Balancing Hormones and Gene Expressions for Rooting Success: Lovastatin Unveils Cytokinin Inhibition in Malus prunifolia var. ringo Apple Stem Cuttings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Determination of Endogenous Hormone Content
2.3. RNA Extraction, cDNA Synthesis, and RT-qPCR Expression Analysis
2.4. Statistical Analysis
3. Results
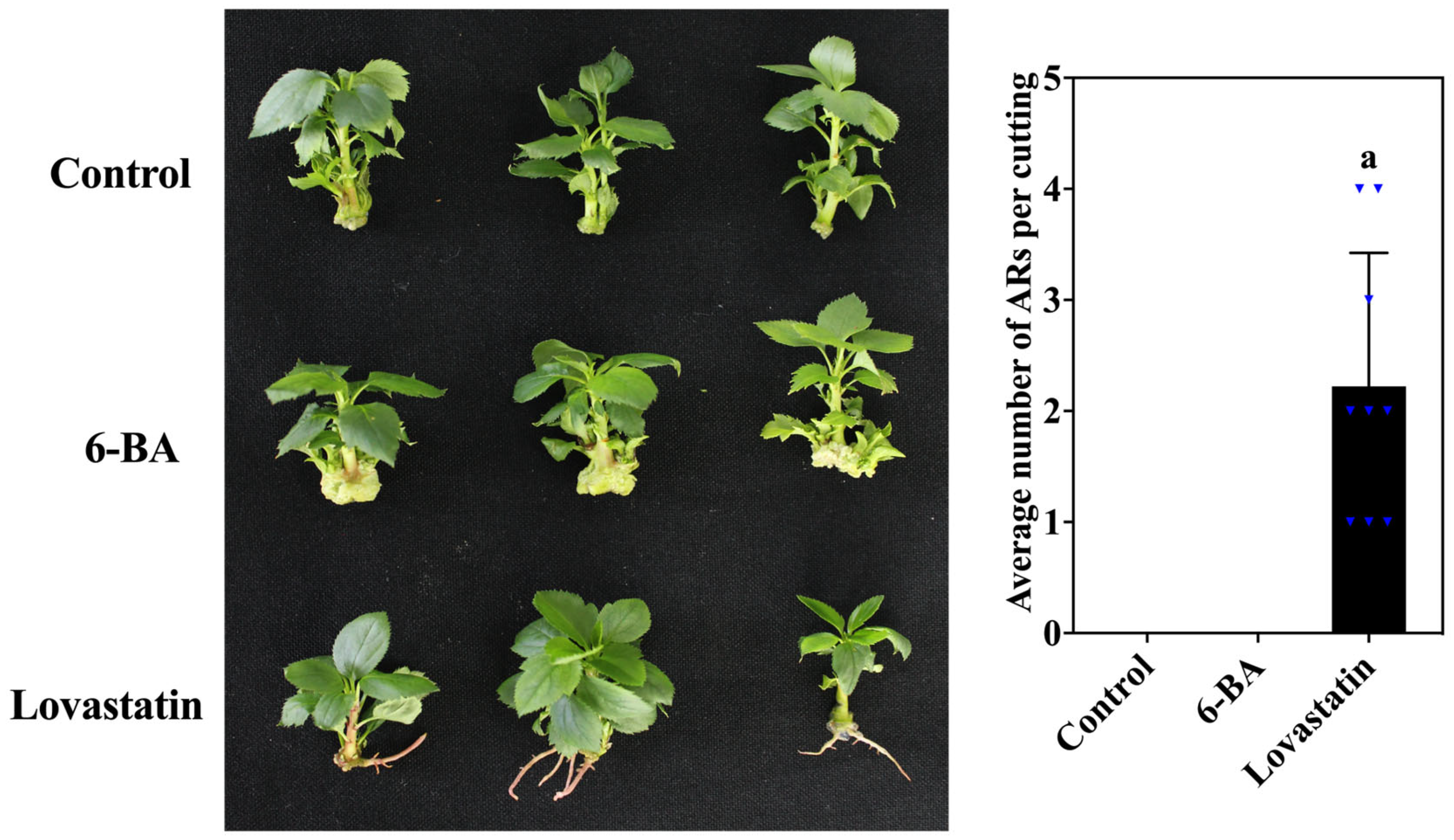
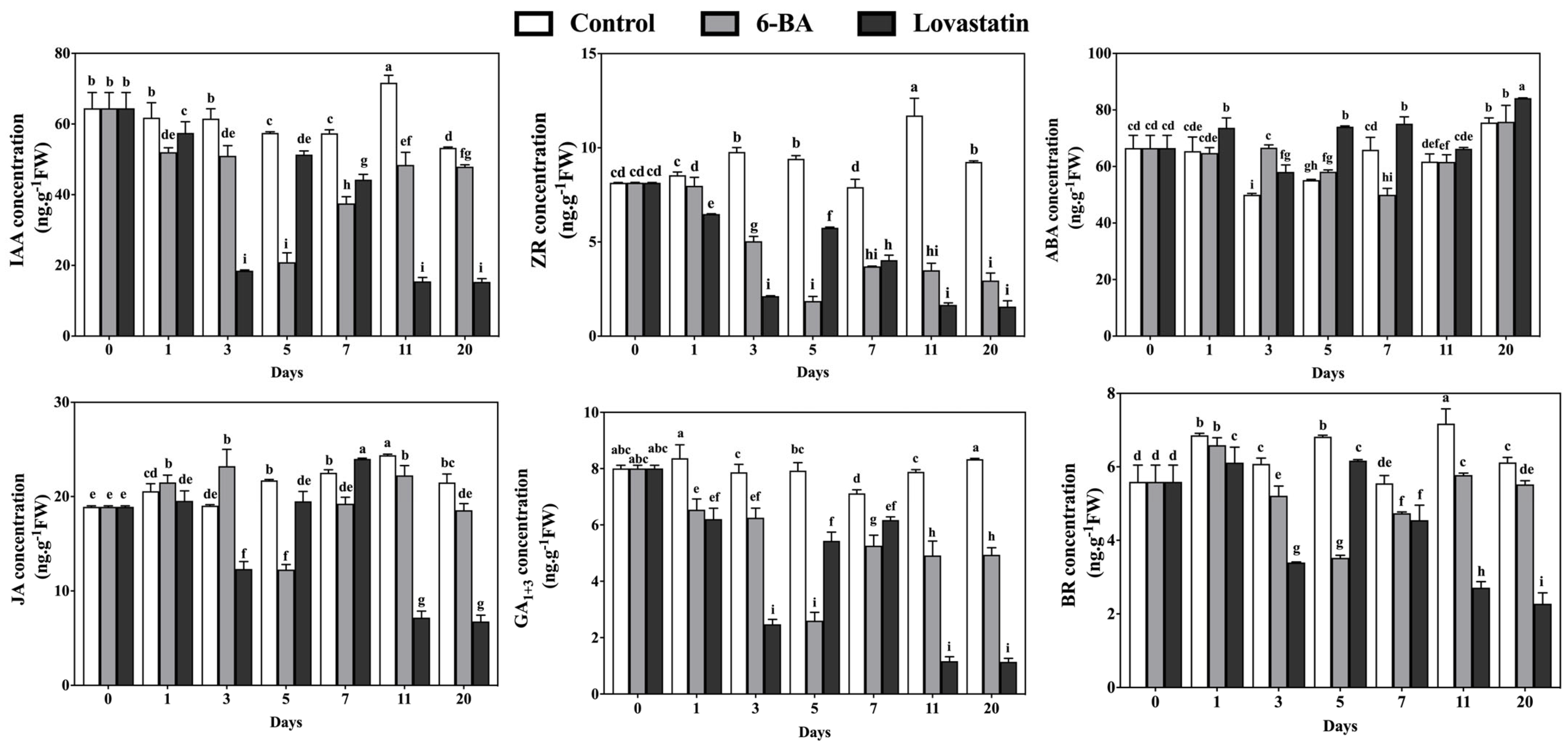
3.1. Effect of 6-BA and Lovastatin Treatments on Adventitious Rooting and Endogenous Hormone Content
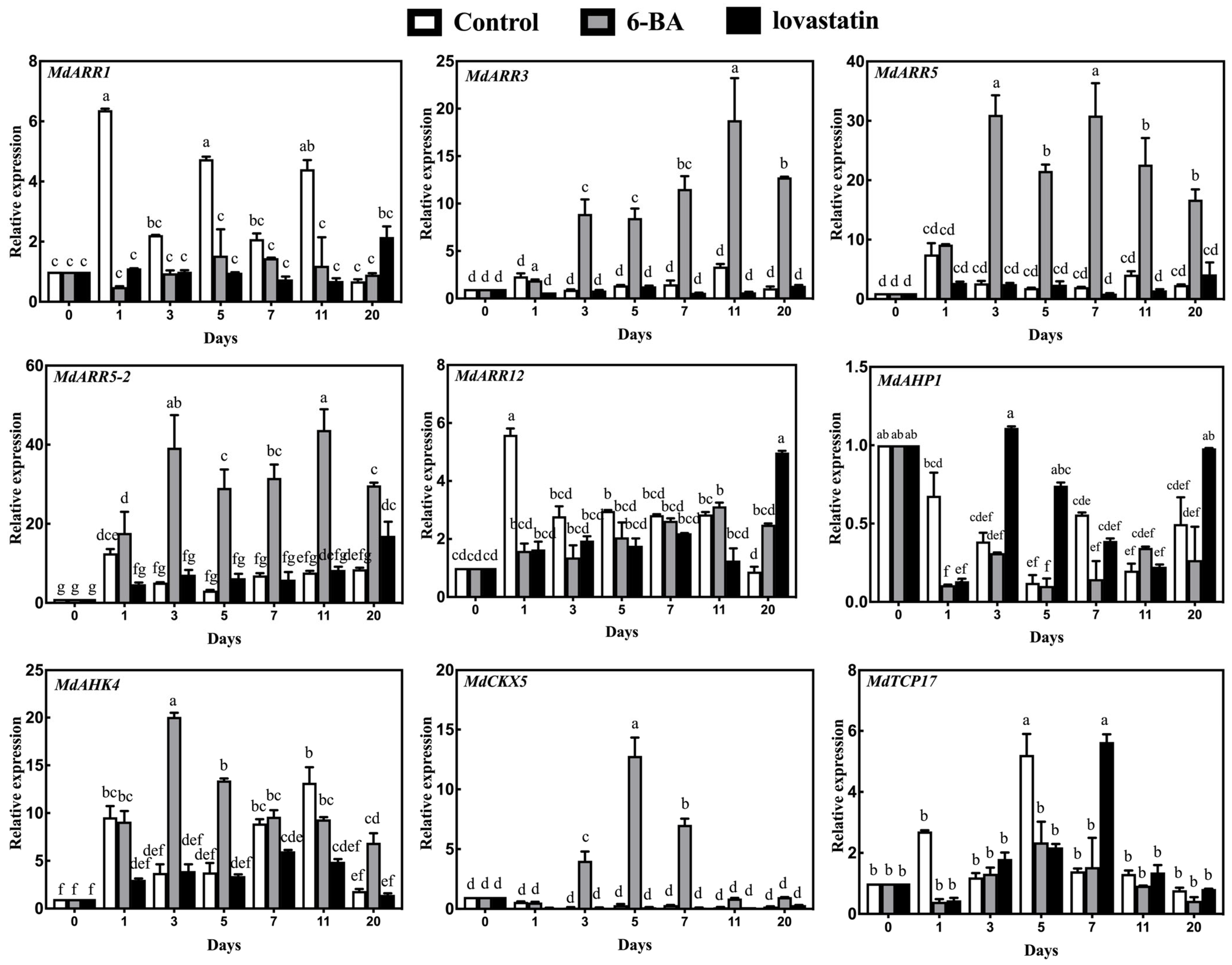
3.2. RT-qPCR Expression Analysis of CK Signal Transduction-Related Genes
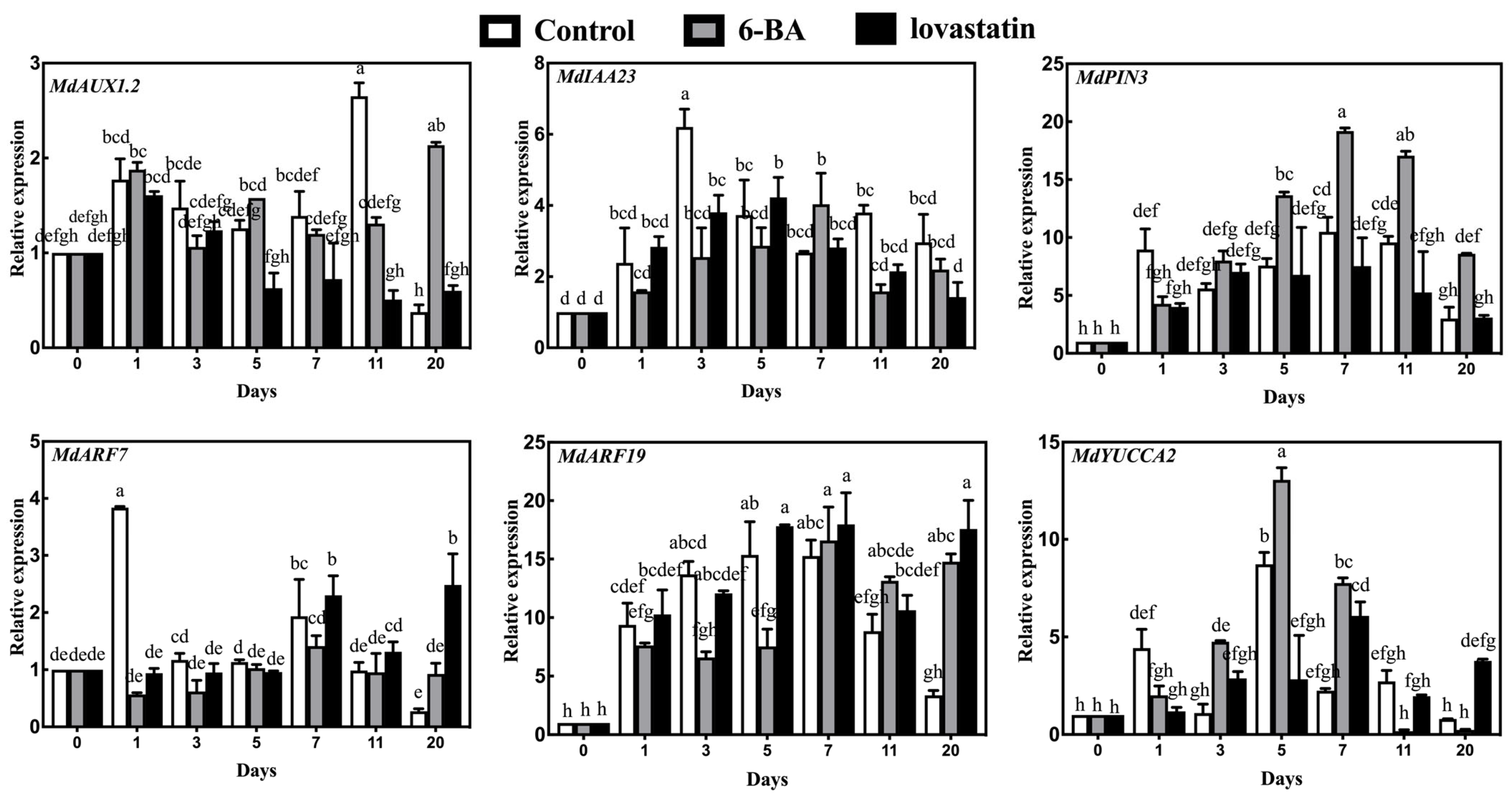
3.3. RT-qPCR Expression Analysis of IAA-Related Genes
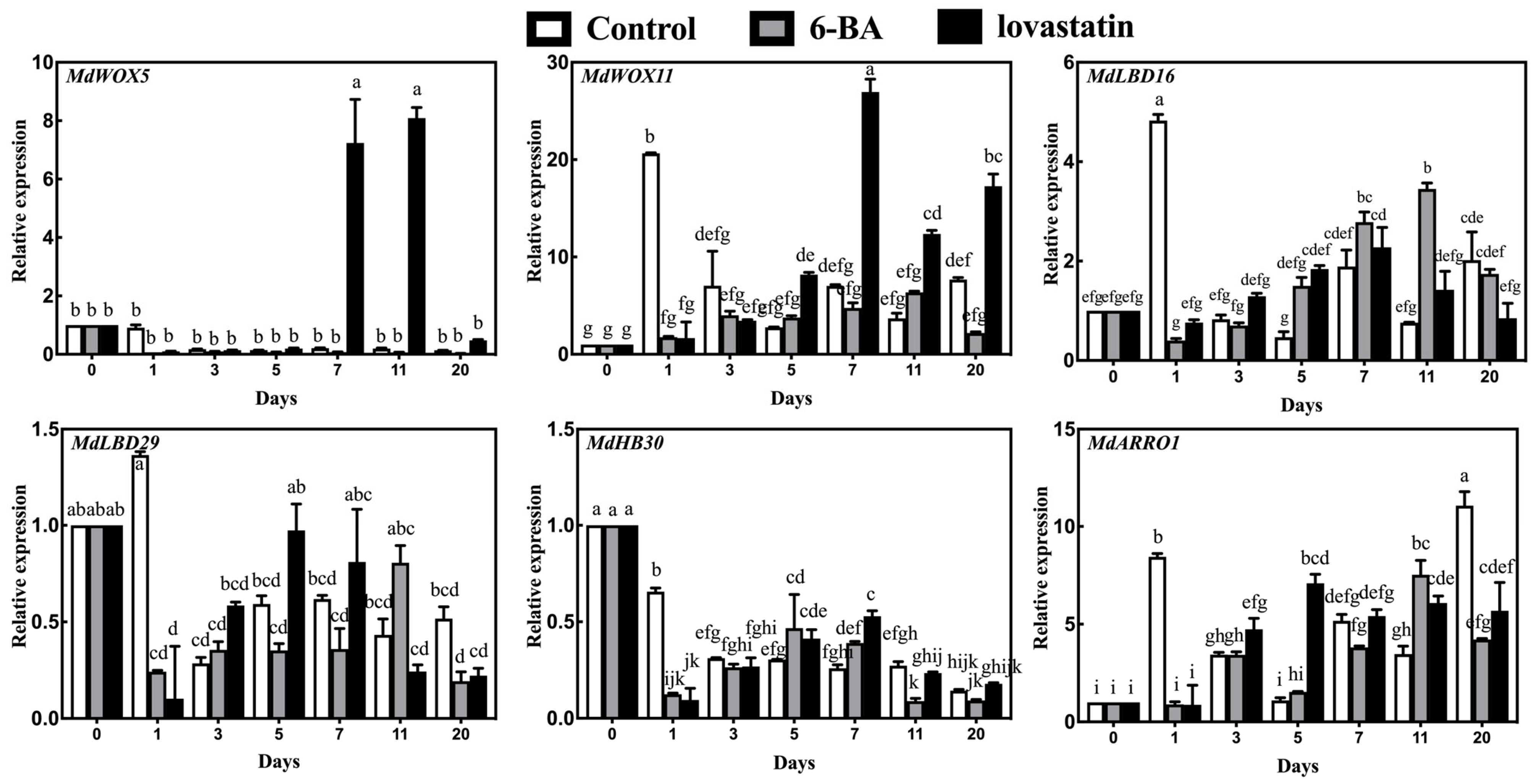
3.4. RT-qPCR Analysis of Root Development-Related Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayat, F.; Qiu, C.; Xu, X.; Wang, Y.; Wu, T.; Zhang, X.; Nawaz, M.A.; Han, Z. Rootstocks influence morphological and biochemical changes in young ‘Red Fuji’apple plants. Int. J. Agric. Biol. 2019, 21, 1097–1105. [Google Scholar]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.; Shi, T.; Gao, Z. An insight into dwarfing mechanism: Contribution of