Heavy Metal Analysis and Health Risk Assessment of Potato (Solanum tuberosum L.) Cultivars irrigated with Fly Ash-Treated Acid Mine Drainage
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Collection and Processing of Soil and Plant Material
2.3. Determination of Concentration of Mineral Nutrients in Tubers
2.4. Heavy Metal Contamination and Health Risk Assessment
2.4.1. Bioaccumulation Factor (BAF) and Translocation Factor (TF) of Toxic Metals
2.4.2. Estimation of Potential Health Risks from Potato Consumption
2.4.3. Estimated Daily Intake (EDI)
2.4.4. Risk from the Intake of Heavy Metals through Ingestion (Target Hazard Quotient, THQ)
2.4.5. Target Cancer Risk (TR)
2.5. Data Analysis
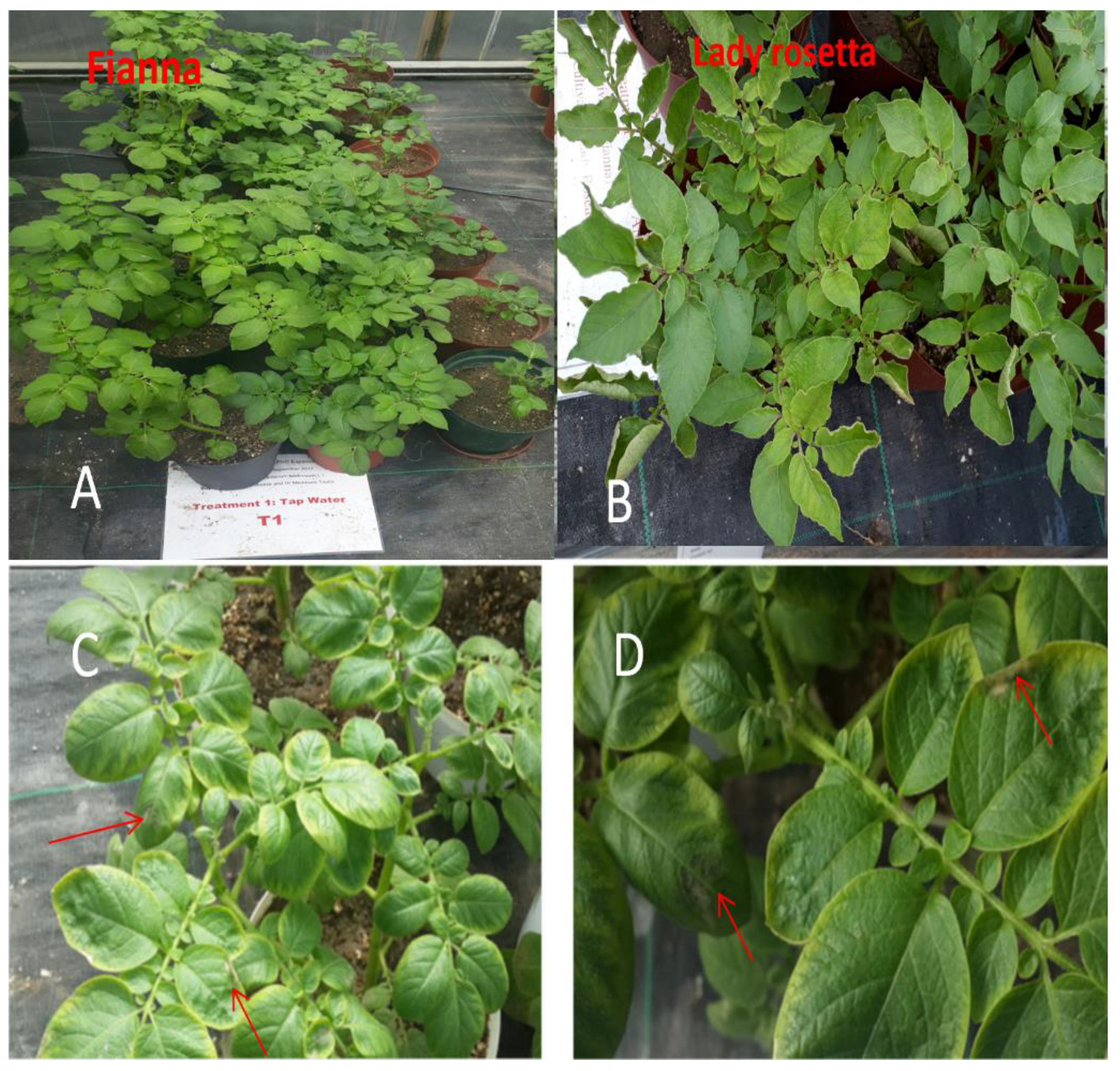
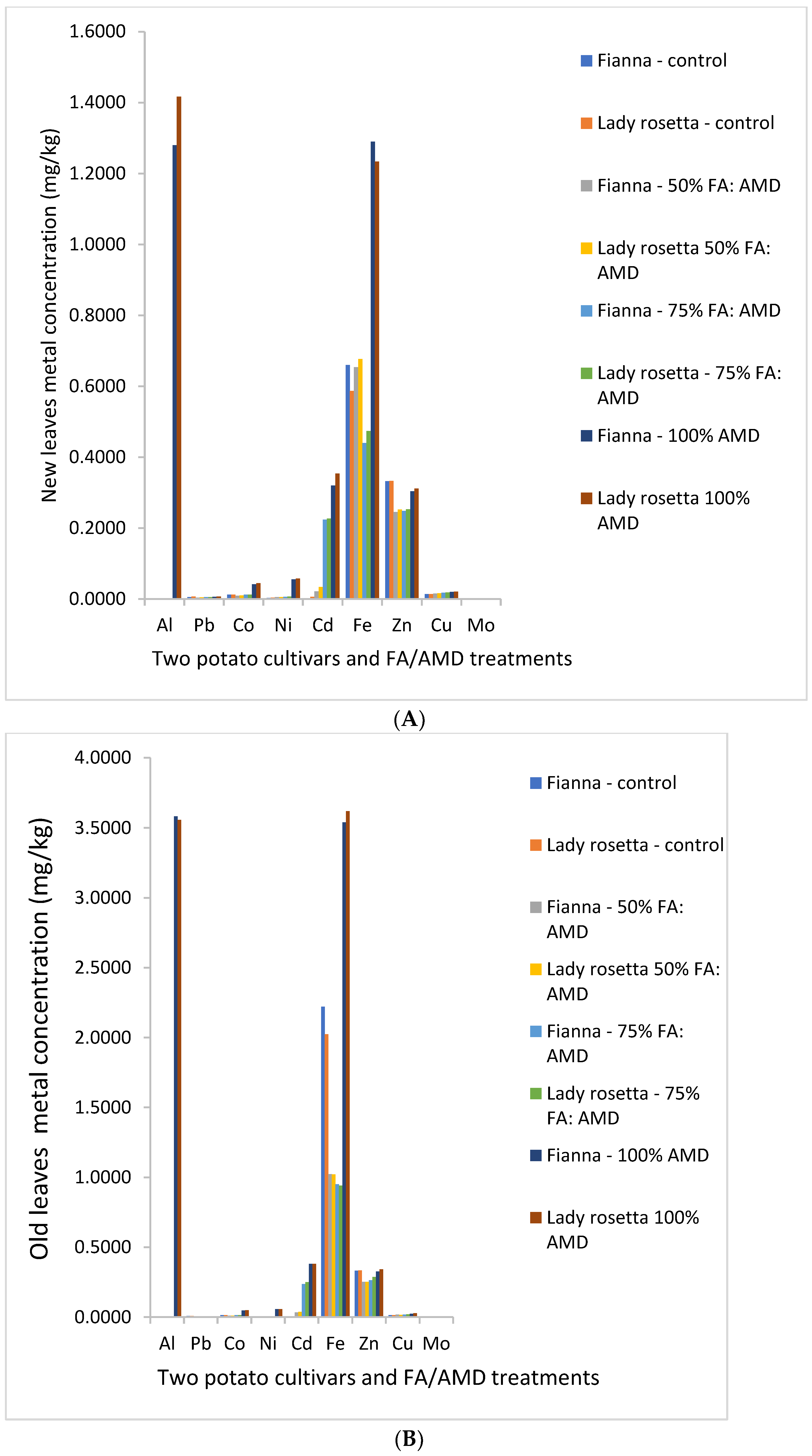
3. Results
| Organ | Ni | Cu | Fe | Zn | Al | Pb | Co | Cd |
|---|---|---|---|---|---|---|---|---|
| Tuber | ||||||||
| Control—F | 0.09 ± 0.00 b | 0.00 ± 0.0 a | 1.88 ± 0.0 b | 0.19 ± 0.0 a | 0.00 ± 0.9 ab | 0.01 ± 0.0 b | 0.07 ± 0.0 b | 0.01 ± 0.0 a |
| Control—LR | 0.09 ± 0.00 b | 0.00 ± 0.0 a | 1.33 ± 0.0 b | 0.18 ± 0.0 a | 0.00 ± 0.9 ab | 0.02 ± 0.0 b | 0.06 ± 0.0 b | 0.07 ± 0.0 a |
| 50% FA: AMD—F | 1.77 ± 0.01 c | 0.12 ± 0.0 a | 0.77 ± 0.0 a | 0.15 ± 0.0 a | 0.00 ± 0.0 a | 0.00 ± 0.0 a | 0.16 ± 0.1 a | 0.98 ± 0.0 b |
| 50% FA: AMD—LR | 1.68 ± 0.00 c | 0.00 ± 0.0 a | 0.65 ± 0.0 a | 0.13 ± 0.0 a | 0.00 ± 0.0 a | 0.00 ± 0.0 a | 0.17 ± 0.1 a | 0.84 ± 0.0 b |
| 75% FA: AMD—F | 0.01 ± 0.00 a | 0.03 ± 0.0 b | 0.98 ± 0.1 a | 0.29 ± 0.0 b | 1.23 ± 0.00 b | 0.00 ± 0.0 a | 0.02 ± 0.0 c | 0.04 ± 0.0 a |
| 75% FA: AMD—LR | 0.01 ± 0.00 a | 0.02 ± 0.0 b | 0.50 ± 0.0 a | 0.22 ± 0.0 b | 1.23 ± 0.01 b | 0.00 ± 0.0 a | 0.01 ± 0.0 c | 0.03 ± 0.0 a |
| 100% AMD—F | 1.89 ± 0.00 d | 1.22 ± 0.0 c | 69.13 ± 0.4 c | 13.23 ± 0.0 c | 5.39 ± 0.3 c | 0.02 ± 0.0 b | 0.02 ± 0.0 c | 1.34 ± 0.0 c |
| 100% AMD—LR | 1.88 ± 0.01 d | 1.23 ± 0.0 c | 67.18 ± 0.2 c | 12.86 ± 0.0 c | 5.74 ± 0.02 c | 0.02 ± 0.0 b | 0.02 ± 0.0 c | 1.01 ± 0.0 c |
| F-Statistics | ||||||||
| Organ | 14.58 *** | 9.00 ** | 6.65 *** | 3.22 * | 20.65 ** | 5.70 ** | 4.35 ** |
| Bioaccumulation Factor (BF) | ||||||
|---|---|---|---|---|---|---|
| Treatments | Cultivars | Cd | Pb | Zn | Ni | Cu |
| Control | Fianna | 0.23 | 0.36 | 0.45 | 0.16 | 0.23 |
| Control | Lady rosetta | 0.20 | 0.38 | 0.45 | 0.17 | 0.23 |
| 50% FA: AMD | Fianna | 0.78 | 1.17 | 0.03 | 0.00 | 0.15 |
| 50% FA: AMD | Lady rosetta | 0.86 | 1.10 | 0.03 | 0.00 | 0.14 |
| 75% FA: AMD | Fianna | 11.83 | 5.43 | 0.19 | 0.00 | 0.01 |
| 75% FA: AMD | Lady rosetta | 12.50 | 5.62 | 0.21 | 0.00 | 0.01 |
| 100% AMD | Fianna | 2.37 | 0.02 | 0.00 | 0.00 | 0.04 |
| 100% AMD | Lady rosetta | 2.37 | 0.02 | 0.00 | 0.00 | 0.04 |
| Translocation Factor (TF) | Estimated Daily Intake (EDI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | Cultivars | Cd | Pb | Zn | Ni | Cd | Pb | Zn | Ni |
| Control | Fianna | 0.30 | 0.37 | 1.72 | 0.03 | 3.48 | 5.13 | 5.15 | 2.45 |
| Control | Lady rosetta | 1.46 | 0.31 | 1.83 | 0.03 | 1.96 | 6.60 | 4.86 | 2.48 |
| 50% FA: AMD | Fianna | 17.74 | 0.87 | 1.67 | 0.00 | 2.62 | 1.31 | 4.02 | 4.74 |
| 50% FA: AMD | Lady rosetta | 16.70 | 0.88 | 1.82 | 0.00 | 2.25 | 1.34 | 3.70 | 4.52 |
| 75% FA: AMD | Fianna | 23.66 | 0.78 | 0.88 | 0.33 | 1.07 | 1.84 | 7.99 | 5.11 |
| 75% FA: AMD | Lady rosetta | 18.75 | 1.01 | 1.29 | 0.36 | 8.03 | 1.48 | 5.90 | 4.95 |
| 100% AMD | Fianna | 19.94 | 0.30 | 0.02 | 0.03 | 3.59 | 5.69 | 3.54 | 5.06 |
| 100% AMD | Lady rosetta | 15.09 | 0.32 | 0.00 | 0.03 | 2.72 | 5.39 | 3.43 | 5.03 |
