1. Introduction
Moringa species (otherwise known as miracle trees or drumstick trees) are tropical deciduous dicotyledonous trees that are distributed throughout Africa and Asia [
1,
2]. All parts of the plant are edible and nutritious, and they are the source of many useful compounds, making them an important famine food and source of medicinal and cleaning compounds for impoverished nations [
3,
4,
5].
The leaves, seeds, and roots of
M. oleifera are well-studied [
1,
6]. The leaves of
M. oleifera are pale-green bipinnate or tripinnate and feathery with opposite ovate leaflets [
1,
6]. The leaves are known to have a broad array of essential nutrients in relatively large concentrations and are, therefore, a common food additive [
3]. The seeds of
M. oleifera are typically brown, roughly almond-shaped, and measure to be roughly 1.9 × 1.1 cm. The seeds are produced in large pods (the ‘drumsticks’) that can grow up to 50 cm in length. The seeds and pods, like the leaves, are high in nutritional quality [
4]. Older roots have a vascular cambium that consists of 6–8 layers; these layers produce roundish vessel elements surrounded by xylem parenchyma cells [
7]. The 3–4 layered phellogen forms rectangular or square-shaped cells, and the walls of the phellum cells are suberized. Additionally, the phelloderm is large and consists of thin-walled parenchymatous cells containing scattered groups of fibers [
7]. The roots contribute to
Moringa’s common classification as a tuber vegetable, as the roots are the most commonly eaten part of the plant [
7]. Furthermore, the root bark is often harvested for various pharmaceutical and ethnobotanical uses [
1,
5,
7].
The stem and bark of
M. oleifera are poorly understood in relation to the other parts of the plant. To date, there has only been one anatomical analysis of mature
M. oleifera stems [
7]. There is, therefore, a gap in the literature regarding the average size and area of stem and cell tissue types across the tree’s stages of growth and a further deficit of anatomical diagrams of stem sections. Each plant tissue type of
M. oleifera has unique anatomical and phytochemical attributes, resulting in unique uses.
Originally native to the sub-Himalayan Mountains of northern India,
M. oleifera has been cultivated for various uses in tropical and subtropical regions around the world [
1]. Some uses of
M. oleifera include biofuel production, water purification, lubrication, leather tanning, and food preparation [
1,
5,
8]. Additionally, this species is a valuable source of phytochemicals, which assist in multiple biological activities, including oxidative DNA damage protection, promoting anti-inflammatory responses, anti-hepatoprotective processes, ulcer recovery, antibiotic immune system responses, antiperoxidative processes, and antiproliferative processes (among others) [
1,
5,
9].
Among the many phytochemicals typically possessed by
M. oleifera are moringine (appears chemically identical to benzylamine) and moringin (4-(α-L-rhamnosyloxy)-benzyl isothiocyanate) [
5]. Moringine is an alkaloid chemical, and its presence in
M. oleifera is the first record of a plant-produced benzylamine [
10]. Moringine is considered to be a toxic compound [
11], with little research elucidating the extent of its toxicity in humans. It has been used in experiments and was found to act as a potassium channel blocker, causing reduced feeding in mice [
12] and resulting in a decrease in plasma-free fatty acids and water intake in rats [
10]. Alternatively, moringin is a sugar derivative that has recently been shown to act medicinally against several pathologies.
M. oleifera bark extracts containing moringin were found to effectively treat rats with aggressive breast and colorectal carcinoma [
13]. Al-Asmari et al. [
13] found that
M. oleifera extracts caused a decrease in cancer cell survival and motility and an increase in malignant cell apoptosis. Moringin has also specifically been shown as promising in the treatment and prevention of ischemic stroke [
14], as well as for increasing apoptosis in neuroblastoma and hepatocarcinoma cells, and as a treatment for multiple sclerosis-induced neuropathic pain [
15,
16,
17].
Paikra et al. [
18] reported that only the leaves of
M. oleifera have been found to contain moringin, while seeds and roots were found to contain moringine. To date, we could not find any specific literature describing the phytochemistry of bark that included both testing for moringin (potentially beneficial) and moringine (potentially harmful).
In natural environments, quantities of secondary metabolites such as moringin and moringine vary with environmental conditions; changes in solar radiation, temperature, nutrient availability, water availability, and biotic competition may all influence chemistry [
5,
19,
20]. In optimal environments, plants tend to invest more energy into growth and reproduction than into protective anti-herbivory measures (such as toxic secondary metabolites) [
5,
19]. Trees growing in optimal environments may produce fewer toxic compounds, such as moringine, as there is little need for anti-herbivory measures. No information currently exists on how levels of moringin or moringine in
M. oleifera may be altered by the environment.
To add to the current literature, we sought to depict the distribution and size of various tissue types within M. oleifera stems grown in a greenhouse under specific environmental conditions (Objective 1). We also sought to evaluate the efficacy of the bark as a source of secondary plant compounds when grown under controlled conditions, considering specifically the presence of moringin and moringine (Objective 2).
