1. Introduction
Cannabis (
Cannabis sativa L.) is one of the oldest annual herbal crops known, whose cultivation originated in Asia and spread to Europe, the Middle East, and America [
1]. It is cultivated for fiber, seed oil, and as a natural medicine herb. The medical potential of cannabis lies in the complex chemical profile, which includes hundreds of secondary metabolites, including cannabinoids, terpenes, and flavonoids. The main known cannabinoids are tetrahydrocannabinol (THC) and cannabidiol (CBD), although cannabigerol (CBG) has been gaining emphasis in recent years. Anti-inflammatory, appetite-stimulant, analgesic, wound-healing, psychotropic, and antiemetic properties are attributed to THC, while CBD has antipsychotic, relaxing, anticonvulsant, and other positive side effects [
2,
3]. CBG serves as the precursor to the most prevalent phytocannabinoids. Research highlights antibacterial, antioxidant, neuroprotective, and anti-inflammatory properties and potential benefits in treating neurological disorders and glaucoma [
3,
4,
5]. Cannabis is listed in Schedule I of the 1961 United Nations Single Convention on Narcotic Drugs, and its cultivation has been banned within the EU. In Europe, only hemp genotypes that comply with the 0.3% THC threshold set by the EU legislation can be cultivated. Since then, hemp breeders have focused their efforts on the increase in seed and fiber yield and in THC reduction without paying particular attention to CBD content, which is low [
2]. Hemp industrial applications are directed towards fiber and seed uses, including bio composite, textile, construction, paper production, bio-fuel, functional food, and animal feed; flowers are not processed [
6]. Indeed, since 1990, the exploitation and marketing of the
C. sativa L. flower has been illegal in the EC. In recent years, there has been increasing interest in the production and marketing of hemp flower extracts, including CBD, due to potential uses in cosmetics, health products, and food. In November 2020, the Court of Justice of the European Union ruled that the marketing of legally produced CBD was authorized by EU law [
6], allowing farmers to cultivate hemp for flower exploitation. Operational costs and yields of hemp flower cultivation for cannabinoid extraction have not yet been investigated, neither in field cultivation nor in soilless cropping systems. Drug-type
C. sativas shows distinct agronomic characteristics in terms of apical height and cannabinoid content and is mainly cultivated using controlled soilless cultivation techniques. Therefore, it makes sense to test this technique for hemp type [
7].
Hemp, grown predominantly for industrial purposes such as fiber and seed production, is typically cultivated outdoors in open fields. This method takes advantage of natural light and larger planting densities to optimize stem elongation and fiber yield. Agronomic practices such as nitrogen fertilization are essential for maximizing biomass, fiber strength, plant growth, and inflorescence size, although excessive nitrogen can compromise fiber quality [
8,
9,
10,
11,
12]. Conversely, the cultivation of drug-type cannabis, particularly for medical purposes, is generally conducted in controlled environments, such as greenhouses or indoor facilities, which use soilless cultivation systems such as nutrient film technique, pot systems, and rockwool with drip irrigation [
13,
14,
15]. These settings enable the precise control of factors like light intensity, temperature, humidity, and nutrient delivery, ensuring that the final product meets high-quality standards [
16]. Recent research indicates that factors such as mineral nutrition, root-zone management, planting density, and light characteristics are important for determining the yield and potency of drug-type cannabis [
12,
17,
18,
19,
20,
21,
22,
23]. However, hemp, cultivated for fiber production, has distinct genetic, morphological, and nutritional needs compared to drug-type cannabis, making it challenging to apply findings from one to the other [
12,
20].
