Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Materials
2.2. Postharvest Treatments
2.3. Color Variations
2.4. Ethylene Production
2.5. Detection of Fruit Firmness
2.6. Cell Membrane Permeability (CMP) and Malondialdehyde (MDA) Content
2.7. Activities of Cell Wall-Degrading Enzymes
2.8. Contents of Protopectin and Water-Soluble Pectin (WSP)
2.9. Mornitering Pulp Cell Structure Using Electron Microscopy
2.10. Chemicals
2.11. Statistical Analysis
3. Results
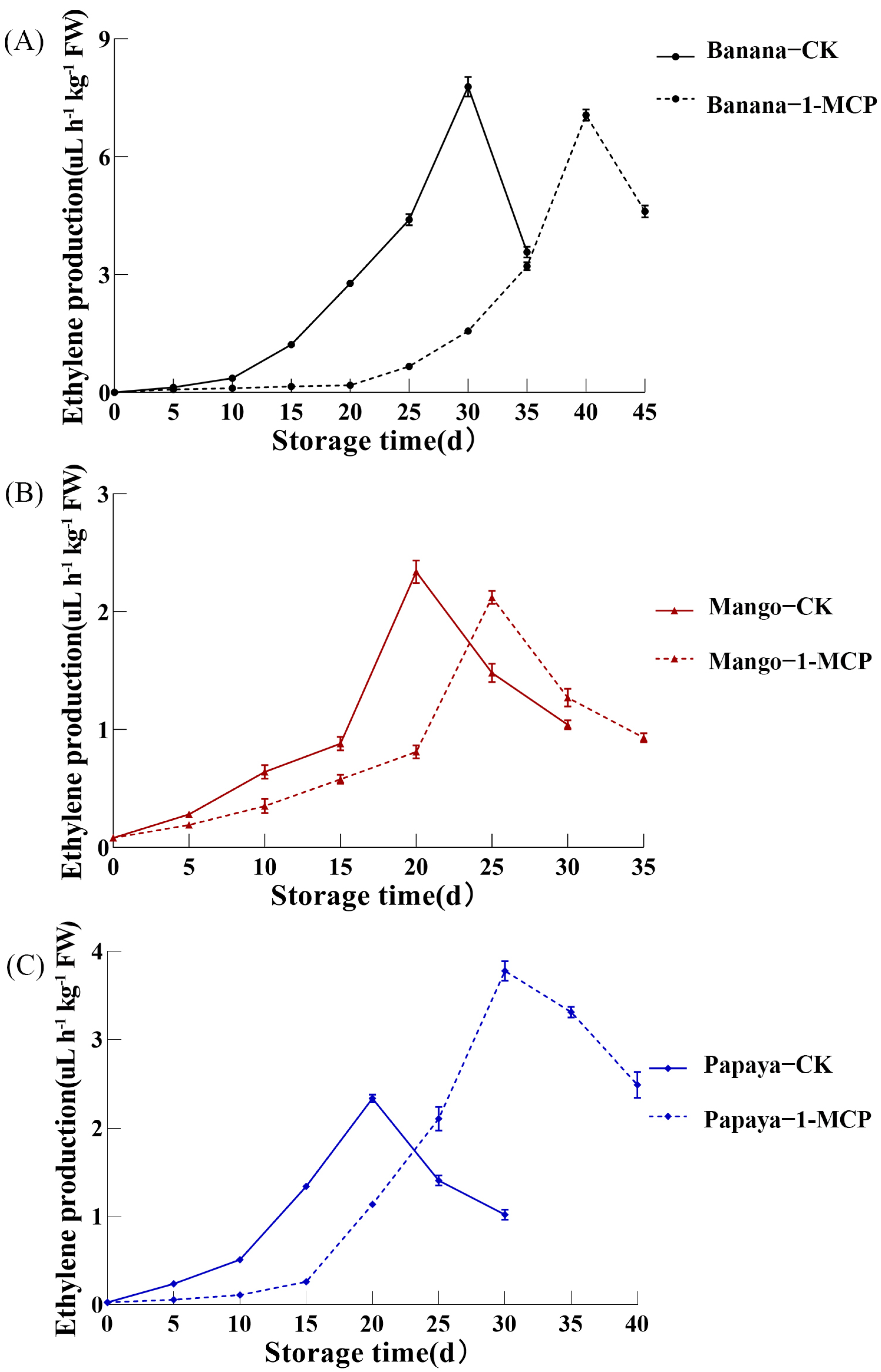
3.1. Effects of 1-MCP Treatment on Production of Ethylene
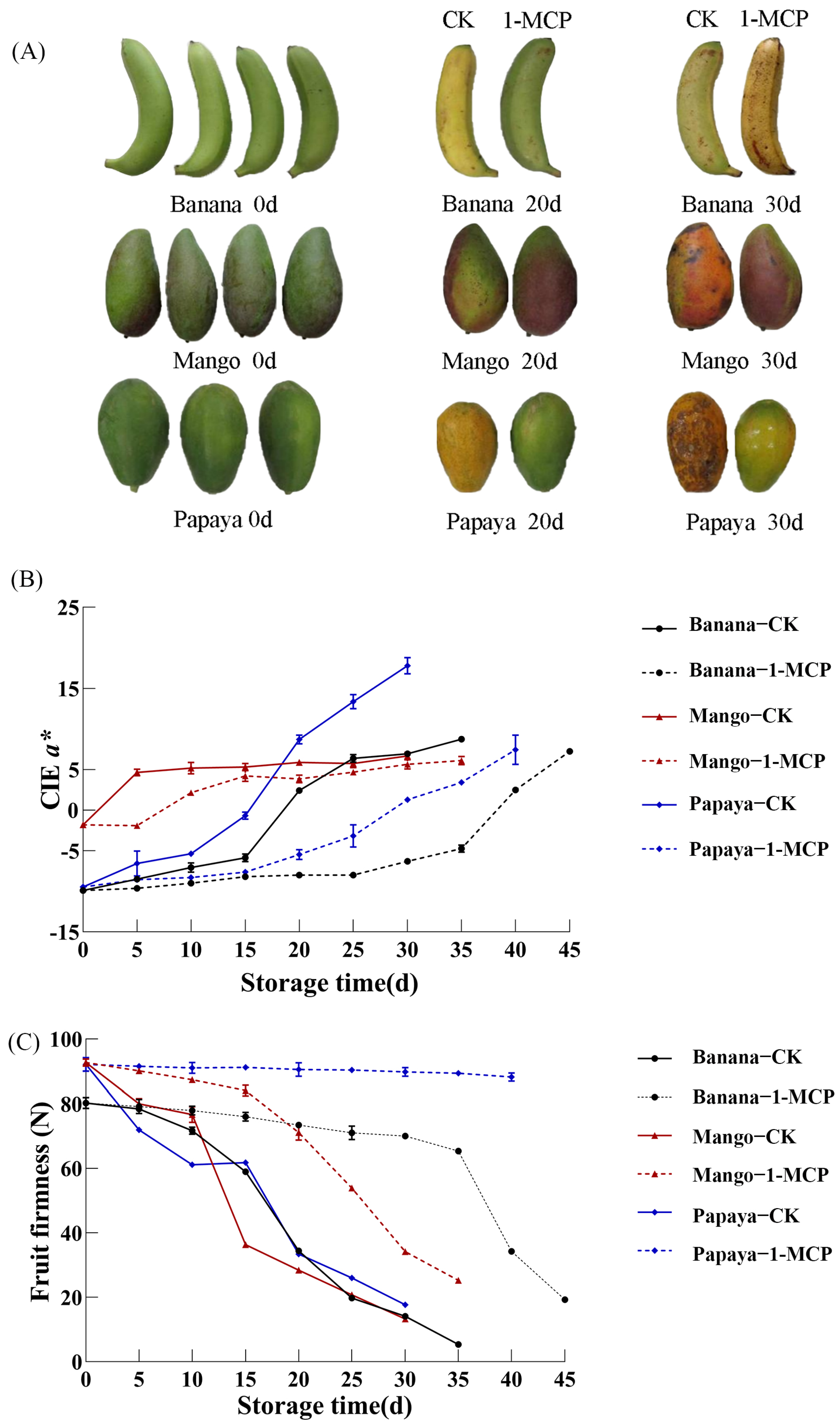
3.2. Effects of 1-MCP on Sensory Quality and Fruit Firmness
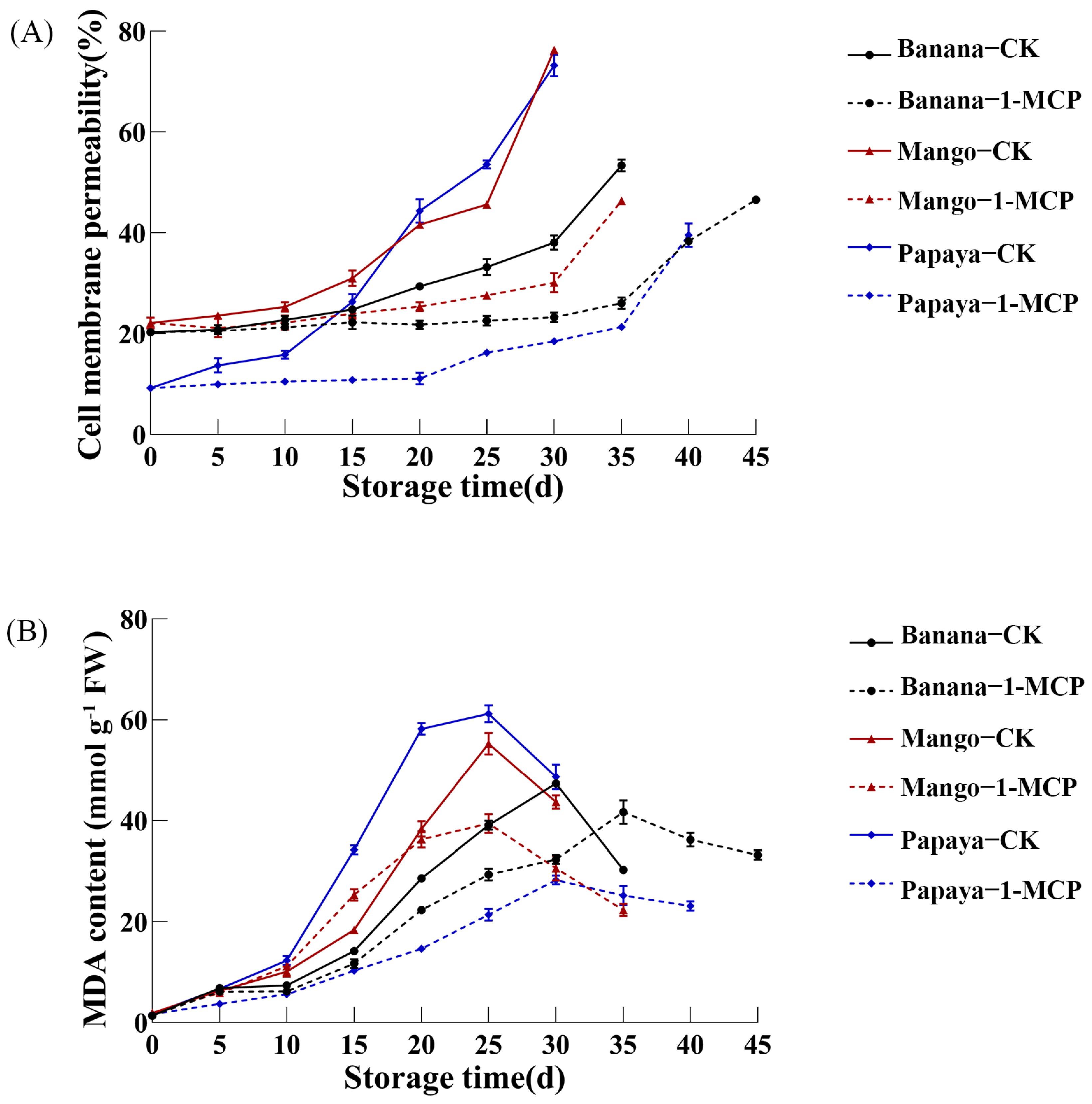
3.3. Effects of 1-MCP Treatment on CMP and MDA Content of Postharvest Fruits
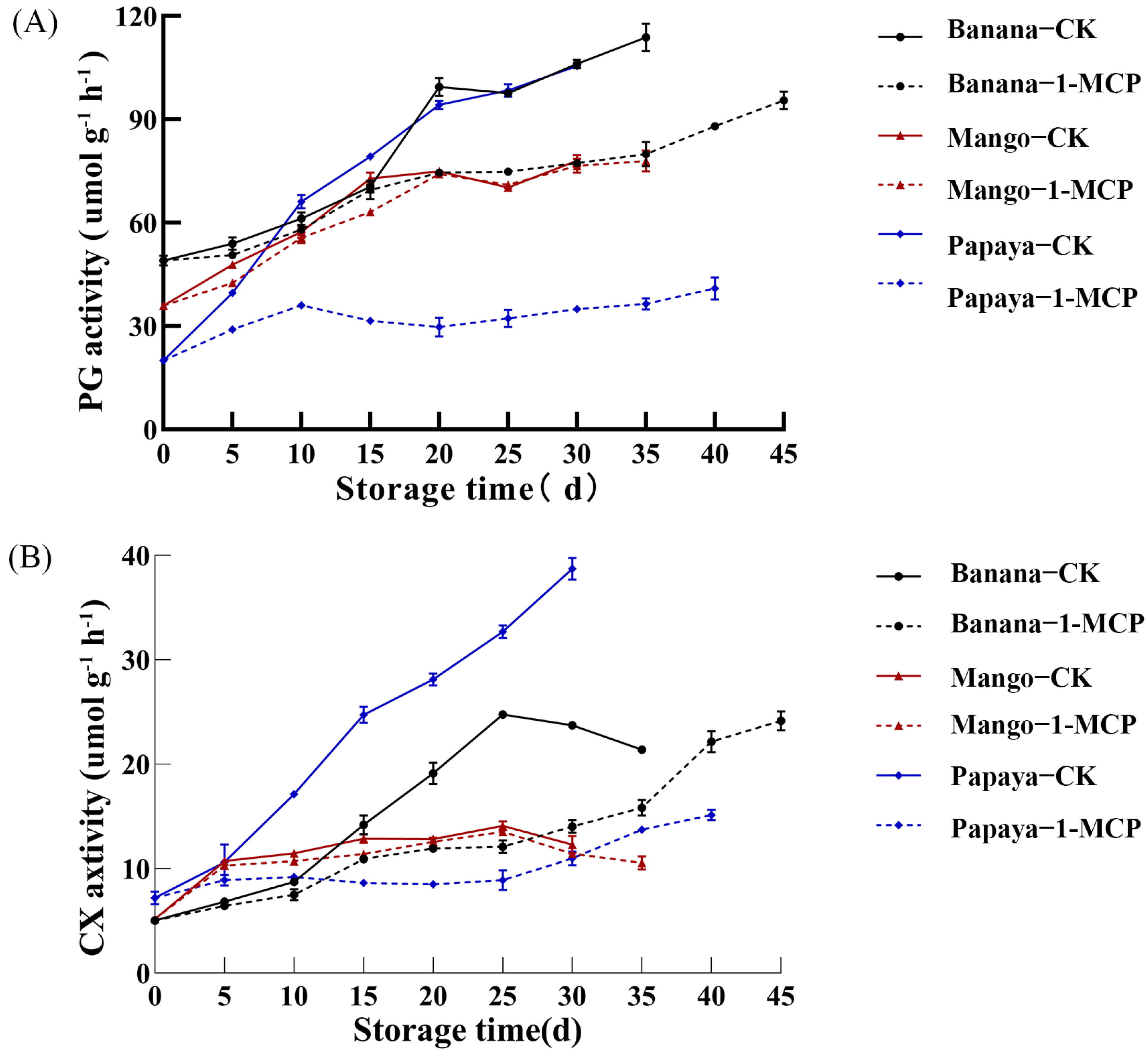
3.4. Effects of 1-MCP Treatment on Activities of PG and CX in Postharvest Fruits
3.5. Effects of 1-MCP Treatment on Contents of Protopectin and WSP in Postharvest Fruits
3.6. Effects of 1-MCP Treatment on Changes of Cell Microstructure in Postharvest Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1-MCP | 1-methylcyclopropene |
| 2-PG | Polygalacturonase |
| PME | Pectin methylesteraselyase |
| EXP | Expansins |
| PL | Pectatelyase |
| GLU | endoglucanase |
| CX | cellulase |
| BGL | Beta-glucosidase |
| XYN | Endo-1,4-beta-xylanase |
| GAL | Beta-galactosidase |
| XTH | Xyloglucan endotransglycosylase |
| PGA | Polygalacturonic acid |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| DNS | 3,5-Dinitrosalicylic acid reagent |
| CMC | Carboxymethyl cellulose |
| FAA | Alcohol formalin acetate mixture |
References
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.; Mohamed, M.T.; Hamzah, M.; Ali, M.M.; Razak, M.F.H. Kinetics of quality changes in papayas (Carica papaya L.) coated with Malaysian stingless bee honey. Sci. Hortic. 2020, 267, 109321. [Google Scholar] [CrossRef]
