Abstract
Essential oils have insecticidal activity against several insects and are composed of numerous compounds. This study investigated the insecticidal effects of the Thuja occidentalis L. (Cupressaceae) essential oil, terpinyl acetate, and bornyl acetate against the peach aphid [Myzus persicae (Sulzer)]. The insecticidal effect of essential oils on three Cupressaceae plants was highest in T. occidentalis. The Thuja occidentalis essential oil was extracted by steam distillation, and the optimum conditions were found. The GC-MS profiling of essential oil components extracted from T. occidentalis leaves identified 16 volatile compounds. The main components were α-thujone (16.58%), β-myrcene (14.62%), bornyl acetate (9.31%), and terpinyl acetate (8.52%). As a result of the metabolite profiling of three Cupressaceae plants, terpinyl acetate and bornyl acetate were present in large amounts in all of the essential oils, and they had an acetate structure, so it was estimated that they had insecticidal activity. The insecticidal activity of these two compounds was stronger than the other individual monoterpene compounds. The addition of surfactants to the terpinyl acetate and bornyl acetate showed strong insecticidal activity. Terpinyl acetate and bornyl acetate can be used as environmentally friendly insecticidal-active compounds.
1. Introduction
The use of synthetic pesticide chemicals puts humans and the environment at risk and contributes to economic and ecological challenges worldwide. The search for new effective pesticidal compounds is essential to combat increasing resistance rates [1].
Plants may provide a potential alternative to currently used insecticides and acaricides because they virtually constitute a rich source of bioactive chemicals [2,3]. It has been well-acknowledged that many plant-derived extracts and phytochemicals, such as alkaloids, phenolics, and terpenoids, are potential alternatives to synthetic insecticides [4]. Plant pesticides are generally known to act specifically on pests, are relatively harmless to non-target organisms, including humans, and are biodegradable and harmless to the environment [5,6,7].
Thuja occidentalis L., commonly known as arbor vitae or white cedar, is indigenous to eastern North America and is growing in Europe as an ornamental tree. In folk medicine, T. occidentalis has been used to treat bronchial catarrh, enuresis, cystitis, psoriasis, uterine carcinomas, amenorrhea, rheumatism, chronic upper respiratory tract infections, and antibiotics [8,9,10].
The green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae), is a pest that sucks the sap of leaves and young shoots, causes severe leaf drying, and excretes a honeydew fungus that grows on and reduces the productivity of major crops while transmitting viruses to plants [11]. The treatment of aphid repellents is efficient and can prevent side effects caused by chemical insecticide treatments [12].
The terpenoids available in relatively large amounts as essential oils, resins, and waxes are important to renewable resources and provide a range of commercially valuable products, including solvents, flavorings, fragrances, adhesives, coatings, and synthetic intermediates [13]. Essential oils possess acute toxicity to insects [14], repellent activity [15], and antifeedant activity [16], as well as acute toxicity against adults and larvae [17].
Metabolic profiling has traditionally focused on two main perspectives when used as a tool to study secondary metabolism. First, it has been used to isolate and identify individual and unknown secondary metabolites [18]. Second, metabolic profiling has been used to study secondary metabolism’s molecular aspects [19]. The profiling of volatile plant metabolites has been applied to identify plant-pathogen-associated compounds [20] and interspecies identification [21]. These efforts have often focused on a limited number of secondary metabolites associated with the particular pathway under study, and studies on the insecticidal activity of single components of essential oils have not been extensively conducted.
Essential oils are made up of various compounds. Although several studies have proven that essential oils have insecticidal effects, studies on specific individual components are very scarce [22,23]. Yong et al. [24] analyzed the chemical composition of essential oils extracted from three conifer leaves by GC-MS and profiled three essential oil components with a high antifungal activity. As a result, the compound present in all three species was bornyl acetate and terpinyl acetate. Then, as a result of assaying the antifungal activity of six monoterpenes, it was revealed that the individual monoterpenes with the highest antifungal activity were bornyl acetate and terpinyl acetate.
The isolation of an individual compound has various advantages. The individual compound can be used to identify the precise insecticidal mechanism for insect toxicity and can be used as an economical biological pesticide and a biomarker of valuable plant resources. In addition, the isolation of individual compounds can enhance insecticidal effects and mixing with other compounds can reduce the likelihood of developing insecticidal resistance by target pest populations [25,26,27]. It is known that the combination of individual active compounds present in essential oils may exhibit synergistic or antagonistic effects [28,29]. Therefore, recently, research on the insecticidal effect produced by combinations of terpenes with insecticidal activity has increased significantly. These individual compounds can be used to maximize the effectiveness and specificity in the design of future insecticides with specific or multiple target sites while ensuring economic and ecological sustainability.
This study was conducted under the hypothesis that bornyl acetate and terpinyl acetate may have a high insecticidal effect. Therefore, in this study, the insecticidal effects of the T. occidentalis essential oil, terpinyl acetate, and bornyl acetate, the main monoterpenes of coniferous species, were investigated against M. persicae.
2. Materials and Methods
2.1. Plant Material and Chemicals
The healthy and mature leaves from 30 to40-year-old Chamaecyparis obtusa (Siebold & Zucc.) Endl., Chamaecyparis pisifera (Siebold & Zucc.) Endl. and T. occidentalis were collected from the Experiment Forest of Gyeongsang National University in Korea (GPS 35°09′11” N, 128°06′04” E, altitude 26 m). Three Cupressaceae leaves were harvested in July 2019. The moisture content of the leaves was 62.3%, and leaves were separated from the branches after cutting off branches at a height of 2 m from the ground. The separated leaves were immediately sealed in PE vinyl, and then the essential oil was extracted immediately. Essential oil extraction was performed using the extraction apparatus reported by Yang et al. [30].
The monoterpene used in this study was (+)-2-carene (97%, Sigma-Aldrich Co., St. Louis, MO, USA), (R)-(+)-limonene (97%, Sigma aldrich), 1-decene (94%, Sigma aldrich), and p-cymene (99%, Acros organics).
2.2. Extraction of Essential Oil
The extraction of essential oils from T. occidentalis leaves was performed using a method by Seo et al. [31]. An amount of 50 g of T. occidentalis leaves was cut into 1–2 cm pieces, put into a 2 L round flask containing 500 mL of distilled water, and distilled at 100 °C for 7 h.
