Abstract
The use of fungicides from the strobilurin and carboxamide groups demonstrates an effect on photosynthetic efficiency by increasing CO2 assimilation and, consequently, plant productivity, due to better a physiological performance. The objective was to evaluate the effect of the application of these fungicides on the physiology and yield of tomato plants. A randomized block design was used with six treatments and five blocks: control, azoxystrobin (75 g ha−1), boscalid (75 g ha−1), pyraclostrobin (75 g ha−1), fluxapyroxad (75 g ha−1) and fluxapyroxad + pyraclostrobin (50.1 g and 99.9 g ha−1). Different physiological, biochemical and antioxidant enzymatic parameters were evaluated. The application of fungicides increased the CO2 assimilation by 64% and the production per plant by 91%. The activity of the nitrate reductase enzyme increased by 1.69 times, the antioxidant system by 3.68 times and photosynthetic pigments by 1.16 times under the action of the studied fungicides with respect to the control. Therefore, the application of fungicides favored the development of the tomato plant, especially with the use of Pyraclostrobin (75 g ha−1).
1. Introduction
Tomato (Solanum lycopersicum L.) is one of the most cultivated vegetables in the world. China is the largest producer, with 64 million tons, and Brazil ranks as the tenth largest producer [1]. Its production in 2021 was 3.88 million tons, and the expectation for 2022 is a negative variation of 9.2% [2].
Worldwide, the group of fungi Alternaria sp. is responsible for significant damage to several crops, including tomatoes, favored mainly by high temperatures and humidity variation [3]. The disease attacks all parts of the plant and causes a total loss of the fruits, with fungicides based on strobilurins and carboxamides being among the groups most widely used to control Alternaria sp. [4]. However, the use of such products for disease control generates additional crop management costs
In a study of production costs for the 2017/2018 harvest, inputs accounted for the largest share of investment by the producer, and, within this item, 55% of the capital is devoted to agricultural pesticides [5]. In view of this, pesticides, including fungicides, in addition to having a disease control function, must also act to improve metabolism, enabling a greater production efficiency and plant yield.
The application of fungicides from the strobilurin group, for example, has the demonstrated effect of increasing the assimilation of nitrite and its incorporation in the chlorophyll molecule; other effects are reported in relation to the photosynthetic efficiency, such as an increase in the assimilation of CO2 and a reduction in the respiratory rate. Effects on plant hormone regulation are also seen, such as reduction in ethylene production, delaying leaf senescence [6,7,8]
As for production, the application of pyraclostrobin or boscalid fungicides to grafted cucumber plants increased plant productivity, probably due to their better physiological performance [9]. In carrot cultivation, biweekly application of boscalid provided greater root length [10], and studies have shown the positive effects of these fungicides on the postharvest quality of tomatoes [11] and melons [12].
In this way, the inclusion of the application of these products in the management program for tomato cultivation, combined with techniques such as cultivation in a protected environment, fertigation and use of hybrids, may provide an increase in the efficiency of the production systems, their productivity and the quality of this vegetable.
Thus, the hypothesis of this research is that fungicides based on strobilurins and carboxamides cause changes in the physiological and biochemical processes of tomato plants that are reflected in productivity gains. To confirm this hypothesis, the present research was carried out with the objective of evaluating the effect of the application of fungicides from the strobilurin and carboxamide groups on the physiology, production and quality of tomato fruits in a protected environment.
2. Materials and Methods
2.1. Design Experimental
The experiment was carried out at the Research and Production Farm of São Manuel, in São Manuel city, São Paulo state, belonging to the School of Agronomic Sciences, Botucatu Campus, Sao Paulo State University, UNESP (22°44′ S, 47°34′ W and 750 m above sea level). The climate is humid subtropical mesothermal with drought in the winter season [13].
The protected ambient used was of the arch type, 30 m long, 7 m wide and 3 m high, covered with a 150 μm low-density polyethylene film with additives and closed on the sides with a 75% shading screen.
The experimental design used consisted of randomized blocks with six treatments and five blocks. Each plot was composed of 6 plants, considering 4 useful plants. The treatments used were: T0—control; T1—azoxystrobin 75 g ha−1; T2—boscalid 75 g ha−1; T3—pyraclostrobin 75 g ha−1; T4—fluxapyroxad 75 g ha−1; T5—fluxapyroxad 50, 1 g ha−1 + pyraclostrobin 99.9 g ha−1.
2.2. Crop Management
The “Conquistador” tomato hybrid was used, sown in expanded polystyrene trays with 128 cells, placing two seeds per cell with the recommended substrate for the production of vegetables. Thinning, to remove plants that had not developed normally, was carried out when the seedlings had cotyledonary leaves.
