify any previous reviews focusing on H. sphondylium. Therefore, our review provides an interdisciplinary and international overview of this species. In the first step, we selected our research questions, the bibliographic article databases and websites, as well as the appropriate search terms. Then, we used practical review criteria for the inclusion or exclusion of the relevant literature. In the third step, we developed and applied methodological review criteria. Finally, we grouped data and information for elaboration in the different sections of the review.
2.1. Step 1: Selecting Research Questions, Databases, Websites, and Appropriate Search Terms
Because we could not identify any comprehensive articles on Heracleum sphondylium, our research questions for the review were rather broad: “How is H. sphondylium used?” or “What are the characteristics of H. sphondylium?”. To search the literature, we chose the search terms “Heracleum AND sphondylium” as key terms for searching within the article title, abstract, and keywords by using the Scopus database. We also used Google Scholar to identify unpublished studies, conference proceedings, and similar publications following the recommendation of Tranfield et al. [18] that searches should not be restricted to bibliographic databases. Using the mentioned search terms, we searched the full text of all documents.
2.2. Step 2: Applying Practical Screening Criteria
We included journal papers, books and book chapters, research reports, and conference papers by accepting empirical publications as well as conceptual/theoretical publications. Quality criteria, such as journal rankings, were not used for exclusion purposes because this review aims to give a comprehensive overview of Heracleum sphondylium.
2.3. Step 3: Applying Methodological Screening Criteria
Within the third step, a review protocol for the content analysis of the publications was determined. The review protocol encompassed three sections. The first section contained the bibliographic data of each publication, such as author(s), year and title of the publication, authors’ affiliations, type of publication, and, if it is a journal, the journal’s name. The second section described the methodology of the publication: theoretical/conceptual, empirical, or practical-solution oriented. The third section focused on the content of the publication. For example, if the subject regarded “chemical analysis”, we graded the document as a key source of chemical characterisation information. If the authors described the “uses”, we graded the document as a key source of ethnobotanical knowledge. Moreover, we separately recorded which terms were included (i.e., aromatic compounds, furocoumarins, etc.).
In Step 4 we grouped data and information to elaborate on further in the next sections, also including authors’ opinions, especially opinions regarding the prospects of this species.
3. Species Description
3.1. Distribution and Habitat
H. sphondylium is a plant with distribution in Europe south of 61° latitude, including Great Britain, up to north-western Africa, and both western and northern Asia. The species has also been introduced to suitable habitats elsewhere, such as in North America [19]. Throughout Europe, the distribution is broadly within mean annual temperatures of 5–15 °C and mean annual precipitation of 700–5000 mm. The Mediterranean region represents the southern limit to the distribution of this species, probably because the minimum winter temperatures are too high for the after-ripening requirements of the seeds (Section 7), and the extended drought periods affect seedling survival. In northern Europe, the distribution of this species may be limited by low temperatures, soil fertility, and pollination requirements. Its most common habitat is that of meadows and woods, especially in mountainous areas up to 2500 m, and is an indicator of soils rich in nitrogen [20]. Table 1 reports the classification of common hogweed habitats according to the CORINE biotopes classification [21].

Table 1.
Habitat classifications of H. sphondylium according to the data specifications retrieved from the CORINE biotopes manual [21].
The broad habitat range of H. sphondylium is largely a result of land management activities. The most “natural” habitats include forest or woodland clearings, coastal cliffs and dunes, riverbanks, and tall herb montane grasslands. Common hogweed occurs at a low density in most deciduous and some coniferous forests. In coastal grassland, H. sphondylium grows near the coastal fringe. It is absent from most maritime grasslands and saltmarshes, except on the non-waterlogged extreme landward side. Common hogweed is widespread on Ammophila arenaria dunes, especially in the north and in east Britain. It is characteristically absent from unimproved rank chalk grasslands and from rank grasslands on soils with a pH of less than approximately 4-3. Typical habitats also include hedgerows, road verges, wastelands, meadows, and abandoned pastures [22].
3.2. Morphology and Biology
The common hogweed plant develops from rhizomatous roots with a furrowed stem and bristly hair, growing up to 2 m high (Figure 3A,B).

Figure 3.
Plant details of H. sphondylium: stem (A,B); leaves (C); and shoots (D). Pictures by Eleonora Matarrese.
The leaves are lobed and pinnate with small, serrated segments (Figure 3C). Hogweed has five-petalled pinkish or white flowers arranged in umbels, usually less than 30 cm in diameter, with 15 to 30 rays. The peripheral flowers have a radial symmetry (zygomorphic). The terminal umbels are flat-topped, and the outermost petals are enlarged. Flowering occurs between June and October. The flowering is phased within the umbel, with the outer row of larger flowers on each umbellet opening first. Self-pollination may occur, but the andromonoecious flowers are protandrous, tending to limit this. Anthesis precedes stigma receptivity within any flower by approximately 8–10 days. Geitonogamy is permitted, however, as filament length is relatively long [22]. The earliest flowers to open (i.e., those on the primary umbel) are the most likely to set seed. Most plants show facultative early abortion of carpels. This may result from the over-production of flowers that, due to limited plant resources, never set seed. The flowers are pollinated by insects, such as beetles, wasps, and especially flies. The small fruits are schizocarps, flattened and winged, elliptical to rounded and glabrous, and up to 1 cm long. The seed dispersal is by wind (anemochory) [20].
The typical number of annual seeds produced per plant is highly variable, on average about 850. Germination is epigeal, but the seeds require about eight weeks’ moist after-ripening at <2 °C before they are ready for germination. The cold germination requirement may limit the spread of H. sphondylium in Southern Europe. The seedlings have panduriform cotyledons. Subsequently, the seedlings develop a rosette of mature leaves and a substantial taproot weighing 5–30 g by the beginning of the second year [22].
Nine subspecies may be recognized: ssp. alpinum (L.) Bonnier and Layens, montanum (Schleicher ex Gaudin) Briq., orsinii (Guss.) H. Neumayer, pyrenaicum (Lam.) Bonnier and Layens, sybiricum (L.) Simonkai, sphondylium, ternatum (Velen.) Brummitt, transsilvanicum (Schur) Brummitt, and verticillatum (Pancic’) Brummitt. Two subspecies are native to Britain: H. sphondylium ssp. sphondylium and H. sphondylium ssp. sybiricum. Other subspecies are found chiefly in the Balkans and in the mountains of Europe [22].
