The Composition of Anthocyanins and Carotenoids Influenced the Flower Color Heredity in Asiatic Hybrid Lilies
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Flower Color Measurement
2.3. Statistical Analysis
3. Results
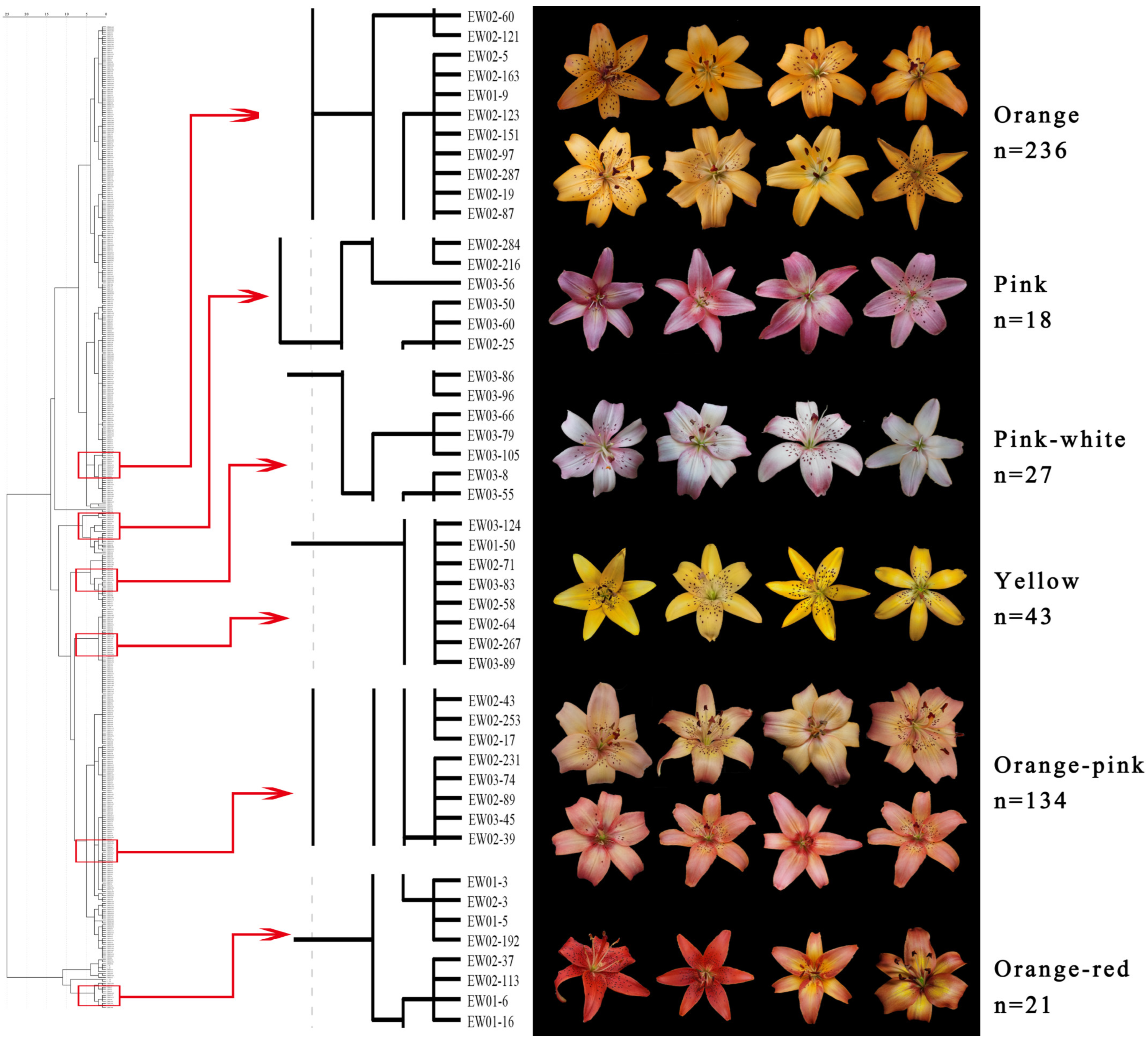
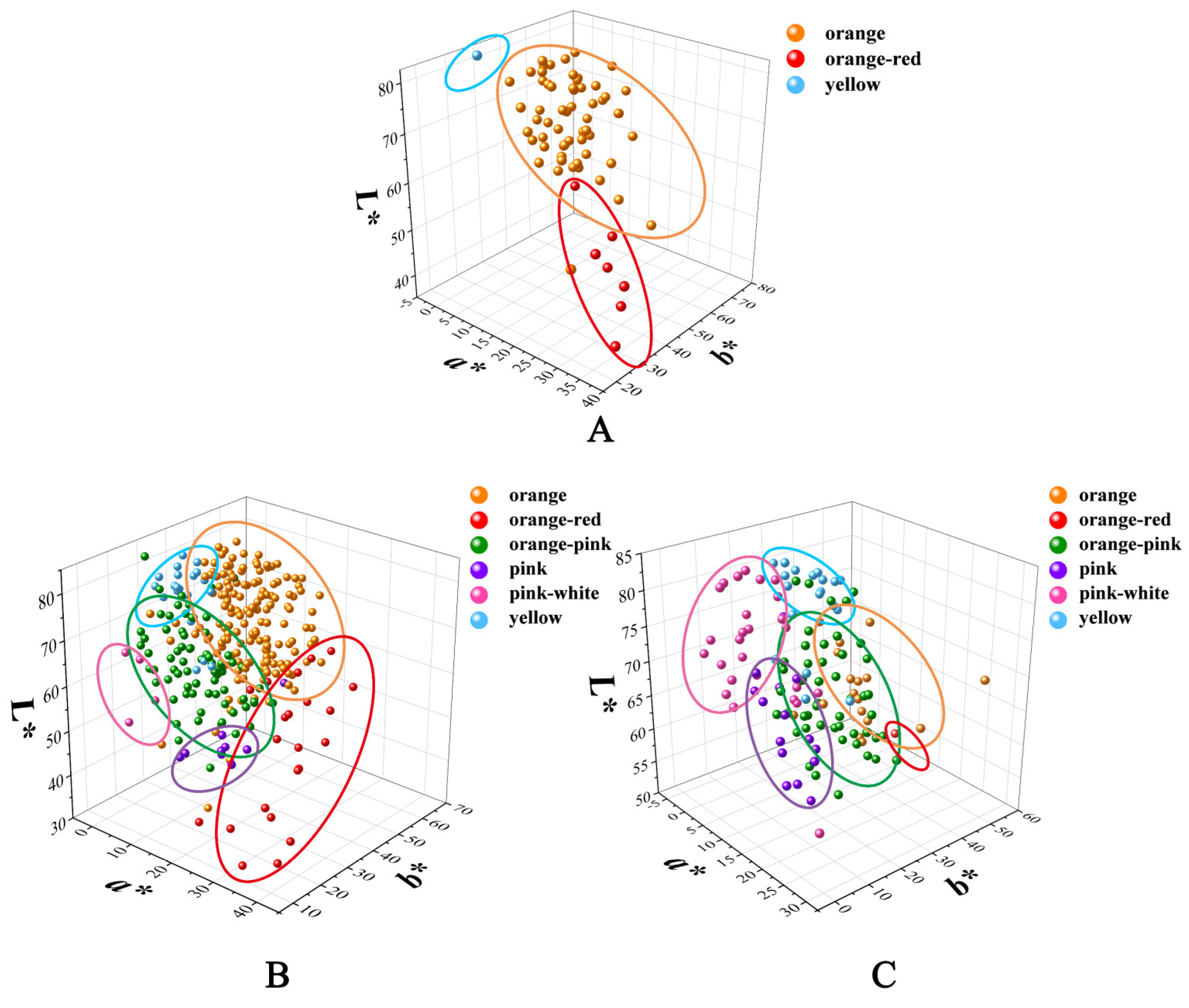
3.1. Colorimetric Evaluation and Numerical Classification of the Hybrids
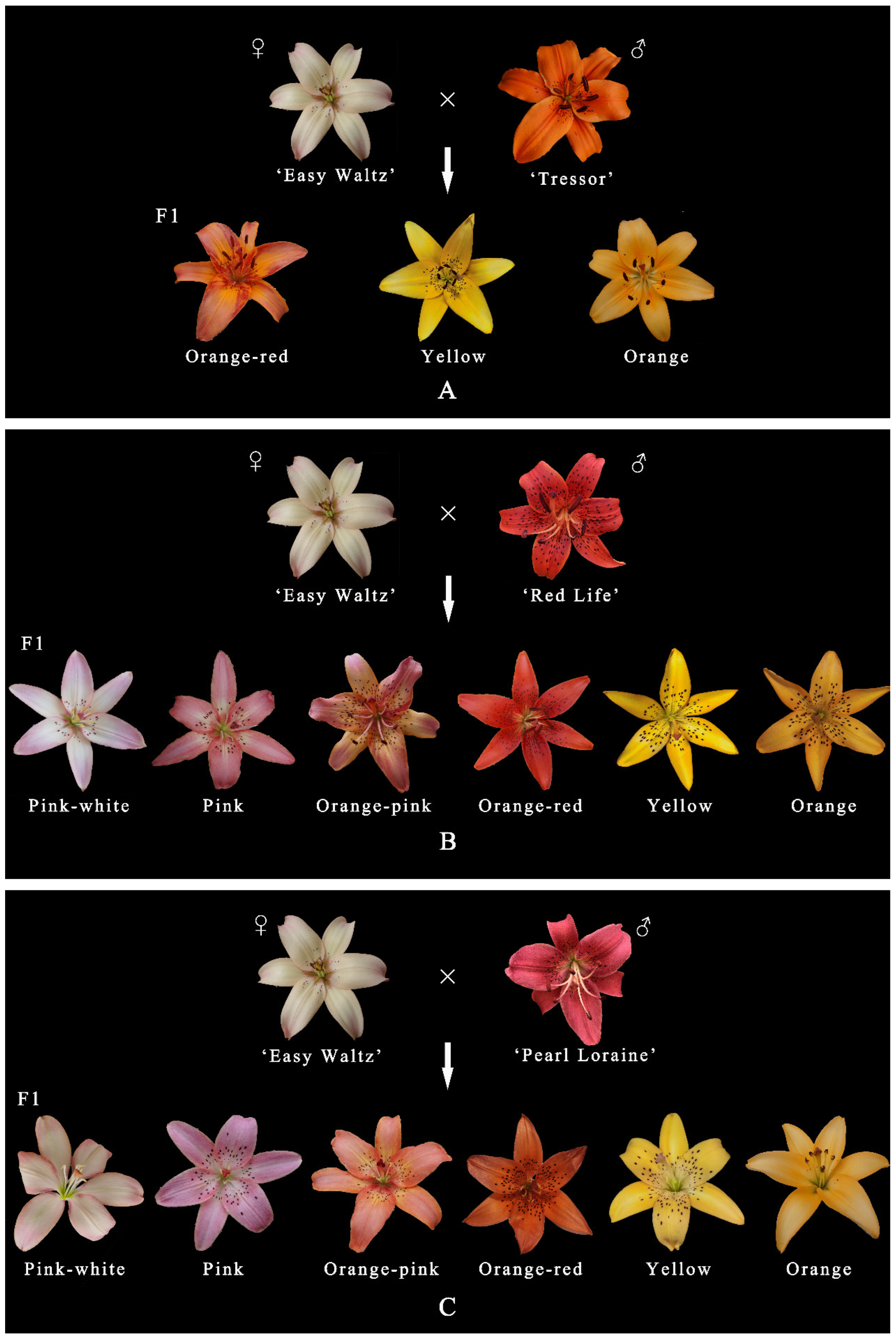
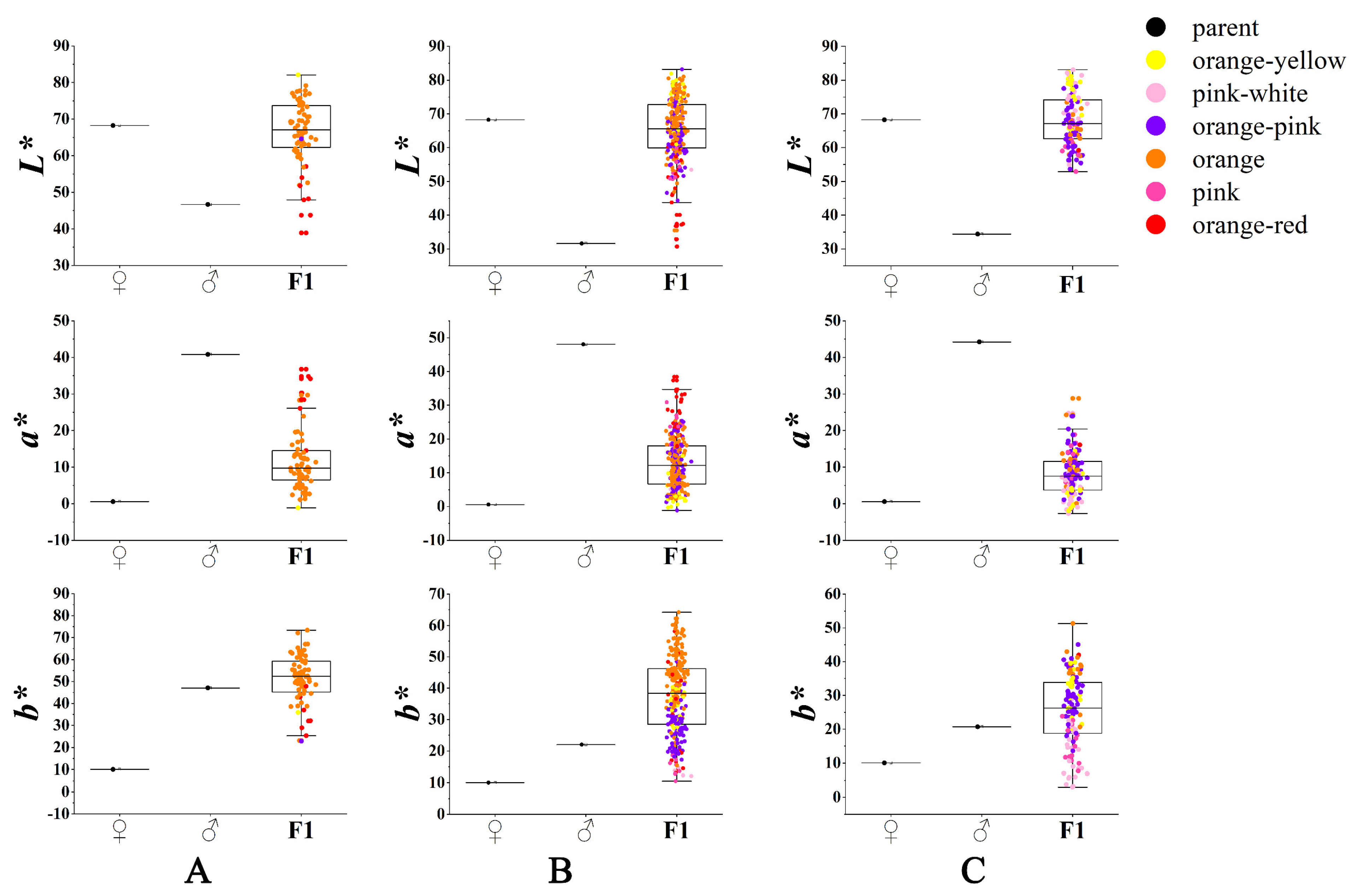
3.2. Variation in Flower Color of the Cross Combinations
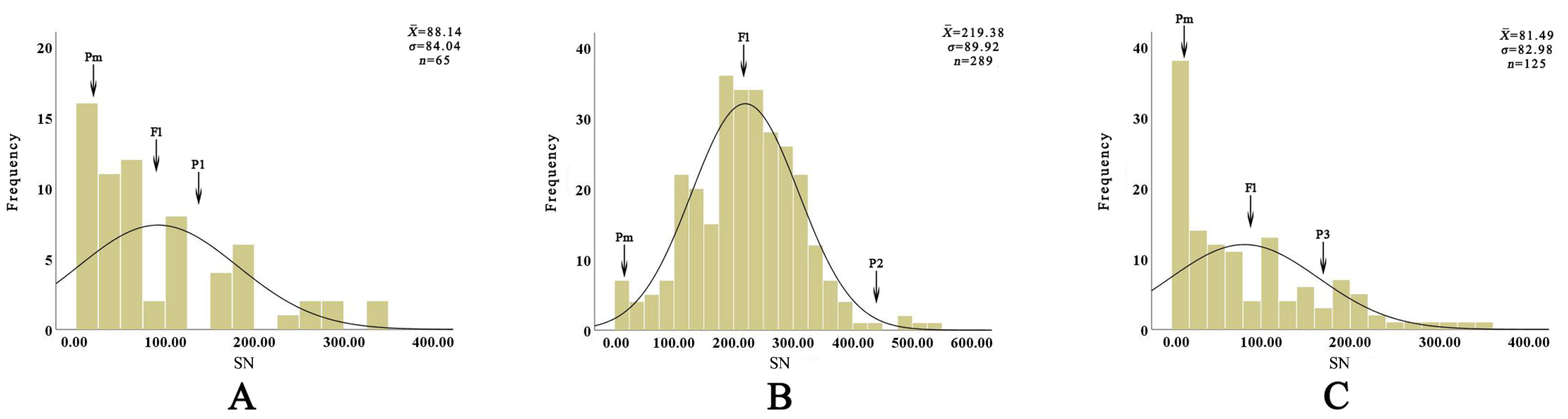
3.3. Variation in Raised Spots on Tepals of Hybrids from Different Cross Combinations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Comber, H.F. A new classification of the genus Lilium. In Royal Horticultural Society Lily Year Book; Chittenden, F.J., Ed.; The Royal Horticultural Society: London, England, 1949; pp. 85–105. [Google Scholar]
- Smyth, D.R.; Kongsuwan, K.; Wisudharomn, S. A survey of C-band patterns in chromosomes of Lilium (Liliaceae). Plant Syst. Evol. 1989, 163, 53–69. [Google Scholar] [CrossRef]
- Du, Y.P.; He, H.B.; Wang, Z.X.; Wei, C.; Li, S.; Jia, G.X. Investigation and evaluation of the genus Lilium resources native to China. Genet Resour. Crop. Ev. 2014, 61, 395–412. [Google Scholar] [CrossRef]
- Leslie, A.C. The International Lily Register, 3rd ed.; The Royal Horticultural Society: London, England, 1982. [Google Scholar]