Transplanting was carried out 30 days after sowing, with one plant per hole spaced at 1.0 × 0.5 m. The beds had a height of 0.20 m above ground level, and each was served by an irrigation and fertirrigation line. The plants were guided with a rod throughout the cycle and vertically tutored.
The first application of the treatments was carried out 3 days after transplanting the seedlings (DAT), and the five subsequent applications at 10, 17, 24 and 31. All fungicide applications were carried out via the leaves using a pressurized CO2 manual sprayer with 0.3 kgf cm−2 and conical nozzles, using a plastic curtain between treatments to prevent drift.
As a source of azoxystrobin (strobilurin), the product Amistar® was used, containing 500 g kg−1 of active ingredient (a.i.) manufactured by Syngenta; for boscalid (carboxamide) the Cantus® product containing 500 g kg−1 (a.i.); for pyraclostrobin (strobilurin) the Comet® product containing 250 g L−1 (a.i.); for fluxapyroxad (carboxamide) the product Xe-mium® was used and for the fluxapyroxad + pyraclostrobin mixture Orkestra® was used. All of them are manufactured by BASF S.A. The applications were carried out via leaf, in the whole plant, using a manual pressurized CO2 sprayer, with 0.3 kgf cm−2, open conical nozzle, using a plastic curtain between treatments to avoid drift.
2.3. Physico-Chemical Properties of the Substrate for Vegetable Production
The commercial substrate Carolina Soil® II (São Paulo, Brazil) was used, composed of Sphagno peat, expanded vermiculite, class A agro-industrial organic waste, dolomitic limestone, agricultural gypsum and traces of NPK fertilizers, with pH 5.5 ± 0.5, EC 0.4 ± 0.3 mScm−1 and a density of 155 kg m−3, placing one seed per cell. When the seedlings had cotyledonary leaves, they were thinned and removed from the plants that had not developed normally.
2.4. Variables Analyzed
Gas exchanges were analyzed at 60 DAT, between 8:00 am and 11:00 am, using equipment with an open photosynthesis system with a CO2 and water vapor analyzer employing infrared radiation (“Infra Red Gas Analyzer—IRGA”) (LI-6400, Photosynthesis Meter, Li-Cor Biosciences, Lincoln, NE, USA). The gas exchange characteristics analyzed were the CO2 assimilation rate (A, μmolCO2 m−2 s−1), stomatal conductance (gs, mol m−2 s−1), internal concentration of CO2 in the leaf (Ci, μmolCO2 mol−1 air) and transpiration rate (E, mmol water vapor m−2 s−1). Water use efficiency (WUE, μmolCO2 (mmol H2O)−1) was determined through the relationship between CO2 assimilation and transpiration rate, and carboxylation efficiency (A/Ci) was determined through the relationship between water use rate and CO2 assimilation and internal CO2 concentration in the leaf.
Three collections were carried out for enzymatic and photosynthetic pigment analysis at 30, 60 and 120 DAT, in which three leaves were collected, later wrapped in aluminum foil and placed in plastic bags, and then frozen in liquid nitrogen. Lipid peroxidation (TBAR) was determined according to the technique described by [14]. The activity of the enzyme superoxide dismutase (SOD) was determined by the methodology described by [15], catalase (CAT) was determined by the methodology described by [16], and the peroxidase enzyme activity (POD) was measured by the spectrophotometric method proposed by [17]. For nitrate reductase activity, the methodology described by [18] was used. The quantification of chlorophylls a and b was performed according to the methodology described by [19], and the levels expressed in µg.g of fresh mass−1.
The activity of the SOD was determined based on the ability of the enzyme to inhibit the photoreduction of NBT (nitrotetrazolium chloride blue). For reaction, 50 µL of crude extract was added to 2950 µL of the reaction medium composed of potassium phosphate buffer (50 mM, pH 7.8), methionine (13.0 mM), EDTA (0.1 μM), NBT (75.0 μM) and riboflavin (2.0 μM). The reaction was started with the aid of a 15 W fluorescent light chamber at 25 °C, the catalysis was ended by interrupting the light at 10 min, and the absorbance of the blue formazan resulting from the photoreduction of the NBT was determined with the aid of a spectrophotometer at 560 nm. The mixture for reading the reaction white consisted of the junction of the crude extract and reaction medium kept in the dark, with a unit of SOD being defined as the activity of the enzyme necessary to inhibit 50% of the photoreduction of the NBT.
To determine the activity of the CAT, 50 µL of crude extract was added to 950 µL of the reaction medium composed of 50 potassium phosphate buffer (mM pH 7.0) supplemented with H2O2 (12.5 mM), with the reading performed on a spectrophotometer with an absorbance of 240 nm after 60 s, and the enzymatic activity was calculated using the molar extinction coefficient ε = 39.4 mM.