Do not confuse the smaller H. sphondylium with the dangerous H. mantegazzianum (giant hogweed) or H. sosnowskyi (Sosnowsky’s hogweed). Giant hogweed typically grows up to 5 m. A mature plant has huge leaves, between 1–1.5 m wide, and a stout, bright green stem with extensive dark reddish-purple splotches and prominent coarse white hairs, especially at the base of the leaf stalk. H. sosnowskyi is also smaller than giant hogweed (growing up to 3 m) and is more commonly found in northern areas of Europe; it is more resilient to harsh conditions than the other two species. The clear watery sap of giant hogweed contains toxins that can cause severe dermatitis (inflammation of the skin). Ultraviolet radiation activates compounds in the sap resulting in severe burns when exposed to the sun. Symptoms occur within 48 h and consist of painful blisters.
H. sphondylium shows a high tolerance to wind exposure and atmospheric salinity. However, plants near the coastal fringe can form semi-succulent leaves due to salt spray, while exposure to grazing and wind reduces the plant to hard stunted rosettes with short leaves [22]. Plants can associate with vesicular-arbuscular mycorrhizas, which have a beneficial role in nutrient uptake, although these associations are facultative [23].
Light requirements are low, considering that H. sphondylium is a common deciduous woodland species, up to light levels of 5% daylight on the forest floor [22].
4. Ethnobotanical Knowledge
The specific term sphondylium means “vertebrate” and refers to the shape of its segmented stem. It was described by Linnaeus in 1753 [3]. Heracleum, on the other hand, is the name of its genus; it derives from ancient Greek Ἡράκλειος (Hērákleios), “of Hera-cles”, with reference to the mythological hero.
The characteristic “farmyard” smell or the observation that pigs can eat hogweed’s foliage and roots are perhaps the origins of its common name in the English language [24]. Hog, “pig”, plus weed, entered the language in 1707; used in various different places for plants eaten by pigs or thought to be suitable for them only.
The small fly Euleia heraclei, known as the “celery fly” or the hogweed “painted-winged fly”, is a species of tephra or fruit fly in the genus Euleia of the Tephritidae family, which is found as the binomial specification suggests on hogweed [25].
Ethnobotanical uses of H. sphondylium L. are summarized in Table 2.

Table 2.
Literature regarding the ethnobotanical uses of H. sphondylium.
In Romania and Morocco, the herbal tea of the aerial parts of hogweed is reputed to be an aphrodisiac and able to treat hypertension [13]. H. sphondylium is known as tavşancılotu and used against dysentery in Turkey [16]. In the 18th century, the inhabitants of the Kamchatka Peninsula distilled a liquor called raka from a “sweet herb” believed to be H. sphondylium [26]; raka was then flavoured with blue berried honeysuckle (Lonicera caerulea).
Borscht is a sour soup common in Eastern Europe and Northern Asia. In English, the word “borscht” is most often associated with the soup’s variant of Ukrainian origin, made with red beetroots as one of the main ingredients, which gives the dish its distinctive red colour [15]. This dish derives from an ancient soup that was originally prepared by using hogweed, which lent the dish its Slavic name. Growing commonly in damp meadows throughout the north temperate zone, hogweed was used not only as fodder (as its English names suggest) but also for human consumption—from Eastern Europe to Siberia to north-western North America. The name borscht ultimately derives from the word бopщ (borshch), which is common to East Slavic languages, such as Ukrainian. Together with cognates in other Slavic languages, it comes from Proto-Slavic *bŭrščǐ, “hogweed”, and ultimately from Proto-Indo-European *bhr ̥ stis, “point, stubble” [15]. Common hogweed was the soup’s principal ingredient before it was replaced with other vegetables, notably beetroot in the Ukrainian version. The English spelling comes from Yiddish (borsht), as the dish was first popularized in North America by Yiddish-speaking Ashkenazi Jews from Eastern Europe. With time, borscht evolved into a diverse array of tart soups, among which the Ukrainian beet-based red version has become the most popular [15]. Its popularity has spread throughout Eastern Europe and—by way of migration away from the Russian Empire—to other continents. In North America, borscht is often linked to either the Jewish or Mennonites, the groups who first brought it there from Europe. Several ethnic groups claim borscht, in its various local guises, as their own national dish consumed as part of ritual meals within Eastern Orthodox, Greek Catholic, Roman Catholic, and Jewish religious traditions [15].
In Eastern European countries and, in particular, Romania, H. sphondylium is used as an aphrodisiac and to treat gynaecological, fertility, and impotence problems. It is also sometimes recommended for epilepsy [19]. In Piedmont, “potions” were commonly prescribed as sedatives for the nervous system. In cooking, spring sprouts were and are used raw in salads or cooked like asparagus. From its seeds, they prepare a liqueur with a pleasant taste [27].
The hogweed was a very common and famous herb in the Renaissance period for fighting depressive crises. The infusion of the root against impotence and frigidity has been known since ancient Egypt [27].
A decoction of roots of the similar species H. asperum or H. leskovii was prepared in Georgia by a local healer in Svaneti as a remedy to purify the body and also used to cure cancer. H. wilhelmsii, another similar species, was foraged, and its roots were used to treat stomach problems [28].
5. Food Uses
Hogweed must be harvested as soon as it blooms but can be dried for later use. It can be used as a fresh vegetable by harvesting its shoots (Figure 3D) as soon as they sprout and before flowering and used in soups or salads. It tastes like asparagus; the crust is somewhat acrid. The stems of the leaves are tied in bunches and dried in the sun until they turn yellow. From these, a sweet substance similar to sugar is formed and is considered a great delicacy. The root can also be eaten cooked; it is usually boiled. This plant can be prepared as an infusion, decoction, or tincture. Hogweed’s young shoots are considered excellent food by many foragers. Hogweed seeds are used as a spice: their flavour is reminiscent of cardamom. If they are harvested unripe, their flavour is reminiscent of mandarins and clementines, with a strong citric scent. The seeds can be macerated in alcohol to prepare a pleasant liqueur. A tincture made from the aerial parts of the plant has also been used to relieve general weakness [20].
In Georgia, Heracleum spp. is harvested and eaten raw, pickled, fermented, and eaten fresh, including the peeled root. The young, fresh stems, called k’ap’i (singular) and k’ap’ebi (plural), are eaten before flowering. The young shoots, just sprouted in spring, are used as fresh food in various alpine regions of western and eastern Georgia. The same is eaten in sour milk (during summer in a recipe called sats’eba). Its leaves have also been put in sour milk and eaten as raw soup. The stems of H. asperum, a similar species, are peeled, and its fresh inner part is eaten, which is said to have a sweet taste. Tradition has it that the shup’q’a, its internal part, had to be eaten within 24 h because it withers the next day [28].