- García-Muñoz, M.C.; Henao-Rojas, J.C.; Moreno-Rodríguez, J.M.; Botina-Azain, B.L.; Romero-Barrera, Y. Effect of rootstock and environmental factors on fruit quality of Persian lime (Citrus latifolia Tanaka) grown in tropical regions. J. Food Compos. Anal. 2021, 103, 104081. [Google Scholar] [CrossRef]
- Chen, J.Q.; Chen, T.C.; Qiu, M.M.; Li, L.; Zhong, Q.G.; Wei, Q.; Deng, Y.J.; Xie, B.G.; Jiang, Y.J.; Chen, B.Z. Identification of ACC synthetase genes in Volvariella volvacea and analysis of their response to ethephon and 1-methylcyclopropene treatments. Sci. Hortic. 2021, 278, 109848. [Google Scholar] [CrossRef]
- Xie, Y.T.; Zhang, F.; Guo, F.Z.; Song, K.Y.; Luo, H.B.; Wang, J.H.; Wang, Y.X.; Jiang, L. Postharvest application of 1-methylcyclopropene delays chloroplast degradation in Gynura bicolor DC under sugar scarcity conditions. Sci. Hortic. 2022, 305, 111342. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.G.; Naing, A.H.; Kwon, J.G.; Kang, I.K. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Q.; Liu, D.H.; Meng, C.X.; Hu, Z.Y.; Ma, L. Identification of the cell wall proteins associated with the softening of Lycium barbarum L. fruit by using iTRAQ technology. Food Chem. Mol. Sci. 2022, 4, 100110. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhu, X.; Hou, Y.Y.; Wang, X.Y.; Li, X.H. Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. cv. Dongzao) fruit during storage. Postharvest Biol. Technol. 2019, 156, 110954. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhang, X.L.; Li, L.L.; Yang, W.T.; Zhang, W.D.; Cheng, S.B.; Guo, M.R.; Chen, G.G. Delaying fruit softening of ‘France’ prune (Prunus domestica L.) using near-freezing temperature storage. LWT 2022, 172, 114165. [Google Scholar] [CrossRef]
