Abstract
Light-emitting diodes (LEDs) have been regarded as the best artificial source of light for a plant factory. However, the effect of light quality on seedling production in such environments requires further study. On the basis of the practical application of light on cucumber seedlings (Ansha Company) in plant factories, the present investigation tracked and recorded the specific effects of red and blue light on the growth of the seedlings by analyzing the photo-biological mechanism involved. The growth parameters, as well as the photosynthetic characteristics of cucumber seedlings, were measured at different variations of light quality. The results showed that when the proportion of red light in the light source was higher than blue light, the height of the seedlings, leaf size, stem diameter, Dixon Quality Index (DQI), relative chlorophyll content, and the net photosynthetic rate were higher than those of the experimental group with a relatively high proportion of blue light. In the case of R7B3 (70% red light and 30% blue light), the stem diameter, DQI, and net photosynthetic rate of seedlings were 14%, 57%, and 22% higher than the minimum value, respectively. The present study analyzed the influence of red and blue light on plant growth characteristics during actual production and provides standardization for it.
1. Introduction
With the arrival of agricultural modernization, facility agriculture has become a very integral part of it, with the light source being a key component. The three most basic variables affecting plant growth are light quality, light intensity, and photoperiod, which not only affect the rate of plant growth [1], morphology [2], and fruit quality [3] but also affect the primary and secondary metabolite content in plants [4]. Therefore, light is not just an important energy source for photosynthesis but is also a critical factor affecting plant growth and development [5].
There are two kinds of light sources—natural and artificial. In the actual production process of the plant factory involving either planting or raising of seedlings [6], the dependence on the natural source (sunlight) for illumination limits the placement position of carriers such as seedbeds or trays. In such a scenario, to avoid mutual shielding, only the single-layer placement mode is possible, which requires a larger use area of the site [7]. In addition, considering the consumption of resources for site control of temperature, humidity, carbon dioxide, etc., the cost of production is also greatly increased. Comparatively, the artificial light source is advantageous, such that it cannot only meet the needs of plants for light but also realize three-dimensional planting, lesser utilization of area, and lower planting costs. Among the existing artificial light sources, light-emitting diodes (LED), being an efficient light source with low energy consumption, small size, and long life, are increasingly becoming the main light sources in the actual production of plant factories [8,9].
It is well known that blue light (400–500 nm) and red light (600–700 nm) are more effective than the light of other wavebands in the photosynthetic process [10]. Red light has a higher quantum efficiency, whereas blue light limits the photosynthetic rate of plants by affecting the photosynthetic system [11,12]. Many researchers have reported that blue light regulates the growth and morphology of plants, for instance, in the inhibition of the extensive growth of stems [13]. When blue light was induced in tomato seedlings, the amount of Rubisco was noted to be higher, along with a reduction in the biomass, while the size of the plant was observed to be more compact [14]. The blue light was also found to improve the activity of beneficial substances or pigment proteins in plants, such as the increase in the antioxidant activity of lettuce or the tolerance achieved as a result of blue light irradiation [15]. By adjusting the response of phytochrome A and cryptochrome 1, the flowering period and the diurnal pattern of the crops could be controlled [16]. The glucose, fructose, and sucrose content in fruits were increased by irradiating the stored fruits with blue light [17]. Red light promotes the elongation of the petioles and stems of tomatoes, thereby loosening the plant structure [18]. It can also induce changes in phytochrome, which affects the opening of cotyledons of young seedlings and causes changes in the morphology of seedlings [19]. The plant height and flowering period of tomato can be regulated by controlling the duration of red light irradiation at night [20]. Far-red light leads to thinning of plant leaves, and the length of the hypocotyl, as well as the dry weight of vegetable seedlings, gradually increase with the increase in its intensity [21]. In a word, light plays a very important role in the influence of plant morphology. It can modulate the gravitropic responses of stems, delay or reduce the generation of tendrils, result in a phenotype with short internodes, and reduce apical dominance, among other factors [22].
Cucumber is one of the most common crops and therefore has received extensive attention. In particular, light quality has always been a major concern in influencing the quality of cucumber seedlings [23,24,25]. A review of existing literature notes that there are differing views on the promotion or inhibition of the growth of cucumber seedlings using the same quality of light because of the complexity of the factors that affect plant growth. For instance, in their study on the effect of light quality on the growth of cucumber seedlings, Hernández et al. [26] reported that the plant height, hypocotyl length, and epicotyl length were decreased with an increase in blue light. This was similar to the study by Su et al. [27], who noted that in comparison to blue light, red light could improve the plant height, leaf area, dry weight, and root activity of the cucumber seedlings. However, Song et al. [28] believed that red light could inhibit hypocotyl elongation by affecting photoreceptors through their gene expression. In terms of photosynthetic characteristics, the investigation by McCree et al. [10] found the net photosynthetic rate was decreased with an increment of blue light. This is because blue light is absorbed by pigments with low energy transfer efficiency, such as carotenoids, or pigments with inactive photosynthesis, such as anthocyanins. This is in contrast to the experimental results obtained by Hogewoning et al. [29], who believed that blue light could stimulate “high-irradiance leaf characteristics” even under constant irradiance, with increased net photosynthetic rate, chlorophyll concentration, and stomatal conductance after the increment of blue light. Wang [30,31] has shown that LED light supplementation can promote the growth and development of cucumbers, thus determining the appropriate time for light supplementation in plants in terms of maximizing yield.
The aforementioned research findings are based on the actual situation of crop seedling breeding in the current scenario of agricultural production. Therefore, the present investigation was designed to study the light supplement system for the breeding of seedlings in plant factories. To analyze the impact of red and blue light on the seedlings breeding in practical applications, the cucumber crop was considered. This was achieved by changing the light quality ratio of the light supplement equipment. The morphological characteristics, growth parameters, net photosynthetic rate, and intercellular carbon-dioxide concentration of cucumber seedlings were measured in the study. Overall, the objective was to determine the best light quality ratio of cucumber seedlings that is the most suitable for actual production, with standardizations for the actual production in plant factories.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Experimental materials: A total of 100 pieces of cucumber fruit with seeds (Ansha Company, Beijing, China) were used for the experiment.
