Abstract
Nitrate accumulation is a major factor for the secondary salinization of greenhouse soil in China. Our previous study pointed out that a low ratio of red:far-red light (R:FR) can improve salt tolerance in pakchoi under excessive nitrate stress. However, the nitrogen metabolism mechanism is still unclear. To detect the effect of a low R:FR ratio on nitrogen metabolism of pakchoi under excessive nitrate stress, two extra additions of nitrogen of 80 mmol·L−1 NO3− (H80) and 160 mmol·L−1 NO3− (H160) with/without a low R:FR ratio (R:FR = 0.7) were set, and the growth index, chlorophyll content, key enzymes in nitrogen metabolism, nitrate and glutamic acid content and NRT gene expression level of pakchoi leaves were examined. The results indicated that a low ratio of R:FR could alleviate the reduction in growth and chlorophyll content in pakchoi under high-level nitrogen stress (H80 and H160). The activity of nitrate reductase (NR), glutamine synthetase (GS) and glutamine synthetase (GOGAT) decreased under H80 and H160 conditions, except of NR with the H80 treatment. The activity of glutamate dehydrogenase (GDH) increased under H80 treatment, but decreased under H160 treatment. However, the activity of GDH decreased further by reducing the ratio of R:FR. Excessive nitrate stress increased the nitrate content, and a low R:FR ratio could inhibit nitrate accumulation. However, the change in glutamic acid content was significantly increased under a low R:FR ratio without stress. Under the high-nitrogen level treatment (H160), the use of a low ratio of R:FR increased NRT gene expression. Therefore, a low R:FR ratio (R:FR = 0.7) could effectively promote the growth of pakchoi and improve its nitrogen metabolism, thus alleviating the stress effect of a high level of nitrogen in pakchoi.
1. Introduction
Pakchoi (Brassica campestris L.) needs an abundance of nitrogen fertilizer in horticultural production. The yield of pakchoi is proportional to nitrogen fertilizer to some extent [1]. However, the nitrogen application level will affect the quality of pakchoi, and a high concentration of nitrogen fertilizer will lead to nitrate stress, which inhibits the growth of pakchoi [2]. In addition, due to the lack of rain shower, excessive artificial fertilization, the secondary salinization of soil in the greenhouse is serious [3]. Previous studies have pointed out that there is excessive nitrate accumulation, which is greater than NaCl accumulation, in secondary salinized soil in the greenhouse [4]. The secondary salinization of soil seriously affects the growth of green vegetables and restricts the development of protected green vegetable production [5]. A high level of nitrogen fertilizer will reduce biomass accumulation, the photosynthetic rate and chlorophyll in pakchoi [6,7] and reduce the antioxidant capacity of tomato seedlings [8]. Excessive nitrate stress also affects the activities of enzymes related to plant nitrogen assimilation, even changing the mechanism of nitrogen assimilation, and affects the utilization of nitrogen by plants [9]. Although there are many studies on the salt resistance of plants, especially the NaCl tolerance of plants, the stress effect of excessive nitrate on nitrate metabolism in plants needs to be further studied.
Light has an important effect on plant growth, salt tolerance [10] and nitrogen metabolism through many metabolic processes. Far-red light promotes the growth of seedlings through leaf expansion and net assimilation of the whole plant [11] and promotes carbohydrate synthesis [12]. Under salt stress, the ratio of R:FR in the light environment of tomato seedlings is reduced appropriately, and the net photosynthetic rate and chlorophyll content of tomato seedlings are significantly increased [13]. The common combination of red and blue light could improve nitrite reductase activity in sprouts, promote carbon assimilation and increase the activities of enzymes related to nitrogen metabolism [14]. Increasing the proportion of red light on the basis of white light can promote carbon assimilation, transformation and nitrogen absorption [15] and accelerate biomass accumulation [16]. Above all, we can speculate that red light and far-red light play an important role in regulating nitrogen metabolism.
In general, key enzymes for nitrogen metabolism include nitrate reductase (NR), glutamine synthetase (GS) and glutamate synthetase (GOGAT). Nitrate finally becomes glutamate to participate in the subsequent metabolism. In addition, glutamate dehydrogenase (GDH), which is involved in another mechanism of assimilating ammonia to produce glutamate, plays an important role in nitrogen metabolism in plants [17].
