Abstract
The objective of this work was to analyze the effect of Thidiazuron (TDZ) treatment on floral initiation, flowering time, ornamental characteristics and physiological metabolism of potted Dendrobium nobile. Three TDZ concentrations (200, 500 and 1000 mg L−1) were applied as solution to water the root zone of the plants. Control plants (plants watered with water) showed a good vegetative development but no floral branches. TDZ greatly influenced the flowering process. For all the tested TDZ concentrations, the first flower bud occurred at 55–60 days after the last irrigation (DAI), the highest TDZ concentration showing the major delay in its occurrence. The initial flowering (30% of flowered plants) began 47 days after the first flower bud initiation with no statistical differences among the treatments. Plants treated with TDZ 500–1000 mg L−1 showed the longest period of flowering (about 32 days) and the single flowers delayed the withering of about 2–3 days compared to the lowest TDZ treatment (200 mg L−1). The number of flowers, floral branches and flowering percentage were distinctly influenced by the TDZ concentration. The highest percentage of flowering (40%) was scored when plants were watered with a TDZ solution at 500 mg L−1 and this was a performant treatment providing the best morphological flower features for the ornamental value of this plant. Among the physiological factors affecting the flowering, this study showed that TDZ increased the relative membrane permeability which facilitated the transport of macromolecular flower-forming substances into and out of the membrane. Therefore, the membrane permeability change could be an indicator of shifts in physiologically active substances during the flowering transition process in Dendrobium nobile plants.
1. Introduction
The regulation of flowering time is an important issue to guarantee the year-round production of ornamental crops and to enable the flower synchronization of cross-parents for successful fertilization in a crossbreeding protocol. In addition, flower initiation is one of the critical life-history phases for ornamental plants. Generally, the environmental response and endogenous pathways stimulate flower bud formation to ensure successful reproduction [,].
Endogenous pathways, including the genes of flowering initiation, hormones, carbohydrate levels, miRNA and floral competence, form an integrated network to regulate the flowering time [,,,,]. Plant growth regulators (PGR) have been proven to act as exogenous hormones and therefore they are widely used to interfere with floral initiation in ornamental crops []. Thidiazuron (TDZ), which is one of the phenylurea derivatives considered as the most active cytokinin-like substance, acts as a multifunctional PGR in regulating floral induction, explant regeneration and dormancy breaking, as well as the leaf postharvest senescence process [,,].
TDZ successfully induced the in vitro florigens of soybean, Pennisetum glaucum, and contributed to early flowering by shortening the required time at the vegetative growth stage [,]. Normally, TDZ addition to Murashige and Skoog Medium (MS media) can stimulate explant in vitro regeneration, resulting in adventitious shoots, callus production and protocorm-like body (PLB) proliferation [,,,].
As a preservative ingredient, 100 µM TDZ applied as a foliar spray at the preharvest stage or added in post-harvest solution significantly prolonged the vase life of Dianthus caryophyllus [], Gladiolus grandiflora [] and Calendula officinalis [] by enhancing solution uptake, superoxide dismutase (SOD) activity and membrane stability index (MSI). Additionally, TDZ could inhibit carotenoid degradation, flower abscission and leaf senescence [,].
Genus Dendrobium, consisting of approximately 1000 species around the world, is mainly distributed in tropical and subtropical areas. China has more than 100 wild species of Dendrobium, with the southwest region being particularly rich; many species have high ornamental value []. D. nobile is one of the appreciated Chinese medicinal plants but it is used in breeding programs to obtain hybrids of D. nobile ‘Spring Dream’.
Previous studies indicated that Dendrobium flowering could be influenced by TDZ directly through in vivo application or indirectly by influencing the flowering of the ex vitro plantlets when applied during the in vitro culture. Wen et al. (2013) proved that TDZ could promote the flowering of D. nobile by affecting the transcriptions of some transcription factors and signal genes []. Supplementing TDZ to the MS medium successfully induced the early flowering in ex vitro plantlets of D. capra and D. nobile [,]. The tissue-cultured plantlets of D. officinale produced a high percentage of inflorescences (83.2%) and normal flowers (73.6%) provided that the in vitro culture was carried out for 9 weeks on MS medium supplemented with TDZ 0.1 mg L−1 []. Some studies indicated that TDZ can further increase the frequency of floral bud formation when used in combination with other PGRs [,]. It was reported that TDZ combined with auxins such as 1-naphthlcetic acid (NAA) and Paclobutrazol (PP333) efficiently improved floral buds compared to its single application []. An optimal in vitro protocol to induce a high rate of flower buds and blossomed flowers in tissue-cultured plants of D. officinale indicated the use of a combination of TDZ with other PGRs (0.3 mg L−1 PP333 + 0.5 mg L−1 6-Benzylaminopurine (6-BA) + 0.5 mg L−1 NAA + 0.06 mg L−1 TDZ) []. Foliar spraying and localized irrigation at root level are appropriate methods for the in vivo application. TDZ (30 mg L−1) when applied as foliar spray and through root zone watering was beneficial in inducing floral bud formation but decreased the flower size and shortened the flowering duration in potted plants of Dendrobium ‘Sunya Sunshine’ []. Generally, the beneficial effect of TDZ on Dendrobium flowering is related to several factors such as the species, application method, the concentration of this growth regulator and if it is used alone or in combination with other PGRs.