- Lim, K.B.; van Tuyl, J.M. Lily: Lilium hybrids. In Flower Breeding and Genetics: Issues, Challenges and Opportunities for the 21st Century; Anderson, N.O., Ed.; Springer: Berlin, Germany, 2006; pp. 513–532. [Google Scholar]
- Barba-Gonzalez, R.; Van Silfhout, A.A.; Visser, R.G.F.; Ramanna, M.S.; Van Tuyl, J.M. Progenies of allotriploids of Oriental × Asiatic lilies (Lilium) examined by GISH analysis. Euphytica 2006, 151, 243–250. [Google Scholar] [CrossRef]
- Van Tuyl, J.M.; Arens, P.; Shahin, A.; Marasek-Ciołakowska, A.; Barba-Gonzalez, R.; Kim, H.T.; Lim, K.-B. Lilium . In Ornamental Crops, Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Berlin, Germany, 2018; pp. 481–512. [Google Scholar] [CrossRef]
- Yamagishi, M. How genes paint lily flowers: Regulation of colouration and pigmentation patterning. Sci. Hortic. 2013, 163, 27–36. [Google Scholar] [CrossRef]
- Nørbæk, R.; Kondo, T. Anthocyanins from flowers of Lilium (Liliaceae). Phytochemistry 1999, 50, 1181–1184. [Google Scholar] [CrossRef]
- Yamagishi, M.; Kishimoto, S.; Nakayama, M. Carotenoid composition and changes in expression of carotenoid biosynthetic genes in tepals of Asiatic hybrid lily. Plant Breed. 2010, 129, 100–107. [Google Scholar] [CrossRef]