The nitrate transporter (NRT) gene family plays a key role in nitrogen absorption, transportation and assimilation [18]. The NRT gene family acts on NO3− uptake and signal transduction in Arabidopsis [19]. The NRT gene is the main research and analysis subject to improve nitrogen use efficiency in crops and deal with and solve nitrate accumulation issues [20]. There are two major types of biological cell plasma membrane NRT in Brassica napus L. [21], namely NRT1 and NRT2. NRT2 is involved in a high-affinity nitrate transfer system (HATs), while most NRT1 is involved in a low-affinity nitrate transfer system (LATs). Previous studies have shown that supplementation with red light can promote the expression of NRT genes [22]. The regulation of NRT gene expression mediated by the ratio of R:FR is not clear.
In our previous study [23], we have found that a low ratio of red light to far-red light (R:FR) can alleviate the harm of salt stress on pakchoi. Therefore, we conducted an in-depth study to determine if low-specific red light and far-red light could enhance pakchoi adaptation to excessive nitrate stress by improving nitrogen metabolism. The accumulation of nitrate is closely related to nitrogen metabolism, and thus, exploring the activities of key enzymes and the expression levels of related genes in nitrogen metabolism can clarify nitrogen assimilation ability to a certain extent. We set three different nitrogen levels through hydroponics. Then, with the different R:FR light ratios, the growth of pakchoi seedlings, activities of key enzymes for nitrogen metabolism (NR, GS, GOGAT, GDH), the content of related substances (nitrate, glutamic acid) and the expression levels of NRT genes were measured under different nitrogen levels.
2. Materials and Methods
The experiment was conducted at Sichuan Agricultural University (Chengdu, China) from September 2021 to August 2022. The seeds of pakchoi (Brassica campestris L. cv. Te Zhong Xiao Bai Cai) were sown in a crypt tray containing a nursery substrate (vermiculite and perlite 3:1) and the nursery experiment was conducted in an artificial climate box. The climate condition was as follows: temperature of 26 ± 1 °C/18 ± 1 °C (day/night), relative humidity of 70%, light intensity of 200 ± 25 μmol·m·−2·s−1, photoperiod of 12 h/12 h (day/night); 1/2 time of Hoagland nutrient solution was changed every 3 days. Because the research indicates that the main salt of secondary saline soil is calcium nitrate [4], the nitrogen source for treatment in this experiment was calcium nitrate. We used a 72-hole plug for seedling cultivation, with a size of 0.54 m × 0.28 m. After 22 days of the seedling period, the seedlings were transplanted into the hydroponics box for 7 days before being stressed, where the environmental condition was not changed.
Except for the filament lamp, the R:FR ratio of all artificial lights was greater than that of natural light [24,25]. The LED white light was used as a control. To decrease the R:FR ratio, based on white light, far-red light tubes were added, and the calculation method for the R:FR index was as follows: the quantum current density of 655~665 nm divided by the quantum current density of 725~735 nm. The ratio of R:FR was set at 4.2 (control) or 0.7, according to our previous study [23]. There were six treatments, shown in Table 1: (1) CK: white light (R:FR = 4.2); (2) L: white light and far-red light, R:FR = 0.7; (3) H80: additional 80 mmol·L−1 NO3− was added, R:FR = 4.2; (4) L80: additional 80 mmol·L−1 NO3− was added, R:FR = 0.7; (5) H160: additional 160 mmol·L−1 NO3− was added, R:FR = 4.2; (6) L160: additional 160 mmol·L−1 NO3− was added, R:FR = 0.7. Moreover, the NO3− concentration was 7.0 mmol·L−1 in the control, while it was 87 and 167 mmol·L−1 after additional 80 mmol·L−1 and 160 mmol·L−1 NO3− were added, respectively. The experimental processing table is as follows:

Table 1.
Experimental treatment.
Samples were collected from randomly selected plants. Leaves were removed from the plants and frozen using liquid nitrogen. The experiment was repeated three times, and 300 seedlings were cultured each time.