- Liu, M.P.; Wang, R.; Sun, W.W.; Han, W.J.; Li, G.; Zong, W.; Fu, J.M. Effects of postharvest calcium treatment on the firmness of persimmon (Diospyros kaki) fruit based on a decline in WSP. Sci. Hortic. 2023, 307, 111490. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, S.; Lin, H.T.; Lu, W.J.; Wang, H.; Chen, Y.H.; Lin, Y.F.; Fan, Z.Q. The role of cell wall polysaccharides disassembly in Lasiodiplodia theobromae-induced disease occurrence and softening of fresh longan fruit. Food Chem. 2021, 351, 129294. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.X.; Li, F.F.; Hong, K.Q.; Yuan, D.B. Effects of procyanidin treatment on the ripening and softening of banana fruit during storage. Sci. Hortic. 2022, 292, 110644. [Google Scholar] [CrossRef]
- Wang, N.; Nian, Y.W.; Li, R.; Shao, Y.Z.; Li, W. Transcription Factor CpbHLH3 and CpXYN1 Gene Cooperatively Regulate Fruit Texture and Counteract 1-Methylcyclopropene Inhibition of Softening in Postharvest Papaya (Carica papaya L.). J. Agric. Food Chem. 2022, 70, 9919–9930. [Google Scholar] [CrossRef]
- Saba, M.K.; Watkins, C.B. Flesh browning development of ‘Empire’ apple during a shelf life period after 1-methylcyclopropene (1-MCP) treatment and controlled atmosphere storage. Sci. Hortic. 2020, 261, 108938. [Google Scholar] [CrossRef]
- Lawson, T.; Lycett, G.W.; Ali, A.; Chin, C.F. Characterization of Southeast Asia mangoes (Mangifera indica L.) according to their physicochemical attributes. Sci. Hortic. 2019, 243, 189–196. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a Novel Edible Composite Coating Based on Gum Arabic and Chitosan on Biochemical and Physiological Responses of Banana Fruits during Cold Storage. J. Agric. Food Chem. 2011, 59, 5474–5482. [Google Scholar] [CrossRef]