The extracted essential oil was measured with a burette attached to the bottom of the extraction apparatus [30]. As for the method used for extracting essential oils, a steam distillation method in which water was heated to volatilize and then cooled to recover the essential oil was used. The equipment used for extraction was the equipment manufactured by Yang et al. [30]. After cutting 100 g of leaves into 2 * 2 cm (width * length), put them into a mixer, grind them for 1 min, and extract 125 g of leaves in an extractor containing 1 L of distilled water for 3 h, then select them, and add them to 1 L of distilled water for 5 h. As heat source equipment, a heating metal with a capacity of 2 kW was used. The essential oil was collected in a burette attached to the extraction device without a separate purification process, and the amount of extracted essential oil was measured with a gauge attached to the extraction device when it had cooled for 1 h after the completion of extraction. The extraction of pre-experimental results was performed once.
The yield of essential oils from the leaves was calculated according to the dry weight of the plant material. The extracted oil was stored in a refrigerator at 4 °C. The resulting oil solution was used for compositional analysis and aphid experiments.
2.3. Chemical Profiling of T. occidentalis Essential Oils through GC-MS
Each essential oil was analyzed on a gas chromatograph (HP5890 SERIES II)—mass spectrometer (GC-MS, HP 5971 SERIES MSD) for metabolite profiling. The GC column was a 60 mm × 0.25 mm × 0.25 μm i. d. HP-1 fused silica capillary column. The GC conditions were as follows: the injector temperature was 250 °C; the column temperature of the isothermal was at 50 °C for 5 min, then programmed to 240 °C for 3 min and held at this temperature for 10 min; the ion source temperature was 230 °C. Helium was used as the carrier gas at 1 mL/min. The effluent of the GC column was introduced directly into the source of the MS. Spectra and was obtained in the EI mode with a 70-eV ionization energy. The sector mass analyzer was set to scan from 50 to 800 amu for 2 s. Compounds were identified by comparison with retention times, and mass spectra were obtained with the authentic standards in the GC-MS system used for analysis. When authentic samples were not available, the identification was carried out by the comparison of mass spectra with those in the mass spectra library [32]. Contents of terpenoid compounds were determined through a relative area (%) on analyzed peaks.
2.4. Insecticidal Activity of Essential Oils of Three Cupressaceae Plants
The insecticidal activity of essential oils, when extracted from the leaves of three Cupressaceae plants (C. obtusa, C. pisifera and T. occidentalis), was tested for the insecticidal effect on aphids by the fumigation method below. As a result, the insecticidal activity of the T. occidentalis essential oil was higher than that of C. obtusa and C. pisifera, so the subsequent study conducted a follow-up study on T. occidentalis essential oils, as follows: the insecticidal activity of essential oils against aphids was measured using the fumigation method.
2.5. Insecticidal Assay of Essential Oil, Terpinyl Acetate, and Bornyl Acetate against M. persicae
For the peach aphid (Myzus persicae Sulzer) used in the insecticidal experiment, the second-generation nymph (wingless aphid) was used. These were provided by the Korea Biochemical Co. (Jinju, Korea), and cabbages were raised as a host. Aphids were reared in acrylic insect breeding cages (L40 × W40 × H50 cm) containing host seedlings under the conditions of a 16 h light: 8 h dark photoperiod, 25 ± 1 °C, and 60 ± 10% relative humidity.
The peach aphids were bred after cabbage leaves more than 6 weeks old after sowing were collected. The M. persicae was reared on a plant leaf under a controlled environmental growth chamber with a 12 h light: 12 h dark photoperiod at 25 °C and 70–80% RH. All bioassays were in the same environmental conditions used to maintain the culture. In all bioassays, the aphids were considered dead when no leg or antennal movements were observed upon gentle prodding with a brush and when aphids showed post-mortem color change.
Six monoterpenes (bornyl acetate, terpinyl acetate, (+)-2-carene, ®-(+)-limonene, 1-decene, and P-cymene) were purchased and tested for their insecticidal effectiveness. The pesticidal activity of essential oils and monoterpenes was tested by fumigation and contact (spray).
For the insecticidal activity test by fumigation, filter paper (Advantec No.2) with a diameter of 70 mm was cut into 2 cm by 4 cm lengths to adsorb the T. occidentalis essential oil along with 6 monoterpene substances at the concentrations of 0, 0.125, 0.25, 0.5, 1, 2, and 5 μL/L.
The impregnated filter paper was attached to a 500 mL plastic glass bottle cap. The plastic cap was then screwed tightly onto the vial. Each bottle contained 20 M. persicae adults. Mortality rates were determined every 3 h after the initiation of exposure until 60 h. All experiments were performed in a growth chamber set at 20 ± 2 °C, 12-= h (dark: bright) photoperiod, and 70% RH. All tests were repeated 5 times, and the insecticidal effect after treatment was measured by counting the immobile dead insects.
Another insecticidal activity was tested with contact toxicity via a spray. T. occidentalis essential oils and monoterpenes (terpinyl acetate and bornyl acetate) were prepared at various concentrations (5%, 10%, 15%, and 20%) in 8% of the Tween-20 solutions. To test the contact toxicity, 20 aphid-attached young cabbage leaves were placed in a plastic container (diameter 15 cm × height 10 cm) lined with filter paper (Advantec No. 2, diameter 90 mm). The insecticide was sprayed 50 cm above the bottom of the plastic container at 15-min intervals. All tests were repeated 5 times, and the insecticidal effect was observed while performing this for 60 min.
2.6. Statistical Analysis
All the above experiments were conducted in five replications. A one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test (DMRT) was applied to evaluate differences among the treatments. When it comes to percentage data, they were transformed into an arcsine value.
3. Results
3.1. Insecticide Activity of Essential Oils of Three Cupressaceae Plants
The insecticidal activity of essential oils extracted from the leaves of three Cupressaceae plants was different for each species (Figure 1). The insecticidal activity of T. occidentalis essential oils was higher than that of C. obtusa and C. pisifera. The T. occidentalis essential oil showed a 60% insecticidal effect for 12 h and a 100% insecticidal effect at 2.0 μL after 18 h of treatment. C. obtusa and C. pisifera essential oils showed a 100% insecticidal effect for 45 h. Therefore, we conducted subsequent studies on T. occidentalis essential oils.