The plant growth index was determined according to the method used by Chen et al. [23]. The fresh weight was weighed with a balance. The material was put into an oven at 105 °C for inactivating enzymes for 0.5 h and then dried to constant temperature at 80 °C, and then, the dried weight was measured. Chlorophyll content was determined via the acetone method [26], including chlorophyll a (Chl a), chlorophyll b (Chl b), carotenoid and total chlorophyll. The key enzyme activities of NR were determined based on the method of Xiong [27]. One unit was defined as 1 µg nitrous nitrogen formed per hour of activity of NR. The activities of GS, GOGAT and GDH were determined via the method of Lin [28]. One unit was defined as 1 pmol L-glutamate y-monohydroxamate formed per min in GS. Moreover a decrease of 0.01 OD340 per min and 1 μmol NADH oxidized per min represented one unit for GOGAT and GDH, respectively. The determination of nitrate content was based on the salicylic acid–sulfuric acid method [27]. The glutamic acid content was determined using the bromothymol blue method [29].
RT-qPCR were performed according to Chen et al. [23]. Gene amplification primers designed for the desired response were obtained from Primer Premier 5.0.0 (Premier Biosoft, Palo Alto, CA, USA). F:5′-CTATATCGGGGTGGCCTCCTCCTA-3′ and R:5′-AGCTTTTTGCATAAGGGAAT-3′ were primer sequences for NRT1. F:5′-GGAGCACAAGCCGCTTGT-3′ and R:5′-AAGGGCTCGCCGAGAAAC-3′ were primer sequences for NRT2 [30]. F:5′-AAACGCCTACCACATCCA-3′ and R:5′ -CCATCGCCATCTCTTTCTTA-3′ were primer sequences for actin [23]. The 2−∆∆Ct method was used for the relative expression determination [31].
Experimental data were analyzed using SPSS 22.0 software, and graphs were created using Origin 2022 software. Statistical analysis of anonymous analysis (ANOVA) data was performed with Duncan’s multifunctional test (p < 0.05).
3. Results
3.1. Effects of R:FR Ratio on Growth of Pakchoi with Excessive Nitrate Stress
Under the non-stress condition, the difference in dry weight and fresh weight of CK and low R:FR treatment (L) was not significant at 10 d (Table 2). After 10 d, the accumulation of dry and fresh weight with high-nitrogen stress under white LED light (H80 and H160) was significantly lower than that of the CK group. However, when the R:FR ratio was reduced under high-nitrogen stress (L80 and L160), the accumulation of dry and fresh weight was significantly increased.

Table 2.
Effects of low R:FR ratio on growth of pakchoi with excessive nitrate stress.
3.2. Effects of Low R:FR on Chlorophyll Content of Pakchoi with Excessive Nitrate Stress
When compared with that in the control, there was a significant reduction in the content of Chl a, Chl b, carotenoid and total Chl by 16.4%, 39.4%, 14.3% and 24.2%, respectively, under L treatment at 10 d (Figure 1A–D). At 10 d, with an increasing nitrate concentration, the chlorophyll contents (content of Chl a, Chl b, total Chl) were increased and then decreased with the normal R:FR ratio (CK, H80, H160). The low ratio R:FR (L, L80, L160) resulted in the same trend. H80 significantly increased the accumulation of Chl a, Chl b, carotenoid and total Chl, while H160 significantly decreased the four parameters mentioned in chlorophyll content on 10 d compared to levels in the CK group. However, diminishing the ratio of R:FR could significantly reduce this effect. There was a similar changing trend among treatment groups at 5 d.


Figure 1.
Effects of low R:FR ratio on content of Chl a, Chl b, carotenoid and total Chl of pakchoi with excessive nitrate stress. (A): Chl a content; (B): Chl b content; (C): Carotenoid content; (D): Total Chl content. The values shown are means ± standard deviations (n = 3). Different letters mean marked differences at p < 0.05 based on Duncan’s multiple range test. 0 d: on day 0 after treatment; 5 d: on the fifth day after treatment; 10 d: on the tenth after treatment. CK: white light (R:FR = 4.2); L: white light and far-red light, R:FR = 0.7; H80: additional 80 mmol·L−1 NO3− was added, R:FR = 4.2; L80: additional 80 mmol·L−1 NO3− was added, R:FR = 0.7; H160: additional 160 mmol·L−1 NO3− was added, R:FR = 4.2; L160: additional 160 mmol·L−1 NO3− was added, R:FR = 0.7. This is similar hereinafter.