scion-rootstock interactions toward fruit crop improvement. Fruit Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Tahir, M.M.; Tong, L.; Fan, L.; Liu, Z.; Li, S.; Zhang, X.; Li, K.; Shao, Y.; Zhang, D.; Mao, J. Insights into the complicated networks contribute to adventitious rooting in transgenic MdWOX11 apple microshoots under nitrate treatments. Plant Cell Environ. 2022, 45, 3134–3156. [Google Scholar] [CrossRef]
- Della Rovere, F.; Fattorini, L.; D’angeli, S.; Veloccia, A.; Falasca, G.; Altamura, M. Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis. Ann. Bot. 2013, 112, 1395–1407. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, D.; Meng, Y.; Li, K.; Wang, H.; Han, M. Inhibition of adventitious root development in apple rootstocks by cytokinin is based on its suppression of adventitious root primordia formation. Physiol. Plant. 2019, 166, 663–676. [Google Scholar] [CrossRef]
- De Klerk, G.-J.; Arnholdt-Schmitt, B.; Lieberei, R.; Neumann, K.-H. Regeneration of roots, shoots and embryos: Physiological, biochemical and molecular aspects. Biol. Plant. 1997, 39, 53–66. [Google Scholar] [CrossRef]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front. Plant Sci. 2016, 7, 381. [Google Scholar] [CrossRef]