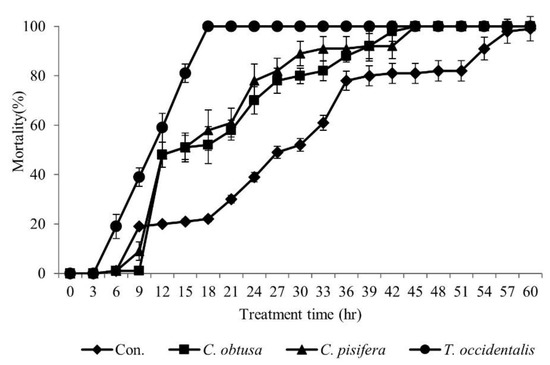
Figure 1.
Insecticidal effect of essential oils from leaves of Thuja occidentalis, Chamaecyparis obtusa, and Chamaecyparis pisifera. The essential oils were prepared at 2.0 μL/L and the insecticidal effect was tested by fumigation method.
3.2. Extraction of T. occidentalis Essential Oils
The essential oil was extracted by the steam distillation method from T. occidentalis leaves. The yield of the essential oil increased according to the extraction time. An optimal extraction was 50 g of leaves added to 500 mL of distilled water and extracted after 7 h at 100 °C (Figure 2).
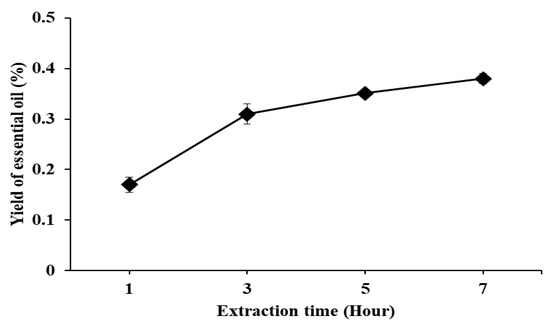
Figure 2.
Yield of Thuja occidentalis essential oil based on extraction time.
3.3. Chemical Profiling of T. occidentalis Essential Oils through GC-MS
The chemical compositions of essential oils obtained from T. occidentalis leaves were analyzed by GC MS (Figure 3). The constituent substances of essential oil were identified by GC-MS in the leaves. (Table 1). The analysis led to the identification of 16 volatiles from essential oils in T. occidentalis leaves. The main constituents were α-thujone (16.58%), β-myrcene (14.62%), bornyl acetate (9.31%), and terpinyl acetate (8.52%). The chemical composition of T. occidentalis essential oils was slightly different compared to the previous study by Yong et al. [24].
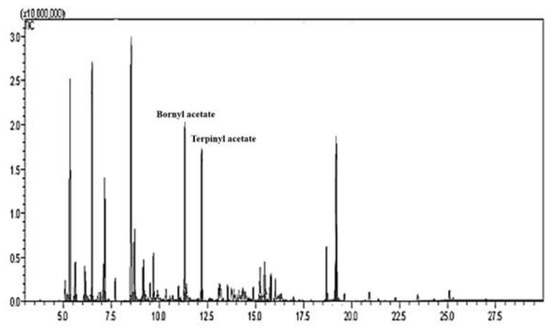
Figure 3.
Gas Chromatogram of essential oils extracted from Thuja occidentalis leaves. The GC column was a 60 mm × 0.25 mm × 0.25 μm i. d. HP-1 fused silica capillary column. The peak numbers correspond to the numbers in Table 1.

Table 1.
Content of volatile compounds in Thuja occidentalis essential oils identified by gas chromatography-mass spectrometry.
3.4. Selection of Putative Insecticidal Compounds from the Essential Oil
An individual compound with insecticidal activity in this study was followed by the metabolite profiling of GC-MS. In a previous study, we investigated the antimicrobial compounds by profiling the essential oil components of Cupressaceae plants [24]. For the measurement of these bioactive compounds, the three terms are designed as follows: the first compound must be contained in all three essential oils; second, the three essential oils must have a large amount of compounds; and thirdly, the biological activity, chemical structure, that is, whether it contains an acetate group was considered. The content of bornyl acetate and terpinyl acetate in the T. occidentalis essential oil was different between C. obtusa and C. pisifera. However, the content of these compounds was high in all three species (Table 1). Through metabolite profiling using GC-MS, terpinyl acetate and bornyl acetate were selected (Figure 4).
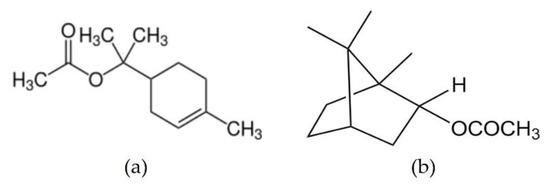
Figure 4.
Putative insecticidal compounds profiled by GC-MS analysis. (a) Terpinyl acetate; (b) Bornyl acetate.
3.5. Insecticidal Activity of T. occidentalis Essential Oils
Different fumigation activities were investigated according to the treatment concentrations (0, 0.125, 0.25, 0.5, 1, 2, 5 μL/L in the air) of the T. occidentalis essential oil. The insecticidal activity of the T. occidentalis essential oil against adults of M. persicae differed according to the treatment concentration (Figure 5). The insecticidal activity of T. occidentalis essential oils was increased concentration-dependently. At the concentration of 0.125 μL/L, the insecticidal effect started to appear after 12 h of treatment, and the mortality rate did not exceed 40%. At concentrations of between 1.0 and 2.0 μL/L, insecticidal activity began to occur 3 h after treatment. The concentration showing the highest insecticidal activity among the treatments was 5.0 μL/L. At 5.0 μL/L, the insecticidal effect appeared immediately after treatment. The mortality rate increased with an increasing treatment concentration.
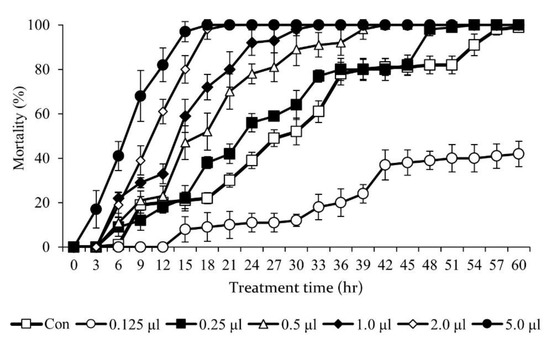
Figure 5.
Insecticidal effect of Thuja occidentalis essential oil at various concentrations against Myzus persicae. The insecticidal effect was tested by fumigation method.