3.3. Effects of Low R:FR on Activities of Key Enzymes of Nitrogen Metabolism in Pakchoi under Excessive Nitrate Stress
H80 could significantly increase the activity of NR, while H160 treatment inhibited the activity of NR significantly, as shown in Figure 2A. Compared with that in the CK group, the activity of NR under H80 was increased by 41% and 49% after 5 d and 10 d, respectively. Moreover the activity of NR after treatment with H160 was decreased by 41% and 59% after 5 d and 10 d, respectively. However, reducing the ratio of R:FR could significantly improve activity of NR further with/without stress treatment.

Figure 2.
Effects of a low R:FR ratio with excessive nitrate stress on activities of NR, GS, GOGAT and GDH. (A): Activity of nitrate reductase (NR), (B): Activity of glutamine synthetase (GS) (C): Activity of glutamate synthetase (GOGAT) (D): Activity of glutamate dehydrogenase (GDH).
It was shown that a high nitrogen level could severely inhibit the activity of GS (Figure 2B), while a low R:FR ratio restrained the decrease in GS. The activities of GS in L were also increased compared with those in the CK group at 5 d and 10 d, respectively.
As shown in Figure 2C, the activity of GOGAT was significantly inhibited by H80 and H160 compared with that in the CK group. However, compared to that with H80, the activity of GOGAT with L80 was increased by 121% and 525% at 5 d and 10 d, respectively. There was a similar trend in the change in GOGAT under L160 treatment, when compared to that with H160. Further, after the R:FR ratio was reduced to 0.7, the activity of GOGAT with L was increased by 67% and 82% compared with that in the CK group at 5 d and 10 d, respectively.
H80 could significantly increase the activity of GDH, while H160 treatment inhibited the activity of GDH significantly, as shown in Figure 2D. When the R:FR ratio was reduced to 0.7, the activity of GDH with L was enhanced.
3.4. Effects of Low R:FR on Nitrate Content and Glutamic Acid Content in Pakchoi under Excessive Nitrate Stress
From Figure 3A, the nitrate content in pakchoi leaves treated with H80 and H160 was promoted markedly compared with that in the CK group. However, there was a reduction in the nitrate content induced by low R:FR, according to a comparison of nitrate content in the L80 vs. H80 and L60 vs. H160 groups after 5 d and 10 d of treatments, respectively.

Figure 3.
Effects of low R:FR ratio on nitrate content and glutamic acid content in pakchoi under excessive nitrate stress. (A): Nitrate content; (B): Glutamic acid content.
It turned out that the glutamic acid content accumulation with H160 was repressed at 5 d after high nitrogen treatment (Figure 3B). However, when the rate of R:FR was reduced to 0.7, the glutamic acid content with L increased significantly compared with that in the CK group at 5 d and 10 d. The results also showed that a low ratio of R:FR could increase the accumulation of glutamic acid content in plants with H80. However, there was no increase with a higher concentration treatment (H160).
3.5. Correlation Analysis of Nitrogen Metabolism-Related Enzymes and Nitrogen Form
The content of NO3− and Glu tended to be stable and the activity of enzymes also tended to be stable after treatment for 10 d. Table 3 shows that the NO3− content was negatively correlated with the activities of NR, GS and GOGAT, while the Glu content was notably positively correlated with the activities of GS and GOGAT. This indicated that improving the enzyme activities of GS and GOGAT and NR in the glutamine–glutamic acid cycle is an effective way to solve NO3− accumulation.

Table 3.
Correlation analysis of nitrogen metabolism-related enzymes and nitrogen forms after 10 d of treatment.
3.6. Effect of Light and Quality Ratio of LED on NRT Gene Expression in Pakchoi under Excessive Nitrate Stress
Under treatment with 160 mmol·L−1NO3−, the expression level of the NRT1 gene was not significantly different within 72 h, except at 6 h and 24 h (Figure 4A). However, there was a trend of first rising and then falling and reaching a peak at 24 h in response to a low R:FR (0.7) under calcium nitrate stress. From Figure 4B, it was observed that under treatment with 160 mmol·L−1 NO3−, NRT2 gene expression significantly exceeded that of the control group within 72 h, and maximum expression was achieved at 24 h. This indicates that the NRT2 gene is also responsive to a low R:FR ratio.

Figure 4.