The activity of the POD enzyme was determined using 100 µL of the crude extract to 4900 µL of the buffer with a reaction medium consisting of potassium phosphate (25 mM pH 6.8), H2O2 (12.5 mM) and pyrogallol (20 mM). The reaction was stopped with 500 µL of sulfuric acid (5%), and the reading was performed on a spectrophotometer with an absorbance of 420 nm. The enzymatic activity was calculated using the molar extinction coefficient ε = 2.47 mM cm−1.
For the determination of nitrate reductase activity, 200 mg of the leaf was collected and inserted into a penicillin tube, and 10 mL of the extracting solution was added. The samples were incubated under vacuum for a duration of 3 cycles of 2 min. After incubation, the samples were placed in a water bath at 30 °C for 1 h. Then, 1 mL of the extracted solution was collected and transferred to tubes, where 1 mL of the sulfanilamide solution and 1 mL of the N-naphthyl solution were added. The readings were performed by spectrophotometry at 540 nm.
In order to evaluate the productive parameters, nine harvests were carried out in two central plants in which the fresh weight of the fruit (g), number of commercial fruits and commercial production (kg plant−1) were evaluated, according to the Tomato Classification Norms [20].
With the fruits of the fourth harvest, physical-chemical analyses and shelf life were carried out. The pH was determined by direct reading in a homogenized pulp solution using a bench pH meter (HI22091-01, Hanna Instruments, Woonsocket, RI, USA), according to the technique described by [21]; the soluble solids content was determined with a portable digital refractometer (PAL-1, Atago, Tokyo, Japan), with the results expressed in °Brix, and the titratable acidity, expressed in grams of citric acid per 100 g of pulp, as recommended from the [22]. The “ratio” was determined through the relationship between the soluble solids (SS) content and the titratable acidity (TA) [23].
To evaluate weight loss, two fruits from each plot were selected, identified and placed on expanded polystyrene trays at room temperature. The fruits were weighed on a precision digital electronic scale (Travola 30, Micheletti, São Paulo, Brazil) every two days until they all lost their commercial aspect.
2.5. Statistical Analysis
The data were previously submitted to the Anderson Darling homogeneity test, through the Minitab program; the number of fruits and weight loss data were transformed into , the normality of the data was verified, the analysis of variance was performed (F test) and the means were compared using the Scott Knott test at 5% of probability, using the AGROESTAT® program. For the variables analyzed over time and which showed a significant effect for the applied treatments, graphs were constructed using the SIGMAPLOT program, version 12.5.
3. Results
3.1. Gas Exchange in Tomato Plants
There was a significant difference at 60 DAT for the gas exchange variables: A, gs, Ci, WUE and A/Ci, with the exception of variable E. In the evaluated period, treatment T2 (Boscalid 75 g ha−1) and T3 (Pyraclostrobin 75 g ha−1) provided, respectively, greater CO2 assimilation rates (A) of 70 and 58% in relation to the control. Stomatal conductance was maintained, while Ci was simultaneously reduced by 25% and 19%, resulting in the efficiency of A in relation to the control treatment (Table 1).

Table 1.
CO2 assimilation rate (A), stomatal conductance (gs), internal CO2 concentration (Ci), water use efficiency (WUE), carboxylation efficiency (A/Ci) and transpiration (E) of plants of tomato plants treated with fungicides with a physiological effect.
In the WUE, treatments T2 (Boscalid 75 g ha−1), T3 (Piraclostrobin 75 g ha−1), T4 (Fluxapiroxad 75 g ha−1) and T5 (Fluxapiroxad + pyraclostrobin (50.1 g and 99.9 g ha−1) resulted in greater water efficiencies of 77%, 61%, 43% and 53% in relation to the control. The A/Ci was significantly higher in T2, with gains above 100% of the value of those obtained in the tomato plants not treated with the groups of fungicides adopted (Table 1).
3.2. Photosynthetic Pigment Content of Tomato Plants
As for the photosynthetic pigments, the plants under the action of the tested fungicides presented contents of chlorophyll a and b, 1.11 and 1.21 times higher, simultaneously, than the control in all the evaluations carried out.
For 30 DAT, the application of associated fungicides (T5—Fluxapyroxad + pyraclostrobin (50.1 g and 99.9 g ha−1) resulted in a greater presence of chlorophyll a, while, for the evaluation of 60 DAT, the treatments T2 (Boscalid 75 g ha−1) and T3 (Pyraclostrobin 75 g ha−1) showed the best results, respectively. As for the evaluation of 120 DAT, the treatments that stood out were the application of Azostrobin and the associated application of Fluxapyroxad and Pyraclostrobin (Table 2). Such results for the treatments with fungicides, when compared with the lower results of the control treatment, demonstrate that both carboxamide and strobirulin positively influenced the levels of chlorophyll a.