The flowering switch in higher plants is profoundly affected by various pathways. Usually, the plant hormone status is a traditional way to check the effects of the growth regulator. Additionally, endogenous carbohydrate levels and some autonomous pathways can modulate the process of floral initiation. There is little information available for TDZ effects on floral induction and the physiological metabolism. Current evidence suggests that carbohydrate and chlorophyll metabolisms involve the molecular regulatory mechanism of floral initiation [,,]. Generally, the transport of macromolecular flower-forming substances in and out of the membrane could lead to the membrane permeability change.
The objective of this research was to analyze the effect of three concentrations of TDZ applied via root irrigation on the floral morphogenesis and flowering time of D. nobile. In particular, the flowering features and the physiological metabolism in response to TDZ rates were studied.
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
The work was carried out in the greenhouses of the Southwest Forestry University Department of Horticulture and Landscape Architecture, Kunming, Yunnan, China.
The experimental plant material was set with 2–3-year-old seedlings of D. nobile obtained through in vivo division. Attention was paid to select homogeneous seedlings (3–4 branches, 22–25 cm height) which were transplanted into pots (diameter: 18 cm) filled with a commercial potting mix, composed of 50% coconut fiber and 50% tree bark (NATURAL COCONUT FIBER, China). From May to September 2020, seedlings were cultivated in the greenhouse according to standard cultivation practices (T/HCHMA 0002-2021 Technical regulation of Dendrobium nobile) and maintained under a 65% polywoven shading net. Then, the plants were moved to the incubator at 25 °C/12 °C (day/night) temperature with a daily light intensity of 90 μ mol m−2 s−1, and 80% relative air humidity (HR). After three months under these conditions, the experiment started.
The plant growth regulator TDZ (Shanghai EKEAR Biotechnology Limited, Shanghai, China) was applied at concentrations of 200, 500 and 1000 mg L−1; plants were watered with 220 mL solution, volume which was considered to saturate the water content of the culture substrate. Plants watered with 220 mL water were used as the control. All samples were subjected to initial watering on 27 December 2020 when the soil water potential dropped at a threshold value equal to 40–50% of saturated water content. Plants were watered every 15 days and were completely irrigated three times during the whole process.
The experimental unit consisted of 8 uniform plants and each treatment had three replicates.
2.2. Flowering and Morphological Characterization of Flowers
Plants were monitored from December 2020 to April 2021 and the morphological observations on floral differentiation and formation were carried out after the last watering. The number of branches and the flowering characteristics were observed in each experimental unit and recorded as number of flower spikes, number of inflorescences per branch, number of flowers per inflorescence and total number of flowers. Early flower bud initiation was recorded and the flowering process was followed from the first flowering opening to the fading flowering.
When plants reached the full bloom, the flower morphology was assessed through the length of flower inflorescence, the length and width of the petal, middle sepal and lateral sepal and the lip height.
2.3. Physiological Characteristics Measurements
Three physiological parameters were examined at 13, 23, 33 and 102 days after the last irrigation (DAI), corresponding to the different stages of transition from vegetative growth to reproductive growth. More precisely, 13, 23, 33 and 102 DAI represented the time related to vegetative bud stage (stage I), transitional stage (stage II), inflorescence development stage (stage III) and bloom stage (stage IV), respectively.
The relative membrane permeability was assessed indirectly by detecting electrolyte leakage or conductivity of the leaf tissues according to the method described by Sairam, 1994 []. The leaf tissue (500 mg) was picked and placed in 20 mL double-distilled water (DDW) in two separate tubes. The first was incubated at 25 °C for 30 min and its conductivity was recorded as C1. The second tube was incubated at 100 °C for 20 min and its conductivity was designated as C2. The conductivity value was measured using an Elico DDS-307 Conductivity meter (INESA Scientific instrument Co., Ltd., Shanghai, China). Membrane stability index (MSI) was determined by a ratio of C1 to C2, expressed in percent.
The relative chlorophyll content (%) was determined using a chlorophyll meter (SPAD-502, Konica Minolta, Japan) by detecting the optical concentration difference at two wavelengths between 650 nm and 940 nm. SPAD value is widely used to stand for the relative amount of chlorophyll in vivo. The fully expanded leaf from the upper one-third of the canopy was considered for the measurement. Three samples were randomly chosen for measurements and 10 leaves were tested for each treatment. The chlorophyll relative content was measured with SPAD (SPAD-502, Konica Minolta, Japan) meter from 11.00 a.m. to 13.00 p.m. at the three above said times (13, 23, 33 DAI) and the additional time (102 DAI) corresponding to the reproductive bloom stage. The measurement of each clean leaf was repeated three times.