- Jeknić, Z.; Morré, J.T.; Jeknić, S.; Jevremović, S.; Subotić, A.; Chen, T.H.H. Cloning and functional characterization of a gene for capsanthin-capsorubin synthase from tiger lily (Lilium lancifolium Thunb. ‘Splendens’). Plant Cell Physiol. 2012, 53, 1899–1912. [Google Scholar] [CrossRef]
- Wang, X.; Yamagishi, M. Mechanisms suppressing carotenoid accumulation in flowers differ depending on the hybrid groups of lilies (Lilium spp.). Sci. Hortic. 2019, 243, 159–168. [Google Scholar] [CrossRef]
- Deli, J.; Molnár, P.; Matus, Z.; Tóth, G.; Steck, A.; Pfander, H. Isolation and characterization of 3,5,6-trihydroxy-carotenoids from petals of Lilium tigrinum. Chromatographia 1998, 48, 27–31. [Google Scholar] [CrossRef]
- Lai, Y.S.; Shimoyamada, Y.; Nakayama, M.; Yamagishi, M. Pigment accumulation and transcription of LhMYB12 and anthocyanin biosynthesis genes during flower development in the Asiatic hybrid lily (Lilium spp.). Plant Sci. 2012, 193, 136–147. [Google Scholar] [CrossRef]
- Yamagishi, M. White with partially pink flower color in Lilium cernuum var. album is caused by transcriptional regulation of anthocyanin biosynthesis genes. Sci. Hortic. 2020, 260, 108880. [Google Scholar] [CrossRef]
- Balode, A. Color analysis of flowers in Lilium sp. breeding. Acta Hortic. 2007, 755, 213–218. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Kang, S.Y.; Rhee, H.Y.; Lim, K.B. The characteristics of progenies derived from Lilium lancifolium and Asiatic hybrid ‘Dreamland’. Korean J. Breed. Sci. 2009, 41, 451–455. [Google Scholar]