2. Methods
2.1. Growing Conditions
All
M. oleifera trees used for this study were grown at the I.K. Barber Enhanced Forestry Lab (EFL), University of Northern British Columbia (UNBC). For the
M. oleifera trees used in anatomical analyses, germination methods were derived from
Moringa Farms [
21]. Seeds of Indian origin were imbibed in a zip-lock bag (left slightly unsealed to allow for airflow) filled with water for 24 h. After the imbibing period, the seeds were removed from the water, dried on paper towel, and placed in a closed paper bag left in a cupboard above a stove to provide a dark warm germination environment. After 14 days, most of the seeds had germinated, and the seeds were planted 2 cm deep into soil. The soil mixture consisted of 107 L peat, 20 L perlite, 20 L vermiculite, 1/3 c coir soil enhancer, 1/3 c dolomitic lime, 1/3 c MicroMax nutrients, and 2/3 L slow-release nutrients (14-14-14). The trees were watered to saturation approximately twice weekly with plain water for the first month and with water containing 14-14-14 fertilizer at every watering thereafter, as signs of nutrient deficiency, including chlorosis, were noticed after 1 month of growth. During watering, the trees were monitored for signs of diseases to ensure none were present. The trees were grown in a growth chamber at 30 °C during the day (0600 to 2200 h) under LED grow lights and cooled to 28 °C between 2200 h and 0600 h with the lights turned off. After 4 months of growth, the trees were cut down and immediately underwent anatomical analysis.
The methods used for growing the trees utilized in chemical analyses were developed by Morgan et al. [