- Lwin, H.P.; Choi, J.H.; Chun, J.P.; Watkins, C.B.; Lee, J.W. 1-Methylcyclopropene treatment alters fruit quality attributes and targeted metabolites in ‘Wonhwang’ pears during shelf life. Sci. Hortic. 2021, 284, 110125. [Google Scholar] [CrossRef]
- Huan, C.; An, X.J.; Yu, M.L.; Li, J.; Ma, R.J.; Tu, M.G.; Yu, Z.F. Effect of combined heat and 1-MCP treatment on the quality and antioxidant level of peach fruit during storage. Postharvest Biol. Technol. 2018, 145, 193–202. [Google Scholar] [CrossRef]
- Luo, S.S.; Wan, B.; Feng, S.H.; Shao, Y.Z. Biocontrol of Postharvest Anthracnose of Mango Fruit with Debaryomyces Nepalensis and Effects on Storage Quality and Postharvest Physiology. J. Food Sci. 2015, 80, 2554–2563. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2011, 62, 258–266. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Mellidou, I.; Boeckx, J.; Kyomugasho, C.; Bessemans, N.; Verlinden, B.E.; Hertog, M.L.; Hendrickx, M.; Nicolai, B.M.; Geeraerd, A.H. Expression analysis of candidate cell wall-related genes associated with changes in pectin biochemistry during postharvest apple softening. Postharvest Biol. Technol. 2016, 112, 176–185. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Rosli, H.G.; Civello, P.M.; Martinez, G.A.; Herrera, R.; Moya-Leon, M.A. Changes in cell wall polysaccharides and cell wall degrading enzymes during ripening of Fragaria chiloensis and Fragaria ananassa fruits. Sci. Hortic. 2010, 124, 454–462. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. The effects of 1-MCP treatment on fruit quality of medlar fruit (Mespilus germanica L. cv. Istanbul) during long term storage in the palliflex storage system. Postharvest Biol. Technol. 2015, 100, 81–90. [Google Scholar] [CrossRef]
- Yang, J.P. The modified method of routine paraffin section. J. Biol. 2006, 23, 45–46. [Google Scholar]