Planting conditions: The seeds were planted in a wet substrate bag (composed of coconut bran with good water retention capacity (80%)) by sowing manually; sprinkled with vermiculite; and placed in a greenhouse for germination, for which the temperature was 23 °C and the humidity was 80%. After their germination (3–4 days), they were transferred to the plant supplementary light seedbed for irradiation with LED light source and were irrigated with EC = 1.5–1.8 mS/cm nutrient solution every day for the entire duration of the seedling growth period.
Treatment group setting: Five control groups were included in the experiment, viz., R9B1 (red light/blue light—9:1), R7B3 (red light/blue light—7:3), R5B5 (red light/blue light- 5:5), R3B7 (red light/blue light—3:7), and R1B9 (red light/blue light- 1:9), with 14 h duration of light every day; the spectrum is shown in Figure 1. As the photosynthetic quantum flux density (PPFD) of a particular light quality varies from another, the PPFD of the lamps of each particular light quality was adjusted to 220–230 in order to ensure the accuracy of the experimental results.
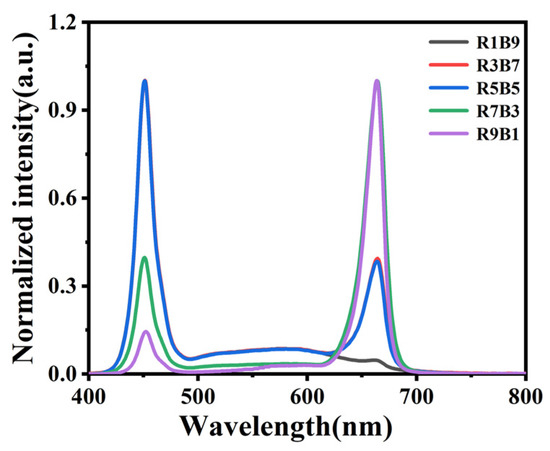
Figure 1.
Spectral distribution of LEDs.
Planting position: The germinated seedlings were placed on the specially designed plant light-compensating frame (Figure 2). Fifteen matrix bags were positioned on each layer of the seedbed, with one seedling planted in each matrix bag. The temperature (23 °C), humidity (80%), and other environmental conditions for each group of treatments were maintained uniformly.

Figure 2.
Specially designed plant light supplement equipment.
Parameters recorded: The growth of the plants was measured on the 13th, 16th, and 19th days after sowing (i.e., in the seedling stage, before being transplanted to a greenhouse at the end of 19 days of seedling growth). The chlorophyll index, whose unit is SPAD; net photosynthetic rate; intercellular carbon dioxide concentration; stomatal conductance; and other parameters were measured during the final transplantation.
2.2. Light Source Design
2.2.1. Spectral Design
The design of the spectrum is an important aspect of the light-filling equipment to meet the needs of the actual production. Current studies have mostly focused on red and blue lights [28,32]. Although red and blue lights are the two light qualities with the highest utilization efficiency, light of other wavelengths also has a considerable impact on plants. The growth of the lettuce crop was found to be the highest under red and blue LED-supplemented light compared to the green light treatment. This is attributed to the fact that the leaves in the lower canopy were able to use the transmitted green light in photosynthesis, thereby decreasing the senescence and abscission in the leaves [33]. Supplementing yellow and green light along with red and blue light was observed to increase the chlorophyll and carotenoid contents in tomato leaves, which in turn increased the photosynthetic rate [34]. Therefore, to ensure the best conditions for raising seedlings in practical production, the current study investigated the effect of red and blue light on seedlings, with the utilization of full spectrum light, in order to enhance their efficiency.
2.2.2. Design of Light Quality Ratio
A review of recent literature [35,36,37,38] reveals that there are different measures of light quality ratios, such as the ratio of chips or the ratio of light intensities of different wavelengths, which are indicated as the red–blue ratio. The LED light source is driven by a constant current. Therefore, in the design of lamps and lanterns, when the same red–blue ratio is considered, a similar ratio of the chip and the light intensity is used as a standard. This results in an inconsistent spectrum emitted by the LED, which affects the accuracy of the experimental results.
Since photosynthesis is a process of converting light energy into chemical energy [39], it is equivalent to the notion that plants absorb energy in light. Therefore, the red–blue ratio mentioned in the present study denotes the proportion of red and blue light energy, which is the proportion of light power. This is denoted using the formula
where is the optical power, is the starting wavelength, is the termination wavelength, and is the relative spectral intensity corresponding to the wavelength . The wavelength range of blue light is 400–499 nm, and the wavelength range of red light is 600–780 nm.
2.2.3. Optical Uniformity Design
To meet the requirement of uniform light, 12 LED lamps were used to fill the light on each layer of the seedbed. The photosynthetic photon flux density (PPFD) of the light source was measured on the plant light-filling surface. The seedbed was placed at a distance of 30 cm from the LED light source. The seedbed had a length of 2.4 m and width of 1.6 m, on which 49 points were marked in seven rows and seven columns, where the row spacing was 0.3 m, while the column spacing was 0.2 m. The PPFD parameters were indicated through a light distribution diagram (Figure 3). The figure is a representation of light distribution when the LED light source is at full power. This ensures that the plant growth rate under the light source is uniform, thereby maintaining the accuracy of the experimental results.
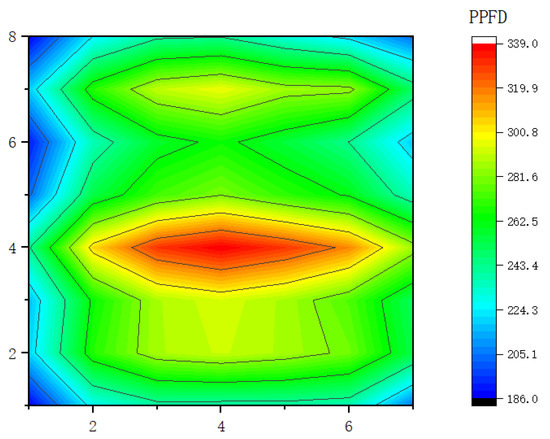
Figure 3.