The soluble sugars were estimated by the anthrone method with glucose as the standard []. The leaf was sampled from the upper branch. Approximately, 10 g of tested and fresh leaves was milled to a fine powder and dried. Each sample was obtained in three portions, and put into graduated Pyrex tubes (150 mm × 25 mm); 5 mL distilled water was added and sealed with sealing film. Then, it was extracted in boiling water for 30 min (twice); the extract was filtered into a 25 mL flask and the tubes and residue were rinsed repeatedly to fix the volume on the scale. The anthrone reagent (5 mL) was pipetted into Pyrex tubes and frozen in ice water. A 1 mL solution was layered on the acid, cooled for 5 min and then thoroughly mixed. Afterwards, it was incubated in boiling water and then cooled in water for 5 min. Absorbance was measured at 620 nm with a UD751GD spectrophotometer (INESA Scientific instrument Co., Ltd., Shanghai, China) using blank as a reference, and the standard curve was plotted with standard solution.
2.4. Data Collection and Statistical Analysis
The data collection started after the last irrigation. An axillary bud was considered to be present when the bud reached 0.20 cm diameter. The time to reach initial flowering and full bloom was assumed to occur when the percentage of blooming plants reached 30% and 60%, respectively. Fading flowering was considered when 80% of the flowers withered. The bloom duration referred to the days from the initial flowering to the fading stage. The duration of a single flower was assessed by the average flowering period of 10 flowers from each treatment.
The influence of TDZ was assessed by evaluating 24 plants used as the population for the observation on the number of flowers/plant, number of flowers/inflorescence, number of inflorescences/branch, number of floral branches/plant and number of branches/plant. Flowering percentage for each treatment was calculated according to the formula: (Total number of floral branches/Total number of branches) × 100. A floral bud of D. nobile usually produces an inflorescence. The number of flowers per inflorescence was determined by the number of flowers emerging from each flower bud.
Measurements on flower morphological characteristics were performed on 10 flowers randomly selected from each treatment. The length of flower inflorescence, the length and width of the petal, middle sepal and lateral sepal and the height of the lip were examined with a vernier caliper.
The leaves for the physiological test were obtained from the upper branch []. Three representative plants were randomly chosen for measurements in each treatment and measurements for each plant were repeated three times repetitively from December 2020 to April 2021.
The significance of TDZ rates on each growth index and physiological parameter was assessed by one-way analysis of variance (ANOVA) using EXCEL (Microsoft Company, Albuquerque, NM, USA). In the case of significance tests of differences, data were analyzed using an ANOVA and Fisher’s LSD test at a significance level of 5% (p = 0.05).
3. Results
3.1. The Influence of TDZ on Flowering
Plants treated with TDZ showed a consistent flowering increase (Table 1). When plants were watered only with water (control), no flowering was observed although plants showed a vigorous vegetative growth. TDZ increased the number of stems bearing flowers, the lower TDZ concentrations (200 and 500 mg L−1) being more effective.

Table 1.
Influence of TDZ treatments on vegetative growth and flowering of Dendrobium nobile plants watered at root level with TDZ solution. The percentage of flowering was calculated according to the formula: (Floral branches per plant/Branches per plant) × 100. Data were collected after the last irrigation. For each variable, values followed by the same small letter are not significantly different at p = 0.05.
Nevertheless, the number of flowers per inflorescence and the number of inflorescences per branch showed no statistical significance for all the tested TDZ concentrations (Table 1).
Generally speaking, 55–60 days were necessary from the last irrigation with TDZ (DAI = days after the last irrigation) to notice the first appearance of the flower buds with significant differences among the TDZ treatments (Table 2). The time span from flower bud appearance to the onset of flowering was the same for all TDZ concentrations tested (47 d) as well as the time required to reach the full bloom was similar for all the TDZ treatments (52–54 d). The highest TDZ concentrations (500 mg L−1 and 1000 mg L−1) significantly retarded the flower withering and prolonged the flowering period, compared to the low TDZ concentration (200 mg L−1) (Table 2). ANOVA showed that TDZ treatment had a significantly positive impact on blooming period of single flowers too.

Table 2.
Influence on flowering of Dendrobium nobile plants watered at root zone with TZD solutions. Initial flowering and full bloom were considered when 30% and 60% of flowered plants were observed, respectively. Fading flowering was when 80% of the flowers withered.
3.2. The Influence of TDZ on Flower Morphology
Flower morphology of D. nobile was greatly influenced by TDZ treatments. Furthermore, the ANOVA analyses revealed that there were significant differences among the three TDZ tested concentrations. Treatment with 500 mg L−1 TDZ had a good inductive effect on the largest size of floral organs, including the length and height of the petal, sepal and lip. Specifically, petal length, middle sepal length and lateral sepal length reached 5.16 cm, 5.12 cm and 4.87 cm, respectively (Figure 1). This TDZ treatment was proven to be the best in enhancing the flower characteristics compared to the other TDZ concentrations (200 and 1000 mg L−1).
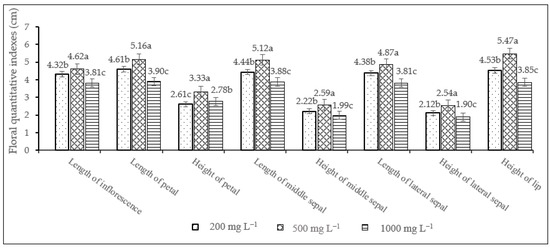
Figure 1.