To date, following our gastronomic experiments aimed at the culinary enhancement of H. sphondylium, this species can be proposed as an ingredient in sweet and savoury dishes: hogweed bread (Figure 4); hogweed gelo with percoca peach and wild strawberries in syrup (Figure 5); hogweed and burdock ‘fake artichokes’ (Figure 6); and “Amaretti” with H. sphondylium (Supplementary Materials). Furthermore, thanks to broader ethnobotanical knowledge, we hypothesized several potential uses for hogweed likened to other wild species, especially of the Apiaceae family.

Figure 4.
Hogweed bread. Picture and recipe by Eleonora Matarrese. The full recipe is available in the Supplementary Materials.

Figure 5.
Hogweed gelo with percoca peach and wild strawberries in syrup. Picture and recipe by Eleonora Matarrese. The full recipe is available in the Supplementary Materials.

Figure 6.
Hogweed and burdock ‘fake artichokes’. Picture and recipe by Eleonora Matarrese. The full recipe is available in the Supplementary Materials.
For example, like Angelica archangelica, hogweed can be used to flavour liqueurs and spirits (such as Chartreuse, Bénédictine, Vermouth, and Dubonnet), flavour fish, and in the preparation of absinthe, brandy, and bitters, as well as other culinary uses, such as jams, compotes, jellies, omelettes, and fillings. Its stems, especially if large enough, can be eaten as they are or added to salads; without leaves, they can be crystallized in sugar syrup and used as a decoration for cakes or to make candies.
Hogweed’s fruits can be used in an infusion to stimulate appetite and facilitate digestion in the same way as the wild carrot (Daucus carota L.). While, like Torilis japonica, its roots can be peeled and cooked, either steamed or boiled, and eaten as a vegetable; they can also be used in purées, stuffed, dried, and powdered.
Leaves and roots can be used as a seasoning and to flavour some kinds of cheese, as with Peucedanum ostruthium. Its root cooked in wine is still used today in Switzerland, while the roots and rhizome can be used to prepare aperitifs and liqueurs for digestive purposes. Like Laserpitium latifolium, hogweed can be used together with cumin to season preserved artichokes, as the ancient Romans did.
Hogweed’s fresh leaves can replace both parsley and celery, as happens with Ligusticum mutellina, while dried leaves can be used as tea. Its seeds, with an intensely aromatic flavour, can be mixed with flour to prepare sweets and bread and in the preparation of some kinds of cheese, as with Carum carvi. They give a particular touch to meat dishes (especially pork), and they can also be used to prepare the well-known kúmmel liqueur, aromatic and digestive. Dried leaves of hogweed can also be used as a new spice colourant in culinary preparations, as with Crithmum maritimum L. [29,30].
Like Meum athamanticum, the root of hogweed can be used as an appetite stimulant and digestive and to reduce menstrual pain. Roots can also be added as an ingredient to soups and stews, while leaves can be used to flavour several dishes. Roots can also be used raw, cut into julienne strips with extra virgin olive oil, salt, and apple cider vinegar (or lemon juice), or sautéed in a pan with garlic or chives, as well as in soups, risottos, omelettes, casseroles, quiches, and as a dip and in fillings, as with Bunium bulbocastanum.
Hogweed’s fruits can be combined with porchetta and fatty meats, while its young shoots and leaves are indicated to flavour salads, fish, sauces, and aromatic vinegars, as made with Foeniculum vulgare.
Finally, like chervil (Anthriscus cerefolium), hogweed can be used as a flavouring in vinegar since it gives a very strong citrus scent. Thus, hogweed can also be used to make syrup to prepare refreshing drinks with a citrus flavour.
6. Phytochemistry and Biological Activity
Phytochemical investigations on H. sphondylium showed the presence, in seeds and roots, of furocoumarins (bergapten, isopimpinellin, and heraclenin) [31,32,33] and essential oils [34] (Figure 7). The essential oils consist mainly of monoterpenes, sesquiterpenes, and phenyl-propanoid compounds [22].
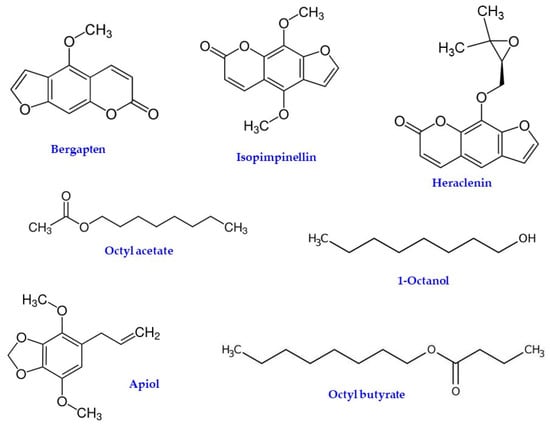
Figure 7.
Chemical structure of some furocoumarins (Bergapten, Isopimpinellin, and Heraclenin) and volatile compounds (Octyl acetate, 1-Octanol, Apiol, and Octyl butyrate) found in H. sphondylium.
Furocoumarins are a class of chemical compounds with phototoxic properties found naturally in many plant species, including some commonly consumed by humans (i.e., grapefruit, carrot, parsnip, turnip, fig, lemon, lime, orange, celeriac, celery, parsley, and dill) [35]. They are synthesized by plants through the fusion of coumarin to a furan ring, generating linear or angular isomers depending on the position of the furan ring. Furocoumarins are produced by plants in response to stress and to defend against predators, such as fungi, bacteria, and insects; furocoumarins react with the DNA of these predators and disrupt replication when exposed to UV light [35]. Furocoumarins have been the focus of much research attention because of their photoactivity. Contact with furocoumarins combined with UV exposure can lead to the development of blistered and burned skin, a reaction known as phytophotodermatitis. However, both the ingestion of and dermal contact with furocoumarin-containing plants enables the absorption of furocoumarins into the bloodstream [35]. The concentration of furanocoumarins in H. sphondylium is much lower than that of the Caucasian hogweeds (H. mantegazzianum—giant hogweed and H. sosnowskyi—Sosnowsky’s hogweed). However, there is evidence that the sap from common hogweed can also produce phytophotodermatitis when contaminated skin is exposed to sunlight [36].
Some biological activities of H. sphondylium L. are reported in Table 3.

Table 3.
Literature regarding the biological activity of H. sphondylium.