- Verstraeten, I.; Schotte, S.; Geelen, D. Hypocotyl adventitious root organogenesis differs from lateral root development. Front. Plant Sci. 2014, 5, 495. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, X.; Pan, R. Relationship between adventitious root formation and plant hormones. Plant Physiol. Commun. 2005, 41, 133–142. [Google Scholar]
- Ramírez-Carvajal, G.A.; Morse, A.M.; Dervinis, C.; Davis, J.M. The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol. 2009, 150, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.A.; Rasmussen, A.; Traini, R.; Voß, U.; Sturrock, C.; Mooney, S.J.; Wells, D.M.; Bennett, M.J. Branching out in roots: Uncovering form, function, and regulation. Plant Physiol. 2014, 166, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.E.; Li, J.; Argueso, C.; Gonzalez, M.; Lee, E.; Lewis, M.W.; Maxwell, B.B.; Perdue, T.D.; Schaller, G.E.; Alonso, J.M. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 2006, 18, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.M.; Mao, J.; Li, S.; Li, K.; Liu, Y.; Shao, Y.; Zhang, D.; Zhang, X. Insights into factors controlling adventitious root formation in apples. Horticulturae 2022, 8, 276. [Google Scholar] [CrossRef]
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin–auxin crosstalk. Trends Plant Sci. 2009, 14, 557–562. [Google Scholar] [CrossRef]
- Chandler, J.W.; Werr, W. Cytokinin–auxin crosstalk in cell type specification. Trends Plant Sci. 2015, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, Y.; Feng, C.; Wei, Y.; Peng, X.; Guo, X.; Guo, X.; Zhai, Z.; Li, J.; Shen, X. Overexpression of MsGH3. 5 inhibits shoot and root development through the auxin and cytokinin pathways in apple plants. Plant J. 2020, 103, 166–183. [Google Scholar] [CrossRef]
- Legué, V.; Rigal, A.; Bhalerao, R.P. Adventitious root formation in tree species: Involvement of transcription factors. Physiol. Plant. 2014, 151, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, L. Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiol. 2016, 172, 2363–2373. [Google Scholar] [CrossRef]
- Wang, H.; Tahir, M.M.; Nawaz, M.A.; Mao, J.; Li, K.; Wei, Y.; Ma, D.; Lu, X.; Zhao, C.; Zhang, D. Spermidine application affects the adventitious root formation and root morphology of apple rootstock by altering the hormonal profile and regulating the gene expression pattern. Sci. Hortic. 2020, 266, 109310. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Guan, L.; Murphy, A.S.; Peer, W.A.; Gan, L.; Li, Y.; Cheng, Z.