The effect of different TDZ concentrations on eight floral quantitative characteristics of D. nobile. LSD0.05 means the least squared difference at the p ≤ 0.05. For each characteristic, different lowercase letters are significantly different at 0.05 level of single-factor ANOVA.
3.3. Effect of TDZ on Soluble Sugar Content during the Flowering Development
According to Zhang et al. (2019), Dendrobium nobile plants sprayed with TDZ (30 mg L−1) were able to switch to the flowering phase after one month of the treatment []. In our experiment, compared to the control (plants watered only with water), TDZ significantly affects the soluble sugar content of Dendrobium at all four developmental stages (Figure 2A). Generally, when TDZ was applied, the soluble sugar content was significantly raised from the vegetative bud stage to the inflorescence development stage. Plants treated with TDZ 200 mg L−1 showed an increase in sugar content over the three developmental stages according to a linear correlation (y= 1.093x − 6.5557 R2 = 1); the highest sugar content value (56.18 ± 0.48 mg g−1) was reached at the inflorescence development stage, and afterwards, a significant decrease was observed (25.11 ± 0.31 mg g−1). When 500 mg L−1 TDZ was used, a slight increase in sugar content was scored between the inflorescence development stage (25.10 ± 0.52 mg g−1) and the bloom stage (29.09 ± 0.97 mg g−1). Plants treated with 1000 mg L−1 TDZ showed a significant decrease in sugar content from the inflorescence development stage (40.58 ± 0.67 mg g−1) to the bloom stage (25.33 ± 0.58 mg g−1).
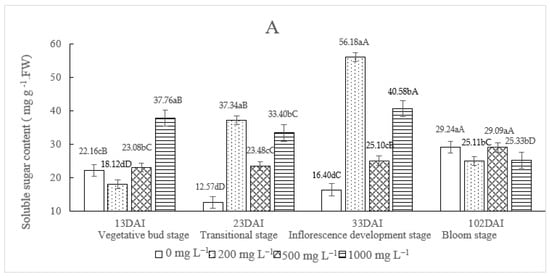
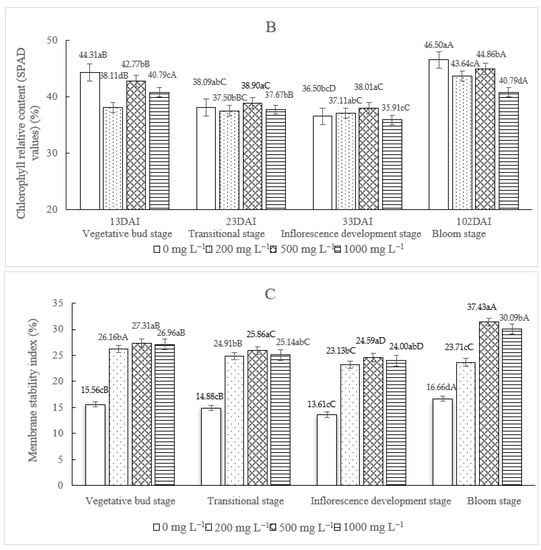
Figure 2.
The effect of different concentrations of TDZ on the soluble sugar content (A), chlorophyll content (B) and relative membrane permeability (C) in Dendrobium nobile. Plants were kept under a controlled temperature in the incubator (temp: 25 °C/12 °C (day/night), light: 90 μ mol m−2 s−1, relative air humidity (HR): 80%). For each stage, different lowercase letters indicated significantly different means (one-way ANOVA, p ≤ 0.05). For each TDZ concentration, different capital letters indicated significantly different means (one-way ANOVA, p ≤ 0.05).
At bloom stage, the sugar content was almost similar in the control plants and in plants treated with the three TDZ concentrations (Figure 2A).
These results suggest that the TZD treatment on D. nobile affected the soluble sugar content already at the lowest TDZ concentration tested (200 mg L−1). A moderate rate (500 mg L−1 TDZ) did not significantly upgrade the soluble sugar at the transitional stage. Taking into consideration the floral characteristics scored when plants were treated with 500 mg L−1 TDZ, it could be argued that at this concentration, no important fluctuation of the soluble sugar content was observed in the critical period from vegetative bud until the inflorescence development stage.
3.4. Effect of TDZ on Chlorophyll Content during the Flower Development
The chlorophyll relative content (SPAD value) in the control plants showed a significant decrease until the inflorescence development stage; at the bloom stage, a significant increase was observed in both the control and TDZ treatments (Figure 2B). Generally speaking, and also in the TDZ-treated plants, a decrease in the SPAD values was scored during the three developmental stages followed by a significant increase at the bloom stage (Figure 2B). However, in the control plants, a more pronounced decrease could be observed until the inflorescence developmental stage (17.63% decrease in SPAD value in the control compared to 2.62%, 11.13% and 11.96% decrease in SPAD value when 200, 500 and 1000 mg L−1 TDZ was applied). At blooming stage, the leaf chlorophyll relative content in the control and the treated plants was not significantly different (Figure 2B).