3.6. Insecticide Activity of Terpinyl Acetate and Bornyl Acetate
The insecticidal activity against M. persicae through the fumigation of monoterpene was investigated. The insecticidal test was carried out for 60 h and was monitored every 3 h after treatment (Figure 6). Six types of monoterpenes showed different insecticidal activities. The insecticidal effect was demonstrated 3 h after treatment, and the insecticidal activity increased as time passed. The terpinyl acetate showed 100% lethality after 21 h, and the bornyl acetate showed 100% lethality after 15 h. Limonene showed a strong insecticidal effect at the beginning, but after 15 h, the insecticidal effect was weaker than both the terpinyl acetate and bornyl acetate. Another monoterpene, in the form of cymene, showed 100% lethality after 36 h. Limonene showed 100% lethality after 48 h. In conclusion, terpinyl acetate and bornyl acetate showed the most vigorous insecticidal activity against M. persicae.
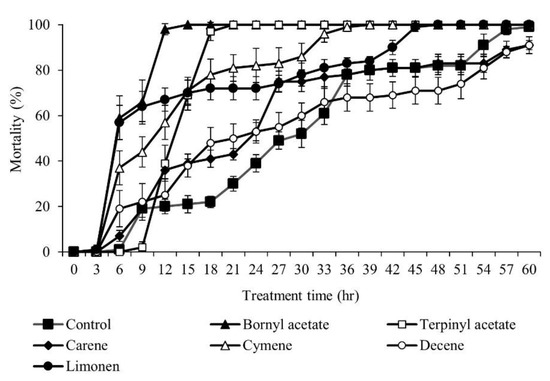
Figure 6.
Insecticidal effect of six monoterpenes against Myzus persicae. Six types of monoterpenes were prepared at 2.0 μL/L and the insecticidal effect was tested by fumigation method.
3.7. Insecticidal Activity of Essential Oil and Monoterpenes with Surfactant
The insecticidal activity was measured for 60 min and monitored every 15 min based on the spray method using various concentrations (5%, 10%, 15%, and 20%) of the T. occidentalis essential oil, terpinyl acetate, and bornyl acetate, each containing 8% Tween 20 (Figure 7). As a result, the treatment agent showed that the highest insecticidal activity was the essential oil. The T. occidentalis essential oil showed an insecticidal effect immediately after spraying, and the mortality rate increased over time. In addition, the insecticidal activity of the T. occidentalis essential oil increased as the treatment concentration increased. The highest insecticidal activity among the treatments was 10% of the T. occidentalis essential oil, which killed 95% in 60 min.
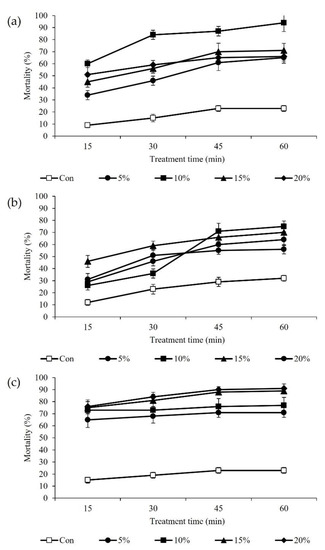
Figure 7.
Insecticidal effect of surfactants mixed with Thuja occidentalis essential oil, terpinyl acetate, and bornyl acetate. (a) Spraying of essential oil with 8% (v/v) tween 20; (b) Spraying of terpinyl acetate with 8% (v/v) tween 20; (c) Spraying of bornyl acetate with 8% (v/v) tween 20; Con: 8% (v/v) tween 20.
The insecticidal activity was investigated according to the concentrations (5%, 10%, 15%, 20%) of terpinyl acetate and bornyl acetate, which are terpenoid components of the T. occidentalis essential oil (Figure 7). These components showed differences in insecticidal activity according to the treatment concentration. In addition, the insecticidal effect on M. persicae increased with increasing concentrations. The terpinyl acetate and bornyl acetate showed an insecticidal effect immediately after spraying, and the longer the treatment time, the higher the activity.
Among the surfactant-containing monoterpenes, the insecticidal effect of bornyl acetate was stronger than that of the terpinyl acetate. In each experiment, the most powerful insecticidal activity was observed in 10% of the terpinyl acetate solution, which killed 75% of the aphids after 60 min, and 20% of the bornyl acetate solution, which killed 90% of the aphids after 60 min. However, there were no significant differences between these treatments (Table 2).

Table 2.
ANOVA test between insecticidal effects of Thuja occidentalis essential oil (10%), terpinyl acetate (10%), and bornyl acetate (20%) with 8% (v/v) tween 20 after 60 min.
4. Discussion
Among the insecticidal effects of the essential oils of three Cupressaceae plants, T. occidentalis had the highest insecticidal effect. The insecticidal activity of the T. occidentalis essential oil has already been studied. Szolyga et al. [34] studied how the insecticidal activity of the essential oil T. occidentalis showed an insecticidal effect when an acetone solution at a concentration of 10 mg/mL was treated to Alphitobius diaperinus Panzer (Coleoptera: Tenebrionidae), and young larvae were more sensitive than older larvae to the tested chemical. In addition, Kéïta et al. [35] reported that the T. occidentalis essential oil had an insecticidal effect on Callosobruchus maculatus.
When the essential oil or monoterpene was fumigated, it started showing insecticidal effects after 3 h, as well as a very large insecticidal effect on aphids after 9–15 h. In the case of T. occidentalis essential oils, the mortality rate was 100% after 15 h, and the C. obtusa and C. pisifera essential oils showed 100% mortality after 45 h. Even in the untreated group, all were killed after 60 h. Often, biological assays for pests are performed in sealed desiccators. In most essential oil insecticidal tests, mortality is usually measured after 24 h [36,37]. In our study, the insecticidal kinetics of insects were identified even after 24 h. After 24 h, the insecticidal activity was less than 40%, which was not significantly different from other studies. However, the 100% mortality in the control treatment after 60 h seems to be due to the small size of the container used at the time of treatment. Pascual-Villalobos et al. [38] also discussed the importance of the experimental container in the insecticidal activity test for essential oils against green peach aphids. However, further research on this is required.
The essential oil from T. occidentalis leaves was extracted by steam distillation, and the yield was found to be affected by extraction time, leaf-to-water ratio, and pretreatment. The highest yield of the essential oil was 0.35%. It is known that the content of essential oils varies from plant to plant by species [39]. The yield of the essential oil of Cupressaceae plants was lower than that of other softwood essential oils. Although the amount of essential oils in the Cupressaceae plants was small, it is known that the insecticidal power was very good [40,41].