Light intensity distribution in cucumber seedling placement plane.
2.3. Methods of Measurement
Measurement of growth: The length of the hypocotyl, plant height, leaf length, and leaf width of the cucumber seedlings were measured using a ruler (accuracy is 0.1 cm), while the diameter of the stem was measured using Vernier calipers [31].
In order to quantify the growth of cucumber seedlings, the Dixon Quality Index (DQI) was introduced, which is given by the formula [40]
Measurement of dry and fresh weight: For the fresh weight, the coconut bran at the root of the seedlings was washed three times, after which the surface water was wiped with absorbent paper and weighed in an accurate electronic scale with a measuring range of 100 g. For the dry weight, the seedlings were placed in an oven at 80 °C for three days, after which they were weighed in a similar manner [21].
Measurement of photosynthetic characteristics: The CIRAS-3 portable photosynthesis/fluorescence meter (Lufthansa Company, Tai’an, China (US PP SYSTEMS and UK Hansatech in China Headquarters)) was used to measure the net photosynthetic rate, stomatal conductance, and intercellular carbon dioxide concentration. SPAD was detected using the chlorophyll analyzer (Kinkolida, Beijing, China). The accuracy of the chlorophyll analyzer was 0.1. The measurement time was 14:00, the first true leaf of the plant was measured, the ambient temperature was 23 °C, and the humidity was 80%.
Statistical Analysis: The experiment was repeated three times, with twenty plants each in a completely randomized block design (there were five groups for each measurement, 20 samples for each group), and twenty plants per treatment were used to determine plant growth parameters. The statistical analysis was carried out using the statistical analysis system program (IBM SPSS Statistics 27, Amonk, New York, NY, USA). The experimental results were subjected to an analysis of variance and the Tukey test.
3. Result and Discussion
3.1. Plant Growth and Morphology
The analysis of data presented in Table 1 indicates significant differences (p < 0.05) in the growth parameters of the seedlings at different ratios of red and blue light. The growth of the hypocotyl is considered as the basis for judging whether a plant is overgrown. In the results obtained in the present study, it can be noted that the different light quality ratios had a significant effect on the length of the hypocotyl. However, compared with plant height, leaf length, and leaf width, the significant effect on the length of the hypocotyl was lower than them. This is in contrast to the results of Jeong et al. [41], which may have been due to the fact that green light promotes the influence of red and blue light on the hypocotyl, thereby inhibiting its elongation [26].

Table 1.
Morphological parameters of cucumber seedlings on the 13th (2022/9/13), 16th (2022/9/16), and 19th (2022/9/19) days after sowing under the effect of blue and red light. Values are means ±standard errors. Means with the same letter within each column were not significantly different at p < 0.05.
Plant height is another important indicator of plant growth. We observed that as the proportion of red light decreased (Figure 4), the height of the seedlings also decreased gradually. This was similar to the results obtained by Hernández et al. [26], who used a blue light chip with a wavelength of 455 nm, unlike the current experiment in which a blue light chip with a wavelength of 450 nm was used. The blue light in this range fell within the maximum activity range of cryptochrome, which is usually active at 420 nm, 450 nm, and 480 nm. Therefore, due to the increase in the proportion of blue light, the decrease in the length of the stem may be attributed to the stimulation of cryptochrome receptors. Red light is known to improve biomass and promote the vertical growth of plants [42]. Therefore, in accordance with this principle, similar changes were observed with regard to the leaf length and width. The stem has a key role in the transportation of water and carbohydrates [43], and the measurement of its diameter is one of the best ways to describe the growth of crops in the vegetative stage. However, observations on the growth of stem diameter revealed a different trend compared to the other parameters. Initially, the stem diameter of R9B1 (6%) was greater than that of R7B3, but with continuous growth, the stem diameter of R7B3 gradually increased to reach 4% higher than that of R9B1 by the 19th day. This indicates that the effect of light quality on the stem diameter of cucumber is not significant at the beginning of germination, but with gradual growth, the physiology of the plant is advanced, resulting in a significant effect on the stem diameter, which is attributed to be a compound effect. Although red light induces an increase in the diameter of the stem [44], exceeding the proportion results in unwanted reactions, indicating that an appropriate amount of blue light can also promote the increase in plant stem diameter.

Figure 4.
(a) Front view of seedlings under different light qualities. (b) Root system diagram of the seedling bottom under different light qualities. (c) Shape of seedling root after cleaning coconut bran (19-day-old seedling; the ratio indicated in the figure is the red–blue light ratio).
The analysis of the fresh weight (Table 2) of the seedlings revealed a trend, wherein initially the fresh weight of the above-ground part was higher, but with decreasing proportion of red light in the spectrum, the fresh weight was also decreased (p < 0.05). Contrastingly, the fresh weight of the underground part was observed to be lower initially but was increased gradually with the decrease in the proportion of red light. The fresh weight of the above-ground and underground plant parts was recorded to be the highest in R7B3 and R9B1 treatments, respectively. This is explained by the fact that red light promotes the growth of the root system. A greater proportion of red light in the spectrum caused an improvement in the growth of the root system. Therefore, the weight of the root system can be said to be positively related to the proportion of red light. The fresh weight of the above-ground part represents not only the accumulation of organic matter but also the amount of water content in the plants. The accumulation of organic matter is dependent on the rate of photosynthesis, while the water content is mainly dependent on the stem diameter. Therefore, the change in the fresh weight of the above-ground part is similar to that of the stem diameter of the seedlings. This phenomenon is similar to the work of Glowacka [45], wherein the effect of inhibiting plant growth in blue light translated into a lower fresh weight of the above-ground parts. This may be related to carotenoids in leaves that affect the efficiency of light conversion into organic matter. The fresh weight of the above-ground part was recorded to be the highest under the R7B3 treatment.

Table 2.