8]. Seeds were planted 2.54 cm deep into a soil mixture prepared by mixing 20 L coir, 20 L coarse sand, and 20 L peat with 60 g of slow-release nutrients (14–14–14); there was also an addition of 3 tablespoons of dolomite to the soil prior to planting. High-pressure sodium (HPS) supplemental lighting was supplied to the trees each day between 0600 to 2200 h. The trees were kept at 24 °C within the housing greenhouse bay and maintained at a relative humidity ranging from 20 to 40%. During the night (2200–0600 h), the lights were turned off and the bay was cooled to 18 °C. The trees were watered approximately three times per week to the point of saturation. These procedures were followed for 7 months, at which point the trees were cut down and frozen until the bark could be peeled from the stems. The bark was then kept frozen until the time of phytochemical analysis.
2.2. Anatomical Analysis
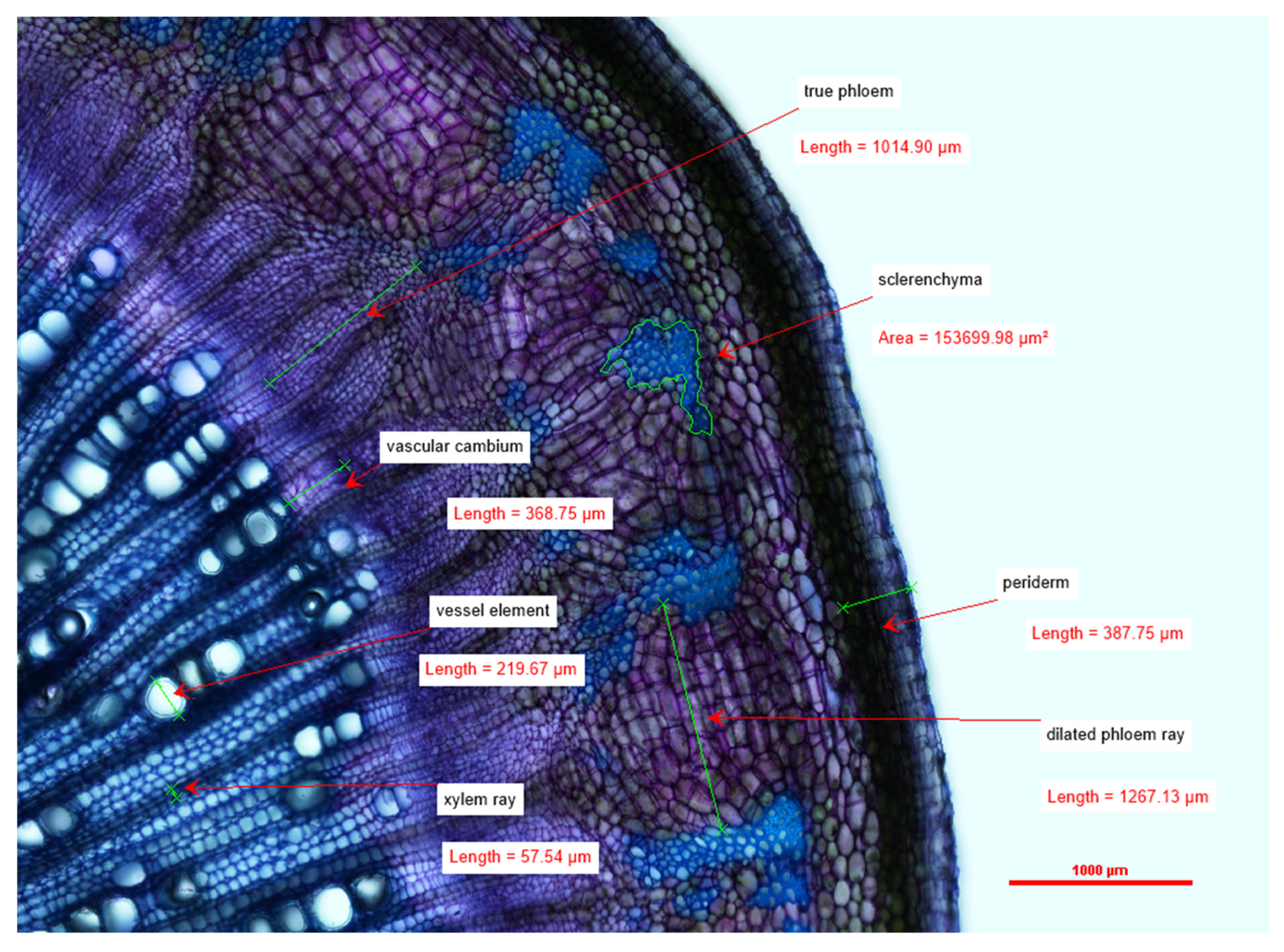
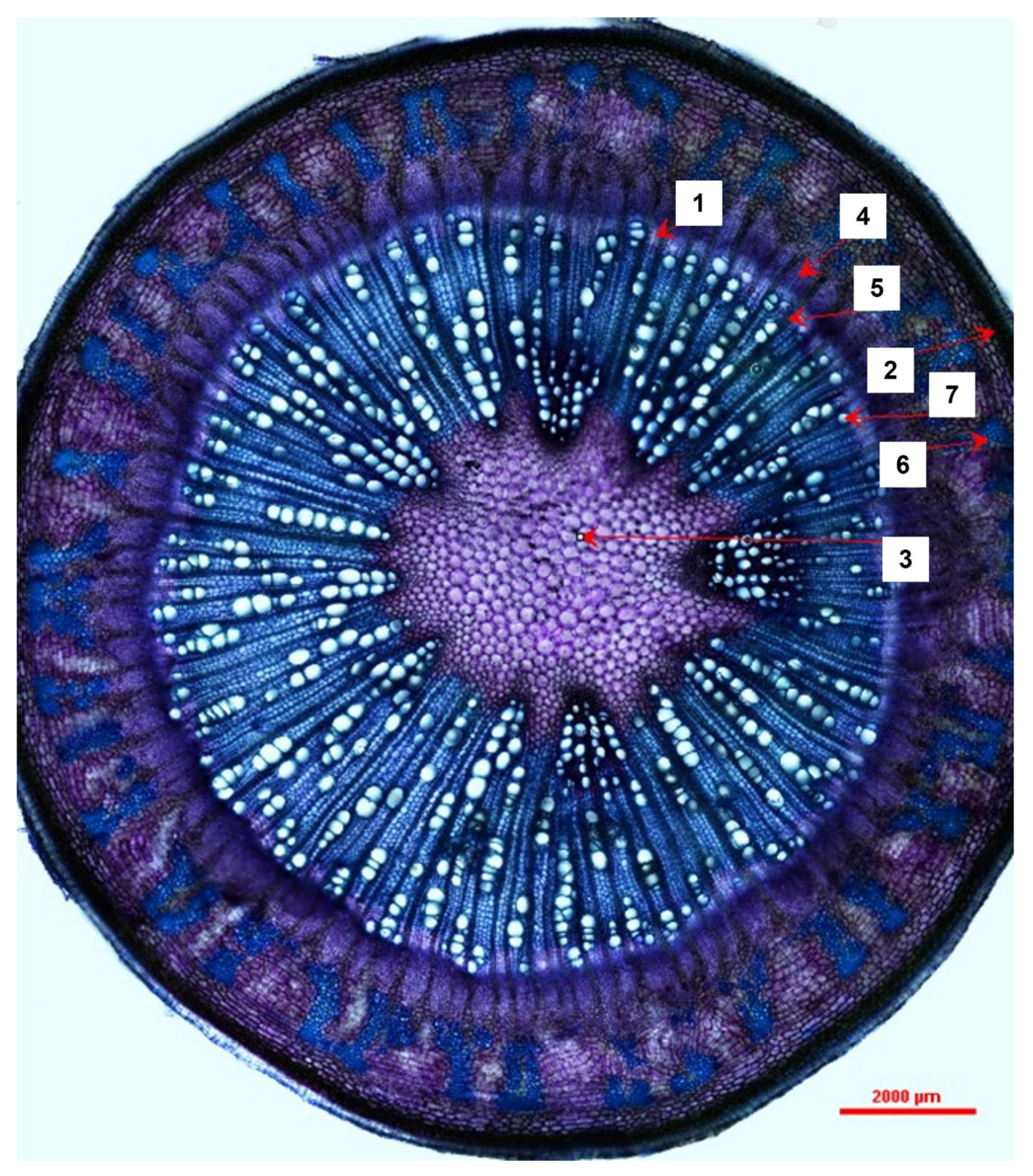
Cross-sections were obtained from five freshly harvested 4-month-old M. oleifera plants by hand using a razor blade. The cross-sections were stained with toluidine blue (TBO) and then photographed and examined using a DS-Ri2 Eclipse FN1 Nikon light microscope under 40× magnification. The cross-section images were analyzed using NIS-Elements Basic Research (v.5.10.01 64-bit) software. Tangential width measurements (at the widest point, from the outside of the cell wall on one side to the other) were recorded for dilated phloem rays, vessel elements, and xylem rays. Radial width measurements were recorded for the periderm, true phloem tissue, and vascular cambium (at the widest point, from the outside of the cell wall on one side to the other). Lastly, small, localized pockets of sclerenchyma tissue within the cortex were measured by tracing the perimeter of the cell walls; the imaging software then algorithmically calculated the estimated area within the tracing. Raw data measurements were collected and loaded into Microsoft Excel.