Expression of NRT1 and NRT2 under additional 160 mmol·L−1 NO3− supplementation treatment with/without a low ratio of R:FR. 4.2: R:FR = 4.2; 0.7:R:FR = 0.7. (A): Relative expression of NRT1; (B): Relative expression of NRT2.
4. Discussion
Salt stress can inhibit plant growth [24,32]. However, a lower proportion of R:FR can promote plant growth under salt stress [10,23,33,34]. Chlorophyll plays a vital role in the capture and transmission of light energy in plant photosynthesis [35]. Studies have shown that salt stress can promote the accumulation of chlorophyll in cucumber [35], but high-concentration salt stress will reduce the chlorophyll content in pakchoi [36]. Meanwhile, the light environment with a low R:FR value can alleviate the salt stress in tomato by promoting chlorophyll synthesis [37]. This experiment showed that with the additional application of 80 mmol·L−1 NO3− (H80), the chlorophyll content of pakchoi was increased, and the promoting effect was more obvious when the ratio of R:FR was lower (L80). Under H160 treatment, the accumulation of chlorophyll content in pakchoi was inhibited, but the inhibition was effectively alleviated by a low ratio of R:FR (L160), which was consistent with previous experimental results [37].
Our previous study [23] pointed that a low ratio of R:FR could promote the activity of NR in pakchoi under both non-stress and excessive nitrate stress, which was further verified in this study. In the process of nitrogen assimilation, NH4+ is assimilated into glutamate through the glutamine glutamate cycle and glutamate dehydrogenase pathways. The glutamine glutamate cycle involves two key enzymes, GS and GOGAT. Glutamine catalyzed by GS is decomposed into glutamic acid by GOGAT. ATP and NADPH are required for these two reactions. When there is too much NH4+ in the plant, the activities of GS and GOGAT increase rapidly. When the ratio of R:FR is reduced, the photosynthetic capacity of the plant increases [34], which can provide energy for reactions and enhance the enzyme activity. Studies have shown that there is no notable difference in GS activity between a processing group and controls when it is stressed by 120 mmol·L−1 nitrate [9]. In this experiment, under treatment with H80, the activity of NR was increased, and activities of GS and GOGAT were reduced, suggesting that the nitrogen concentration was too high and caused serious injury to the plants. However, the activities of these two enzymes increased under treatment with a low ratio of R:FR. As a leucine zipper transcription factor, HY5 is sensitive to light signals and can induce the expression levels of genes related to nitrogen metabolism to promote nitrogen metabolism in plants [38]. Whether a low ratio of R:FR promotes the increase in the activity of enzymes related to nitrogen metabolism by stimulating HY5 needs to be further verified.
When the glutamine glutamic acid cycle was blocked and the concentration of NH4+ was too high, GDH was induced to catalyze NH4+. In this study, under H80 treatment, NR enzyme activity was increased and GS and GOGAT activities were decreased, resulting in the accumulation of a large amount of ammonium chloride and increased GDH activity. While under the H160 treatment, the GDH activity was also inhibited (Figure 2D) due to the harmful stress. However, the activity of GDH was inhibited further when the R:FR ratio was decreased. This might be due to the enhancement of plant photosynthesis, which provided ATP and reducing hydrogen for the enzymes GS and GOGAT, with strong affinity for NH4+, and enhanced the circulation of glutamine and glutamic acid [39]. In addition, studies have also shown that when plants are under stress and lack an organic carbon source, GDH will begin to catalyze the decomposition of glutamic acid to provide energy for the plants [40], thus enhancing the activity of GDH. Reducing the R:FR ratio would enhance photosynthesis, improve the plant’s ability to obtain carbon sources and reduce the need for GDH to decompose glutamic acid, thereby reducing its activity. Thus, reducing the ratio of R:FR reduces the requirement of plants for the decomposition of glutamic acid and reduces the ability of GDH to decompose glutamic acid.
The transcription level of NRT1 can reflect the absorption and utilization of N [41]. Results indicate that after a period of cultivation, expression of the NRT gene was significantly increased in the plants treated with a low ratio of R:FR, and expression of the NRT1 gene, promoted by a low ratio of R:FR, was higher than that of the NRT2 gene under higher-concentration calcium nitrate stress. Studies have shown that sucrose can be used as a signaling molecule to induce HY5 transcription factor binding to the nitric acid transporter gene NRT2.1 in roots and promote the expression of NRT2.1 [40]. A low ratio of R:FR could promote plant photosynthesis and increase sugar accumulation [23]. It was speculated that the low ratio of R:FR promoted the expression of genes related to nitrogen metabolism by inducing HY5.