Table 2.
Chlorophyll a (Cl a) and chlorophyll b (Cl b) content of tomato plants treated with fungicides with physiological effect, at different collection times.
As for the chlorophyll b content, greater differences between treatments occurred at 30 and 120 DAT, and, in both, the control showed lower results than the strobilurins and carboxamides applied separately (with the exception of Azoxystrobin at 30 DAT). At 60 DAT, there was no statistical difference between the treatments, with the exception of Azoxystrobin, which presented a lower result than the others (Table 2).
3.3. Antioxidant Defense System of Tomato Plants
The application of fungicides to the plants favored the production of the enzymes that regulate antioxidant metabolism and reduced membrane damage on the analyzed dates (Table 3).

Table 3.
Lipid peroxidation (TBARS), superoxide dismustase (SOD), catalase (CAT), peroxidase (POD) and nitrate reductase (NR) activity of tomato plants treated with fungicides with physiological effect, at different collection times.
Lipid peroxidation was significantly reduced after the application of Azoxistrobin (T1): by 77%, at 60 DAT, when compared to control plants. This reduction in membrane damage was also observed at 120 DAT in the T3 treatment (Piraclostrobin 75 g ha−1), which inhibited lipid peroxidation by 36% compared to the control (Table 3).
The SOD activity was higher in the treatments based on Boscalid (T2), Pyraclostrobin (T3) and Fluxapyroxad (T4), with increases of 1.26, 5.94 and 1.02 times, respectively, in relation to the control. The same behavior was observed for the CAT activity, in which plants treated with fungicide, in general, showed greater enzymatic activity, while the application of Fluxapyroxad (T4) and Fluxapyroxad + Piraclostrobin (T5) increased it on average 6.30 and 1.87 times, respectively, in relation to T0, demonstrating the positive effect of strobirulins and carboxamides in increasing the activity of these antioxidant enzymes throughout the tomato cycle (Table 3).
The POD enzyme activity increased 5.68 times under application of 75 ha−1 of Fluxapyroxad in relation to the control at 120 DAT (Table 3).
The application of Fluxapyroxad increased 1.69 times the activity of the nitrate reductase enzyme in relation to the control at 120 DAT. This result demonstrates the promising impact of fungicides with a physiological effect from different chemical groups throughout the entire crop cycle (Table 3).
3.4. Productive Parameters of Tomato Plants
The productive parameters, including the weight, number and production of fruits of the tomato, were positively influenced by the application of the tested fungicides in relation to the control. Plants under application of Azoxystrobin (T1), Pyraclostrobin (T3), Fluxapiroxad (T4) and Fluxapyroxad + pyraclostrobin (T5) presented an average fruit weight higher than that of the other treatments. Likewise, the application of Pyraclostrobin (T3) provided a greater number of fruits and, consequently, greater production per plant at the end of the cycle, with gains of 26% and 91% over the control, respectively (Table 4).

Table 4.
Fruit weight (FW), production per plant (PP) and number of fruits (NF) of tomato plants treated with physiological effect fungicides.
3.5. Postharvest Quality of Tomato Fruits
The postharvest characteristics of soluble solids and acidity were altered depending on the application of the treatments (Table 5); however, the pH did not differ statistically between the treatments. As for acidity, plants under the application of Boscalid (T2), Pyraclostrobin (T3) and Fluxapyroxad+pyraclostrobin (T5) did not differ from the control. Meanwhile, for soluble solids, only Pyraclostrobin (T3) and the control showed higher °Brix content.

Table 5.
Soluble solids content (°Brix), pH (pH), titratable acidity (Ac) and the ratio between °Brix and acidity (Ratio) of tomato fruits treated with fungicides with physiological effect.
The ratio values of the tomatoes were higher in the plants treated with Fluxapyroxade (T4), showing an increase of 94% in relation to the control. This result reflects a lower acidity in the fruits under this treatment and a medium sugar content, providing better flavor for the palates of the consumers.
In the dynamics of mass loss, a gradual decrease in the tomato fruits was observed during the days of storage. At eight days, most of the fruits from all treatments already showed physiological and phytosanitary damage. For this reason, the weight loss was computed up to six days after harvesting (Figure 1). In the treatments where Azoxystrobin (T1) and Boscalida (T2) were applied, the fruits maintained good visual and texture characteristics, and, thus, showed less mass loss six days after harvesting, with a reduction of 27% and 32%, simultaneously, in relation to the control.