The essential oil of H. sphondylium seeds, rich in 1-octanol and octyl butyrate, showed significant antimicrobial activity [16], while octyl acetate and octyl butyrate were the most abundant aliphatic esters identified in the essential oil of the fruits [34].
Senejoux et al. [13] investigated the vasorelaxant effects of a dichloromethane extract of H. sphondylium and the mechanisms involved. The authors showed that this extract exhibited vasorelaxant properties through endothelium-independent mechanisms involving the inhibition of Ca2+ mobilisation and changes in K+ channels conductivity.
In a study aimed to evaluate the phenolic composition in different parts of the H. sphondylium plant, Benedec et al. [40] found high amounts of rutin in flowers (984 mg/100 g) and in leaves (477 mg/100 g). Regarding other flavonoids, quercitrin was found in leaves (15 mg/100 g), and quercetin in flowers (13 mg/100 g); ferulic acid (13.04 mg/100 g) and chlorogenic acid (4.32 mg/100 g) were found in roots. The same authors also found that flower and leaf extracts exhibited the highest antioxidant capacity, according to the phenolic content. In this respect, this plant shows immense potential to be domesticated and used industrially [17].
Uysal et al. [17] investigated the inhibitory action of different extracts of H. sphondylium on key enzymes involved in Alzheimer’s disease, type 2 diabetes, and epidermal hyperpigmentation conditions. Additionally, these authors studied in silico molecular docking to provide additional insight into the interaction of the phenolic compounds identified in the different extracts with the analysed enzymes. The methanol extract, rich in chlorogenic acid, was revealed to be a good inhibitor of AChE. However, docking studies showed that rutin and quercetin possess high binding energy when docked to AChE. Thus, the authors concluded that the observed inhibitory action of the methanol extract on AChE might be due to the synergistic action of the phenolic compounds present [17]. AChE activity and oxidative stress play a major role in the onset and progression of Alzheimer’s disease. In addition, the methanol extract showed strong antimutagenicity against 4-nitro-O-phenylenediamine, a powerful direct-acting mutagen, and 2-amino anthracene, a pro-mutagen [17]. The methanol extract also showed potent antioxidant properties on a battery of in vitro assays as well as moderate antifungal activity against Candida albicans and C. parasilopsis [17].
Antimicrobial activity was also described by other authors. Effectively, the ethanol and aqueous extracts of H. sphondylium showed antibacterial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, Listeria monocytogenes, Shigella, Streptococcus pyogenes, and Corynebacterium diphtheriae, as well as antifungal activity against Candida albicans and C. krusei [37].
The essential oils of H. sphondylium showed good antifungal activity against Candida glabrata, highlighting an inhibitory effect higher than the antifungal agent ketoconazole [38].
Fierascu et al. [39] found that the hydroalcoholic extract of H. sphondylium showed good antioxidant potential and antifungal activity against Aspergillus niger and Penicillium hirsutum, suggesting its use as a natural antifungal agent for the treatment of fruits and vegetables in the postharvest period. The results of this study also suggested the use of the extract as a bio-herbicide [39].
In a study aimed to evaluate the effect of the essential oil of H. sphondylium against four human tumour cell lines, Maggi et al. [34] found that the essential oil showed moderate cytotoxic effects against a human malignant melanoma cell line and a human colon carcinoma cell line.
7. Domestication
Both ethnobotanical knowledge and food use suggest a good potential for the domestication of hogweed, although literature information relating to its cultivation is very scarce and fragmentary.
Seeds of H. sphondylium show an underdeveloped embryo (morphological dormancy—MD), as well as physiological inhibiting mechanisms of germination (physiological dormancy—PD), and thus, it can be described as a species with morphophysiological (MDP) dormancy. In particular, Baskin and Baskin [41] described common hogweed as a species with deep complex MPD, and its seeds would require only cold stratification for loss of PD and MD of the embryo. Effectively, Stokes [42] demonstrated that low temperatures were necessary to make food reserves in the endosperm of H. sphondylium seeds available to the growing embryo. The author found that seeds of H. sphondylium need a period of 9–12 weeks at 2–5 °C to germinate, while at 15 °C, seeds remain dormant. This is because low temperatures stimulate the rapid hydrolysis of endosperm proteins into soluble nitrogenous compounds and the formation of the amino acid glycine and arginine, which, together with soluble sugars, are beneficial for the growth and germination of embryos. On the other hand, at high temperatures (15 °C), soluble nitrogenous compounds are not available, and alanine, which does not stimulate embryo growth, results as the most abundant amino acid [42].
It is important to highlight that the application of gibberellic acid did not stimulate the germination of seeds of H. sphondylium [41]. Therefore, the domestication of hogweed must include a protocol for cold stratification of its seeds in order to overcome dormancy and optimize the percentage of germination. To this end, future research activities could carry out protocols already used for similar species starting from propagation. For example, the propagation protocol for the production of H. maximum provides a treatment of a 72 h water soak of fresh seeds, with the water changed daily. Then, seeds are placed into 100 days of cold, moist stratification, i.e., in fine mesh bags and buried in moist peat moss in a ventilated container under refrigeration at 1 to 3 °C [43]. This protocol describes soaking in water as necessary to leach out inhibitors on seed coats. It is well known that breaking MPD requires embryo growth and/or differentiation, and the seed must be imbibed for this to happen. It is not a case that MPD is frequent in parts of the world with moist seasonal climates and particularly common in plants (i.e., Heracleum genera) of woodlands or damp grasslands. In our opinion, for H. sphondylium, the seeds soaking in water could also be compared to other treatments, such as their priming into solutions containing glycine and arginine, with the aim to verify a possible faster overcome of dormancy. Furthermore, future research activities could be aimed at evaluating the effects of the photoperiod, light intensity, and different temperatures on hogweed seed germination, as for other species of the Apiaceae family [44,45].
H. sphondylium usually grows in fertile, clay-rich soil with a pH of 6–7, although it can also grow in soils with a pH of about 4.3, provided that they contain adequate amounts of nitrogen, phosphorous, and potassium [22]. Effectively, this species is typically absent on chalk heath and limestone heath soils and also from soils on dolerite and basalt since the availability of nitrogen and phosphorous in these soils is low [22]. Williams [46] first found that the nutrient status of the soil is the most important factor affecting the distribution and yield of this species; the author observed greater biomass levels of hogweed when the soil was enriched using a potassium-based fertilizer. In this regard, future research activities could be carried out to evaluate the effect of fertilisation techniques on the yield and quality of this species. Indeed, some aspects, such as the optimal amounts of fertilizers per area and the different forms of nitrogen, need to be studied with the aim of full domestication of this species. At the same time, we think that the possible implementation of sustainable management of fertilisation (i.e., organic fertilisation or living mulch) [47,48] would be desirable in the context of organic farming and, more generally, from an environmentally friendly point of view. On the other hand, since data about open-field cultivation of hogweed is not present in the literature, other growing aspects, such as plant density and irrigation, should be necessarily studied by experimental trials.