- Jo, Y.K.; Ramzan, F.; Son, B.G.; Kim, H.Y.; Lim, K.B. Crossing of Asiatic hybrids for breeding of new Lily cultivars. Korean J. Breed. Sci. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Wang, H.; Kong, Y.; Dou, X.Y.; Lang, L.X.; Bai, J.R. Genetic variation of phenotypic traits in F1 generation between Asiatic hybrid lily ‘Renoir’ and Lilium davidii var. willmottiae. Mol. Plant Breed. 2021, 19, 6111–6119. [Google Scholar] [CrossRef]
- Hanbury, A.; Serra, J. Mathematical morphology in the CIELAB space. Image. Anal. Stereol. 2002, 21, 201–206. [Google Scholar] [CrossRef]
- Afonso, T.; Moresco, R.; Uarrota, V.G.; Navarro, B.B.; Nunes, E.D.C.; Maraschin, M.; Rocha, M. UV-vis and CIELAB based chemometric characterization of Manihot esculenta carotenoid contents. J. Integr. Bioinform. 2017, 14, 20170056. [Google Scholar] [CrossRef]
- Wang, N.H.; Dai, M.Y.; Zheng, G.; Chang, P.J.; Xuan, L.J.; Liu, Z.G.; Wang, Y.L.; Cheng, S.Y.; Wang, Z.W.; Wang, H.L. Flavonoid components and gene expression analysis reveal flower pigmentation difference between Magnolia biondii and its variety M. biondii var. purpurascens. Trees-Struct. Funct. 2021, 36, 583–591. [Google Scholar] [CrossRef]
- Donoso, A.; Rivas, C.; Zamorano, A.; Pena, A.; Handford, M.; Aros, D. Understanding Alstroemeria pallida flower colour: Links between phenotype, anthocyanins and gene expression. Plants 2021, 10, 55. [Google Scholar] [CrossRef]