The yield of the essential oil was also different from previous research. The yield of T. occidentalis was as low as 0.35%, much lower than that of C. obtusa (4.5%) and C. pisifera (5.3%) [30]. Tumen et al. [42] also stated that the yield of the essential oil was 0.13–0.48 mL/100 g in a pinecone tree, 0.42–0.59 mL/100 g in fir, 0.36 mL/100 g in spruce, and 0.37 mL/100 g in cedar. The difference in refinery yield seems to be a characteristic of endemic species, and it is estimated that there are factors at play, such as the environment.
Secondary metabolites are usually synthesized in plants because they represent the most important defense mechanisms against pathogens. The quantity produced along with their quality may vary depending on plant species, habitat, the organ of production and climatic conditions [43,44].
The main components of the essential oil in T. occidentalis were α-thujone (16.58%), β-myrcene (14.62%), bornyl acetate (9.31%), and terpinyl acetate (8.52%). Szolyga et al. [34] also analyzed the chemical composition of the T. occidentalis essential oil by GC-MS. As a result, the monoterpenoid ketones, including α-thujone (69.8%), β-thujone (9.5%), and fenchone (7.8%), were the main components. The monoterpenoid alcohols, including terpinene-4-ol, p-cymene-8-ol, and diterpenoid beyerene, were present only in small amounts (<3.0%). In addition, Kéïta et al. [35] reported the existence of 22 compounds by analyzing the T. occidentalis essential oil used for the fumigation of insects by gas chromatography. These results showed that the composition of essential oils in this study was somewhat different.
The concentrations of bornyl acetate and terpinyl acetate were different for each essential oil. The T. occidentalis essential oil contained terpinyl acetate (8.52%) and bornyl acetate (9.31%). This content is significantly lower than that of the C. obtusa essential oil (terpinyl acetate at 12.04% and bornyl acetate at 14.45%) and the C. pisifera essential oil (terpinyl acetate at 4.61% and bornyl acetate at 20.43%). Moreover, the chemical composition of the T. occidentalis essential oil in this study was somewhat different from that reported by other researchers. EMEA [45] reported that α-thujone and l-fenchone were the main ingredients, and Wallach [46] reported that α-pinene and 1-borneol (free and acetate) were the main ingredients. Although the concentrations of bornyl acetate and terpinyl acetate in the T. occidentalis essential oil were low, the insecticidal effect was high, indicating the potential of these two substances as strong insecticides.
Among the essential oil components, the strongest insecticidal component was judged to be an acetic acid-based substance. It indicated that both the bornyl acetate and terpinyl acetate had insecticidal activity. Xie et al. [36] also reported that the geranyl acetate showed the strongest insecticidal activity, followed by neryl acetate (LC50 = 0.19 μL/L) and bornyl acetate (LC50 = 0.38 μL/L) for monoterpenes, which showed an insecticidal effect on subterranean termites [Reticulitermes chinensis Synder (Isoptera: Rhinotermitidae)].
Terpinyl acetate and other short-chain esters are natural flavors widely used in food, cosmetic and pharmaceutical industries as food additives, perfumes, odorants, and disinfectants [47]. Bornyl acetate is the acetate ester of borneol. It is used as a food additive as well as a flavoring and odorant. It is a component of essential oils extracted from pine needles and is primarily responsible for odor [48]. The content of bornyl acetate was reported to be as high as about 30% in Pinus uncinata Ramond ex DC. (Pinaceae) essential oil and fir trees [Abies balsamea (L.) Mill. (Pinaceae), A. sibirica Ledeb.] [49]. However, in general, it has been reported that the content of bornyl acetate in essential oils of Pineaceae varies from trace amounts to 15.7% [50].
This variation in the type and concentration of terpenoid components in essential oils can be attributed to environmental factors (climate/weather, soil/nutrient) that can affect the regulation of essential oil biosynthesis [51]. On the other hand, it should be considered that different proportions of the main constituents of various essential oils may vary depending on several factors such as origin, age, plant part, temperature, extraction method, and storage [52]. Indeed, the adaptive metabolism of plants affects the quality, quantity, and chemical composition of plant essential oils, possibly contributing to the creation of unique and specific chemical compositions [53].
Essential oils are compounds of many ingredients. There are few studies on the insecticidal effect of specific components among essential oils extracted from the leaves of the coniferous species. Liu et al. [54] reported that among the components of Artemisia rupestris L. (Asteraceae) essential oils, four constituent compounds, α-terpinyl acetate, α-terpineol, 4-terpineol acetate, and linalool, exhibited contact toxicity to booklice (Psocoptera). Among them, α-terpinyl acetate was reported to have nearly 4.5 times more insecticidal effect than crude essential oil against booklice.
In addition, it was reported that bornyl acetate, a major component of the essential oil in the root of Valeriana officinalis L. (Caprifoliaceae), had insecticidal and repellent effects against Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae) and Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) [37]. Furthermore, bornyl acetate was tested for fumigant activity against adults of Callosobruchus chinensis L. (Coleoptera: Chrysomelidae) and Sitophilus oryzae L. (Coleoptera: Curculionidae) [55].
According to the treatment method of essential oils, it was found that insecticidal activity was different. In the case of this study, it was applied in two ways: fumigation and spray (contact). As a result, the spray had an insecticidal effect in a short time at a high concentration, and the fumigation showed the insecticidal effect slowly while the insecticidal power was continued. The investigation of the pesticidal pathways of natural insecticides is important to explore the most appropriate formulations and delivery means suitable for future commercialization [56]. The insecticidal activity by fumigation and contact seems to be different depending on the type of essential oil. It has been reported that the insecticidal activity of the A. rupestris essential oil is higher by fumigation than by spray (contact) [53]. Many essential oils primarily act as fumigants with additional contact actions. However, in this study, fumigation and spray treatments were not conducted at the same concentration. Further research on the dual fumigant + contact action of the T. occidentalis essential oil is also required.
In general, essential oils can be inhaled, ingested, or absorbed through the cuticle by insects [57]. According to previous studies, the fumigation toxicity of essential oils and its main component, volatile monoterpene, has been reported. Insects were also susceptible to topical application and died within at least 2–3 min.
The insecticidal effects of fumigation on individual ingredients other than essential oils have also been reported. It has also been reported that bornyl acetate has an insecticidal effect upon fumigation and contact. Feng et al. [37] reported that the bornyl acetate, a major component of essential oils from the root of Valeriana officinalis L., had insecticidal and repellent effects on Liposcelis bostrychophila and Tribolium castaneum through fumigation and contact.