-M. Physiological and molecular regulation of adventitious root formation. Crit. Rev. Plant Sci. 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Li, S.-W.; Shi, R.-F.; Leng, Y. De novo characterization of the mung bean transcriptome and transcriptomic analysis of adventitious rooting in seedlings using RNA-Seq. PLoS ONE 2015, 10, e0132969. [Google Scholar] [CrossRef] [PubMed]
- Kuroha, T.; Kato, H.; Asami, T.; Yoshida, S.; Kamada, H.; Satoh, S. A trans-zeatin riboside in root xylem sap negatively regulates adventitious root formation on cucumber hypocotyls. J. Exp. Bot. 2002, 53, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, E.; Di Mambro, R.; Dello Ioio, R.; Costantino, P.; Sabatini, S. Acidic cell elongation drives cell differentiation in the Arabidopsis root. EMBO J. 2018, 37, e99134. [Google Scholar] [CrossRef] [PubMed]
- Mähönen, A.P.; Higuchi, M.; Törmäkangas, K.; Miyawaki, K.; Pischke, M.S.; Sussman, M.R.; Helariutta, Y.; Kakimoto, T. Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr. Biol. 2006, 16, 1116–1122. [Google Scholar] [CrossRef]
- Ioio, R.D.; Linhares, F.S.; Scacchi, E.; Casamitjana-Martinez, E.; Heidstra, R.; Costantino, P.; Sabatini, S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007, 17, 678–682. [Google Scholar] [CrossRef]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef]