3.5. The Change in Relative Membrane Permeability
Relative membrane permeability increased significantly under the three TDZ concentrations and it was significantly higher than the control at all the developmental stages (Figure 2C). Generally, the MSI% slightly decreased over the three stages until the bloom stage; at the bloom stage, a significant increase in MSI% was scored both for the control and the three TDZ applications; in the case of TDZ 200 mg L−1, the MSI% was stable between the inflorescence development stage and the bloom stage. Looking at the bloom stage, it is interesting to highlight that the MSI% significantly increased from the control until the 500 mg L−1 TDZ treatment. At this TDZ concentration, the highest values were scored and a consequent significant decrease was then observed at 1000 mg L−1 TDZ.
4. Discussion
Flowering is a critical event which implies the transition from the vegetative to the reproductive phase. This process is influenced by several environmental and endogenous signals []. Among them, the effects of phytohormones on orchid flowering have been studied []. TDZ applied as the only growth regulator during the in vitro propagation of D. capra and D. nobile caused a low rate of flower bud formation in the ex vitro plantlets [,]. Some researchers considered that a combination of various hormones was more effective to induce the flower transition in Dendrobium. An inflorescence induction of 100% was observed when microshoots of D. wangliangii were cultured on 1/2 MS medium with 2 mg L−1 TDZ and 0.5 mg L−1 NAA []. Besides NAA, paclobutrazol (PP333) was found to be beneficial for a high frequency of floral induction. Wang et al. (2014) proved that a combination of 0.05–0.1 mg L−1 TDZ and 1.0 mg L−1 NAA could produce more than 50% floral bud induction and 84% normal flowers in D. officinale []. Similarly, they could successfully induce the early flowering if the plantlets of D. capra were in vitro cultured in MS medium containing TDZ and NAA over an 11-month period []. High TDZ rates with a warm temperature (exceeding 25 °C) usually led to flower deformation.
When the in vivo irrigation technique was applied, the TDZ effect could be influenced mostly by the watering method and cultivation environment, such as substrates, air temperature and humidity. Sphagna moss and coconut shell could achieve a relatively better water holding capacity than pine bark. D. ‘Sunya Sunshine’ treated with 30–120 mg L−1 TDZ in a combination of foliar spaying and root irrigation exhibited no significant difference in floral induction rate, which was more than 80% in all treatments. Compared with the control group, the flower number per inflorescence and flower size decreased and the flowering duration was shortened []. Nevertheless, the floral bud formation percentage in nodes of D. wardianum was 84.3% after in vivo spraying twice with 200 mg L−1 Paclobutrazol (PP333) and 200 mg L−1 TDZ mixed solution in vivo []. It was presumed that the species greatly determined the TDZ concentration response. Undoubtably, an appropriate rate of TDZ would enhance the Dendrobium flower traits and the early flowering. Our research found that a moderate rate of TDZ (500 mg L−1) could induce a high percentage of flower formation, retard the flower withering and prolong the flowering period. Moreover, TDZ significantly improved the number of flowers, number of floral branches and the size of floral organs.
Our study showed that TDZ greatly improve the blooming rate of D. nobile but, compared to previous results (Zhang et al., 2019), the percentage of flowering (about 20–40% depending on TDZ concentration) was poor []. Several reasons could account for that. Firstly, the substrate greatly affected the TDZ effect because the pine bark reduced TDZ adsorption. Secondly, foliar spraying combined with root irrigation, as used in the previous studies, could better enhance the TDZ uptake and utilization with an effect on flowering percentage. The results suggested that an integrated network constructed by different ways of application and the use of several growth regulators could effectively regulate the flowering transition of Dendrobium.
However, some studies revealed the adverse effect of TDZ application on abnormal bud appearance [,]. No abnormal flowers of D. nobile were observed under our experimental conditions. Endogenous regulation involves many crucial chemical signals to modulate the repression and activation of florigen gene expression, such as FLC (FLOWERING LOCUS C), FLD (FLOWERING LOCUS D) and FT (FLOWERING LOCUS T) [,,]. The previous studies revealed that TDZ upregulated flowering genes’ expression [,]. In orchids, a low temperature (10 °C) was beneficial for the vernalization of D. nobile by upregulating the expression of DnFT and, DnVRN1 expression but decreasing DnMFT (Dendrobium nobile FLOWERING LOCUS T) and DnVRN1 (Dendrobium nobile VERNALIZATION 1) expression [,].
Zheng et al. 2017 reported that D. spring could not finish the floral differentiation at 26/21 °C (day/night). A relative low night temperature (≤17 °C) was conductive to its floral initiation []. Tian et al. 2007 found that its floral differentiation rate could reach 64.74% at 10 °C for 30 d []. A continuous temperature of 12 °C at night could enhance the flowering induction and early flowering of D. nobile in our experiment, although it produced a relatively low flower formation rate. Further research should be carried out to discuss whether a lower temperature could improve a high rate of floral differentiation in Dendrobium.