Dry weight and fresh weight of cucumber seedlings at 19 days (2022/9/19) after sowing. Values are means ±standard errors. Means with the same letter within each column were not significantly different at p < 0.05.
Regarding dry weight, the trend of change in the underground part was similar to that in the above-ground part, where initially it was found to be decreased but later increased with a decrease in the proportion of red light. This is due to the elimination of moisture from the seedlings during the baking process, which is an indication of the accumulation of organic matter alone. This confirms that the influence of light quality on the fresh weight is similar to that on the stem diameter. Red light increases the accumulation of organic matter by causing an increase in the growth of roots, thereby speeding up the growth rhythm of plants. This is different from the opinion of Song [46], wherein a high blue light intensity was seen to potentially be required in favor of the root dry weight. This may be due to different types of light sources, resulting in different spectra.
3.2. Healthy Seedling Coefficient of Seedlings
The DQI of seedlings under different light qualities is represented in Figure 5. From the figure, we observe that when the proportion of red light was higher than that of blue light, the DQI value was relatively larger. The DQI index of R5B5 was the smallest among all the treatments. The calculation based on the proportion of plant height and stem diameter provided values of 11.2, 10.0, 10.2, 9.5, and 11.1 as the proportion of red light decreased, which shows that there was no significant difference among the five groups of treatments (R9B1, R7B3, R5B5, R3B7, R1B9, respectively). However, the total dry weights were recorded as 4.2, 3.8, 1.8, 2.4, and 2.3 g, respectively, where the trend of change was similar to that of DQI. Conversely, the calculation based on the proportion of the dry weight of the above-ground part and underground part provided values of 1.6, 1.4, 1.8, 1.6, and 1.3, respectively, wherein the difference between them was not significant, but its change trend was opposite to that of DQI. Therefore, it can be said that the changing trend of DQI was similar to that of the total dry weight of plants. By analyzing the cause of changes in DQI, the cause of changes in the total dry weight can be explained. On the basis of this inference, we can say that red light promotes the growth of roots as well as the accumulation of plant organic matter, which is equivalent to the conclusion that red light affects the dry weight of plants. Blue light causes the stems to grow thicker, resulting in the acceleration of the transmission of water and nutrients. Moreover, it is similar to the work of Eliamara [47]. The higher the value of DQI, the higher the seedling quality, and they are more vigorous when they are transplanted in the field. A value greater than or equal to 0.10 for the DQI has been cited to identify seedlings of high-quality vegetables. In this experiment, the DQIs of all the treatments were higher than 0.10. It can be seen that LED is a correct choice for vegetable lighting.
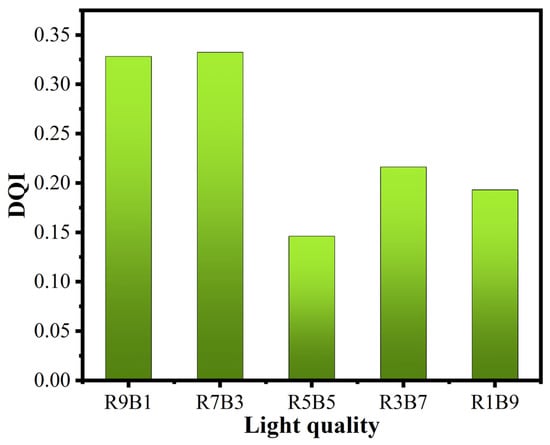
Figure 5.
DQIs of cucumbers under different light qualities.
3.3. Changes in Chlorophyll Content and Photosynthetic Parameters of Seedlings
The SPAD value (Figure 6) indicates the degree of green color in the leaf that is essential to determine the chlorophyll content. This reflects the demand for nitrogen, photomorphogenesis processes, and light availability in plants. Similar nutrient solution components were maintained in the control experiment for all the seedlings; therefore, one of the functions of SPAD is considered to reflect the absorption of nutrients by the plants. The SPAD values of R9B1 and R7B3 did not have a significant difference but were higher than other treatments groups (R5B5, R3B7, R1B9), with a large proportion of blue light (R9B1 was only higher than R7B3, but R9B1 was 10%, 12%, and 15% higher than R5B5, R3B7, and R1B9, respectively). This is in agreement with the experimental results of Chu Zhongxi et al. [48]. The chlorophyll content in the leaves of cucumber grown under blue light was lower than that under red and white light. This was inconsistent with the observation by Sæbø et al. [49], who noted that in the tissue of birch leaf, in comparison with white and red light, the chlorophyll content under blue light was higher by almost two times that under red light. This may have been due to the fact that under the blue light source, the net area of chloroplasts in each cell of the leaves was the largest, and the starch accumulated in such cells was lower than that in cells exposed to red light; therefore, the inhibition on the synthesis of chlorophyll was lower, leading to higher chlorophyll content. For cucumber seedlings, the difference may lie in the difference in phytochrome, which can upregulate the synthesis of ascorbate peroxidase (APX), thereby inhibiting the degradation and senescence of chlorophyll by absorbing red light [50].
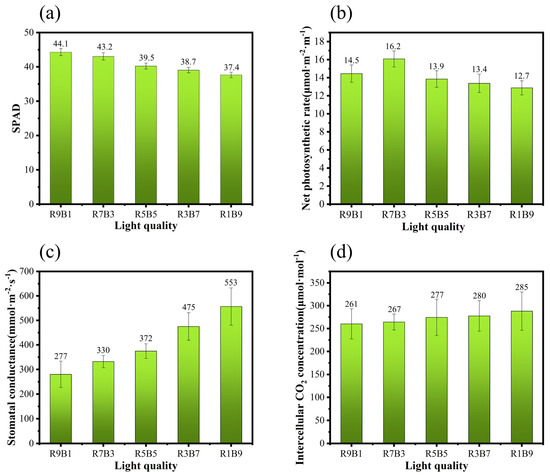
Figure 6.
(a) Relative chlorophyll content of seedlings under different light qualities. (b) Net photosynthetic rate of seedlings under different light qualities. (c) Stomatal conductance of seedlings under different light qualities. (d) Intercellular carbon dioxide concentration of seedlings under different light qualities.