2.3. Chemical Analysis
The extraction methods used were derived from Oluduro et al. [
22]. The bark was removed from 19 frozen samples of 7-month-old
Moringa. Once the bark had been removed, it was ground using liquid nitrogen and an IKA A11 basic analytical mill. The mill was cleaned between samples to avoid cross-contamination. Once the bark was ground, ethanolic extracts were prepared for each of the 19 samples. A total of 5 g (±0.10 g) of plant tissue (with an average moisture content of 39.7 % (SE = 1.39 %, CV = 0.111) were mixed with 100 mL of 90% ethanol. The solutions were then left at room temperature and shaken for 30 min a day at 120 rpm for 3 days and then left undisturbed at room temperature for an additional 2 days. Once the extraction time had fully elapsed, the suspension solutions underwent gravity filtration through #1 Whatman paper until clear (at least two filtrations were necessary). The extractions were then rotovapped at room temperature to remove some of the excess ethanol and water. The concentrated extracts were then filtered through 0.45 µm microfilters via syringe. The finished extracts were then stored in air-tight 2 mL vials at 4 °C.
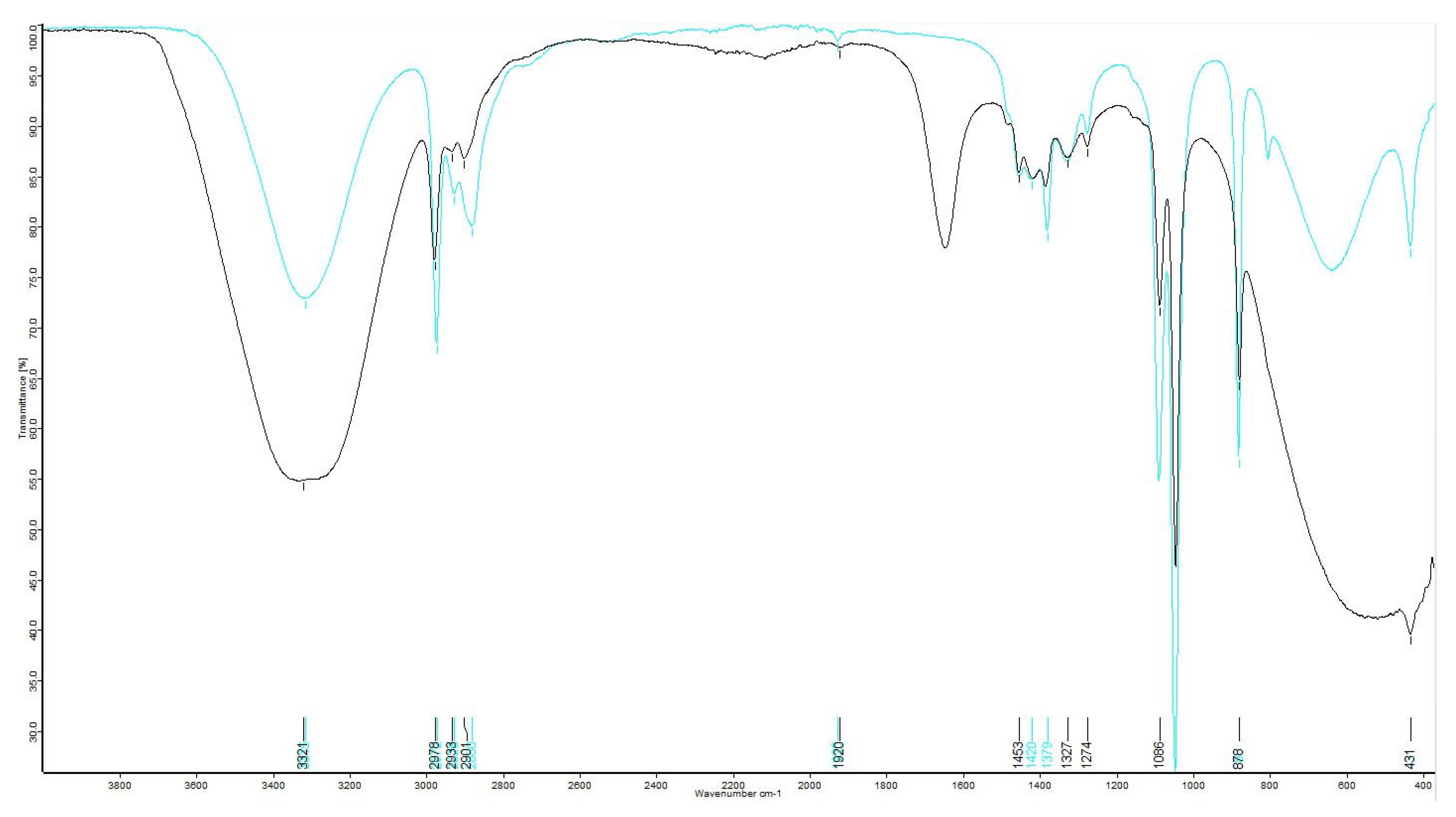
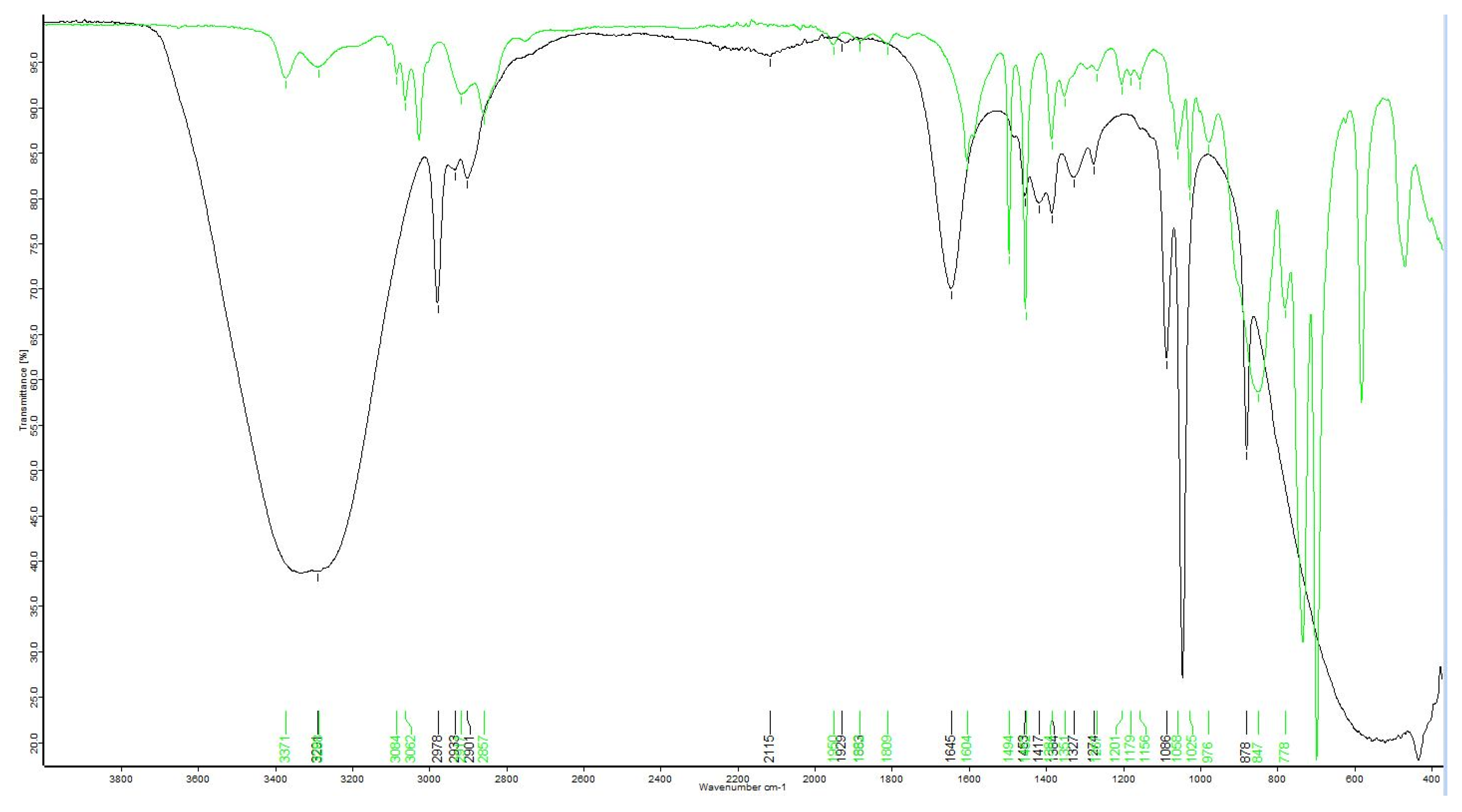