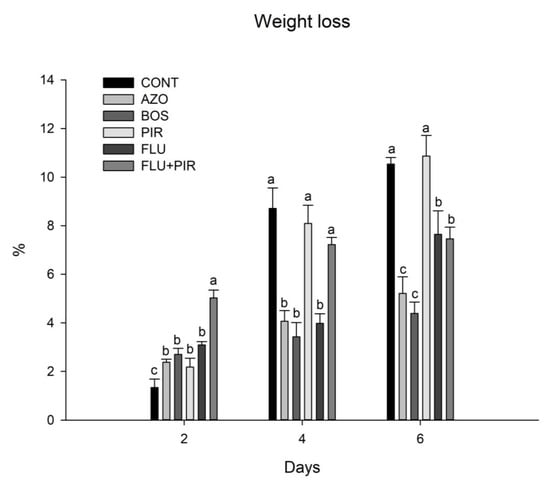
Figure 1.
Weight loss on different evaluation days of tomato fruits treated with physiological effect fungicides. Different lowercase letters in the columns in the same evaluation period differ statistically using the Scott Knott test at 5% probability. CONT—Control; AZO—azoxystrobin; BOS—boscalid; PIR—pyraclostrobin; FLU—fluxapyroxad; FLU + PIR—fluxapyroxad + pyraclostrobin.
4. Discussion
Several studies have proved the positive physiological effect on plants resulting from the application of strobilurins and carboxamides, in addition to their fungicidal activity [6,9,24]. The positive influence on photosynthesis of the application of boscalid and pyraclostobin was reflected in the increase in A. One of the reasons for the increase in A in plants subjected to the application of pyraclostrobin is the change in the CO2 compensation point and the decrease in respiration that these compounds cause in the plant, reflected in the increase of the photosynthetic rate [25,26] as also observed in soybeans [10]. Although the mode of action of strobilurins and carboxamides is similar, there is still a lack of information explaining the beneficial physiological action of carboxamides [27].
Our WUE results are in line with what has been observed in the literature, where the application of strobilurins favored the WUE in tomatoes under conditions of water deficit [28] and salinity [29]. A lower availability of carbon in the leaf mesophyll in plants treated with fungicide, associated with little variation in stomatal conductance and a higher A, reflects a greater efficiency of action of the rubisco enzyme (A/Ci). This demonstrates the physiological activity of the products used, since the control had the lowest A/Ci value, but the highest Ci value.
The application of strobilurin and carboxamide, mainly separately, resulted in a better performance of the gas exchange variables, corroborating [9]. However, the physiological action of carboxamides, although proven, has not yet been fully elucidated [30].
Strobilurins and carboxamides have a physiological effect that results in the so-called ’green effect’ [27]. This effect consists of reducing the rate of ethylene production through a decrease in the activity of the enzyme ACC synthase, thus reflected in the maintenance of photosynthetically active green tissues [25,31]. Leaf senescence is an activity strictly linked to oxidative physiological processes, mainly through the action of reactive oxygen species (ROS) [32]. Since the SOD, POD and CAT enzymes are involved in the control and elimination of ROS, their action is related to the plant’s ability to remain healthy [33].
Boari et al. observed that the application of the fungicide pyraclostrobin contributed to a 61% increase in the activity of the SOD, POD, CAT and APX enzymes in tomato plants under saline stress [29]. Also, in cucumber plants (Cucumis sativus L.), Amaro et al. observed greater activity of the CAT enzyme in grafted plants treated with boscalid, pyraclostrobin and both associated [9]. In ginseng (Panax ginseng Mey. cv. “Ermaya”), it was observed that the application of azoxystrobin also favored the presence of the SOD, POD, CAT and APX enzymes, decreasing the presence of the O2•− radical in the leaves [34].
SOD is the enzyme responsible for the dismutation of superoxide (O2•−), a highly reactive free radical, into hydrogen peroxide (H2O2), another free radical, but one with less damage potential [35]. After the action of SOD, the POD enzyme comes into play and eliminates the H2O2 produced by the dismutation of O2•− by SOD [36]. Similarly, CATs (CAT) are enzymes that rapidly demutate H2O2 into H2O and O2, with a significant effect on peroxisomes [37].
Fungicides with side effects help to increase the activity of nitrate reductase [38] and favor the assimilation of nitrogen and the conversion of nitrate into nitrite, a conversion that occurs precisely due to the action of nitrate reductase in the cytosol [39]. Plants subjected to treatments with fungicides, either isolated or associated, generally showed a greater performance of the antioxidant enzymes and nitrate reductase. Such results demonstrate the physiological effect of stimulating the expression of these enzymes, reflected in the delay of tissue degradation and its consequent senescence.