- Marchant, A.; Bhalerao, R.; Casimiro, I.; Eklöf, J.; Casero, P.J.; Bennett, M.; Sandberg, G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 2002, 14, 589–597. [Google Scholar] [CrossRef]
- Lee, H.W.; Cho, C.; Kim, J. Lateral organ boundaries domain16 and 18 act downstream of the AUXIN1 and LIKE-AUXIN3 auxin influx carriers to control lateral root development in Arabidopsis. Plant Physiol. 2015, 168, 1792–1806. [Google Scholar] [CrossRef]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef]
- Wilmoth, J.C.; Wang, S.; Tiwari, S.B.; Joshi, A.D.; Hagen, G.; Guilfoyle, T.J.; Alonso, J.M.; Ecker, J.R.; Reed, J.W. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005, 43, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Niu, C.; Li, K.; Fan, L.; Liu, Z.; Li, S.; Ma, D.; Tahir, M.M.; Xing, L.; Zhao, C. Cytokinin-responsive MdTCP17 interacts with MdWOX11 to repress adventitious root primordium formation in apple rootstocks. Plant Cell 2023, 35, 1202–1221. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Tahir, M.M.; Xie, Z.; Wei, P.; Yu, J.; Liu, H.; He, Y.; Ren, X.; Ma, Y.; Mao, J. Balancing Hormones and Gene Expressions for Rooting Success: Lovastatin Unveils Cytokinin Inhibition in Malus prunifolia var. ringo Apple Stem Cuttings. Horticulturae 2023, 9, 1341. https://doi.org/10.3390/horticulturae9121341
Sun S, Tahir MM, Xie Z, Wei P, Yu J, Liu H, He Y, Ren X, Ma Y, Mao J. Balancing Hormones and Gene Expressions for Rooting Success: Lovastatin Unveils Cytokinin Inhibition in Malus prunifolia var. ringo Apple Stem Cuttings. Horticulturae. 2023; 9(12):1341. https://doi.org/10.3390/horticulturae9121341
Chicago/Turabian StyleSun, Sinuo, Muhammad Mobeen Tahir, Zushu Xie, Pengyan Wei, Jianing Yu, Hangkong Liu, Yinnan He, Xiaoying Ren, Yuanyuan Ma, and Jiangping Mao. 2023. "Balancing Hormones and Gene Expressions for Rooting Success: Lovastatin Unveils Cytokinin Inhibition in Malus prunifolia var. ringo Apple Stem Cuttings" Horticulturae 9, no. 12: 1341. https://doi.org/10.3390/horticulturae9121341
APA StyleSun, S., Tahir, M. M., Xie, Z., Wei, P., Yu, J., Liu, H., He, Y., Ren, X., Ma, Y., & Mao, J. (2023). Balancing Hormones and Gene Expressions for Rooting Success: Lovastatin Unveils Cytokinin Inhibition in Malus prunifolia var. ringo Apple Stem Cuttings. Horticulturae, 9(12), 1341. https://doi.org/10.3390/horticulturae9121341