| Target Hazard Quotient (THQ) | Target Cancer Risk (TR) | Cancer Risk | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Cultivars | Cd | Pb | Zn | Ni | Cd | Pb | Zn | Ni | |
| Control | Fianna | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 |
| Control | Lady rosetta | 0.03 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.04 |
| 50% FA: AMD | Fianna | 0.45 | 0.00 | 0.00 | 0.02 | 0.45 | 0.00 | 0.00 | 0.02 | 0.50 |
| 50% FA: AMD | Lady rosetta | 0.39 | 0.00 | 0.00 | 0.01 | 0.39 | 0.00 | 0.00 | 0.02 | 0.41 |
| 75% FA: AMD | Fianna | 0.01 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.20 |
| 75% FA: AMD | Lady rosetta | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 |
| 100% AMD | Fianna | 0.62 | 0.00 | 0.01 | 0.02 | 0.63 | 0.00 | 0.01 | 0.02 | 0.70 |
| 100% AMD | Lady rosetta | 0.47 | 0.00 | 0.01 | 0.02 | 0.50 | 0.00 | 0.01 | 0.02 | 0.51 |
| Potential Carcinogenic Effects | ||||
|---|---|---|---|---|
| Treatments | Cultivars | Cd | Pb | Ni |
| Control | Fianna | 0.00 | 3.71 | 0.00 |
| Control | Lady rosetta | 0.00 | 2.89 | 0.00 |
| 50% FA: AMD | Fianna | 0.00 | 14.51 | 0.00 |
| 50% FA: AMD | Lady rosetta | 0.00 | 14.26 | 0.00 |
| 75% FA: AMD | Fianna | 0.00 | 10.36 | 0.02 |
| 75% FA: AMD | Lady rosetta | 0.00 | 12.88 | 0.02 |
| 100% AMD | Fianna | 0.00 | 3.35 | 0.00 |
| 100% AMD | Lady rosetta | 0.00 | 3.54 | 0.00 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and avenues for acid mine drainage treatment, beneficiation, and valorisation in circular economy: A review. Ecol. Eng. 2022, 183, 1–24. [Google Scholar] [CrossRef]
- Masood, N.; Hudsone-Edwards, K.; Farooqi, A. True cost of coal: Coal mining industry and its associated environmental impacts on water resource development. J. Sustain. Min. 2020, 19, 35–149. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable resolutions for environmental threat of the acid mine drainage. Sci. Total Environ. 2020, 717, 1–9. [Google Scholar] [CrossRef]
- Acharya, B.S.; Kharel, G. Acid mine drainage from coal mining in the United States–An overview. J. Hydrol. 2020, 588, 1–14. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Genty, T.; Bussière, B.; Potvin, R.; Benzaazoua, M.; Zagury, G.J. Dissolution of calcitic marble and dolomitic rock in high iron concentrated acid mine drainage: Application to anoxic limestone drains. J. Environ. Earth Sci. 2012, 66, 2387–2401. [Google Scholar] [CrossRef]
- Payus, C.; Ann Huey, L.; Adnan, F.; Besse Rimba, A.; Mohan, G.; Kumar Chapagain, S.; Roder, G.; Gasparatos, A.; Fukushi, K. Impact of extreme drought climate on water security in North Borneo: Case study of Sabah. Water 2020, 12, 1135. [Google Scholar] [CrossRef]
- Matabane, D.L. Identification Determination of Potentially Toxic Elements in Water and Sediments from Blood and Mokolo rivers in Limpopo Province, South Africa. Ph.D. Thesis, University of Limpopo, Polokwane, South Africa, 2019. [Google Scholar]
- Chen, G.; Ye, Y.; Yao, N.; Hu, N.; Zhang, J.; Huang, Y. A critical review of prevention, treatment, reuse, and resource recovery from acid mine drainage. J. Clean. 2021, 329, 1–23. [Google Scholar] [CrossRef]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J. Environ. Manag. 2021, 278, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Atkinson, S.A. Calcium, magnesium, phosphorus and vitamin D fortification of complementary foods. J. Nutri. 2003, 133, 1–6. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. J. Extr. Ind. Soc. 2019, 6, 241–249. [Google Scholar]
- Mbamba, C.K. Using Froth Flotation to Mitigate Acid Rock Drainage Risks while Recovering Valuable Coal from Ultrafine Colliery Waste. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2011. [Google Scholar]