Apart from clay-rich soils, H. sphondylium also grows on the fertile brown loams of Scottish mountains and in continental Europe, in soils rich in nitrogen, as well as in soils of coastal habitats, with moderate salinity derived from sea spray and not by saltwater from inundation [22]. The different habitats in which hogweed grows spontaneously suggest the possibility of its cultivation in marginal areas, from the coast to mountainous areas. In this context, a way to domesticate hogweed without exploiting forest lands could be the forest farming of this species; that is, its cultivation in the forest understory of either established or developing forests [49]. To this end, it is important to cultivate plants that can grow under a forest canopy without negative effects due to the shade of the trees. In a study aiming to evaluate the effects of shade and planting methods on the growth of Heracleum moellendorffii [50], the authors found that shading significantly improved the height growth of the plants (10–20 cm increase) in both unfertilized and fertilized plots with highly moist soil conditions. Results also highlighted that shading improved aboveground production in unfertilized plots, in agreement with the characteristics typical of shade-tolerant species [50]. According to Sheppard [22], light requirements are low for H. sphondylium, although shaded plants have low seed production, producing tall elongate individuals with relatively few umbels. Therefore, future research activities could be aimed at evaluating the effects of shading and other treatments on the growth and yield of H. sphondylium, as for other species of the same botanical genera [50].
However, we cannot overlook the potential risks regarding the domestication of H. sphondylium in the open field due to possible hybridisation between common hogweed and Caucasian ones, especially H. mantegazzianum [51,52,53]. Giant hogweed is native to the western Caucasus region of Eurasia. It was introduced to Britain as an ornamental plant in the 19th century and has also spread to other areas in Western Europe, the United States, and Canada. Its close relatives, Sosnowsky’s hogweed and Persian hogweed, have similarly spread to other parts of Europe. It is important to highlight that the sap of giant hogweed is phototoxic and causes phytophotodermatitis in humans, resulting in blisters and scars. These serious reactions are due to the furanocoumarin derivatives in the leaves, roots, stems, flowers, and seeds of the plant. Consequently, it is considered to be a noxious weed in many jurisdictions.
To this end, the domestication of common hogweed by using a controlled environment (such as greenhouses) could be an effective way to reduce the risk of hybridisation between common hogweed and Caucasian hogweeds. Moreover, the evaluation of soilless cultivation systems for the full domestication of hogweed could also be useful since several studies report the application of these systems to wild edible species. For example, the domestication of Urospermum dalechampii and U. picroides was tested for the first time, using a floating system to evaluate the yield and quality of these species as potential new vegetables for the ready-to-eat production chain [54]. Montesano et al. [55] evaluated the cultivation of potted Crithmum maritimum L., a wild edible halophyte of the Apiaceae family, applying a closed cycle ebb and flow hydroponic system and using a seaweed-based compost as a sustainable peat substitute for the formulation of media mixtures, even up to a complete peat replacement. The protocol for the production of potted H. maximum provides a growing medium composed of 50% milled sphagnum peat, perlite, and vermiculite with controlled release fertilizer for macronutrients (13N:13P2O5:13K2O) and fertilizer for micronutrients (12% S, 0.1% B, 0.5% Cu, 12% Fe, 2.5% Mn, 0.05% Mo, and 1% Zn) [43]. Soilless cultivation systems were primarily developed in response to the excessive spread of soil pathogens; however, they also allow for optimal control of plant growth, high productivity, and product quality, as well as very highly efficient water and fertilizer use. At the same time, consumers remain critical towards soilless-cultivated vegetables, mainly due to the perception of these techniques as unnatural, resulting from artificial growth and consequently characterized by low quality [56]. In our opinion, for H. sphondylium, like for other Apiaceae species, the experimental evaluation of alternative growing media to peat and perlite could be a way towards the domestication and, at the same time, increased sustainability of soilless systems in the perspective of a circular economy [56].
Some studies also proposed the application of soilless cultivation systems for the biofortification of wild edible species. For example, D’Imperio et al. [57] described the boron biofortification of Portulaca oleracea L. through soilless cultivation for a new tailored crop. An increase in the nutritional value of P. oleracea L., also through biofortification with zinc [58] and silicon [59], was carried out using a floating system. Puccinelli et al. [60] described the selenium biofortification of Rumex acetosa L., Plantago coronopus L., and P. oleracea L., grown as microgreens. Other authors investigated the effect of selenium on the growth, yield, and biofortification of Eruca sativa L. grown in a hydroponic system [61]. Biofortification is a process that can be applied to fresh, uncooked vegetables with the primary aim of improving their nutritional quality. Considering the increasing research activities that have been directed towards this technique in the last few years [62], the prospect of soilless cultivation for H. sphondylium, and also for biofortification objectives, could be a further option in the context of biodiversity enhancement.
The soilless cultivation of wild edible plants under an agrivoltaic greenhouse represents another important research topic. The process of co-developing photovoltaic (PV) electricity generation and crop cultivation on the same land is called “agrivoltaics”, where the prefix “agri” refers to the science and technology of producing crops in agriculture, and “voltaic” refers to PV power generation. To this end, Buttaro et al. [63] evaluated the soilless production of the wild rocket (Diplotaxis tenuifolia L.) as affected by greenhouse coverage with PV modules. In this study, the authors suggested the possibility of combining soilless cultivation and solar energy production, highlighting the importance of choosing species that are not negatively affected by shading in terms of the yield and quality of the cultivated product. Effectively, the integration of traditional opaque PV modules in crop cultivation environments caused adverse impacts on crop growth due to the shadow effect, especially when high shading ratios have occurred. H. sphondylium can be considered a species with low light requirements [22]. Therefore, we hypothesize that future research activities can also be aimed at evaluating the adaptability of common hogweed to cultivation under agrivoltaic systems.
8. Prospect
Apart from the traditional and innovative food uses of H. sphondylium, several biological activities (Figure 8) are reported in the literature regarding their non-food uses potentially being exploitable by the pharmaceutical and agri-food industries.