- Tilahun, S.; Lee, Y.M.; Choi, H.R.; Baek, M.W.; Lee, J.S.; Park, D.S.; Kang, H.M.; Jeong, C.S. Modified atmosphere packaging combined with CO2 and 1-methylcyclopropene prolong the storability and maintain antioxidant properties of cherry tomato. Sci. Hortic. 2021. 288, 110401. [CrossRef]
- Chai, J.X.; Wang, Y.T.; Liu, Y.F.; Yong, K.; Liu, Z.D. 1-MCP extends the shelf life of ready-to-eat ‘Hayward’ and ‘Qihong’ kiwifruit stored at room temperature. Sci. Hortic. 2021, 289, 110437. [Google Scholar] [CrossRef]
- Duan, X.W.; Cheng, G.P.; Yang, E.; Yi, C.; Ruenroengklin, N.; Lu, W.J.; Luo, Y.B.; Jiang, Y.M. Modification of pectin polysaccharides during ripening of postharvest banana fruit. Food Chem. 2008, 111, 144–149. [Google Scholar] [CrossRef]
- Zerbini, P.E.; Vanoli, M.; Rizzolo, A.; Grassi, M.; Pimentel, R.M.D.A.; Spinelli, L.; Torricelli, A. Optical properties, ethylene production and softening in mango fruit. Postharvest Biol. Technol. 2015, 101, 58–65. [Google Scholar] [CrossRef]
- Chang, L.Y.; Brecht, J.K. Responses of 1-methylcyclopropene (1-MCP)−treated banana fruit to pre− and post−treatment ethylene exposure. Sci. Hortic. 2023, 309, 111636. [Google Scholar] [CrossRef]
- Jung, S.K.; Watkins, C.B. Internal ethylene concentrations in apple fruit at harvest affect persistence of inhibition of ethylene production after1-methylcyclopropene treatment. Postharvest Biol. Technol. 2014, 96, 1–6. [Google Scholar] [CrossRef]
- Langer, S.E.; Marina, M.; Francese, P.; Civello, P.M.; Martínez, G.A.; Villarreal, N.M. New insights into the cell wall preservation by 1-methylcyclopropene treatment in harvest-ripe strawberry fruit. Sci. Hortic. 2022, 299, 111032. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Wu, F.; Zhao, Y.T.; Shi, L.; Zhu, X. Cloning and expression analysis of polygalacturonase and pectin methylesterase genes during softening in apricot (Prunus armeniaca L.) fruit. Sci. Hortic. 2019, 256, 108607. [Google Scholar] [CrossRef]