Amaro et al., testing the same fungicides, also found a beneficial effect on nitrate reductase activity, and, similarly, there was variation of the different chemical groups throughout the cycle with a positive effect on enzyme expression [9]. Ruske et al. also observed that strobilurins favored the assimilation of nitrogen, as well as its displacement to wheat grains [40].
All plants under the application of the tested fungicides showed higher A, WUE and A/Ci in relation to the control, reflected in a greater production per plant, since these presented a greater production of photoassimilates, especially under the application of pyraclostrobin. Therefore, it is possible to affirm that the use of this fungicide increased the photosynthetic apparatus of the plants, increasing the assimilation of CO2 and reducing possible stresses, and thereby reducing the floral abortion, resulting in a greater number of fruits.
Similar to our production results, the application of boscalid, pyraclostrobin alone and the two together promoted greater melon fruit weight [12], and, for tomato fruit production, the application of fluxpyrad + pyraclostrobin and metiram + pyraclostrobin provided higher yields [41]. These results demonstrate that the positive effects of the application of these fungicides on the physiology and production of the tomato plant may be linked to the active principle, cultivar used, edaphoclimatic conditions, number and interval between applications.
Fruit flavor is determined by sensory aspects such as sweetness, acidity, aroma, texture and others [42]. The ratio, the relation between soluble solids content and the titratable acidity, is one of the best parameters for evaluating the flavor, demonstrating a balance between the sugars and acids of the fruits, which is more important than the isolated measurement of sugars or acidity [43].
Our results demonstrate that the application of fungicides reduced the degradation of chlorophyll and, together with a better use of nitrogen due to the greater activity of the enzyme nitrate reductase, caused a delay in the senescence of the leaves of the tomato plants. Furthermore, since chlorophyll is an essential component for photosynthesis, and absorbs energy and directs it to the photosystems [44], the application of the tested fungicides also increased the rate of CO2 assimilation.
Increased CO2 assimilation, in turn, increased fruit production in the plants treated with pyraclostrobin. While the activity of the antioxidant enzymes, which were also positively affected by the application of fungicides, may have reduced the environmental stress that caused less fruit abortion, evidenced by the greater production in the plants under application of the fungicides.
The physiological performance of these groups (strobilurins and carboxamides) is probably related to their fungitoxic mode of action, as they depend more on the fungal defense reactions, which interferes, at least partially, with the mitochondria of the plant cell being affected in its respiratory process, thus being able to benefit the liquid photosynthesis of the plant and, consequently, potentiate the assimilation of carbon. It is worth noting that the temporary reduction in respiration may not mean phytotoxicity, depending on the importance of mitochondrial respiration for the energy supply, which will vary according to the plant phenology and environmental conditions [9,41,45].
5. Conclusions
The application of the tested fungicides promoted beneficial changes in the physiology and biochemistry of the tomato plant in a protected environment, in addition to an increase in fruit production, corroborating our hypothesis.
The plants under fungicide application showed a higher activity of the enzyme nitrate reductase and higher rates of CO2 assimilation and carboxylation efficiency.
The application of 75 g ha−1 of pyraclostrobin in tomato plants in a protected environment can be recommended, due to the increase in fruit production per plant.
Author Contributions
Investigation, W.J.J., E.S.A., A.K.L.F., I.C.d.S.M., F.G.B.F.F.J. and D.M.R.S.; methodology, W.J.J., E.S.A., A.K.L.F., I.C.d.S.M., F.G.B.F.F.J. and D.M.R.S.; project administration, E.S.A.; data curation, E.S.A.; formal analysis, W.J.J., E.S.A., A.K.L.F., I.C.d.S.M., F.G.B.F.F.J. and D.M.R.S.; writing—original draft preparation, E.S.A. and W.J.J.; conceptualization, visualization and editing, J.D.R., E.O.O.; supervision, validation, writing—review, J.D.R. and E.O.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant number 001.
Data Availability Statement
All data included in the main text.
Acknowledgments
The authors would like to express their gratitude to the School of Agronomy Science of Universidade Estadual Paulista “Júlio de Mesquita Filho” (UNESP), Botucatu campus, and all its servers, who contributed to the development of this study and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, for the financial support for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. Countries by Commodity 2020. Available online: https://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 23 April 2022).
- SIDRA—Sistema Ibge de recuperação automática. Levantamento Sistemático da Produção Agrícola. Available online: https://sidra.ibge.gov.br/home/lspa/brasil (accessed on 23 April 2022).
- Töfoli, J.G.; Domingues, R.J.; Ferrari, J.T. Requeima e mancha de alternaria nas culturas da batata e tomate: Divulgação técnica 76. Inst. Biológico 2013, 75, 33–40. [Google Scholar]
- AGROFIT—Sistema de Agrotóxicos Fitossanitário. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 23 April 2022).