Carbohydrates are believed to play a crucial role in the regulation of flowering. TPS1 (TREHALOSE-6-PHOSPHATE SYNTHASE 1) could inhibit its floral initiation regardless of favorably inductive environmental conditions []. Seo et al. (2011) proved that the INDETERMINATE DOMAIN transcription factor AtIDD8 regulated photoperiodic flowering by modulating the sugar transport and metabolism of Arabidopsis thaliana [].
In our experiment, the soluble sugar content (SSC) was correlated with the occurrence of flower buds, whereas there was no distinct fluctuation at the transition stage. The 500 mg L−1 TDZ treatment sustained a stable level of soluble sugar content. We could speculate that during the reproductive development, a sugar signal regulation occurred mainly at the inflorescence differentiation and blooming phase under this average TDZ concentration. An intensive study should be performed to obtain a sequential and dynamic examination of its content based on the precise scheduling of the whole transition phase.
A previous study indicated that chloroplasts worked as essential sensors to cause nuclear transcriptional changes at the developmental transition stage. In Arabidopsis, PTM, a PHD transcription factor modulating chloroplast retrograde signaling, could mediate the transcriptional repression of FLC through the recruitment of FVE (a component of the histone deacetylase complex) []. In our research, TDZ irrigation decreased in the chlorophyll content in D. nobile from the vegetative phase to the inflorescence development stage (13 DAI, 23 DAI and 33 DAI). However, a more pronounced decrease was shown in the control plants. These results seemed to be irrelevant to the flower transition. We speculated that the chloroplast regulatory network might be involved in multiple responses including carbohydrate synthesis and flowering gene expression. Further research is required regarding the chloroplast signaling regulatory mechanism to determine the tradeoff between vegetative growth and reproductive growth.
TDZ used as a preservative solution could improve the membrane integrity of cut flowers [,]. In contrast to the control group, TDZ increased the cell membrane permeability and had a positive rate effect. TDZ facilitated the transport of macromolecular flower-forming substances into and out of the membrane. Therefore, the membrane permeability change could be an indicator of shifts in physiologically active substances during the flowering transition process.
5. Conclusions
Investigations addressed to promote flowering in Dendrobium are well appreciated due to the long vegetative period scored in these plants. In our experiments, we found that the TDZ applied to water the root zone of D. nobile plants was beneficial in affecting the flowering. In fact, the untreated plants were able to show a good vegetative growth but no flowering. The TDZ-treated plants flowered and the highest percentage of flowering (40%) was recorded when this growth regulator was applied at 500 mg L−1. No significant differences were scored in the time frame necessary to reach the initial and full bloom stage when different TDZ concentrations were tested. On the contrary, when 500 mg L−1 TDZ was applied, the important flower features able to enhance the ornamental value, flower number and flowering period were improved. Among the different physiological parameters studied to elucidate the positive effect of TDZ on D. nobile flowering, the relative membrane permeability seems to account for a more efficient transport of nutrients and macromolecules during the flowering process and this could explain the best performing flowering.
Author Contributions
Investigation, Q.W. and Z.C.; methodology, L.W.; data curation, H.G. and Z.C.; draft preparation, S.R.; writing—editing, M.H. and S.R.; writing—review and supervision, Z.L.; resources, Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
The project is funded by the National Key Research and Development Project of the Ministry of Science and Technology (2019YFD10010003). This research belongs to the sub-project of efficient breeding technology and variety creation of Orchid (2019YFD10010003). This item is also funded by Innovation and Entrepreneurship Training for College students of the Ministry of Education (20180554027) and Yunnan Postgraduate Adviser Team Construction Project (No. 2019-101).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank anonymous reviewers for comments on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Song, Y.H.; Ito, S.; Imaizumi, T. Flowering time regulation: Photoperiod-and temperature-sensing in leaves. Trends Plant Sci. 2013, 18, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Verhage, L.; Angenent, G.C.; Immink, R.G.H. Research on floral timing by ambient temperature comes into blossom. Trends Plant Sci. 2014, 19, 583–591. [Google Scholar] [CrossRef]