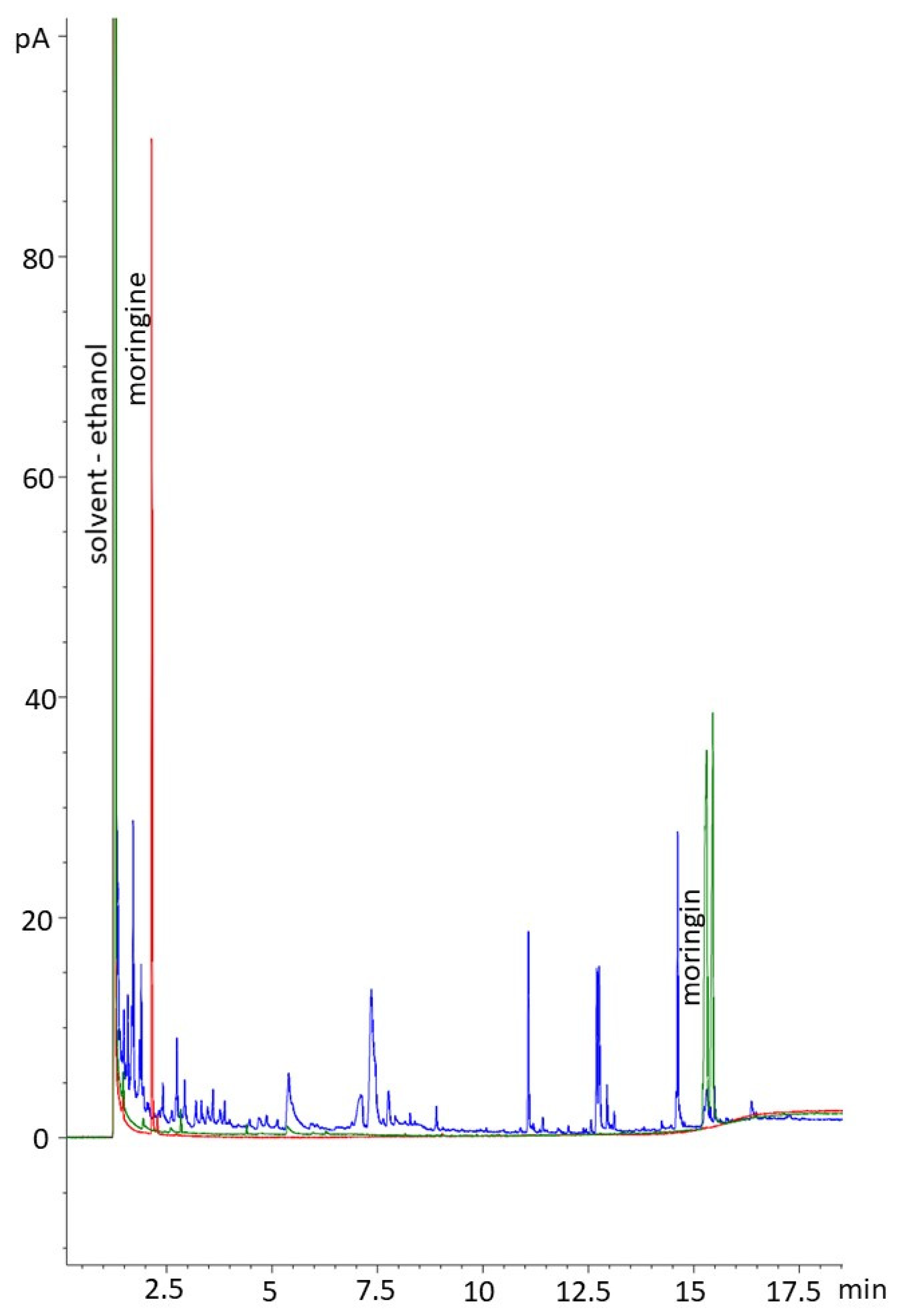
Extracts were analyzed using Fourier-transform infrared spectroscopy (FTIR) on a Bruker ALPHA II with a platinum ATR module to detect if moringin or moringine were present. Determining the presence of the two compounds consisted of comparisons to FTIR of standard-grade moringin (molecular weight = 311.35 g/mol) and moringine (molecular weight = 107.15 g/mol). The moringin standard was >98.0% pure, obtained from ChemFaces (CAS No. 73255-40-0, Catalog No. CFN89445), and the moringine standard, in the form of benzylamine >99.0% pure, was obtained from Sigma-Aldrich (CAS No. 100-46-9, Catalog No. 13180). Once the qualitative phytochemical presence of the two compounds of interest were determined, the extracts were quantitatively analyzed using gas chromatography (GC) on an Agilent 6890GC at Northern Analytical Laboratory Services (NALS) at the University of Northern British Columbia’s Prince George campus. The 19 samples were run with a ValcoBond VB5, 30 m × 0.25 mm × 0.25 µm film thickness column, with a constant flow rate of 1.7 mL/min using helium carrier gas at a 20:1 split ratio, with an oven programmed at 120 °C for 1 min, ramped 10 °C/min to 250 °C then ramped 25 °C/min to 300 °C and then held for 4 min at 300 °C.
2.4. Data Analysis
Descriptive statistics were calculated for the anatomical measurements across the 4-month-old trees using Microsoft Excel 2020 and IBM SPSS version 26 statistical analytics software. When possible, all 5 samples were used in the calculation; unfortunately, in some of the samples, the phloem rays were interconnected and/or the sclerenchyma tissues ran the circumference of the stem and were not possible to measure.
A distribution report was produced to summarize the presence–absence data resulting from the FTIR analysis; sample peaks were matched to the standard outputs and to the output of pure ethanol to determine if either moringine or moringin were present in the ethanolic extracts. Using GC, compound determinations were based on retention times with standards. The peaks of the moringin standard were integrated over the whole range of peaks to acquire concentration data; standard concentration data were plotted against area to determine correlation and for inaccuracy correction (R2 = 0.992).
Since this was discovery-based research and not a true experiment, we did not make any statistical comparisons aside from obtaining average measurements of anatomy and chemical concentrations.