Above all, under the same nitrogen level, compared to those with white light, the NR, GS and GOGAT enzyme activities of pakchoi were enhanced with a low R:FR ratio. The model of the effect of excessive nitrate stress and/or the R:FR ratio on nitrogen metabolism in pakchoi is shown in Figure 5. Moreover, the expression of the NRT gene was also increased under treatment with a low R:FR ratio, which led to the decrease in the nitrate content and increase in the glutamic acid content in pakchoi.
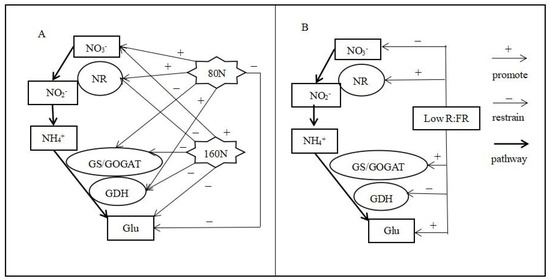
Figure 5.
Pattern of nitrogen assimilation in pakchoi with different nitrogen levels regulated by a low R:FR. H80 represents the extra addition of 80 mmol·L−1 NO3− nitrogen treatment, and H160 represents the extra addition of 160 mmol·L−1 NO3− nitrogen treatment. (A) represents the effect of nitrogen treatment on the nitrogen metabolism pathway, and (B) represents the effect of reducing the red:far-red (R:FR) ratio on the nitrogen metabolism pathway.
5. Conclusions
In summary, excessive nitrate stress inhibited the growth of pakchoi. However, a low ratio of red light to far-red light can alleviate this inhibition. Activities of NR and GDH were increased, while activities of GS and GOGAT were decreased, with the H80 treatment. Under H160 treatment, the activities of the four enzymes were decreased. A low ratio of R:FR could improve the activities of NR, GS and GOGAT, but restrain GDH, involved in nitrogen metabolism, and enhance the expression level of the NRT genes. Thus, it could improve the nitrogen metabolism of pakchoi and promote nitrogen assimilation under excessive nitrate stress.
Author Contributions
Conceptualization, X.Z.; Data curation, Y.G.; Investigation, Y.G., L.C. and C.J.; Methodology, Q.C.; Validation, X.C.; Writing—original draft, X.Z.; Writing—review and editing, X.Z., Y.G. and C.J.; Funding acquisition: Z.H., X.Z., Y.G. and C.J. contributed equally to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Study on Vegetable Science of Farmland System in Qinghai-Tibei Plateau (2019QZKK0303). And The APC was also funded by Study on Vegetable Science of Farmland System in Qinghai-Tibei Plateau (2019QZKK0303).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, P.; Zhao, Z.H. Effects of nitrogen fertilizer on growth and nitrate accumulation of pakchoi. China Fruit Veget. 2017, 37, 25–28. [Google Scholar]
- Yang, X.Y.; Yang, J.S. The impact of nitrogen supply level on the growth and nitrate accumulation of pakchoi. Plant Nutr. Fert. Sci. 2007, 1, 160–163. [Google Scholar]
- Chen, S.Q.; Jiang, Y.T. Cause, harm and treatment measures of secondary salinization of facility soil. Liaoning Huagong. 2018, 47, 1146–1148. [Google Scholar]
- Sun, Z.D.; Han, X.R.; Peng, J.; Fan, F.; Zhang, Q.G. Effect of exogenous nitric oxide on PSII function and distribution and utilization of luminous energy in tomato seedlings under stress of Ca(NO3)2. J. Nucl. Agric. Sci. 2016, 30, 2451–2459. [Google Scholar]
- Qi, X.L.; Zou, Z.R.; Yang, R. Alleviative effect of exogenous ALA on lettuce plants under NaCl stress. Acta Agric. Boreali-Occident. Sin. 2008, 17, 202–206. [Google Scholar]
- Zhou, C.; Zheng, C. Effects of different nitrogen levels on yield and quality of pakchoi. Inform.Agric.Sci.Tech. 2006, 2, 36–37. [Google Scholar]
- Lu, Y.; Shu, S.; Zhu, W.M.; Guo, S.R. Effects of exogenous spermidine on growth, photosynthesis and quality of Chinese cabbage under calcium nitrate stress. Xibei Zhiwu Xuebao 2015, 35, 0787–0792. [Google Scholar]