- Yunusa, I.A.M.; Erasmus, D.; DeSilva, D.L.; Murray, B.R.; Burchett, M.D.; Skilbeck, G.C.; Heidrich, C. Fly-ash: An exploitable resource for management of Australian agricultural soils. Fuel 2006, 85, 2337–2344. [Google Scholar] [CrossRef]
- Rai, G.K.; Bhat, B.A.; Mushtaq, M.; Tariq, L.; Rai, P.K.; Basu, U.; Dar, A.A.; Islam, S.T.; Dar, T.U.; Bhat, J.A. Insights into decontamination of soils by phytoremediation: A detailed account on heavy metal toxicity and mitigation strategies. J. Physiol. Plant. 2021, 173, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci. Total Environ. 2020, 749, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.A.; Williams, C.D.; Roberts, C.L. Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker, and synthetic zeolites. J. Hazard. Mater. 2008, 156, 23–35. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Osibote, O.A.; Darmokoesoemo, H.; Kusuma, H.S. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: A review. J. Mater. 2021, 14, 2751–2774. [Google Scholar] [CrossRef]
- Nemutanzhela, M.V.; Modise, D.M.; Siyoko, K.J.; Kanu, S.A. Assessment of Growth, Tuber Elemental Composition, Stomatal Conductance, and Chlorophyll Content of Two Potato Cultivars Under Irrigation with Fly Ash-Treated Acid Mine Drainage. Am. J. Potato Res. 2017, 94, 1–12. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Emenike, E.C.; Iwuozor, K.O.; Okoro, H.K.; Ige, O.O. Acid mine drainage: The footprint of the Nigeria mining industry. J. Chem. Africa 2022, 5, 1–14. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements. A review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Bouazza, M. The use of coal combustion fly ash as a soil amendment in agricultural lands (with comments on its potential to improve food security and sequester carbon). Fuel 2013, 109, 400–408. [Google Scholar] [CrossRef]
- Frossard, E.; Bucher, M.; Mächler, F.; Mozafar, A.; Hurrell, R. Potential for increasing the content and bioavailability of Fe, Zn, and Ca in plants for human nutrition. J. Sci. Food Agric. 2000, 80, 861–879. [Google Scholar] [CrossRef]
- Elfnesh, F.; Tekalign, T.; Solomon, W. Processing quality of improved potato (Solanum tuberosum L.) cultivars as influenced by growing environment and blanching. Afr. J. Food. Sci. 2011, 5, 324–332. [Google Scholar]
- Chatterjee, C.; Gopal, R.; Dube, B.K. Impact of iron stress on biomass, yield, metabolism, and quality of potato (Solanum tuberosum L.). Sci. Hortic. 2006, 108, 1–6. [Google Scholar] [CrossRef]
- Farooq, M.; Anwar, F.; Rashid, U. Appraisal of heavy metal contents in different vegetables grown in the vicinity of an industrial area. Pak. J. Bot. 2008, 40, 2099–2106. [Google Scholar]
- Balistrieri, L.S.; Seal II, R.R.; Piatak, N.M.; Paul, B. Assessing the concentration, speciation, and toxicity of dissolved metals during mixing of acid-mine drainage and ambient river water downstream of the Elizabeth Copper Mine, Vermont, USA. Appl. Geochem. 2007, 22, 930–952. [Google Scholar] [CrossRef]
- Harmanescu, M.; Alda, L.M.; Bordean, D.M.; Gogoasa, I.; Gergen, I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem. Cent. J. 2011, 5, 1–10. [Google Scholar] [CrossRef]
- Gibson, R.S.; Hotz, C. Dietary diversification/modification strategies to enhance micronutrient content and bioavailability of diets in developing countries. Br. J. Nutr. 2001, 85, 159–166. [Google Scholar] [CrossRef]
- Onder, S.; Caliskan, M.E.; Onder, D.; Caliskan, S. Different irrigation methods, and water stress effects on potato yield and yield components. Agric. Water. Manag. 2005, 73, 73–86. [Google Scholar] [CrossRef]