Pesticide formulations contain pesticidal active ingredients that control target pests, carriers such as organic solvents or mineral clays, surface-active ingredients such as stickers and dusting agents and/or stabilizers, dyes, and other components such as chemicals for improvement or enhancement [58]. In our study, tween 20 was applied as a surfactant, but if a specialized surfactant is applied in the future, the insecticidal power will be more substantial.
Figure 8 schematically shows the process of searching for a single monoterpene which would have an insecticidal effect on Myzus persicae through chemical profiling from the essential oil of T. occidentalis, which is known to have high antibacterial and insecticidal effects. The essential oil was extracted from T. occidentalis leaves by crushing and steam distillation, and terpinyl acetate and bornyl acetate, which have excellent insecticidal effects, were detected through chemical profiling using GC-MS. These two monoterpenes showed strong insecticidal effects as a result of two insecticidal activity assays: direct contact (spraying) and fumigation. As a result, it was possible to discover an individual compound with biological activity among the essential oil components composed of more than dozens of compounds. These substances showed similar activity to the insecticidal effect of the essential oil and showed an excellent insecticidal effect even with the addition of the surfactant. Finally, this study requires the development of formulations (e.g., sprays, fumigants etc.) that can improve insecticidal power and safety and reduce costs.
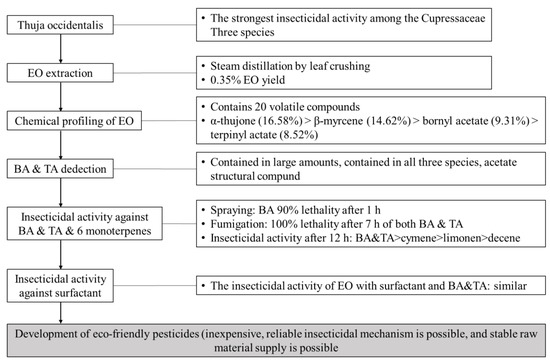
Figure 8.
Schematic diagram for the analysis of the insecticidal effect of Thuja occidentalis essential oil, terpinyl acetate, and bornyl acetate on Myzus persicae. EO—essential oil, BA—bornyl acetate, and TA—terpinyl acetate.
5. Conclusions
This study aimed to identify monoterpenes with potent insecticidal activities against peach aphids (Myzus persicae) through the metabolite profiling of T. occidentalis essential oils composed of various substances. Until now, the development of plant-derived insecticides has been studied for essential oils. However, in the future, for the delivery of natural insecticides and to increase the insecticidal activity through mixing with other insecticides, strong individual insecticidal ingredients will begin to be developed. Terpinyl acetate and bornyl acetate, which were detected through metabolite profiling using GC-MS, showed strong insecticidal activities against peach aphids. It was confirmed that these two compounds can be applied to eco-friendly components through mixing with surfactants. However, although our experiments have demonstrated efficacy on a small scale in the laboratory, a commercial scale analysis will be required. In addition, it is necessary to verify the insecticidal effect of terpinyl acetate and bornyl acetate on various pests and the off-target effect on beneficial insects (bees, parasitoids, and predators). It is also essential to verify a more accurate insecticidal mechanism and efficacy, chemical injury, persistence, environmental impacts, and correlation between cost and efficacy. However, this study is significant in that the T. occidentalis essential oil and individual monoterpenes, namely terpinyl acetate and bornyl acetate, have shown potential as new biological pesticide sources which are expected to be developed as eco-friendly biopesticides in the future.
Author Contributions
Conceptualization, H.-J.S. and M.-S.C.; methodology, S.-H.Y., H.-G.K. and D.-H.K.; resources, K.-B.P.; writing—review and editing, K.-C.S.; visualization, S.-H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This study was carried out with the support of the “Forest BioResource Collection, Conservation and Characteristic Evaluation” of the National Forest Seed and Variety Center” and “The Forestry Science and Technology Research and Development Project (Forest Convergence Specialist Training Project, Project No. FTIS task number 2020186A00-2022-AA02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Harborne, J. Introduction to Ecological Biochemistry, 4th ed.; Academic Press: London, UK, 1993. [Google Scholar]
- Marcic, D. Acaricides in modern management of plant-feeding mites. J. Pest Sci. 2012, 85, 395–408. [Google Scholar] [CrossRef]
- Koul, O. Insect Antifeedants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Adeyemi, M.M.H. The potential of secondary metabolites in plant material as deterents against insect pests: A review. Afr. J. Pure Appl. Chem. 2010, 4, 243–246. [Google Scholar]
- Costa, E.M.; Araujo, E.L.; Maia, A.V.P.; Silva, F.E.L.; Bezerra, C.E.S.; Silva, J.G. Toxicity of insecticides used in the Brazilian melon crop to the honey bee Apis mellifera under laboratory conditions. Apidologie 2013, 45, 34–44. [Google Scholar] [CrossRef]
- Da Silva, I.M.; Zanuncio, J.C.; Brügger, B.P.; Soares, M.A.; Zanuncio, A.J.V.; Wilcken, C.F.; Tavares, W.D.S.; Serrão, J.E.; Sediyama, C.S. Selectivity of the botanical compounds to the pollinators Apis mellifera and Trigona hyalinata (Hymenoptera: Apidae). Sci. Rep. 2020, 10, 4820. [Google Scholar] [CrossRef]
- Johnston, W. Thuja occidentalis L.: Northern White-Cedar. In Silvics of North America; Burns, R., Honkalla, B., Eds.; USDA: Washington, DC, USA, 1990. [Google Scholar]
- Naser, B.; Bodinet, C.; Tegtmeier, M.; Lindequist, U. Thuja occidentalis (Arbor vitae): A Review of its Pharmaceutical, Pharmacological and Clinical Properties. Evid.-Based Complement. Altern. Med. 2005, 2, 69–78. [Google Scholar] [CrossRef]