- Liu, S.W.; Wang, N.; Irfan, M.; Xu, J.Y.; Zhang, Y.J.; Cai, G.X. Effect of fermentation broth of endoph ytic fungi on physiological and biochemical characteristics of tomato seedling under calcium nitrate stress. Iran. J. Sci. Technol. Trans. Sci. Trans. A Sci. 2019, 43, 1427–1432. [Google Scholar]
- Pang, Q.Q.; Chen, R.Y.; Liu, H.C.; Song, S.W.; Su, W.; Wen, G.W. Effects of fulvic acid on the growth of pakchoi and the activities of enzymes related to nitrogen metabolism under nitrate stress. J. Zhejiang Agric. Univ. 2015, 27, 2136–2140. [Google Scholar]
- Cao, K.; Yu, J.; Xu, D.W.; Ai, K.Q.; Bao, E.C.; Zou, Z.R. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedings by increasing leaf expansion and whole plant net assessment. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Duong, T.N.; Takamura, T.; Watanabe, H.; Okamoto, K.; Tanaka, M. Responses of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Appl. Fundam. Aspects Plant Cell Tissue Organ. Cult. 2003, 73, 43–52. [Google Scholar]
- Gao, D.G.; Yu, J.; Zou, Z.R.; Cao, K.; Bao, E.C.; Meng, L.L.; Li, S.Z.; Ye, L. Effects of different ratios of red light to far-red light on photosynthetic capacity of tomato under salt stress. J.Anhui Agric.Univ. 2021, 48, 578–583. [Google Scholar]
- Zhang, L.W. Effects of Light Quality on Physiological Characteristics and Quality of Three Kinds of Sprouting Vegetables. Master’s Thesis, Shandong Agricultural University, Jinan, China, 2010. [Google Scholar]
- Zhen, S.Y.; Iersel, M.V. Far-red light is needed for efficient photochemistry and photosynthesis. Plant Physiol. 2017, 209, 115–122. [Google Scholar] [CrossRef]
- Ning, Y.; Deng, H.H.; Li, Q.M.; Mi, Q.H.; Han, B.; Ai, X.Z. Effects of red and blue light quality on carbon and nitrogen metabolism and key enzyme activities of celery. Acta Phytopsoil. Sin. 2015, 1, 112–118. [Google Scholar]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Zhi, L.; Shi, H.Z.; Liu, S.G.; Wang, D.Z.; Zu, C.L.; Yang, Y.F. Changes of earbon nitrogen metabolism of flue-cured tobacco with sweet aroma in south anhui under different fertilization rates. J. Exp. Bot. 2015, 66, 2055–2065. [Google Scholar]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate trends. Plant Sci. 2012, 17, 458–467. [Google Scholar]
- Zhao, S.P.; Zhang, R.L.; Xu, M.F.; Zheng, J.C. Study on the mechanism of the effect of ammonium nitrate ratio on nitrate accumulation in different genotypes of pakchoi. Soils. 2011, 43, 32–38. [Google Scholar]
- Sandrine, F.R.; ‚Erwan, L.D.; ‚Philippe, L.; James, H.M.; Alain, O. Effect of nitrate pulses on BnNRT1 and BnNRT2 genes: mRNA levels and nitrate in flux rates in relation to the duration of N deprivation in Brassica napus L. J. Exp. Bot. 2002, 53, 1711–1721. [Google Scholar]
- Dong, S.J. Effect of LED Light Supplement on Pepper Seedling Growth and Colonization of Arbuscular Mycorrhizal Fungi. Master’s Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Chen, L.B.; Huang, J.; Liu, Q.L.; Li, Z.L.; Chen, X.; Han, J.X.; Gan, Y.R.; He, Y.X.; Jiang, C.X.; Tang, Y.X.; et al. Low R:FR ratio affects pakchoi’s growth and nitrate content under excess nitrate stress. Horticulturae 2022, 8, 186. [Google Scholar] [CrossRef]
- Yu, J. Effect of Different Ratio of Red Light to Far Red Light on Salt Tolerance of Tomato. Northwest. Master’s Thesis, Agriculture and Forestry University, Shanxi, China, 2018. [Google Scholar]