- Liu, S.M.; Huang, H.; Huber, G.J.; Pan, Y.G.; Shi, X.Q.; Zhang, Z.K. Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Sane, V.A.; Chourasia, A.; Nath, P. Softening in mango (Mangifera indica cv. Dashehari) is correlated with the expression of an early ethylene responsive, ripening related expansin gene, MiExpA1. Postharvest Biol. Technol. 2005, 38, 223–230. [Google Scholar] [CrossRef]
- Ong, M.K.; Forney, C.F.; Alderson, P.G.; Ali, A. Postharvest profile of a Solo variety ‘Frangi’during ripening at ambient temperature. Sci. Hortic. 2013, 160, 12–19. [Google Scholar] [CrossRef]
- Singh, V.; Jawandha, S.K.; Gill, P.P.S.; Gill, M.S. Suppression of fruit softening and extension of shelf life of pear by putrescine application. Sci. Hortic. 2019, 256, 108623. [Google Scholar] [CrossRef]
- Thumdee, S.; Manenoi, A.; Chen, N.J.; Paull, R.E. Papaya Fruit Softening: Role of Hydrolases. Trop. Plant Biol. 2010, 3, 98–109. [Google Scholar] [CrossRef]
- Chiriboga, M.A.; Saladié, M.; Bordonaba, J.G.; Recasens, I.; Garcia-Mas, J.; Larrigaudière, C. Effect of cold storage and 1-MCP treatment on ethylene perception, signalling and synthesis: Influence on the development of the ever green behaviour in ‘Conference’ pears. Postharvest Biol. Technol. 2013, 86, 212–220. [Google Scholar] [CrossRef]
- Nunes, C.; Santos, C.; Pinto, G.; Silva, S.; Lopes-da-Silva, J.A.; Saraiva, J.A.; Coimbra, M.A. Effects of ripening on microstructure and texture of ‘‘Ameixad Elvas” candied plums. Food Chem. 2009, 115, 1094–1101. [Google Scholar] [CrossRef]