Regarding the net photosynthetic rate, we observed that initially it increased but gradually decreased with a decrease in the proportion of red light. The net photosynthetic rate under R7B3 treatment was recorded to be the highest (16.1). The reason for this phenomenon lies in the understanding of the nature of photosynthesis. Photosynthesis is carried out in a series of protein supermolecules that are embedded in the photosynthetic membrane. Through charge separation and photosynthetic electron transfer of photosystem I (PSI) and photosystem II (PSII) reaction centers, light energy is converted into chemical energy forms, such as adenosine triphosphate (ATP) and reduced coenzyme II (NADHP), for the fixation of carbon dioxide in the dark reaction [51] (Figure 7). However, the change in the light quality ratio also has a certain conversion effect on the state of the optical system. It is generally believed that red light is helpful for the transformation of light energy from PSII to PSI, alleviating the damage of light energy to PSII, which causes an imbalance between the two optical systems [52]. In other words, red light accelerates the transport of e- from PSII to PSI, which speeds up the process of photoreaction (specifically, the growth morphology and net photosynthetic rate of R9B1 and R7B3 are all higher than R5B5, R3B7, and R1B9). However, the plant can alleviate this imbalance by adjusting the ratio of PSI and PSII to ensure that its growth rate is not affected [53].
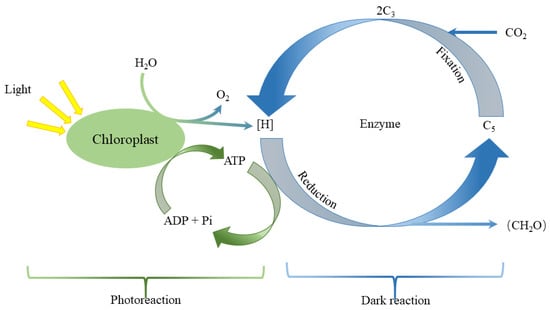
Figure 7.
Mechanism of plant photosynthesis.
PSII is very sensitive to light quality. Red light affects the degradation and replacement of D1 protease, while the content of PSII core antenna proteins CP47 and CP43 also inhibits the light [54,55]. Therefore, the photoinhibition of red light on PSII has always been considered a harmful reaction in photosynthesis [29]. As we can see in Figure 7 and Figure 8, the photoinhibition of red light on PSII weakens the water absorption and utilization by plants and it is due to the reduction of H+. The reduction of H+ slows down the conversion process from ADP to ATP and reduces photoreaction efficiency (specifically, the net photosynthetic rate of R7B3 is higher than R9B1), finally affecting the dark reaction (formation of (CH2O)). Miao et al. [56] noted that the light suppression in PSII induced by red light can be reduced by supplementing an appropriate proportion of blue light. This is because blue light upregulates the expression of CP47 and CP43, which are indispensable in the repair of PSII. This is the reason for the higher net photosynthetic rate of R7B3 compared to that of R9B1 in our experiment. Apart from PSII and PSI, the chlorophyll content also has a significant impact on the photosynthetic rate of plants. Light impacts the electron transport rate in PSII, resulting in modifications in the photosynthetic characteristics, which are also influenced by the chlorophyll content [48]. In the present investigation, in cucumber seedlings, a higher proportion of blue light resulted in the decline of chlorophyll content. Therefore, when the proportion of blue light gradually increases (light quality from R7B3 to R1B9), its net photosynthetic rate will continue to decline. This is similar to the results of the study by Kim et al. [57], who noted that blue light had a negative impact on the growth and photosynthesis of chrysanthemum plants.
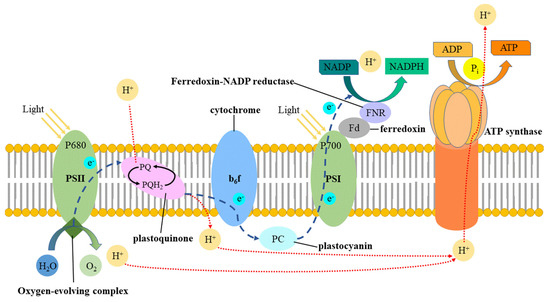
Figure 8.
Schematic diagram of the light system participating in plant growth [58].
Although the net photosynthetic rate decreased with the increase in the proportion of blue light, its stomatal conductance was consistently increased, reaching the maximum under the R1B9 treatment. McCree [10] described the effect of light quality on the photosynthetic rate of plants in the field and growth room and created a relative quantum efficiency curve (RQE). The RQE curve shows that photons with wavelengths within the standard number range were not equally effective in driving photosynthesis. According to the RQE curve, the efficiency of red light (600–700 nm) was 25–30% higher than that of blue (400–500 nm) and green light (500–600 nm). The low RQE of blue light is due to its absorption by carotenoids or anthocyanins. Therefore, by comparing the results of the report, we can conclude that the photosynthetic rate of plants decreases with the increase in the proportion of blue light and is contrary to the change rule of stomatal conductance, which is consistent with the results of the current research. On the one hand, the specific reason for the expansion of stomatal conductance can be attributed to the fact that blue light activates the plasma membrane ATPase to continuously pump out protons, forming a transmembrane electrochemical gradient. In other words, the power of K+ passing through its channel increases, thereby promoting the absorption of K+ by the guard cells, leading to a decrease in the intracellular osmotic potential, water absorption and expansion of the guard cells, and the opening of the stomata [59]. On the other hand, there is a special blue light receptor, known as zeaxanthin, in the chloroplast. This pigment generally exists in chloroplasts but is not limited to the guard cells. Blue light promotes zeaxanthin, resulting in higher quantum efficiency in the stomata [60,61]. Frechilla et al. [62] reported that blue light promotes stomatal opening in monocotyledons and dicotyledons, but green light reverses this effect. They believed that the reversibility of stomatal opening and closing caused by blue–green light might be derived from two structural dissimilations of zeaxanthin, namely, physiological living bodies that mainly absorb green light and inactive bodies that absorb blue light, which is similar to the reversibility of photosensitive pigments to red and far red light. In a word, although red light can greatly promote the growth of plants and increase their photosynthesis, excessive red light will cause the unbalance of PSI and PSII. Blue light is needed to balance the relationship between PSI and PSII. This is why R7B3 treatment has the highest net photosynthetic rate. However, a higher proportion of blue light resulted in the decline of chlorophyll content, and red light is helpful for the transformation of light energy from PSII to PSI, alleviating the damage of light energy to PSII, improving the photosynthetic rate. This is why the growth morphology, DQI, and net photosynthetic rate of higher red light (R9B1,R7B3) is higher than the lower red light (R5B5,R3B7,R1B9).