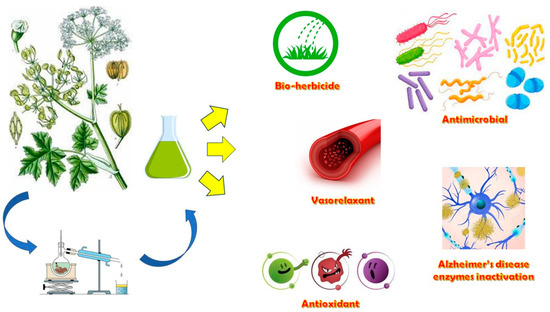
Figure 8.
Graphical representation of the different biological activities attributable to H. sphondylium.
Therefore, in order to evaluate whether hogweed can be regarded as a new horticultural crop with a concrete chance to succeed, a SWOT analysis was performed. SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis comprises the analysis of the strengths and weaknesses of a project, product, place, or person and their relationship with the opportunities and threats of the surroundings. In short, it is a framework for identifying and analysing the internal and external factors that can have an impact on the viability of a project, product, place, or person. SWOT analysis is considered an important decision-making tool and is often used to systematically analyse the internal and external environments of projects, products, and organisations. Table 4 reports the SWOT analysis regarding the exploitation of hogweed as a new cash crop.

Table 4.
SWOT analysis related to the exploitation of H. sphondylium as a new cash crop.
Regarding the strengths, it is important to first specify that hogweed can be considered an ancient and traditional wild vegetable used for making various dishes and processed products. This species of the Apiaceae family shows interesting organoleptic traits and a great diffusion in different habitats, from meadows and woods of mountainous areas to coastal areas, showing good adaptability to different pedoclimatic conditions. This strength makes hogweed a good candidate as a crop for the exploitation of marginal areas such as forest understory and coastal habitats. Of course, its richness in chemical compounds is not of secondary importance. Therefore, the development of a specific agri-food chain based on both fresh and processed hogweed products could favour the development of new markets able to meet the growing demand for functional products. However, it must be highlighted that: (i) there is currently no widespread market for hogweed, and (ii) hogweed knowledge is limited to local areas and a few researchers. These weaknesses, therefore, require specific activities to be carried out in order to disseminate knowledge, promote potential uses, and boost consumer demand. To this end, hogweed exploitation could require multi-disciplinary activities and integrated projects [64].
Thanks to its richness of useful chemical compounds, hogweed seems to be a very promising candidate for both the pharmaceutical and agri-food industries for the production of new functional products. At the same time, the presence of some specific compounds makes this species also interesting for the industrial production of natural herbicides and crop protection products for the post-harvest sector. At any rate, according to Petropoulos et al. [1], a multi-step approach could be needed for hypothesizing the full commercialisation of these new products. In this context, the evaluation of several hogweed populations from different geographical areas should be first carried out in order to select the best chemotypes for specific uses. At the same time, also needed could be an evaluation of the potential differences regarding the bioactive compounds content of plants under cultivation conditions with respect to wild plants, although currently, there are no tested protocols for its cultivation.
Some threats may arise due to the potential resistance of consumers and farmers regarding this species as a functional food and/or new cash crop. This may require a few preventive activities, including clinical trials for evaluating effects on health and a specific marketing project to achieve increased consumer acceptance. Therefore, the establishment of consortia between research institutes, business companies, and governmental organisations aiming to carry out these research and development activities could be a good opportunity. Other threats may arise due to possible hybridisation between common hogweed and the dangerous H. mantegazzianum. For this reason, the domestication of hogweed in controlled environments could be an effective way to reduce this risk. In this context, the application of soilless cultivation systems may be a further opportunity.
9. Conclusions
Current knowledge suggests that H. sphondylium shows good potential as a new horticultural crop, being a refined food and also an interesting source of health compounds, as well as for non-food products. Furthermore, hogweed could be an alternative for both horticultural and industrial crops in the presence of soils in marginal areas, from the coast to mountainous regions. This review also suggests that ethnobotany may offer a source of inspiration for agriculture, as hogweed and, more generally, all wild edible plants have the potential to lead food systems to be healthier, more sustainable, and resilient to climate change in the context of biodiversity enhancement. On the other hand, the interesting characteristics of hogweed and its several uses are known by researchers only through scientific literature and/or by a low percentage of people in niche areas. Therefore, a multi-disciplinary approach and integrated projects should be used for hypothesising the commercialisation of this potential new horticultural crop. Finally, several cultivation aspects will need to be examined before hypothesizing the full domestication of this wild edible plant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9020246/s1, Hogweed recipes related to Figure 4, Figure 5, and Figure 6, as well as to “Amaretti” with H. sphondylium.
Author Contributions
Funding
This study was carried out within the Agritech National Research Center (Spoke 7) and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them. This research was also supported by the Regione Puglia Administration under Rural Development Program 2014–2020, Project ‘Biodiversity of Apulian vegetable species (BiodiverSO Veg)’, Measure 10, Sub measure 10.2, Operation 1 “Program for the conservation and the valorization of the genetic resources in agriculture” (DDS n. 04250182807, CUP: B97H22003760009)—n. 3.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to Patrizia Renna Fray for manuscript editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Licata, M.; Tuttolomondo, T.; Leto, C.; Virga, G.; Bonsangue, G.; Cammalleri, I.; Gennaro, M.C.; La Bella, S. A survey of wild plant species for food use in Sicily (Italy)—Results of a 3-year study in four Regional Parks. J. Ethnobiol. Ethnomed. 2016, 12. [Google Scholar] [CrossRef]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mattirolo, O.; Gallino, B.; Pallavicini, G. Phytoalimurgia Pedemontana.; Blu edizioni: Pevegnano, Italy, 2011; ISBN 9788879041218. [Google Scholar]
- Elia, A.; Santamaria, P. Biodiversity in vegetable crops, a heritage to save: The case of Puglia region. Ital. J. Agron. 2013, 8, 4. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Santoro, R.F.; Heinrich, M. Food for two seasons: Culinary uses of non-cultivated local vegetables and mushrooms in a south Italian village. Int. J. Food Sci. Nutr. 2005, 56, 245–272. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef]
- Benincasa, P.; Tei, F.; Rosati, A. Plant density and genotype effects on wild asparagus (Asparagus acutifolius L.) spear yield and quality. HortScience 2007, 42, 1163–1166. [Google Scholar] [CrossRef]
- Dorais, M.; Papadopoulos, A.P.; Luo, X.; Leonhart, S.; Gosselin, A.; Pedneault, K.; Angers, P.; Gaudreau, L. Soilless greenhouse production of medicinal plants in north Eastern Canada. Acta Hortic. 2001, 554, 297–303. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; La Rotonda, P.; Elia, A. Weed control in lampascione - Muscari comosum (L.) Mill. Crop Prot. 2012, 36, 65–72. [Google Scholar] [CrossRef]
- Branca, F.; Fisichella, A. Response of Brassica fruticulosa Cyr. to greenhouse cultivation. In Proceedings of the VI International Symposium on Protected Cultivation in Mild Winter Climate: Product and Process Innovation 614, Ragusa, Sicily, 8 March 2003; Volume 614, pp. 89–93. [Google Scholar]
- Senejoux, F.; Demougeot, C.; Cuciureanu, M.; Miron, A.; Cuciureanu, R.; Berthelot, A.; Girard-Thernier, C. Vasorelaxant effects and mechanisms of action of Heracleum sphondylium L. (Apiaceae) in rat thoracic aorta. J. Ethnopharmacol. 2013, 147, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Heracleum sphondylium L.—USDA Plants Database. Available online: https://plants.sc.egov.usda.gov/home/plantProfile?symbol=HESP6 (accessed on 24 December 2022).