- Li, W.; Shao, Y.Z.; Chen, W.X.; Jia, W.J. The Effects of Harvest Maturity on Storage Quality and Sucrose-Metabolizing Enzymes During Banana Ripening. Food Bioprocess Technol. 2011, 4, 1273–1280. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Xiao, X.; Cao, S.F.; Chen, W.; Yang, Z.F.; Shi, L.Y. Effect of 1-MCP on the regulation processes involved in ascorbate metabolism in kiwifruit. Postharvest Biol. Technol. 2021, 179, 111563. [Google Scholar] [CrossRef]
- Song, Z.Y.; Qin, J.J.; Yao, Y.L.; Lai, X.H.; Zheng, W.; Chen, Y.X.; Zhu, X.Y.; Li, X.P. A transcriptomic analysis unravels key factors in the regulation of stay-green disorder in peel of banana fruit (Fenjiao) caused by treatment with 1-MCP. Postharvest Biol. Technol. 2020, 168, 111290. [Google Scholar] [CrossRef]
- Shi, L.Q.; Liu, Q.; Qiao, Q.H.; Zhu, Y.L.; Huang, W.; Wang, X.M.; Ren, Z.X. Exploring the effects of pectate and pectate lyase on the fruit softening and transcription profiling of Solanum lycopersicum. Food Control 2022, 133, 108636. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, Y.; Li, W.; Shao, Y. Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage. Horticulturae 2023, 9, 126. https://doi.org/10.3390/horticulturae9020126
Li R, Wang Y, Li W, Shao Y. Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage. Horticulturae. 2023; 9(2):126. https://doi.org/10.3390/horticulturae9020126
Chicago/Turabian StyleLi, Rui, Ying Wang, Wen Li, and Yuanzhi Shao. 2023. "Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage" Horticulturae 9, no. 2: 126. https://doi.org/10.3390/horticulturae9020126
APA StyleLi, R., Wang, Y., Li, W., & Shao, Y. (2023). Comparative Analyses of Ripening, Texture Properties and Cell Wall Composition in Three Tropical Fruits Treated with 1-Methylcyclopropene during Cold Storage. Horticulturae, 9(2), 126. https://doi.org/10.3390/horticulturae9020126