Compared with the obvious change rule of stomatal conductance, the change rule of intercellular carbon dioxide concentration is not as obvious as the intercellular carbon-dioxide concentration that is almost similar under different light qualities (intercellular carbon dioxide concentration is almost between 260 and 290 ). This shows that the change rule of net photosynthesis is not completely affected by CO2. The influence of concentration may be caused by the insufficient supply of ATP and NADPH due to the influence on the photosynthetic system under the aforementioned red–blue light ratio; that is to say, the insufficient assimilation ability restricts the light assimilation, which was the similar observation by Li et al. [63].
4. Conclusions
Light quality is an important factor in regulating plant morphology and photosynthetic characteristics. When the proportion of red light was higher than that of blue light, the growth, DQI, and net photosynthetic rate of cucumber seedlings were higher than those treatments where the blue light was higher, with a higher proportion of blue light indicating that red light was beneficial to the growth of cucumber seedlings and promoted the healthy growth of the plants. Specifically, the DQI and net photosynthetic rate of the R9B1 control group were not the highest.
In a word, red and blue light have their separate role in regulating plant growth and photosynthesis. A higher proportion of red and blue light can be used to irradiate seedlings of cucumber, but it has to be ensured that the proportion of red light should not be too high so as to provide more appropriate lighting conditions for seedling growth under protective cultivation conditions. Further studies are required to understand the coupling phenomenon of red light and blue light on their effect on plant growth.
Author Contributions
Y.L., J.Z., and M.S. conceived the idea, designed the experiments, and supervised the work. D.J., X.S., B.Y., and W.W. carried out the experiments and characterization, and wrote the draft of the manuscript. D.J., X.W., S.Y., and X.D. conducted the measurements. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (grant no. 2021YFB3501700) and Shanghai Science and Technology Committee (STCSM) Science and Technology Innovation Program (grant no. 22N21900400) and Municipal Science and Technology Commission Project: Research and development of efficient tomato seedling technology and LED light control system in artificial light plant factory (22N21900200).
Data Availability Statement
The data presented in this study are available on request from the authors.
Acknowledgments
The authors are extremely appreciative and grateful to Shanghai Youyou Agricultural Technology Co., Ltd., as well as to the Shanghai Academy of Agricultural Sciences for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, X.; Xu, H.; Shao, L.; Li, T.; Wang, Y.; Wang, R. Response of Photosynthetic Capacity of Tomato Leaves to Different LED Light Wavelength. Environ. Exp. Bot. 2018, 150, 161–171. [Google Scholar] [CrossRef]
- Särkkä, L.E.; Jokinen, K.; Ottosen, C.-O.; Kaukoranta, T. Effects of HPS and LED Lighting on Cucumber Leaf Photosynthesis, Light Quality Penetration and Temperature in the Canopy, Plant Morphology and Yield. AFSci 2017, 26, 102–110. [Google Scholar] [CrossRef]
- Xu, H.; Xu, Q.; Li, F.; Feng, Y.; Qin, F.; Fang, W. Applications of Xerophytophysiology in Plant Production—LED Blue Light as a Stimulus Improved the Tomato Crop. Sci. Hortic. 2012, 148, 190–196. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Tanybayeva, Z.; Kydyrbekova, A.; Turbekova, A.; Aytkhozhin, S.; Zhantasov, S.; Taukenov, A. The Efficiency of LED Irradiation for Cultivating High-Quality Tomato Seedlings. Sustainability 2021, 13, 9426. [Google Scholar] [CrossRef]
- Goto, E. Plant Production in a closed plant factory with artificial lighting. Acta Hortic. 2012, 956, 37–49. [Google Scholar] [CrossRef]
- Ding, B.-J.; Hofvander, P.; Wang, H.-L.; Durrett, T.P.; Stymne, S.; Löfstedt, C. A Plant Factory for Moth Pheromone Production. Nat. Commun. 2014, 5, 3353. [Google Scholar] [CrossRef] [PubMed]
- Seedapalee, T.; Inkham, C.; Ruamrungsri, S.; Jogloy, S.; Hongpakdee, P. Physiological Responses of Sun Choke’s Seedlings under Different Wavelength LED Lighting. Sci. Hortic. 2021, 282, 110029. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chang, T.T.; Guo, S.R.; Xu, Z.G.; Li, J. Effect of different light quality of LED on growth and photosynthetic character in cherry tomato seedling. Acta Hortic. 2011, 907, 325–330. [Google Scholar] [CrossRef]
- McCree, K.J. The Action Spectrum, Absorptance and Quantum Yield of Photosynthesis in Crop Plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Legris, M.; Ince, Y.Ç.; Fankhauser, C. Molecular Mechanisms Underlying Phytochrome-Controlled Morphogenesis in Plants. Nat. Commun. 2019, 10, 5219. [Google Scholar] [CrossRef] [PubMed]
- Sng, B.J.R.; Mun, B.; Mohanty, B.; Kim, M.; Phua, Z.W.; Yang, H.; Lee, D.-Y.; Jang, I.-C. Combination of Red and Blue Light Induces Anthocyanin and Other Secondary Metabolite Biosynthesis Pathways in an Age-Dependent Manner in Batavia Lettuce. Plant Sci. 2021, 310, 110977. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific Functions of Red, Far Red, and Blue Light in Flowering and Stem Extension of Long-Day Plants. Jashs 2001, 126, 275–282. [Google Scholar] [CrossRef]