- Zimmer, M. Gezielte konservative Therapie der akuten Sinusitis in der HNO-Praxis. Therapiewoche 1985, 35, 4024–4028. [Google Scholar]
- Park, B.; Lee, M.-J.; Lee, S.-K.; Lee, S.-B.; Jeong, I.-H.; Park, S.-K.; Jeon, Y.-J.; Lee, H.-S. Insecticidal activity of coriander and cinnamon oils prepared by various methods against three species of agricultural pests (Myzus persicae, Teyranychus urticae and Plutella xylostella). J. Appl. Biol. Chem. 2017, 60, 137–140. [Google Scholar] [CrossRef]
- Kim, K.W.; Jeong, H.C. Repellency of herb plants and essential oils against the green peach aphid, Myzus persicae. J. Korean Soc. Tob. Sci. 2003, 25, 7–11. [Google Scholar]
- Dawson, F.A. The amazing terpenes. Nav. Stores Rev. 1994, 104, 6–12. [Google Scholar]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badawy, M.; El-Arami, S.A.A. Fumigant and Contact Toxicities of Monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their Inhibitory Effects on Acetylcholinesterase Activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, S. Bioactivity of essential oil from Ailanthus altissima bark against 4 major stored-grain insects. Afr. J. Microbiol. Res. 2010, 4, 154–157. [Google Scholar] [CrossRef]
- Huang, Y.; Lam, S.; Ho, S. Bioactivities of essential oil from Elletaria cardamomum (L.) Maton. to Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J. Stored Prod. Res. 2000, 36, 107–117. [Google Scholar] [CrossRef]
- Deb, M.; Kumar, D. Bioactivity and efficacy of essential oils extracted from Artemisia annua against Tribolium casteneum (Herbst. 1797) (Coleoptera: Tenebrionidae): An eco-friendly approach. Ecotoxicol. Environ. Saf. 2020, 189, 109988. [Google Scholar] [CrossRef]
- Blount, J.W.; Masoud, S.; Sumner, L.W.; Huhman, D.; Dixon, R.A. Over-expression of cinnamate 4-hydroxylase leads to increased accumulation of acetosyringone in elicited tobacco cell-suspension cultures. Planta 2002, 214, 902–910. [Google Scholar] [CrossRef]
- Frydman, A.; Weisshaus, O.; Bar-Peled, M.; Huhman, D.V.; Sumner, L.W.; Marin, F.R.; Lewinsohn, E.; Fluhr, R.; Gressel, J.; Eyal, Y. Citrus fruit bitter flavors: Isolation and functional characterization of the gene Cm1,2RhaT encoding a 1,2 rhamnosyltransferase, a key enzyme in the biosynthesis of the bitter flavonoids of citrus. Plant J. 2004, 40, 88–100. [Google Scholar] [CrossRef]
- Prithiviraj, B.; Vikram, A.; Kushalappa, A.; Yaylayan, V. Volatile Metabolite Profiling for the Discrimination of Onion Bulbs Infected by Erwinia carotovora ssp. carotovora, Fusarium oxysporum and Botrytis allii. Eur. J. Plant Pathol. 2004, 110, 371–377. [Google Scholar] [CrossRef]
- Schulz, H.; Krüger, H.; Liebmann, A.J.; Peterka, H. Distribution of Volatile Sulfur Compounds in an Interspecific Hybrid between Onion (Allium cepa L.) and Leek (Allium porrum L.). J. Agric. Food Chem. 1998, 46, 5220–5224. [Google Scholar] [CrossRef]
- Aydin, T.; Bayrak, N.; Baran, E.; Cakir, A. Insecticidal effects of extracts of Humulus lupulus (hops) L. cones and its principal component, xanthohumol. Bull. Èntomol. Res. 2017, 107, 543–549. [Google Scholar] [CrossRef]
- Negahban, M.; Moharramipour, S.; Sefidkon, F. Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J. Stored Prod. Res. 2007, 43, 123–128. [Google Scholar] [CrossRef]
- Yong, S.H.; Song, H.J.; Park, D.J.; Kim, D.H.; Park, K.B.; Choi, M.S. Chemical compositions and antifungal activity against Botrytis cinerea of the essential oils from the leaves of three conifer species. For. Sci. Technol. 2021, 17, 169–179. [Google Scholar] [CrossRef]
- Nauen, R. Insecticide resistance in disease vectors of public health importance. Pest Manag. Sci. 2007, 63, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Combinations of Plant Essential Oil Based Terpene Compounds as Larvicidal and Adulticidal Agent against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2019, 9, 9471. [Google Scholar] [CrossRef] [PubMed]
- Soonwera, M.; Moungthipmalai, T.; Takawirapat, W.; Sittichok, S. Ovicidal and repellent activities of several plant essential oils against Periplaneta americana L. and enhanced activities from their combined formulation. Sci. Rep. 2022, 12, 12070. [Google Scholar] [CrossRef] [PubMed]
- Hummelbrunner, L.A.; Isman, M.B. Acute, Sublethal, Antifeedant, and Synergistic Effects of Monoterpenoid Essential Oil Compounds on the Tobacco Cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef]
- Pavela, R.; Vrchotová, N.; Tříska, J. Mosquitocidal activities of thyme oils (Thymus vulgaris L.) against Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2009, 105, 1365. [Google Scholar] [CrossRef]
- Yang, J.K.; Kang, B.K.; Kim, T.H.; Hong, S.C.; Seo, W.T.; Choi, M.S. Efficient extraction methods and analysis of essential oil from softwood leaves. Korean J. Biotechnol. Bioeng. 2002, 17, 357–364. [Google Scholar]
- Seo, W.T.; Yang, J.K.; Kang, B.K.; Park, W.J.; Hong, S.C.; Kang, Y.M.; Jung, H.Y.; Kim, Y.D.; Kang, S.M.; Kim, S.W.; et al. Extraction and biological activities of essential oil from Thuja occidental Leaves. Korean J. Med. Crop Sci. 2003, 11, 364–370. [Google Scholar]
- McLafferty, F.W. Wiley Registry of Mass Spectral Data, 6th ed.; John Wiley and Sons: New York, NY, USA, 1994. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Szołyga, B.; Gniłka, R.; Szczepanik, M.; Szumny, A. Chemical composition and insecticidal activity of Thuja occidentalis and Tanacetum vulgare essential oils against larvae of the lesser mealworm, Alphitobius diaperinus. Èntomol. Exp. Appl. 2014, 151, 1–10. [Google Scholar] [CrossRef]