4. Discussion
We sought to depict the distribution and size of various tissue types within
M. oleifera stems grown in a greenhouse under specific environmental conditions (Objective 1). In a study by Vyas [
7], a brief overview of
Moringa oliefera’s stem anatomy was previously documented. They described young stems as having 16–18 vascular bundles, a large parenchymous pith, and a pericycle composed of alternate groups of parenchyma cells and fibers; these groups eventually form a circular band in mature stems [
7]. Vyas [
7] further describes mature stems’ vascular cambia (VC) that produce large amounts of secondary xylem, which consists of uniseriate xylem rays, roundish vessel elements, and lignified thick-walled fibers; the VC also produces small amounts of secondary phloem. We add to this description by providing measurements for a variety of tissue and cell types in juvenile–mature transitional tissues of
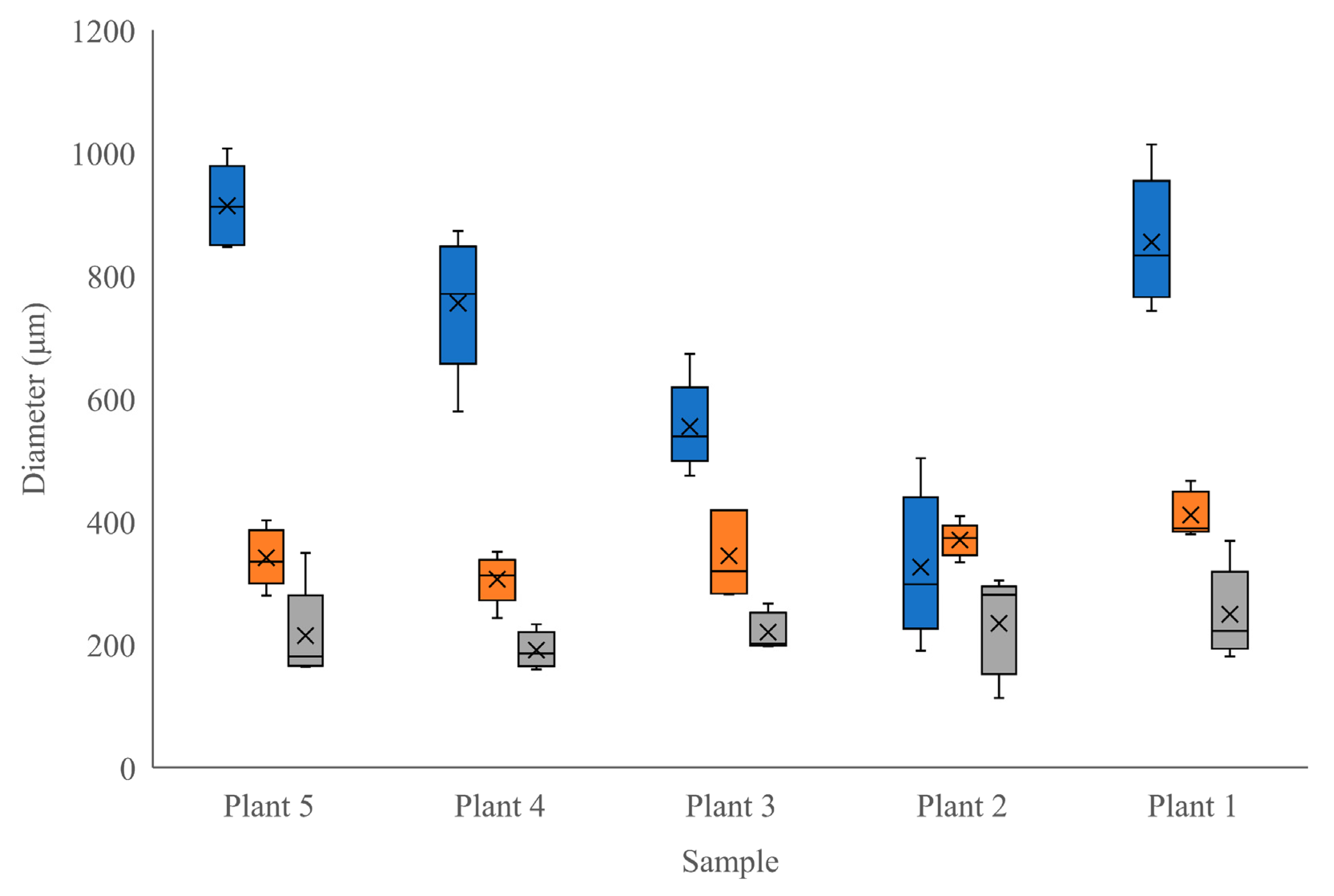
Moringa oliefera. This growth stage highlights the development of the ring of secondary tissues, including sclerenchyma. Interestingly, the radial diameter of the true phloem showed much more variability between individuals than did other tissue types. Phloem is the main transport tissue for photosynthates, metabolites, and other compounds and generally displays a high level of plasticity in response to environmental factors [
20]. Given that these trees were all grown in the same, highly controlled environment, it is unlikely that atmospheric conditions were the reason for this variability. Seeds were also all from the same source, although they could have been derived from different parent trees. Since our plants did suffer from a nutrient deficiency at one point during their growth period, it is possible that the severity of this stress was different among individuals and, therefore, created a difference in phloem development [
20], contributing to the variability shown among phloem tissues between our samples (
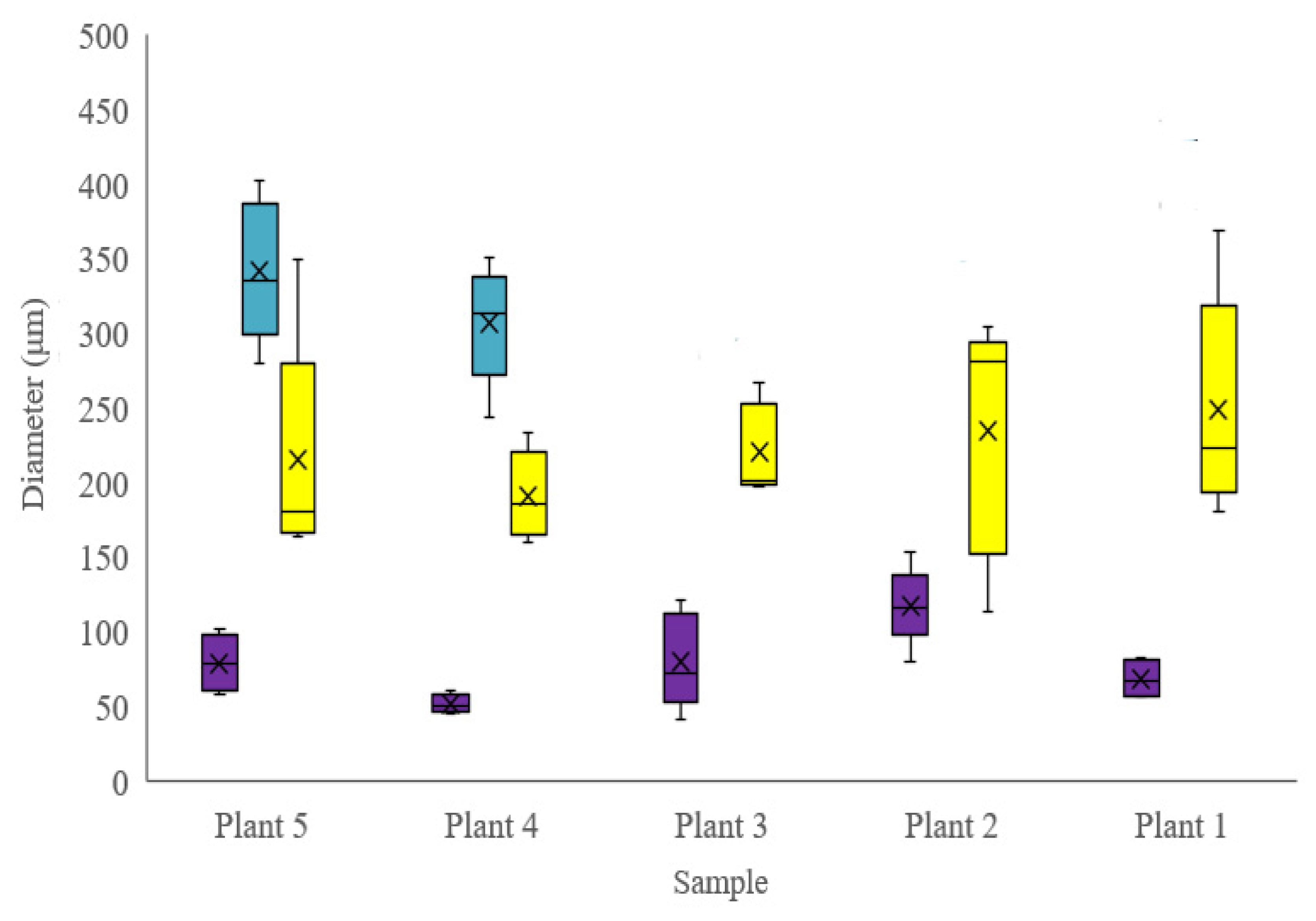
Figure 3). The higher degree of consistency in the other tissue and cell sizes between individuals indicates that tissues other than phloem were relatively unaffected by the period of mineral deficiency (
Figure 3 and
Figure 4). Our description of the stem anatomy of juvenile–mature transitioning
M. oleifera grown under controlled conditions (Objective 1) adds to the currently available literature describing the anatomy of this species.