- Lerna, A. Influence of harvest date on nitrate contents of three potato varieties for off-season production. J. Food. Compos. Anal. 2009, 22, 551–555. [Google Scholar]
- Maier, N.A.; McLaughlin, M.J.; Heap, M.; Butt, M.; Smart, M.K. Effect of nitrogen source and calcitic lime on soil pH and potato yield, leaf chemical composition, and tuber cadmium concentrations. J. Plant Nutr. 2002, 25, 523–544. [Google Scholar] [CrossRef]
- Ogbonna, O.; Jimoh, W.L.; Awagu, E.F.; Bamishaiye, E.I. Determination of some trace elements in water samples within Kano Metropolis. Adv. Appl. Sci. Res. 2011, 2, 62–68. [Google Scholar]
- Oguh, C.E.; Obiwulu, E.N.O. Human risk on heavy metal pollution and bioaccumulation factor in soil and some edible vegetables around active auto-mechanic workshop in Chanchaga Minna Niger state, Nigeria. Ecol. Environ. Sci. 2020, 4, 12–22. [Google Scholar]
- Ranjbar, G.; Kariminejad, F.; Jamali, J.; Shams, M.; Najafpoor, A.A.; Dehghan, A. Heavy metal concentration in water, soil and cultivated vegetables at the edge of Kashaf Roud River, Mashhad, Iran: Ecological risk assessment and bioaccumulation factor. J. Environ. Anal. Chem. 2022, 25, 1–22. [Google Scholar] [CrossRef]
- Hussain, A.; Alamzeb, S.; Begum, S. Accumulation of heavy metals in edible parts of vegetables irrigated with wastewater and their daily intake to adults and children, District Mardan, Pakistan. Food Chem. 2013, 136, 1515–1523. [Google Scholar]
- Maharia, R.S.; Dutta, R.K.; Acharya, R.; Reddy, A.V.R. Heavy metal bioaccumulation in selected medicinal plants collected from Khetri copper mines and comparison with those collected from fertile soil in Haridwar, India. J. Environ. Sci. Health B. 2010, 45, 174–181. [Google Scholar] [CrossRef]
- Abdu, N.; Agbenin, J.O.; Buerkert, A. Phytoavailability, human risk assessment and transfer characteristics of cadmium and zinc contamination from urban gardens in Kano, Nigeria. J. Sci. Food Agric. 2011, 91, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, S.; Ronaghi, A.; Karimian, N. Effect of cadmium toxicity on micronutrient concentration, uptake and partitioning in seven rice cultivars. Arch. Agron. Soil Sci. 2013, 59, 231–245. [Google Scholar] [CrossRef]
- Saha, N.; Mollah, M.Z.I.; Alam, M.F.; Rahman, M.S. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control 2016, 70, 110–118. [Google Scholar] [CrossRef]
- Yoon, E.; Park, K.; Lee, H.; Yang, J.H.; Lee, C. Estimation of excess cancer risk on time-weighted lifetime average daily intake of PAHs from food ingestion. Hum. Ecol. Risk Assess. (HERA) 2007, 13, 669–680. [Google Scholar] [CrossRef]
- Arcury, T.A.; Nguyen, H.T.; Summers, P.; Talton, J.W.; Holbrook, L.C.; Walker, F.O.; Chen, H.; Howard, T.D.; Galván, L.; Quandt, S.A. Lifetime and current pesticide exposure among Latino farmworkers in comparison to other Latino immigrants. Am. J. Ind. Med. 2014, 57, 776–787. [Google Scholar] [CrossRef]
- Amin, M.; Rahman, M.; Hossain, S.; Rahman, M.; Rahman, M.M.; Jakariya, M.; Sikder, M. Trace metals in vegetables and associated health risks in industrial areas of Savar, Bangladesh. J. Health Pollut. 2020, 10, 200905. [Google Scholar] [CrossRef] [PubMed]
- Naughton, D.P.; Petróczi, A. Heavy metal ions in wines: Meta-analysis of target hazard quotients reveal health risks. Chem. Cent. J. 2008, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miranzadeh Mahabadi, H.; Ramroudi, M.; Asgharipour, M.R.; Rahmani, H.R.; Afyuni, M. Assessment of heavy metals contamination and the risk of target hazard quotient in some vegetables in Isfahan. Pollution 2020, 6, 69–78. [Google Scholar]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef]