- Borscht. Available online: https://en.wikipedia.org/wiki/Borscht (accessed on 25 January 2023).
- Işcan, G.; Demirci, F.; Kürkçüoǧlu, M.; Kivanç, M.; Başer, K.H.C. The bioactive essential oil of Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2003, 58, 195–200. [Google Scholar] [CrossRef]
- Uysal, A.; Ozer, O.Y.; Zengin, G.; Stefanucci, A.; Mollica, A.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Multifunctional approaches to provide potential pharmacophores for the pharmacy shelf: Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Comput. Biol. Chem. 2019, 78, 64–73. [Google Scholar] [CrossRef]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a Methodology for Developing Evidence-Informed Management Knowledge by Means of Systematic Review. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Heracleum sphondylium. Available online: https://en.wikipedia.org/wiki/Heracleum_sphondylium (accessed on 26 January 2023).
- Heracleum sphondylium: Sistematica, Etimologia, Habitat, Coltivazione. Available online: https://antropocene.it/2021/05/08/heracleum-sphondylium/ (accessed on 27 December 2022).
- Devillers, P.; Devillers-Tcrschuren, J.; Ledant, J.-P. CORINE Biotopes Manual. Habitats of the European Community. Data Specifications—Part 2.; Office for Official Publications of the European Communities: Luxemburg, 1991; ISBN 92-826-3228-8. [Google Scholar]
- Sheppard, A.W. Heracleum sphondylium L. J. Ecol. 1991, 79, 235–258. [Google Scholar] [CrossRef]
- Dominik, T.; Pachlewski, R. Badanie mykotrofizmu zespolow roslinnych regla dolnego w Tatrac. Acta Soc. Bot. Corum Pol. 1956, 25, 3–26. [Google Scholar] [CrossRef]
- Common Hogweed—Identification, Edibility, Distribution—Galloway Wild Foods. Available online: https://gallowaywildfoods.com/hogweed/ (accessed on 24 December 2022).
- Euleia heracleid. Available online: https://www.eakringbirds.com/eakringbirds4/insectinfocuseuleiaheraclei.htm (accessed on 24 December 2022).
- Anderson, H.A. Berries: A Global History; Reaktion Books: New York, NY, USA; Dover, DE, USA, 2018; ISBN 9781780239385. [Google Scholar]
- Colombo, M.L.; Luciano, R. Ombrellifere Della Provincia di Cuneo; ArabaFenice, 2008; ISBN 9788895853000. Available online: https://www.amazon.co.uk/Ombrellifere-della-provincia-Cuneo-Colombo/dp/8895853008 (accessed on 29 January 2023).
- Bussmann, R.W. Ethnobotany of the Caucasus; Bussmann, R.W., Ed.; Springer: Cham, Switzerland, 2017; ISBN 9783-319494111. [Google Scholar]
- Renna, M.; Gonnella, M. The use of the sea fennel as a new spice-colorant in culinary preparations. Int. J. Gastron. Food Sci. 2012, 1, 111–115. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M.; Caretto, S.; Mita, G.; Serio, F. Sea fennel (Crithmum maritimum L.): From underutilized crop to new dried product for food use. Genet. Resour. Crop Evol. 2017, 64, 205–216. [Google Scholar] [CrossRef]
- Bicchi, C.; D’Amato, A.; Frattini, C.; Cappelletti, E.M.; Caniato, R.; Filippini, R. Chemical diversity of the contents from the secretory structures of Heracleum sphondylium subsp. sphondylium. Phytochemistry 1990, 29, 1883–1887. [Google Scholar] [CrossRef]
- Muckensturm, B.; Duplay, D.; Robert, P.C.; Simonis, M.T.; Kienlen, J.C. Substances antiappétantes pour insectes phytophages présentes dans Angelica sylvestris et Heracleum sphondylium. Biochem. Syst. Ecol. 1981, 9, 289–292. [Google Scholar] [CrossRef]
- Brazdovicova, B.; Kostalova, D.; Strocinska, E.; Tomko, J. Isolation and identification of oroselol and other furocoumarin derivatives from Heracleum sphondylium L. roots. Cesk. Farm. 1982, 31, 346–347. [Google Scholar] [PubMed]
- Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; Vittori, S. Composition and biological activities of hogweed [Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt] essential oil and its main components octyl acetate and octyl butyrate. Nat. Prod. Res. 2014, 28, 1354–1363. [Google Scholar] [CrossRef]
- Melough, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef]
- Paathak, M.A.; Farrington, D.; Fitzpatrick, T.B. The presently known distribution of furocoumarins (psoralens) in plants. J. Investig. Dermatol. 1962, 39, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Dinparast, L.; Zengin, G. The Genus Heracleum: A Comprehensive Review on Its Phytochemistry, Pharmacology, and Ethnobotanical Values as a Useful Herb. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1018–1039. [Google Scholar] [CrossRef] [PubMed]
- Iscan, G.; Ozek, T.; Ozek, G.; Duran, A.; Baser, K.H.C. Essential oils of three species of Heracleum. Anticandidal activity. Chem. Nat. Compd. 2004, 40, 544–547. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Padure, I.M.; Avramescu, S.M.; Ungureanu, C.; Bunghez, R.I.; Ortan, A.; Dinu-Pirvu, C.; Fierascu, I.; Soare, L.C. Preliminary assessment of the antioxidant, antifungal and germination inhibitory potential of Heracleum sphondylium L. (Apiaceae). Farmacia 2016, 64, 403–408. [Google Scholar]
- Benedec, D.; Hanganu, D.; Filip, L.; Oniga, I.; Tiperciuc, B.; Olah, N.K.; Gheldiu, A.M.; Raita, O.; Vlase, L. Chemical, antioxidant and antibacterial studies of Romanian Heracleum sphondylium. Farmacia 2017, 65, 252–256. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press Inc.: San Diego, CA, USA, 1998; ISBN 978-0-12-080260-9. [Google Scholar]
- Stokes, P. A Physiological Study of Embryo Development in Heracleum sphondylium L. Ann. Bot. 1952, 16, 441–447. [Google Scholar] [CrossRef]
- Luna, T. Propagation Protocol for Production of Container (Plug) Heracleum maximum Bartr. Plants 172 mL Conetainers. Available online: https://npn.rngr.net/renderNPNProtocolDetails?selectedProtocolIds=apiaceae-heracleum-5 (accessed on 29 January 2023).