- Li, X.; Lu, M.; Tang, D.Q.; Shi, Y.M. Composition of carotenoids and flavonoids in Narcissus cultivars and their relationship with flower color. PLoS ONE 2015, 10, e0142074. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.Y.; Wang, S.L.; Niu, X.Y. Analysis of anthocyanins and flavonols in petals of 10 Rhododendron species from the Sygera Mountains in Southeast Tibet. Plant Physiol. Bioch. 2016, 104, 250–256. [Google Scholar] [CrossRef]
- Cui, H.L.; Zhang, Y.A.; Shi, X.L.; Gong, F.F.; Xiong, X.; Kang, X.P.; Xing, G.M.; Li, S. The numerical classification and grading standards of daylily (Hemerocallis) flower color. PLoS ONE 2019, 14, e0216460. [Google Scholar] [CrossRef]
- Lu, C.F.; Li, Y.F.; Wang, J.Y.; Qu, J.P.; Chen, Y.; Chen, X.Y.; Huang, H.; Dai, S.L. Flower color classification and correlation between color space values with pigments in potted multiflora chrysanthemum. Sci. Hortic. 2021, 283, 110082. [Google Scholar] [CrossRef]
- Lei, T.; Song, Y.; Jin, X.H.; Su, T.Y.; Pu, Y.W. Effects of pigment constituents and their distribution on spathe coloration of Zantedeschia hybrida. Hortscience 2017, 52, 1840–1848. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Yin, M.; Abbas, F.; Sun, Y.; Gao, T.; Yan, F.L.; Li, X.Y.; Yu, Y.Y.; Yue, Y.C.; Yu, R.C. Classification and association analysis of Gerbera (Gerbera hybrida) flower color traits. Front. Plant Sci. 2022, 12, 779288. [Google Scholar] [CrossRef]