- Bortey-Sam, N.; Nakayama, S.M.; Ikenaka, Y.; Akoto, O.; Baidoo, E.; Yohannes, Y.B.; Mizukawa, H.; Ishizuka, M. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol. Environ. Saf. 2015, 111, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Ishak, N.S. Estimation of target hazard quotients and potential health risks for metals by consumption of shrimp (Litopenaeus vannamei) in Selangor, Malaysia. Sains Malays. 2017, 46, 1825–1830. [Google Scholar] [CrossRef]
- Han, B.C.; Jeng, W.L.; Chen, R.Y.; Fang, G.T.; Hung, T.C.; Tseng, R.J. Estimation of target hazard quotients and potential health risks for metals by consumption of seafood in Taiwan. Arch. Environ. Contam. Toxicol. 1998, 35, 711–720. [Google Scholar] [CrossRef]
- Ahmed, M.; Baki, M.A.; Islam, M.; Kundu, G.K.; Habibullah-Al-Mamun, M.; Sarkar, S.K.; Hossain, M. Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environ. Sci. Pollut. Res. 2015, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Hurrell, R.F. Improving iron, zinc and vitamin A nutrition through plant biotechnology. Curr. Opin. Biotechnol. 2002, 13, 142–145. [Google Scholar] [CrossRef]
- Brown, P.H.; Welch, R.M.; Cary, E.E.; Checkai, R.T. Micronutrients: Beneficial effects of nickel on plant growth. J. Plant Nutr. 1987, 10, 2125–2135. [Google Scholar] [CrossRef]
- Chen, C.; Huang, D.; Liu, J. Functions and toxicity of nickel in plants: Recent advances and prospects. Clean Soil Air Water 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Liang, M.U.; Zhou, T.H.; Zhang, R.F.; Sun, Q.H.; Xu, Y.W. Nutritional evaluation of different cultivars of potatoes (Solanum tuberosum L.) from China by grey relational analysis (GRA) and its application in potato steamed bread making. J. Integr. Agric. 2019, 18, 231–245. [Google Scholar]
- Aberoumand, A. Assay of Nutritional Potential of the Fruits of Solanum indicum L. in Iran. J. Agric. Technol. 2012, 8, 923–929. [Google Scholar]
- Dunbar, K.R.; Mclaughlin, M.J.; Reid, R.J. The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L). J. Exp. Bot. 2003, 54, 349–354. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Williams, C.M.J.; McKay, A.J.K.G.; Kirkham, R.J.K.G.; Gunton, J.J.K.G.; Jackson, K.J.; Thompson, R.; Dowling, B.; Partington, D.; Smart, M.K.; et al. Effect of cultivar on uptake of cadmium by potato tubers. Aust. J. Agric. Res. 1994, 45, 1483–1495. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Tripathy, S.; Panigrahi, M.K.; Equeenuddin, S. Evaluation of the use of an alkali modified fly ash as a potential adsorbent for the removal of metals from acid mine drainage. Appl. Water Sci. 2013, 3, 567–576. [Google Scholar] [CrossRef]
- Keller, V.; Stopić, S.; Xakalashe, B.; Ma, Y.; Ndlovu, S.; Mwewa, B.; Simate, G.S.; Friedrich, B. Effectiveness of fly ash and red mud as strategies for sustainable acid mine drainage management. Minerals 2020, 10, 707. [Google Scholar] [CrossRef]
- Barrutia, O.; Epelde, L.; García-Plazaola, J.I.; Garbisu, C.; Becerril, J.M. Phytoextraction potential of two Rumex acetosa L. accessions collected from metalliferous and non-metalliferous sites: Effect of fertilization. Chemosphere 2009, 74, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Yalamanchali, R. Lithium, an Emerging Environmental Contaminant, is Mobile in the Soil-Plant System. Ph.D. Thesis, Lincoln University, Philadelphia, PA, USA, 2012. [Google Scholar]
- Rao, K.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Mengist, M.F.; Milbourne, D.; Griffin, D.; McLaughlin, M.J.; Creedon, J.; Jones, P.W.; Alves, S. Cadmium uptake and partitioning in potato (Solanum tuberosum L.) cultivars with different tuber-Cd concentration. Environ. Sci. Pollut. Res. 2017, 24, 27384–27391. [Google Scholar] [CrossRef]