- Nowruzian, A.; Masoumian, M.; Ebrahimi, M.A.; Bakhshi Khaniki, G.R. Effect of Breaking Dormancy Treatments on Germination of Ferula assa-foetida Seed. Iran. J. Seed Res. 2017, 3, 155–169. [Google Scholar] [CrossRef]
- Renna, M. Reviewing the prospects of sea fennel (Crithmum maritimum L.) as emerging vegetable crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef]
- William, E. Botanical Composition of the Park Grass Plots at Rothamsted 1856–1976; Rothamsted Experimental Station: Hertfordshire, UK, 1978. [Google Scholar]
- Fracchiolla, M.; Renna, M.; D’Imperio, M.; Lasorella, C.; Santamaria, P.; Cazzato, E. Living Mulch and Organic Fertilization to Improve Weed Management, Yield and Quality of Broccoli Raab in Organic Farming. Plants 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Fracchiolla, M.; Renna, M.; Durante, M.; Mita, G.; Serio, F.; Cazzato, E. Cover crops and manure combined with commercial fertilizers differently affect yield and quality of processing tomato (Solanum lycopersicum L.) organically grown in puglia. Agriculture 2021, 11, 757. [Google Scholar] [CrossRef]
- Mudge, K.; Gabriel, S. Farming the Woods: An Integrated Permaculture Approach to Growing Food and Medicinals in Temperate Forests; Chelsea Green Publishing: White River Junction, VT, USA, 2014. [Google Scholar]
- Bin, Y.W.; Hernandez, J.O.; Park, B.B. Effects of shade and planting methods on the growth of Heracleum moellendorffii and adenophora divaricata in different soil moisture and nutrient conditions. Plants 2021, 10, 2203. [Google Scholar] [CrossRef]
- Henry, P.; Provan, J.; Goudet, J.; Guisan, A.; Jahodová, Š.; Besnard, G. A set of primers for plastid indels and nuclear microsatellites in the invasive plant Heracleum mantegazzianum (Apiaceae) and their transferability to Heracleum sphondylium. Mol. Ecol. Resour. 2008, 8, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Grzędzicka, E. Invasion of the Giant Hogweed and the Sosnowsky’s Hogweed as a Multidisciplinary Problem with Unknown Future—A Review. Earth 2022, 3, 287–312. [Google Scholar] [CrossRef]
- Niinikoski, P.; Korpelainen, H. Population genetics of the invasive giant hogweed (Heracleum sp.) in a northern European region. Plant Ecol. 2015, 216, 1155–1162. [Google Scholar] [CrossRef]
- Anaclerio, M.; Renna, M.; di Venere, D.; Sergio, L.; Santamaria, P. Smooth golden fleece and prickly golden fleece as potential new vegetables for the ready-to-eat production chain. Agriculture 2021, 11, 74. [Google Scholar] [CrossRef]
- Montesano, F.F.; Gattullo, C.E.; Parente, A.; Terzano, R.; Renna, M. Cultivation of Potted Sea Fennel, an Emerging Mediterranean Halophyte, Using a Renewable Seaweed-Based Material as a Peat Substitute. Agriculture 2018, 8, 96. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M. The evolution of soilless systems towards ecological sustainability in the perspective of a circular economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- D’Imperio, M.; Parente, A.; Montesano, F.F.; Renna, M.; Logrieco, A.F.; Serio, F. Boron Biofortification of Portulaca oleracea L. through Soilless Cultivation for a New Tailored Crop. Agronomy 2020, 10, 999. [Google Scholar] [CrossRef]
- D’Imperio, M.; Durante, M.; Gonnella, M.; Renna, M.; Montesano, F.F.; Parente, A.; Mita, G.; Serio, F. Enhancing the nutritional value of Portulaca oleracea L. by using soilless agronomic biofortification with zinc. Food Res. Int. 2022, 155, 111057. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Santamaria, P.; Serio, F. Silicon biofortification of leafy vegetables and its bioaccessibility in the edible parts. J. Sci. Food Agric. 2016, 96, 751–756. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F. Selenium biofortification of three wild species, Rumex acetosa L., Plantago coronopus L., and Portulaca oleracea L., grown as microgreens. Agronomy 2021, 11, 1155. [Google Scholar] [CrossRef]
- Nascimento, C.S.; Nascimento, C.S.; Lopes, G.; Carrasco, G.; Lupino, P.; Bernardes, A. Biofortified Rocket (Eruca sativa) with Selenium by Using the Nutrient Film Technique. Horticulturae 2022, 8, 1088. [Google Scholar] [CrossRef]
- Renna, M.; D’Imperio, M.; Maggi, S.; Serio, F. Soilless biofortification, bioaccessibility, and bioavailability: Signposts on the path to personalized nutrition. Front. Nutr. 2022, 9, 1–16. [Google Scholar] [CrossRef]
- Buttaro, D.; Renna, M.; Gerardi, C.; Blando, F.; Santamaria, P.; Serio, F. Soilless production of wild rocket as affected by greenhouse coverage with photovoltaic modules. Acta Sci. Pol. Cultus. 2016, 15, 129–142. [Google Scholar]
- Renna, M.; Montesano, F.; Gonnella, M.; Signore, A.; Santamaria, P. BiodiverSO: A Case Study of Integrated Project to Preserve the Biodiversity of Vegetable Crops in Puglia (Southern Italy). Agriculture 2018, 8, 128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).