- Amasino, R.M.; Michaels, S.D. The timing of flowering. Plant Physiol. 2010, 154, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Zeng, L. The critical role of miRNAs in regulation of flowering time and flower development. Genes 2020, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, S.; Yustis, J.C.; Chávez-Hernández, E.C.; Martínez, T.; Sanchez, M.D.; Garay-Arroyo, A.; Álvarez-Buylla, E.R.; García-Ponce, B. Beyond the genetic pathways, flowering regulation complexity in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 5716. [Google Scholar] [CrossRef]
- Higuchi, Y. Florigen and anti-florigen: Flowering regulation in horticultural crops. Breed. Sci. 2018, 68, 109–118. [Google Scholar] [CrossRef]
- Kim, J.J. The miR156-SPL3 module regulates ambient temperature-responsive flowering via FT in Arabidopsis thaliana. Plant Physiol. 2012, 159, 461–478. [Google Scholar] [CrossRef]
- Anawal, V.V.; Narayanaswamy, P.; Yallesh Kumar, H.S. Effects of plant growth regulators on induction of flowering in pomegranate (Punica granatum L.) cv. Bhagwa. Int. J. Sci. Res. 2015, 4, 7–9. [Google Scholar]
- Chen, L.R.; Chen, J.T.; Chang, W.C. Efficient production of protocorm-like bodies and plant regeneration from flower stalk explants of the sympodial orchid Epidendrum radicans. In Vitr. Cell. Dev. Biol. Plant 2002, 38, 441–445. [Google Scholar] [CrossRef]
- Kurumwanshi, R.S.; Jadhav, P.V.; Surbhaiyya, S.D.; Moharil, M.P.; Nandanwar, R.S.; Wandhare, M.R.; Manjaya, J.G. In vitro florigenesis: An efficient regeneration system avoiding time consuming vegetative phase in popular Indian soybean variety JS-335. Indian J. Exp. Biol. 2021, 59, 458–466. [Google Scholar]
- Singh, M.; Tiwari, N. Thidiazuron outpaces 6-benzylamino purine and kinetin in delaying flower senescence in Gladiolus grandiflora by alleviating physiological and biochemical responses. J. Appl. Biol. Biotechnol. 2021, 9, 56–62. [Google Scholar]
- Maity, P.J.; Kulkarni, V.M.; Vishnu, B. Thidiazuron-induced multiple shoot regeneration and in vitro flowering in Pennisetum glaucum (L.). R. Br. Phytomorphol. 2016, 66, 45–50. [Google Scholar]
- Sujjaritthurakarn, P.; Kanchanapoom, K. Efficient direct protocorm-like bodies induction of dwarf Dendrobium using Thidiazuron. Not. Sci. Biol. 2011, 3, 88–92. [Google Scholar] [CrossRef]
- Celikel, F.G.; Reid, M.S.; Jiang, C.Z. Water uptake and vase life of cut Gardenia jasminoides flowers. In Proceedings of the XXX International Horticultural Congress IHC 2018: International Symposium on Ornamental Horticulture and XI International 1263, Istanbul, Turkey, 12–16 August 2018; pp. 335–342. [Google Scholar]
- Hajong, S.; Kumaria, S.; Tandon, P. Effect of plant growth regulators on regeneration potential of axenic nodal segments of Dendrobium chrysanthum Wall. ex Lindl. J. Agric. Sci. Technol. 2013, 15, 1425–1435. [Google Scholar]
- Chamani, E.; Esmaeilpour, B. Thidiazuron effects on physiochemical characteristics of carnation during pre and postharvest periods. J. Appl. Hort. 2007, 9, 115–117. [Google Scholar] [CrossRef]
- Lone, M.L.; Farooq, S.; Parveen, S.; Tahir, I. 6-Benzylamino purine outperforms Kinetin and Thidiazuron in ameliorating flower longevity in Calendula officinalis L. by orchestrating physiological and biochemical responses. Ornam. Hortic. 2021, 27, 183–195. [Google Scholar] [CrossRef]
- Ferrante, A.; Vernieri, P.; Serra, G.; Tognoni, F. Changes in abscisic acid during leaf yellowing of cut stock flowers. Plant Growth Regul. 2004, 43, 127–134. [Google Scholar] [CrossRef]
- Sankhla, N.; Mackay, W.A.; Davis, T.D. Reduction of flower abscission and leaf senescence in cut phlox inflorescences by thidiazuron. In Proceedings of the XXVI International Horticultural Congress: Issues and Advances in Postharvest Horticulture 628, Toronto, ON, Canada, 11–17 August 2002; pp. 837–841. [Google Scholar]
- Chen, S.C.; Tsi, Z.H.; Luo, Y.B. Flora Reipublicae Popularis Sinicae Tomus 19. In Dendrobium; Science Press: Beijing, China, 1999; pp. 75–146. [Google Scholar]
- Wen, Z.Z.; Zhang, E.; Liu, Y.G.; Liu, W. Primary screening of differentially expressed genes during TDZ induced floral initiation with SSH in Dendrobium nobile. Acta Hortic. Sin. 2013, 40, 1591–1599. [Google Scholar]
- Lawrie, M.D.; Layina, Z.; Ningtias, D.R.; Alifianto, F.N.; Indrianto, A.; Purwantoro, A.; Semiarti, E. In vitro germination and flowering of Dendrobium capra JJ smith, an endemic orchid of java. Hayati J. Biosci. 2021, 28, 172. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zeng, S.; Chen, Z. Preliminary report on the study of in vitro flowering and fruiting of Dendrobium officinale. Guizhou Agric. Sci. 2014, 42, 34–37. [Google Scholar]