- Pandey, N.; Sharma, C.P. Effect of heavy metals Co2+, Ni2+, and Cd2+ on growth and metabolism of cabbage. Plant. Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Akubugwo, I.E.; Obasi, N.A.; Chinyere, G.C.; Ugbogu, A.E. Nutritional and chemical value of Amaranthus hybridus L. leaves from Afikpo, Nigeria. Afr. J. Biotechnol. 2007, 6, 2833–2839. [Google Scholar]
- Gajewska, E.; SkŁodowska, M. Differential effect of equal copper, cadmium and nickel concentration on biochemical reactions in wheat seedlings. Ecotoxicol. Environ. Saf. 2010, 73, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Öncel, I.; Keleş, Y.; Üstün, A. Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Pollut. 2000, 107, 315–320. [Google Scholar] [CrossRef] [PubMed]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters, and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–76. [Google Scholar]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- Hafeez, B.M.K.Y.; Khanif, Y.M.; Saleem, M. Role of zinc in plant nutrition-a review. Am. J. Exp. Agric. 2013, 3, 1–18. [Google Scholar] [CrossRef]
- Ragályi, P.; Takács, T.; Füzy, A.; Uzinger, N.; Dobosy, P.; Záray, G.; Szűcs-Vásárhelyi, N.; Rékási, M. Effect of Se-Enriched Irrigation Water and Soil Texture on Biomass Production and Elemental Composition of Green Pea and Carrot and Their Contribution to Human Se Intake. Agriculture 2022, 12, 496. [Google Scholar] [CrossRef]
- Gebreselassie, H.; Wahassu, M.; Shimelis, B. Evaluation of potato (Solanum tuberosum L.) varieties for yield and yield components in Eastern Ethiopia. Evaluation 2016, 6, 1–9. [Google Scholar]
- Solomon, F.; Asrat, A.; Daniel, T.; Zenebe, G.M.; Eshetu, A. Evaluation of potato (Solanum tuberosum L.) varieties for yield and yield components. J. Hortic. 2019, 11, 48–53. [Google Scholar] [CrossRef]
- Tessema, L.; Mohammed, W.; Abebe, T. Evaluation of potato (Solanum tuberosum L.) varieties for yield and some agronomic traits. Open Agric. 2020, 5, 63–74. [Google Scholar] [CrossRef]
- Alemayehu, T.G.; Miilion, P.M.; Seman, A.S. Evaluation of growth, yield and quality of potato (Solanum tuberosum L.) varieties at Bule, Southern Ethiopia. Afr. J. Plant Sci. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- Bilate, B.; Mulualem, T. Performance evaluation of released and farmers’ potato (Solanum tuberosum L.) varieties in eastern Ethiopia. J. Agric. Res. 2016, 5, 1–8. [Google Scholar]
- Cabello, R.; De Mendiburu, F.; Bonierbale, M.; Monneveux, P.; Roca, W.; Chujoy, E. Large-scale evaluation of potato improved varieties, genetic stocks and landraces for drought tolerance. Am. J. Potato Res. 2012, 89, 1–11. [Google Scholar] [CrossRef]
- Saleh, S.R.; Kandeel, M.M.; Ghareeb, D.; Ghoneim, T.M.; Talha, N.I.; Alaoui-Sossé, B.; Aleya, L.; Abdel-Daim, M.M. Wheat biological responses to stress caused by cadmium, nickel and lead. Sci. Total Environ. 2020, 706, 1–10. [Google Scholar] [CrossRef]
- Bor, M.; Seckin, B.; Ozgur, R.; Yılmaz, O.; Ozdemir, F.; Turkan, I. Comparative effects of drought, salt, heavy metal and heat stresses on gamma-aminobutryric acid levels of sesame (Sesamum indicum L.). Acta Physiol. Plant. 2009, 31, 655–659. [Google Scholar] [CrossRef]
- Nazima, B.; Manoharan, V.; Miltonprabu, S. Grape seed proanthocyanidins ameliorates cadmium-induced renal injury and oxidative stress in experimental rats through the up-regulation of nuclear related factor 2 and antioxidant responsive elements. Biochem. Cell Biol. 2015, 93, 1–16. [Google Scholar] [CrossRef]
- Jackson, A.P.; Alloway, B.J. The transfer of cadmium from agricultural soils to the human food chain. JTEMIN 2017, 28, 121–170. [Google Scholar]