- Izzo, L.G.; Hay Mele, B.; Vitale, L.; Vitale, E.; Arena, C. The Role of Monochromatic Red and Blue Light in Tomato Early Photomorphogenesis and Photosynthetic Traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue Light-Emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. Horts 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Weller, J.L.; Perrotta, G.; Schreuder, M.E.L.; Van Tuinen, A.; Koornneef, M.; Giuliano, G.; Kendrick, R.E. Genetic Dissection of Blue-Light Sensing in Tomato Using Mutants Deficient in Cryptochrome 1 and Phytochromes A, B1 and B2: Cryptochrome 1 in Tomato. Plant J. 2001, 25, 427–440. [Google Scholar] [CrossRef]
- Fantini, E.; Facella, P. Cryptochromes in the Field: How Blue Light Influences Crop Development. Physiol. Plantarum 2020, 169, 336–346. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca Sativa, L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Xin, X.; Chen, W.; Wang, B.; Zhu, F.; Li, Y.; Yang, H.; Li, J.; Ren, D. Arabidopsis MKK10-MPK6 Mediates Red-Light-Regulated Opening of Seedling Cotyledons through Phosphorylation of PIF3. J. Exp. Bot. 2018, 69, 423–439. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Ye, L.; Zhao, H.; Zou, Z. Red Light Treatments at Night during Seedling Stage in Greenhouse Promoting Tomato Vegetative Growth and Improving Yield. Trans. Chin. Soc. Agric. Eng. 2016, 32, 180–186. [Google Scholar]
- Hwang, H.; An, S.; Lee, B.; Chun, C. Improvement of Growth and Morphology of Vegetable Seedlings with Supplemental Far-Red Enriched LED Lights in a Plant Factory. Horticulturae 2020, 6, 109. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Scopel, A.L.; Sánchez, R.A.; Radosevich, S.R. Photomorphogenic processes in the agricultural environment. Photochem. Photobiol. 1992, 56, 777–788. [Google Scholar] [CrossRef]
- Hamedalla, A.M.; Ali, M.M.; Ali, W.M.; Ahmed, M.A.A.; Kaseb, M.O.; Kalaji, H.M.; Gajc-Wolska, J.; Yousef, A.F. Increasing the Performance of Cucumber (Cucumis Sativus, L.) Seedlings by LED Illumination. Sci. Rep. 2022, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Ménard, C.; Dorais, M.; Hovi, T.; Gosselin, A. Developmental and physiological responses of tomato and cucumber to additional blue light. Acta Hortic. 2006, 711, 291–296. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Growth and Morphological Response of Cucumber Seedlings to Supplemental Red and Blue Photon Flux Ratios under Varied Solar Daily Light Integrals. Sci. Hortic. 2014, 173, 92–99. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological Responses of Cucumber Seedlings under Different Blue and Red Photon Flux Ratios Using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Cui, J. Effects of Supplemental Lighting with LED Light Quality on Growth and Photosynthetic Characteristics of Cucumber Seedlings. China Veg. 2012, 24, 48–54. [Google Scholar]
- Song, J.; Cao, K.; Hao, Y.; Song, S.; Su, W.; Liu, H. Hypocotyl Elongation Is Regulated by Supplemental Blue and Red Light in Cucumber Seedling. Gene 2019, 707, 117–125. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis Sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Wang, S.; Fang, H.; Xie, J.; Wu, Y.; Tang, Z.; Liu, Z.; Lv, J.; Yu, J. Physiological Responses of Cucumber Seedlings to Different Supplemental Light Duration of Red and Blue LED. Front. Plant Sci. 2021, 12, 709313. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Tang, Z.; Wu, Y.; Xiao, X.; Zhang, G.; Hu, L.; Liu, Z.; Lyu, J.; Yu, J. Red and Blue LED Light Supplementation in the Morning Pre-Activates the Photosynthetic System of Tomato (Solanum Lycopersicum, L.) Leaves and Promotes Plant Growth. Agronomy 2022, 12, 897. [Google Scholar] [CrossRef]
- Novičkovas, A.; Brazaitytė, A.; Duchovskis, P.; Jankauskienė, J.; Samuolienė, G.; Virsilė, A.; Sirtautas, R.; Bliznikas, Z.; Zukauskas, A. Solid-state lamps (LEDs) for the short-wavelength supplementary lighting in greenhouses: Experimental results with cucumber. Acta Hortic. 2012, 927, 723–730. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-Light Supplementation for Enhanced Lettuce Growth under Red- and Blue-Light-Emitting Diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.; Chang, T.; Guo, S. Growth and Photosynthesis of Cherry Tomato Seedling Exposed to Different Low Light of LED Light Quality. Acta Bot. Boreali-Occident. Sin. 2010, 30, 725–732. [Google Scholar]
- Li, Z.; Chen, G.; Gao, F.; Luo, J.; Li, C.; He, Z.; Zhou, X. Effects of Different Light Quality Ratio on Growth and Photosynthesis Capacity in Pakchoi under Excess Nitrate Stress. IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012105. [Google Scholar] [CrossRef]
- Dyśko, J.; Stanisław, K. Effects of LED and HPS Lighting on the Growth, Seedling Morphology and Yield of Greenhouse Tomatoes and Cucumbers. Hort. Sci. 2021, 48, 22–29. [Google Scholar] [CrossRef]
- Matysiak, B.; Kaniszewski, S.; Dyśko, J.; Kowalczyk, W.; Kowalski, A.; Grzegorzewska, M. The Impact of LED Light Spectrum on the Growth, Morphological Traits, and Nutritional Status of ‘Elizium’ Romaine Lettuce Grown in an Indoor Controlled Environment. Agriculture 2021, 11, 1133. [Google Scholar] [CrossRef]