- Wang, H.; Fan, Y.W.; Yang, Y.; Zhang, H.; Li, M.F.; Sun, P.; Zhang, X.Z.; Xue, Z.; Jin, W.M. Classification of rose petal colors based on optical spectrum and pigment content analyses. Hortic. Environ. Biote. 2022. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.F.; Wang, L.; Qi, X.Y.; Song, M.; Cao, Y.W.; He, G.R.; Tang, Y.C.; Yang, P.P.; Ming, J. The numerical classification of flower color phenotype in lily. Acta Hortic. Sin. 2021, 49, 571–580. [Google Scholar] [CrossRef]
- Abe, H.; Nakano, M.; Nakatsuka, A.; Nakayama, M.; Koshioka, M.; Yamagishi, M. Genetic analysis of floral anthocyanin pigmentation traits in Asiatic hybrid lily using molecular linkage maps. Theor. Appl. Genet. 2002, 105, 1175–1182. [Google Scholar] [CrossRef]
- Yamagishi, M. MYB19LONG is involved in brushmark pattern development in Asiatic hybrid lily (Lilium spp.) flowers. Sci. Hortic. 2020, 272, 109570. [Google Scholar] [CrossRef]
- He, X.F.; Xu, S.F.; Leng, P.S.; Wang, W.H. Transcriptome sequencing reveals genes involved in petal spot formation of Asiatic hybrid lily cultivar ‘Easy Dance’. Int. J. Agric. Biol. 2018, 20, 939–944. [Google Scholar] [CrossRef]
- Yamagishi, M.; Akagi, K. Morphology and heredity of tepal spots in Asiatic and Oriental hybrid lilies (Lilium spp.). Euphytica 2013, 194, 325–334. [Google Scholar] [CrossRef]