- Qian, X.; Wang, C.; Ouyang, T.; Tian, M. In vitro flowering and fruiting in culture of Dendrobium officinate kimura et migo. (Orchidaceae). Pak. J. Bot. 2014, 46, 1877–1882. [Google Scholar]
- Cen, X.F.; Huang, C.H.; Wei, P.X. Effects of hormone factors on the in vitro culture flowering induction of Dendrobium officinate Kimura et Migo. Agric. Sci. Technol. Hunan 2010, 11, 75–79. [Google Scholar]
- Zhang, D.; Liao, Y.; Lu, S.; Li, C.; Shen, Z.; Yang, G.; Yin, J. Effect of thidiazuron on morphological and flowering characteristics of Dendrobium ‘Sunya Sunshine’ potted plants. N. Z. J. Crop Hortic. Sci. 2019, 47, 170–181. [Google Scholar] [CrossRef]
- Sairam, R.K. Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 1994, 32, 594. [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [PubMed]
- Alkio, M.; Schubert, A.; Diepenbrock, W.; Grimm, E. Effect of source-sink ratio on seed set and filling in sunflower (Helianthus annuus L.). Plant Cell Environ. 2003, 26, 1609–1619. [Google Scholar] [CrossRef]
- Wahl, V.; Ponnu, J.; Schlereth, A.; Arrivault, S.; Langenecker, T.; Franke, A.; Feil, R.; Lunn, J.E.; Stitt, M.; Schmid, M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 2013, 339, 704–707. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, L.; Wei, Q.; Ju, Y.; Zou, X.; Wan, X.; Liu, X.; Yin, Z. A new insight into flowering regulation: Molecular basis of flowering initiation in Magnolia× soulangeana ‘Changchun’. Genes 2019, 11, 15. [Google Scholar] [CrossRef]
- Zhao, D.; Hu, G.; Chen, Z.; Shi, Y.; Zheng, L.; Tang, A.; Long, C. Micropropagation and in vitro flowering of Dendrobium wangliangii: A critically endan- gered medicinal orchid. J. Med. Plants Res. 2013, 7, 2098–2110. [Google Scholar]
- Wang, Z.H.; Zhu, G.F.; OUM, C.; Wang, B.Q. Induction of plant growth regulators on early in vivo flowering of nobile-type Dendrobium. Guangdong Agric. Sci. 2008, 10, 37–39. [Google Scholar]
- Wu, H.H.; Chen, F.C. Effect of plant growth regulators on shoot multiplication from flower stalk nodal buds of Phalaenopsis and Doritaenopsis. J. Taiwan Soc. Hortic. Sci. 2008, 54, 151–159. [Google Scholar]
- Wang, Z.H.; Wang, L.; Ye, Q.S. High frequency early flowering from in vitro seedlings of Dendrobium nobile. Sci. Hortic. 2009, 122, 328–331. [Google Scholar] [CrossRef]
- Xiang, L.; Li, X.; Qin, D.; Guo, F.; Wu, C.; Miao, L.; Sun, C. Functional analysis of FLOWERING LOCUST orthologs from spring orchid (Cymbidium goeringii Rchb. f.) that regulates the vegetative to reproductive transition. Plant Physiol. Biochem. 2012, 58, 98–105. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Song, S.; Li, Y.; Shen, L.; Yu, H. DOFT and DOFTIP1 affect reproductive development in the orchid Dendrobium Chao Praya Smile. J. Exp. Bot. 2017, 68, 5759–5772. [Google Scholar] [CrossRef]
- Lee, P.L.; Chen, J.T. Plant regeneration via callus culture and subsequent in vitro flowering of Dendrobium huoshanense. Acta Physiol. Plant. 2014, 36, 2619–2625. [Google Scholar] [CrossRef]
- Wen, Z.; Guo, W.; Li, J.; Lin, H.; He, C.; Liu, Y.; Zhang, Q.; Liu, W. Comparative transcriptomic analysis of vernalization-and cytokinin-induced floral transition in Dendrobium nobile. Sci. Rep. 2017, 7, 45748. [Google Scholar] [CrossRef]
- Wang, H.M.; Tong, C.G.; Jang, S. Current progress in orchid flowering/flower development research. Plant Signal. Behav. 2017, 12, e1322245. [Google Scholar] [CrossRef]
- Wang, S.L.; An, H.R.; Tong, C.G.; Jang, S. Flowering and flowering genes: From model plants to orchids. Hortic. Environ. Biotechnol. 2021, 62, 135–148. [Google Scholar] [CrossRef]
- Zheng, B.Q.; Deng, X.M.; Li, K.; Miao, K.; Wang, Y. Effects of temperature treatment on flower bud differentiation and development of Dendrobium. For. Res. 2017, 30, 460–464. [Google Scholar]
- Tian, D.Q.; Cao, Q.Y.; Ding, H.Q.; Yu, L.J. A preliminary study on flower forcing of Dendrobium spring at low temperature. Zhejiang Agric. Sci. 2007, 1, 38–40. [Google Scholar]
- Seo, P.J.; Ryu, J.; Kang, S.K.; Park, C.M. Modulation of sugar metabolism by an INDETERMINATE DOMAIN transcription factor contributes to photoperiodic flowering in Arabidopsis. Plant J. 2011, 65, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Guo, H.; Chi, W.; Chai, X.; Sun, X.; Xu, X.; Ma, J.; Rochaix, J.D.; Leister, D.; Wang, H.; et al. Chloroplast retrograde signal regulates flowering. Proc. Natl. Acad. Sci. USA 2016, 113, 10708–10713. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).