- Matysiak, B.; Kowalski, A. The growth, photosynthetic parameters and nitrogen status of basil, coriander and oregano grown under different LED light spectra. Asphc 2021, 20, 13–22. [Google Scholar] [CrossRef]
- Barber, J.; Tran, P.D. From Natural to Artificial Photosynthesis. J. R. Soc. Interface. 2013, 10, 20120984. [Google Scholar] [CrossRef]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Jeong, H.W.; Lee, H.R.; Kim, H.M.; Kim, H.M.; Hwang, H.S.; Hwang, S.J. Using Light Quality for Growth Control of Cucumber Seedlings in Closed-Type Plant Production System. Plants 2020, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ji, F.; Xu, L.; He, D.; Key Lab. Agricultural Engineering in Structure and Environment of Ministry of Agriculture and Rural Affairs, College of Water Resources & Civil Engineering, China Agricultural University, Beijing 100083, China Effects of LED Light Quality on the Growth of Pepper Seedling in Plant Factory. Int. J. Agric. Biol. Eng. 2019, 12, 44–50. [Google Scholar]
- Baek, S.; Jeon, E.; Park, K.S.; Yeo, K.-H.; Lee, J. Monitoring of Water Transportation in Plant Stem With Microneedle Sap Flow Sensor. J. Microelectromech. Syst. 2018, 27, 440–447. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Song, S.; Su, W.; Liu, H. Morphological and Physiological Responses of Cucumber Seedlings to Supplemental LED Light under Extremely Low Irradiance. Agronomy 2020, 10, 1698. [Google Scholar] [CrossRef]
- Głowacka, B. The Effect of Blue Light on the Height and Habit of the Tomato (Lycopersicon esculentum Mill.) Transplant. Folia Hortic. 2004, 16, 3–10. [Google Scholar]
- Jinxiu, S.; Qingwu, M.; Weifen, D.; Dongxian, H. Effects of Light Quality on Growth and Development of Cucumber Seedlings in Controlled Environment. Biol. Eng. 2017, 10, 312–317. [Google Scholar]
- Silva, E.M.; Costa, G.G.S.; Andrade, A.F.; Ferreira, H.C.P.; Steiner, F. Light Spectral Quality on Production of Lettuce, Cucumber and Sweet Pepper Seedlings. Sci. Agrar. Parana. 2016, 15, 446–452. [Google Scholar] [CrossRef]
- Zhong-Xi, C.H.U.; Zhe, T.; Li-Jie, F.; Qun, Z.; Xiao-Gang, W.E.N.; Sen-Tian, S.; Xiao-Feng, Z.H.U. Effect of Different Light Quality on Photosynthetic Characteristics of Cucumber Leaves. J. Integr. Plant Biol. 1999, 41, 867–870. [Google Scholar]
- Sæbø, A.; Krekling, T.; Appelgren, M. Light Quality Affects Photosynthesis and Leaf Anatomy of Birch Plantlets in Vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Shen, Z.; Xia, K.; Cui, J. Effects of Light Quality on the Chloroplastic Ultrastructure and Photosynthetic Characteristics of Cucumber Seedlings. Plant Growth Regul. 2014, 73, 227–235. [Google Scholar] [CrossRef]
- Omasa, K.; Shimazaki, K.-I.; Aiga, I.; Larcher, W.; Onoe, M. Image Analysis of Chlorophyll Fluorescence Transients for Diagnosing the Photosynthetic System of Attached Leaves. Plant Physiol. 1987, 84, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.S.; Telfer, A.; Chapman, D.J.; Barber, J. State 1-State 2 Transition in Leaves and Its Association with ATP-Induced Chlorophyll Fluorescence Quenching. Biochim. Biophys. Acta-Bioenerg. 1981, 638, 60–68. [Google Scholar] [CrossRef]
- Chow, W.S.; Melis, A.; Anderson, J.M. Adjustments of Photosystem Stoichiometry in Chloroplasts Improve the Quantum Efficiency of Photosynthesis. Proc. Natl. Acad. Sci. USA 1990, 87, 7502–7506. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Photosystem-II Damage and Repair Cycle in Chloroplasts: What Modulates the Rate of Photodamage in Vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Rajagopal, S.; Murthy, S.D.S.; Mohanty, P. Effect of Ultraviolet-B Radiation on Intact Cells of the Cyanobacterium Spirulina Platensis: Characterization of the Alterations in the Thylakoid Membranes. J. Photochem. Photobiol. B Biol. 2000, 54, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wang, X.; Gao, L.; Chen, Q.; Qu, M. Blue Light Is More Essential than Red Light for Maintaining the Activities of Photosystem II and I and Photosynthetic Electron Transport Capacity in Cucumber Leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Kim, S.-J.; Hahn, E.-J.; Heo, J.-W.; Paek, K.-Y. Effects of LEDs on Net Photosynthetic Rate, Growth and Leaf Stomata of Chrysanthemum Plantlets in Vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Thylakoid. Available online: https://www.wikidoc.org/index.php/Thylakoid (accessed on 6 September 2012).
- Zeiger, E. The Biology of Stomatal Guard Cells. Annu. Rev. Plant. Physiol. 1983, 34, 441–447. [Google Scholar] [CrossRef]
- Frechilla, S.; Zhu, J.; Talbott, L.D.; Zeiger, E. Stomata from Npql, a Zeaxanthin-Less Arabidopsis Mutant, Lack a Specific Response to Blue Light. Plant Cell Physiol. 1999, 40, 949–954. [Google Scholar] [CrossRef]
- Zeiger, E.; Zhu, J. Role of Zeaxanthin in Blue Light Photoreception and the Modulation of Light–CO2 Interactions in Guard Cells. J. Exp. Bot. 1998, 49, 433–442. [Google Scholar] [CrossRef]
- Frechilla, S.; Talbott, L.D.; Bogomolni, R.A.; Zeiger, E. Reversal of Blue Light-Stimulated Stomatal Opening by Green Light. Plant Cell Physiol. 2000, 41, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Li, X.U.; Shiqi, L.I.U.; Liandong, Q.; Qingling, L.; Wenyan, Y.U. Effect of Light Quality on Leaf Lettuce Photosynthesis and Chlorophyll Fluorescence. 2010, 26, 96–100. Available online: https://europepmc.org/article/CBA/647442 (accessed on 7 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).