- Yamagishi, M. Isolation and identification of MYB transcription factors (MYB19Long and MYB19Short) involved in raised spot anthocyanin pigmentation in lilies (Lilium spp.). J. Plant Physiol. 2020, 250, 153164. [Google Scholar] [CrossRef]
- Shahin, A.; Arens, P.; Van Heusden, A.W.; Van Der Linden, G.; Van Kaauwen, M.; Khan, N.; Schouten, H.J.; Van De Weg, W.E.; Visser, R.G.F.; Van Tuyl, J.M. Genetic mapping in Lilium: Mapping of major genes and quantitative trait loci for several ornamental traits and disease resistances: Genetic mapping in Lilium. Plant Breed. 2011, 130, 372–382. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wang, S.D.; Wang, W.H.; Zhang, K.; Liu, C.Y. Tingyuan Baihe Shiyong Jishu, 1st ed.; China Agriculture Press: Beijing, China, 2016; pp. 69–78. [Google Scholar]
- Wang, Q.; Lv, T.; Lv, Y.M. The impact of tepal structure and pigment composition on the flower colour of lily. Acta Hortic. Sin. 2021, 48, 1873–1884. [Google Scholar] [CrossRef]
- UPOV. Guidelines for the conduct of tests for distinctness, uniformity and stability, Lilium, TG/59/7. In Proceedings of the International Union for the Protection of New Varieties of Plants, Geneva, Switzerland, 24 March 2010; Available online: https://www.upov.int/portal/index.html.en (accessed on 10 October 2022).