- Thévenod, F.; Wolff, N.A. Iron transport in the kidney: Implications for physiology and cadmium nephrotoxicity. Metallomics 2016, 8, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Vaidya, V.S.; Liu, J.; Waalkes, M.P.; Edwards, J.R.; Lamar, P.C.; Bernard, A.M.; Dumont, X.; Bonventre, J.V. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ren, Z.; Zhao, J.; Peprah, F.A.; Xie, Y.; Cheng, D.; Wang, Y.; Liu, H.; Wong, C.K.C.; Zhou, Y.; et al. Calcimimetic compound NPS R-467 protects against chronic cadmium-induced mouse kidney injury by restoring autophagy process. Ecotoxicol. Environ. Saf. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Pan, J.; Plant, J.A.; Voulvoulis, N.; Oates, C.J.; Ihlenfeld, C. Cadmium levels in Europe: Implications for human health. Environ. Geochem. Health 2010, 32, 1–12. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Bernard, A.; Diamond, G.L.; Duffus, J.H.; Illing, P.; Nordberg, M.; Bergdahl, I.A.; Jin, T.; Skerfving, S. Risk assessment of effects of cadmium on human health (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 755–808. [Google Scholar] [CrossRef]
- Prasad, B.; Mortimer, R.J. Treatment of acid mine drainage using fly ash zeolite. Water Air Soil Pollut. 2011, 218, 1–13. [Google Scholar] [CrossRef]
- Jones, S.N.; Cetin, B. Evaluation of waste materials for acid mine drainage remediation. Fuel 2017, 188, 294–309. [Google Scholar] [CrossRef]
- Roy, M. Phytoreclamation of abandoned acid mine drainage site after treatment with fly ash. In Phytorestoration of Abandoned Mining and Oil Drilling Sites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 167–206. [Google Scholar]
- Bäckström, M.; Sartz, L. Mixing of acid rock drainage with alkaline ash leachates—Fate and immobilisation of trace elements. Water Air Soil Pollut. 2011, 222, 1–13. [Google Scholar] [CrossRef]
- Gitari, W.M.; Petrik, L.F.; Etchebers, O.; Key, D.L.; Iwuoha, E.; Okujeni, C. Passive neutralisation of acid mine drainage by fly ash and its derivatives: A column leaching study. Fuel 2008, 87, 1–14. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Ogundiran, M.B.; Togun, A.O. Soil amendment with compost and crop growth stages influenced heavy metal uptake and distribution in maize crop grown on lead-acid battery waste contaminated soil. J. Environ. Chem. Eng. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Kurniawan, S.B.; Iwuozor, K.O.; Aniagor, C.O.; Ajala, O.J.; Oba, S.N.; Iwuchukwu, F.U.; Ahmadi, S.; Igwegbe, C.A. A review of treatment technologies for the mitigation of the toxic environmental effects of acid mine drainage (AMD). Process Saf. Environ. Prot. 2022, 157, 37–58. [Google Scholar] [CrossRef]
- Gibson, R.S.; Perlas, L.; Hotz, C. Improving the bioavailability of nutrients in plant foods at the household level. Proc. Nutr. Soc. 2006, 65, 160–168. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1–37. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Zubillaga, M.; Lysionek, A.; Sarabia, M.I.; Caro, R.; De Paoli, T.; Hager, A.; Weill, R.; Boccio, J. Zinc as an essential micronutrient: A review. Nutr. Res. 2000, 20, 1–19. [Google Scholar] [CrossRef]
- Pandey, V.C.; Abhilash, P.C.; Singh, N. The Indian perspective of utilizing fly ash in phytoremediation, phytomanagement and biomass production. J. Environ. Manag. 2009, 90, 2943–2958. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Wessels, I.; Rink, L. Micronutrients in autoimmune diseases: Possible therapeutic benefits of zinc and vitamin D. J. Nutr. Biochem. 2020, 77, 1–23. [Google Scholar] [CrossRef]