- Kéïta, S.M.; Vincent, C.; Schmidt, J.-P.; Arnason, J.T. Insecticidal effects of Thuja occidentalis (Cupressaceae) essential oil on Callosobruchus maculatus [Coleoptera: Bruchidae]. Can. J. Plant Sci. 2001, 81, 173–177. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, K.; Huang, Q.; Lei, C. Evaluation toxicity of monoterpenes to subterranean termite, Reticulitermes chinensis Snyder. Ind. Crops Prod. 2014, 53, 163–166. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Wang, Y.; Chen, Z.-Y.; Guo, S.-S.; You, C.-X.; Du, S.-S. Efficacy of bornyl acetate and camphene from Valeriana officinalis essential oil against two storage insects. Environmental Science amd Polluion Research. 2019, 26, 16157–16165. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Guirao, P.; López, M.D. Fumigant Toxicity in Myzus persicae Sulzer (Hemiptera: Aphididae): Controlled Release of (E)-anethole from Microspheres. Plants 2020, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Salehi, B.; Sharifi-Rad, J.; Matthews, K.R.; Ayatollahi, S.A.; Kobarfard, F.; Ibrahim, S.A.; Mnayer, D.; Zakaria, Z.A.; et al. Plants of the Genus Zingiber as a Source of Bioactive Phytochemicals: From Tradition to Pharmacy. Molecules 2017, 22, 2145. [Google Scholar] [CrossRef] [PubMed]
- Giatropoulos, A.; Pitarokili, D.; Papaioannou, F.; Papachristos, D.P.; Koliopoulos, G.; Emmanouel, N.; Tzakou, O.; Michaelakis, A. Essential oil composition, adult repellency and larvicidal activity of eight Cupressaceae species from Greece against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2013, 112, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.; Rostaefar, A. Insecticidal activity of essential oil from Juniperus communis L. subsp. hemisphaerica (Presl) nyman against two stored product beetles. Ecol. Balk. 2014, 6, 87–93. [Google Scholar]
- Tümen, I.; Hafizoglu, H.; Kilic, A.; Dönmez, I.E.; Sivrikaya, H.; Reunanen, M. Yields and Constituents of Essential Oil from Cones of Pinaceae spp. Natively Grown in Turkey. Molecules 2010, 15, 5797–5806. [Google Scholar] [CrossRef]
- Sonboli, A.; Kanani, M.R.; Yousefzadi, M.; Mojarad, M. Chemical composition and antibacterial activity of the essential oil of Salvia hydrangea from two localities of Iran. J. Med. Plants 2009, 8, 20–28. [Google Scholar]
- Kotan, R.; Kordali, S.; Cakir, A.; Kesdek, M.; Kaya, Y.; Kilic, H. Antimicrobial and insecticidal activities of essential oil isolated from Turkish Salvia hydrangea DC. ex Benth. Biochem. Syst. Ecol. 2008, 36, 360–368. [Google Scholar] [CrossRef]
- The European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Veterinary Medicinal Products—Thuja occidentalis; Summary Report; EMEA: London, UK, 1999. [Google Scholar]
- Wallach, O. Zur Kenntniss der Terpene und der ätherischen Oele. Justus Liebigs Ann. Chem. 1893, 277, 105–154. [Google Scholar] [CrossRef]
- Banthorpe, D.V. Terpenoids. In Natural Products: Their Chemistry and Biological Significance; Mann, J.R., Davidson, S., Hobbs, J.B., Banthorpe, D.V., Harborne, J.B., Eds.; Longman Scientific & Technical, Longman Group: Harlow, UK, 1994; pp. 289–359. [Google Scholar]
- Lee, N.-H.; Lee, S.-M.; Lee, T.-M.; Chung, N.; Lee, H.-S. GC-MS Analyses of the Essential Oils Obtained from Pinaceae Leaves in Korea. J. Essent. Oil Bear. Plants 2015, 18, 538–542. [Google Scholar] [CrossRef]
- Bonikowski, R.; Celiński, K.; Wojnicka-Półtorak, A.; Maliński, T. Composition of essential oils isolated from the needles of Pinus uncinata and P. uliginosa grown in Poland. Nat. Prod. Commun. 2015, 10, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The genus Pinus: A comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Masotti, V.; Juteau, F.; Bessière, J.M.; Viano, J. Seasonal and Phenological Variations of the Essential Oil from the Narrow Endemic Species Artemisia molinieri and Its Biological Activities. J. Agric. Food Chem. 2003, 51, 7115–7121. [Google Scholar] [CrossRef]
- Oliveira, M.; Brugnera, D.; Cardoso, M.; Guimarães, L.; Piccoli, R. Rendimento, composição química e atividade antilisterial de óleos essenciais de espécies de Cymbopogon. Rev. Bras. Plantas Med. 2011, 13, 8–16. [Google Scholar] [CrossRef]
- Liu, X.C.; Li, Y.P.; Li, H.Q.; Deng, Z.W.; Zhou, L.; Liu, Z.L.; Du, S.S. Identification of Repellent and Insecticidal Constituents of the Essential Oil of Artemisia rupestris L. Aerial Parts against Liposcelis bostrychophila Badonnel. Molecules 2013, 18, 10733–10746. [Google Scholar] [CrossRef]
- Zouari-Bouassida, K.; Trigui, M.; Makni, S.; Jlaiel, L.; Tounsi, S. Seasonal Variation in Essential Oils Composition and the Biological and Pharmaceutical Protective Effects of Mentha longifolia Leaves Grown in Tunisia. BioMed Res. Int. 2018, 2018, 7856517. [Google Scholar] [CrossRef]
- Park, I.-K.; Lee, S.-G.; Choi, D.-H.; Park, J.-D.; Ahn, Y.-J. Insecticidal activities of constituents identified in the essential oil from leaves of Chamaecyparis obtusa against Callosobruchus chinensis (L.) and Sitophilus oryzae (L.). J. Stored Prod. Res. 2003, 39, 375–384. [Google Scholar] [CrossRef]
- Kim, S.-I.; Chae, S.-H.; Youn, H.-S.; Yeon, S.-H.; Ahn, Y.-J. Contact and fumigant toxicity of plant essential oils and efficacy of spray formulations containing the oils against B- and Q-biotypes of Bemisia tabaci. Pest Manag. Sci. 2011, 67, 1093–1099. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A. Fumigant toxic activity and reproductive inhibition induced by monoterpenes on Acanthoscelides obtectus (Say) (coleoptera), a bruchid of kidney bean (Phaseolus vulgaris L.). J. Stored Prod. Res. 1995, 31, 291–299. [Google Scholar] [CrossRef]
- Herzfeld, D.; Sargent, K. Chapter 4: Pesticide Formulations. In Private Pesticide Applicator Safety Education Manual, 19th ed.; University of Minnesota Extension: Falcon Heights, MN, USA, 2011; pp. 85–108. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).