We qualitatively and quantitatively confirmed that 100% of the samples contained moringin using FTIR and GC, indicating that moringin is consistently produced in
Moringa oleifera bark in trees grown under controlled greenhouse conditions. Thus far, moringin has largely been extracted from the seeds of
Moringa for medicinal use [
14,
24,
25]. The bark extracts we produced contained an average of 0.08 mg/mL and up to 0.15 mg/mL of moringin, but the samples consisted of an average of 39.7% water; therefore, the concentration of moringin in solution was diluted. Further refinement of the extraction process from bark could likely yield concentrations that are useful in medicine, for example, for the treatment of spinal cord injury and ischemic stroke [
14,
25]. Moringin was administered to rats at 3.5 mg/mL daily, producing neuroprotective properties and reducing oxidative stress and inflammation [
14].
The double confirmation (through both FTIR and GC) that none of the bark samples contained moringine indicates that a greenhouse environment did not encourage the production of the potentially toxic compound [
5,
10,
11,
12]. In outdoor conditions, there are greater temperature, moisture, and nutrient fluctuations than under greenhouse conditions. Growing under optimal conditions and not being exposed to the dangers of herbivory, plants are less likely to produce defensive or stress-related secondary metabolites. Furthermore, plant chemical defenses vary greatly with age, and there is a particular reduction in chemical defenses during the transitional phase between being a juvenile and maturity [
26]. Given that it can take up to 8 months for
M. oleifera to mature [
23], our research supports that growing
M. oleifera in stable greenhouses for 4–7 months yields plants that do not have a risk of containing moringine. This is an important conclusion for those looking to produce
Moringa plants for the treatment of pathologies.
We sought to evaluate the efficacy of the bark as a source of secondary plant compounds when grown under controlled conditions, considering specifically the presence of moringin and moringine (Objective 2). This research suggests that by producing fast-growing M. oleifera in optimal greenhouse conditions, trees can produce bark within 4–7 months, from which the extract is of good quality for use in medicinal treatments. This production can be conducted anywhere in the world, as illustrated by our location in northern Canada, and is not limited to tropical growing conditions, as would be the case in outdoor cultivation.