- Christian, P. Micronutrients and reproductive health issues: An international perspective. Nutr. J. 2003, 133, 1–5. [Google Scholar] [CrossRef]
- Brengi, S.H.; El-Gindy, A.G.M.; El-Sharkawy, I.; Abouelsaad, I.A. Variation in cadmium accumulation among potato cultivars grown on different agricultural sites: A potential tool for reducing cadmium in tubers. Horticulturae 2021, 7, 377. [Google Scholar] [CrossRef]
- Larsson Jönsson, E.H.; Asp, H. Influence of nitrogen supply on cadmium accumulation in potato tubers. J. Plant Nutr. 2011, 34, 1–16. [Google Scholar] [CrossRef]
- Arévalo-Hernández, C.O.; Arévalo-Gardini, E.; Barraza, F.; Farfán, A.; He, Z.; Baligar, V. Growth and nutritional responses of wild and domesticated cacao genotypes to soil Cd stress. Sci. Total Environ. 2021, 763, 1–11. [Google Scholar] [CrossRef]
- Mengist, M.F.; Milbourne, D.; Griffin, D.; McLaughlin, M.J.; Creedon, J.; Jones, P.W.; Alves, S. Zinc uptake and partitioning in two potato cultivars: Implications for biofortification. Plant Soil. 2021, 463, 1–13. [Google Scholar] [CrossRef]
- Yan, B.F.; Nguyen, C.; Pokrovsky, O.S.; Candaudap, F.; Coriou, C.; Bussiere, S.; Robert, T.; Cornu, J.Y. Contribution of remobilization to the loading of cadmium in durum wheat grains: Impact of post-anthesis nitrogen supply. Plant Soil. 2018, 424, 1–16. [Google Scholar] [CrossRef]
- Leonardi, C.; Toscano, V.; Genovese, C.; Mosselmans, J.F.W.; Ngwenya, B.T.; Raccuia, S.A. Evaluation of cadmium and arsenic effects on wild and cultivated cardoon genotypes selected for metal phytoremediation and bioenergy purposes. Environ. Sci. Pollut. Res. 2021, 28, 1–14. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Matus, I.; Walter, I.; Hirzel, J. Absorption and distribution of cadmium of three maize hybrids in three environments. J. Plant Nutr. Soil Sci. 2017, 17, 1–13. [Google Scholar] [CrossRef]
- Brown, S.P.; Grillo, M.A.; Podowski, J.C.; Heath, K.D. Soil origin and plant genotype structure distinct microbiome compartments in the model legume Medicago truncatula. Microbiome 2020, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Long-distance transport in the xylem and phloem. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 49–70. [Google Scholar]
- Sterckeman, T.; Thomine, S. Mechanisms of cadmium accumulation in plants. Crit. Rev. Plant Sci. 2020, 39, 1–38. [Google Scholar] [CrossRef]
- Martinka, M.; Vaculík, M.; Lux, A. Plant cell responses to cadmium and zinc. Plant Cell Physiol. 2014, 2014, 1–36. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raletsena, M.V.; Mongalo, N.I.; Munyai, R. Heavy Metal Analysis and Health Risk Assessment of Potato (Solanum tuberosum L.) Cultivars irrigated with Fly Ash-Treated Acid Mine Drainage. Horticulturae 2023, 9, 192. https://doi.org/10.3390/horticulturae9020192
Raletsena MV, Mongalo NI, Munyai R. Heavy Metal Analysis and Health Risk Assessment of Potato (Solanum tuberosum L.) Cultivars irrigated with Fly Ash-Treated Acid Mine Drainage. Horticulturae. 2023; 9(2):192. https://doi.org/10.3390/horticulturae9020192
Chicago/Turabian StyleRaletsena, Maropeng Vellry, Nkoana Ishmael Mongalo, and Rabelani Munyai. 2023. "Heavy Metal Analysis and Health Risk Assessment of Potato (Solanum tuberosum L.) Cultivars irrigated with Fly Ash-Treated Acid Mine Drainage" Horticulturae 9, no. 2: 192. https://doi.org/10.3390/horticulturae9020192
APA StyleRaletsena, M. V., Mongalo, N. I., & Munyai, R. (2023). Heavy Metal Analysis and Health Risk Assessment of Potato (Solanum tuberosum L.) Cultivars irrigated with Fly Ash-Treated Acid Mine Drainage. Horticulturae, 9(2), 192. https://doi.org/10.3390/horticulturae9020192