- Zhou, Q.F.; Wang, E.Z. High-efficient horticultural facilities and horticultural lighting source. J. Chang. Veg. 1999, 1, 1–4. [Google Scholar]
- Lichtenhaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Xiong, Q.E. A Experimental Course of Plant Physiology; Sichuan Science and Technology Press: Chengdu, China, 2003. [Google Scholar]
- Lin, C.C.; Kao, C.H. Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by NaCl. Plant Growth Regul. 1996, 18, 233–238. [Google Scholar] [CrossRef]
- Jin, O.; Chen, B. Comparison of determination methods of glutamate content. Chin. J. Biochem. Pharm. 1993, 59–60. [Google Scholar]
- Zhao, S.P.; Zhang, Y.Z.; Ye, X.Z.; Zheng, J.C. Research on the mechanism of different nitrate accumulationin Chinese cabbage with different genotypes. Plant Nutr. Fert. Sci. 2021, 16, 681–688. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR methods. Acta Agron. Sci. 2002, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Li, H.R.; Guo, S.Y.; Wang, S.M.; Shi, H.Z.; Han, Q.Q.; Bao, A.K.; Qing, M.A. Research advances in higher plant adaptation to salt stress. Acta Prataculturae Sin. 2015, 24, 220–236. [Google Scholar]
- Zhou, X.T.; Li, Z.L.; He, J.J.; Wang, X.Y.; Liu, Q.L.; Huang, J.; Xie, Y.D.; He, Z.Q. Effects of red to far-red light ratio on growth and photosynthetic characteristics of tomato seedlings under calcium nitrate stress. Photosynthetica 2021, 59, 625–632. [Google Scholar] [CrossRef]
- Li, Z.L.; Chen, G.Y.; Gao, F.Q.; Luo, J.P.; Li, C.W.; He, Z.Q.; Zhou, X.T. Effects of different light quality ratio on growth and photosynthesis capacity in pakchoi under excess nitrate stress. IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012105. [Google Scholar] [CrossRef]
- Gao, X.X.; Li, R.C.; Wen, X.Z.; Miao, Y.X. Effect of the ratio of red light and far-red light on the growth and photosynthetic characteristics of cucumber seedlings under salt stress. Shandong Agric. Sci. 2021, 53, 36–41. [Google Scholar]
- Xu, F.F.; Xu, P.; Hu, Z.T.; Zhao, J. Photosynthetic Physiological Response of pakchoi to Salt Stress. Mol. Plant Breed. 2018, 16, 3327–3332. [Google Scholar]
- Ai, K.Q.; Su, H.; Zh, H.J.; Zhou, B.; Cao, K.; Zou, Z.R. Effects of different red and far-red light on chlorophyll synthesis of tomato under salt stress. North. Hortic. 2019, 1, 14–22. [Google Scholar]
- Wang, Z.R.; Wang, H.Y.; Deng, H.F.; Xv, C.Q. Regulation of transcription factor HY5 on plant light morphogenesis and nitrogen metabolism. China Veget. 2018, 5, 20–27. [Google Scholar]
- Chi, S.L.; Yang, Y.; Xu, W.H.; Chen, X.G.; Chen, Y.Q.; Xie, W.W.; Xiong, S.X.; Wang, Z.Y.; Xie, D.T. Correlation between nitrate content in pakchoi and NO3-: NH4+and key enzymes of nitrogen metabolism. Food Sci. 2015, 36, 70–77. [Google Scholar]
- Zhang, Y.Y.; Song, X.F.; Xu, J.B.; Zhou, Q.; Liu, H.Q.; Wang, Y.; Zhang, Z.Y.; Song, H.L. Effects of different nitrogen concentrations on the growth and physiological indicators of water hyacinth, especially the activities of key enzymes of nitrogen metabolism. Plant Resour. Environ. 2021, 30, 39–46. [Google Scholar]
- Yu, Y.C.; Xu, T.; Li, X.; Tang, J.; Ma, D.F.; Li, Z.Y.; Sun, J. NaCl-induced changes of ion homeostasis and nitrogen metabolism in two sweet potato (Ipomoea batatas L.) cultivars exhibit different salt tolerance at adventitious root stage. Environ. Exp. Bot 2016, 129, 23–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).