- HORTIFRUTI BRASIL. Hortaliças—Gestão Sustentável. Available online: https://www.hfbrasil.org.br/br/revista/acessar/completo/especial-hortalicas.aspx (accessed on 25 April 2022).
- Venancio, W.S.; Rodrigues, M.A.T.; Begliomini, E.; Souza, N.L.D. Physiological effects of strobilurin fungicides on plants. Publ. UEPG Ciências Exatas E Da Terra Agrárias E Eng. 2003, 9, 59–68. [Google Scholar]
- Töfoli, J.G.; Domingues, R.J. Severa Pinta Preta. Available online: http://www.biologico.sp.gov.br/artigos_ok.php?id_artigo=59 (accessed on 25 April 2022).
- Fagan, E.B.; Dourado Neto, D.; Vivian, R.; Franco, R.B.; Yeda, M.P.; Massignam, L.F.; Martins, K.V. Efeito da aplicação de piraclostrobina na taxa fotossintética, respiração, atividade da enzima nitrato redutase e produtividade de grãos de soja. Bragantia 2010, 69, 771–777. [Google Scholar] [CrossRef]
- Amaro, A.C.E.; Ramos, A.R.P.; Macedo, A.C.; Ono, E.O.; Rodrigues, J.D. Effects of the fungicides azoxystrobin, pyraclostrobin and boscalid on the physiology of Japanese cucumber. Sci. Hortic. 2018, 228, 66–75. [Google Scholar] [CrossRef]
- Colombari, L.F.; Baldini, L.F.G.; Baldini, V.; Cardoso, A.I.I.; Goto, R. Efeito fisiológico de fungicidas sistémicos em parâmetros agronômicos da cenoura. Rev. Bras. Cienc. Agrar. 2015, 38, 366–371. [Google Scholar] [CrossRef]
- Ramos, R.P.; Amaro, A.C.E.; Macedo, A.C.; Souza, E.R.; Rodrigue, J.D.; Ono, E.O. Acúmulo de carboidratos no desenvolvimento de tomateiro tratado com produtos químicos. Semin. Ciên. Agrár. 2015, 36, 705–718. [Google Scholar] [CrossRef]
- Macedo, A.C.; Amaro, A.C.E.; Ramos, A.R.P.; Ono, E.O.; Rodrigues, J.D. Strobilurin and boscalid in the quality of net melon fruits. Semin Ciên Agrár. 2017, 38, 543–550. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Rama Devi, S.; Prasad, M.N.V. Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1998, 138, 157–165. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Peixoto, H.P.P.; Cambraia, J.; Santana, R.; Mosquim, P.R.; Moreira, M.A. Aluminium effects on lipid peroxidation and the activities of enzymes of oxidative metabolism in sorghum. Rev. Bras. De Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Teisseire, H.; Guy, V. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Streeter, J.G.; Bosler, M.E. Comparison of in vitro and in vivo assays for nitrate reductase in soybean leaves. Plant Physio. 1972, 49, 448. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Cqh/Ceagesp. Programa Brasileiro para Modernização da Horticultura. In Normas Técnicas de Classificação do Tomate; Centro de qualidade em Horticultura (CQH. Documentos, 26): São Paulo, Brazil, 2003.
- Pregnolatto, W.; Pregnolatto, N.P. Normas Analíticas do Instituto Adolfo Lutz: Métodos Químicos e Físicos Para Análise de Alimentos; Instituto Adolfo Lutz: São Paulo, Brasil, 1985; 533p. [Google Scholar]
- Instituto Adolfo Lutz. Normas Analíticas: Métodos Químicos e Físicos Para Análise de Alimentos, 2nd ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008; 371p. [Google Scholar]
- Tressler, D.K.; Joslyn, M.A. Fruits and Vegetables Juice Processing Technology; Conn. AVI: Westport, CT, USA, 1961; 1028p. [Google Scholar]
- Ruela, V.M.; Silva, A.B.; Veiga, A.D.; Souza, T.C.; Marques, D.M.; Costa, C.E.M.; Rezende, T.T. Growth and physiological response of coffee seedlings treated with fungicides. Coffee Sci. 2019, 14, 138–146. [Google Scholar] [CrossRef]
- Grossmann, K.; Retzlaff, G. Bioregulatory Effects of the Fungicidal Strobilurin Kresoxim-methyl in Wheat (Triticum aestivum). Pestic. Sci. 1997, 50, 11–20. [Google Scholar] [CrossRef]
- Bryson, R.J.; Leandro, L.; Jones, D.R. The physiological effects of kresoxim-methyl on wheat leaf greenness and the implication for crop yield. In Proceedings of the Righton Crop Protection Conference—Pests and Diseases, Brighton, UK, 13–16 November 2000. [Google Scholar]