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Color and phenolic content changes during flower development in groundcover rose. J. Am. Soc. Hortic. Sci. 2010, 135, 195–202. [Google Scholar] [CrossRef]
- Akbari, R.; Hatamzadeh, A.; Sariri, R.; Bakhshi, D. Relationship of flower color parameters and metal ions of petal tissue in fully opened flowers of gerbera. J. Plant Stud. 2013, 2, 89–96. [Google Scholar] [CrossRef][Green Version]
- Itle, R.A.; Kabelka, E.A. Correlation between L*a*b* color space values and carotenoid content in pumpkins and squash (Cucurbita spp.). Hortscience 2009, 44, 633–637. [Google Scholar] [CrossRef]
- Abdelaali, S.B.; Rodrigo, M.J.; Saddoud, O.; Zacarías, L.; Hajlaoui, M.R.; Mars, M. Carotenoids and colour diversity of traditional and emerging Tunisian orange cultivars (Citrus sinensis (L.) Osbeck). Sci. Hortic. 2018, 227, 296–304. [Google Scholar] [CrossRef]
- Xue, L.; Wang, Z.G.; Zhang, W.; Li, Y.X.; Wang, J.; Lei, J. Flower pigment inheritance and anthocyanin characterization of hybrids from pink-flowered and white-flowered strawberry. Sci. Hortic. 2016, 200, 143–150. [Google Scholar] [CrossRef]


| No. | Cross Combination | ♀ | Pigment Composition and Content (μg/g, DW) | ♂ | Pigment Composition and Content (μg/g, DW) | Hybrid Number |
|---|---|---|---|---|---|---|
| 1 | EW01 | ‘Easy Waltz’ | no carotenoid medium anthocyanin (94.36 ± 7.36) | ‘Tresor’ | high carotenoid (141.38 ± 5.32) no anthocyanin | 65 |
| 2 | EW02 | ‘Easy Waltz’ | no carotenoid medium anthocyanin (94.36 ± 7.36) | ‘Red Life’ | high carotenoid (120.78 ± 9.56) medium anthocyanin (91.33 ± 3.94) | 289 |
| 3 | EW03 | ‘Easy Waltz’ | no carotenoid medium anthocyanin (94.36 ± 7.36) | ‘Pearl Loraine’ | low carotenoid (4.66 ± 0.16) high anthocyanin (144.43 ± 2.51) | 125 |
| Total | 479 |
| Cross Combination | Pink-White | Pink | Orange-Pink | Orange-Red | Yellow | Orange |
|---|---|---|---|---|---|---|
| EW01 | 0 | 0 | 0 | 6 | 2 | 57 |
| EW02 | 4 | 11 | 80 | 14 | 21 | 159 |
| EW03 | 23 | 7 | 54 | 1 | 20 | 20 |
| Cross Combination | The Average Spots Number of Parents | Hybrids | |||||
|---|---|---|---|---|---|---|---|
| ♀ | SN | ♂ | SN | No. without Spots | No. with Spots | Proportion | |
| EW01 | ‘Easy Waltz’ | 15 | ‘Tresor’ | 147 | 11 | 54 | 1:5 |
| EW02 | ‘Easy Waltz’ | 15 | ‘Red Life’ | 437 | 0 | 289 | —— |
| EW03 | ‘Easy Waltz’ | 15 | ‘Pearl Loraine’ | 164 | 27 | 98 | 1:4 |
| Cross Combination | Parent | F1 | Hi/% | Hybrid Ratio/% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ♀ | ♂ | MPs | ± σ | Range | CV/% | BLP/% | BP/% | OHP/% | ||
| EW01 | 15.00 | 147.00 | 81.00 | 88.14 ± 84.04 | 0.00~339.00 | 95.35 | 108.81 | 20.00 | 55.38 | 24.62 |
| EW02 | 15.00 | 437.00 | 226.00 | 219.38 ± 89.92 | 2.00~526.00 | 40.99 | 97.07 | 0.35 | 98.27 | 1.38 |
| EW03 | 15.00 | 164.00 | 90.00 | 81.49 ± 82.98 | 0.00~341.00 | 101.83 | 90.54 | 29.60 | 52.80 | 17.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, J.; Zhang, Q.; Yu, P.; Zhou, Y.; Jia, G. The Composition of Anthocyanins and Carotenoids Influenced the Flower Color Heredity in Asiatic Hybrid Lilies. Horticulturae 2022, 8, 1206. https://doi.org/10.3390/horticulturae8121206
Li J, Chen J, Zhang Q, Yu P, Zhou Y, Jia G. The Composition of Anthocyanins and Carotenoids Influenced the Flower Color Heredity in Asiatic Hybrid Lilies. Horticulturae. 2022; 8(12):1206. https://doi.org/10.3390/horticulturae8121206
Chicago/Turabian StyleLi, Jiewen, Jiawei Chen, Qian Zhang, Pengcheng Yu, Yanping Zhou, and Guixia Jia. 2022. "The Composition of Anthocyanins and Carotenoids Influenced the Flower Color Heredity in Asiatic Hybrid Lilies" Horticulturae 8, no. 12: 1206. https://doi.org/10.3390/horticulturae8121206
APA StyleLi, J., Chen, J., Zhang, Q., Yu, P., Zhou, Y., & Jia, G. (2022). The Composition of Anthocyanins and Carotenoids Influenced the Flower Color Heredity in Asiatic Hybrid Lilies. Horticulturae, 8(12), 1206. https://doi.org/10.3390/horticulturae8121206