- Amaro, A.C.E.; Baron, D.; Ono, E.O.; Rodrigues, J.D. Physiological effects of strobilurin and carboxamides on plants: An overview. Acta Physiol. Plant. 2020, 42, 4. [Google Scholar] [CrossRef]
- Giuliani, M.M.; Carucci, F.; Nardella, E.; Francavilla, M.; Ricciardi, L.; Lotti, C.; Gatta, G. Combined effects of deficit irrigation and strobilurin application on gas exchange, yield and water use efficiency in tomato (Solanum lycopersicum L.). Sci. Hortic. 2018, 233, 149–158. [Google Scholar] [CrossRef]
- Boari, F.; Cantore, V.; Di Venere, D.; Sergio, L.; Candido, V.; Schiattone, M.I. Pyraclostrobin can mitigate salinity stress in tomato crop. Agric. Water Manag. 2019, 222, 254–264. [Google Scholar] [CrossRef]
- Ajigboye, O.O.; Murchie, E.; Ray, R.V. Foliar application of isopyrazam and epoxiconazole improves photosystem II efficiency, biomass and yield in winter wheat. Pestic. Biochem. Physiol. 2014, 114, 52–60. [Google Scholar] [CrossRef]
- Köhle, H.; Grossmann, K.; Jabs, T.; Gerhard, M.; Kaiser, W.; Glaab, J.; Conrath., U.; Seehaus, K.; Herms, S. Physiological Effects of the Strobilurin Fungicide F 500 on Plants; Dehne, H.W., Gisi, U., Kuck, K.H., Russell, P.E., Lyr, H., Eds.; Modern Fungicides and Antifungal Compounds III. Andover; AgroConcept GmbH: Bonn, Germany, 2003. [Google Scholar]
- Scandalios, J.G. Oxygen Stress and Superoxide Dismutases. Plant Physiol. 1993, 101, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zkiewicz, M.D.; Ska-Polit, E.S.; Krupa, Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. BioMetals 2004, 17, 379–387. [Google Scholar] [CrossRef]
- Liang, S.; Xu, X.; Lu, Z. Effect of azoxystrobin fungicide on the physiological and biochemical indices and ginsenoside contents of ginseng leaves. J. Ginseng. Res. 2018, 42, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, L.A.; Corpas, F.J.; Lopez–Huertas, E.; Palma, J.M. Plant Superoxide Dismutases: Function under Abiotic Stress Conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Tuzet, A.; Rahantaniaina, M.S.; Noctor, G. Analyzing the function of catalase and the ascorbate-glutathione pathway in H2O2 processing: Insights from an experimentally constrained kinetic model. Antioxid. Redox. Signal. 2019, 30, 1238–1268. [Google Scholar] [CrossRef] [PubMed]
- Factor, T.L.S.L.; Júnior, S.L.; Purquerio, L.F.V. Secondary effects of fungicides in tomato seedlings production. Acta Hortic. 2011, 923, 269–275. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer Nature Singapore Pte Ltd.: Singapore, 2018. [Google Scholar]
- Ruske, R.E.; Gooding, M.J.; Jones, S.A. The effects of triazole and strobilurin fungicide programmes on nitrogen uptake, partitioning, remobilization and grain N accumulation in winter wheat cultivars. J. Agric. Sci. 2003, 140, 395–407. [Google Scholar] [CrossRef]
- Marek, J.; Azevedo, D.; Ono, E.O.; Rodrigues, J.D.; Faria, C.M.D.R. Photoynthetic and productive increase in tomato plants treated with strobilurins and carboxamides for the control of Alternaria solani. Sci. Hortic. 2018, 242, 76–89. [Google Scholar] [CrossRef]
- Antunes, M.C.; Cuquel, F.L.; Zawadneak, M.A.C.; Mogor, A.F.; Resende, J.T.V. Postharvest quality of strawberry produced during two consecutive seasons. Hortic. Bras. 2014, 32, 168–173. [Google Scholar] [CrossRef]
- Chitarra, M.I.F.; Chitarra, A.B. Pós-Colheita de Frutas e Hortaliças: Fisiologia e Manuseio, 2nd ed.; UFLA: Lavras, Brasil, 2005; 785p. [Google Scholar]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta BBABioenerg 2005, 1807, 977–988. [Google Scholar] [CrossRef]
- Rodrigues, M.A.T. Avaliação do Efeito Fisiológico do Uso de Fungicidas na Cultura de Soja. In Tese de Doutorado em Fitotecnia—Escola Superior de Agricultura “Luiz de Queiroz”; Universidade de São Paulo: Piracicaba, Brazil, 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).