The use of growth regulators that stimulate flowering, promote ripening, and control shoot apex could be useful in the case of growing hemp in a soilless cultivation system. Ethephon (R1150, 2-chloroethylphosphonic acid) is a growth regulator used for several years on crops such as tomatoes, beetroot, and coffee to stimulate fruit development and ripening. When broken down, this molecule is transformed into a volatile phytohormone ethylene. The ethylene signaling system is an important component of the innate immune system of plants, influencing both its morphology and physiology [
24]. Growth studies of etiolated pea plants revealed elongation inhibition, increased diameter, and horizontal shoot growth [
25]. This molecule also appears to have a significant inhibitory effect on the height of the wheat crop [
26]. Ethylene also has an inhibitory effect on root growth, with this effect occurring within 20 min of application and growth resuming within 20 min of removal [
27]. In some plants, such as several varieties of cucurbits, the induction of female flowers by ethylene has been demonstrated [
27]. In cannabis cultivation, the ability to feminize male plants is particularly advantageous for improving yields in both dioecious and monoecious cannabis varieties. Ethephon application has been shown to induce female flowers on male plants, reducing reliance on expensive feminized seeds and mitigating unwanted pollination, which negatively impacts cannabinoid-rich resin production in female inflorescences [
28,
29,
30,
31,
32]. Studies have further highlighted the potential of ethephon to enhance cannabinoid content during the flowering phase. For instance, ethephon sprays increased THC, CBD, and α-tocopherol levels, with specific concentrations influencing cannabinoid profiles: 1 μM and 100 μM increased THC, while CBD levels peaked at 10 μM but decreased at 5 μM and 100 μM [
33,
34]. Another study found that applying ethephon 7–14 days after floral primordia formation induced female flowers on male plants at a concentration of 500 mg/L [
32]. However, a study concluded that there was no evidence that 75 mM ethephon increased THC concentration or the ratio of THC to CBD at harvest [
35]. The conclusions are rather mixed, but the effects are potentially relevant for large-scale cultivation. A recent study examined the impact of ethephon sprays during the flowering period on cannabinoid production in both monoecious and dioecious hemp varieties [
36]. A specific foliar application of ethephon enhanced CBD production in Félina 32. In the dioecious variety Kompolty, a positive linear correlation was observed between increasing foliar concentrations of ethephon and CBD content. However, ethephon application in Santhica 27 resulted in a reduction in CBG levels [
36]. Finally, ethephon broadly influences the physiology and biochemistry of cannabis, while its availability and proven success in a variety of crops make it a cost-effective option for improving cannabis cultivation.
This study compares open-field cultivation, which is traditionally used for hemp production (excluding flowers), with greenhouse-based cultivation specifically focused on flower production. While open-field cultivation is primarily used for fiber, seed, and straw, greenhouse cultivation offers more controlled conditions that may enhance flower yield and cannabinoid content. In the greenhouse, a growth regulator, ethephon, is applied to test its effect on plant size and cannabinoid levels. The study focuses on two varieties: Félina 32, known for its CBD content, and Santhica 27, valued for its CBG content. The objective is to assess the potential of incorporating flower production into open-field cultivation, in contrast to a dedicated greenhouse system, and to evaluate differences in yield, agronomic characteristics, and the economic feasibility of both systems for the flower market and its untapped potential in hemp production.
2. Materials and Methods
2.1. Chemicals and Substrates
Potassium hydroxide and sulfuric acid were purchased from VWR International (Leuven, Belgium). Rockwool cubes (25 mm × 25 mm × 40 mm) for the germination of seeds were purchased from Grodan (Roermond, The Netherlands). Cultivation substrates were Grodan Delta Rockwool® blocks (75 mm × 75 mm) placed on Grodan rockwall Gro-Lab® (100 mm × 150 mm × 1000 mm). Hydroponic nutrient solutions were ready-to-use solutions designed for hydroponic culture from Plagron (Weert, The Netherlands) with the following characteristics:
- -
Hydro A: NPK fertilizer (3-0-1). With a total of 3.2% total nitrogen (N), including 3.2% nitric nitrogen (NO3) and 1.3% water-soluble potassium oxide (K2O), and 5.9% calcium oxide (CaO).
- -
Hydro B: NPK fertilizer solution (1-3-6). With a total of 1.2% total nitrogen (N), including 1.2% nitric nitrogen (NO3), 3.2% water-soluble phosphorus pentoxide (P2O5), 5.8% water-soluble potassium oxide (K2O), and 1.4% magnesium oxide (MgO).
2.2. Plant Material
Two monoecious hemp genotypes, one with a cannabidiolic chemotype (Félina 32) and one with a cannabigerolic chemotype (Santhica 27), were evaluated in this study. All genotypes were approved by the European Union for commercial use and certified with a THC content below 0.2%. The seeds were provided by Hemp-it (Beaufort-en-Anjou, France) under the commercial name FELINA 32 and SANTHICA 27.
2.3. Greenhouse Hydroponic Soilless Cultivation Trial and Ethylene Treatment
The experimental trial was carried out at the “SERR’URE” rooftop half-chapel bioclimatic greenhouse on the TERRA building from the University of Liège–Gembloux Agro-Bio Tech located in Gembloux (Walloon Region, Belgium; lat. 50.562643° N, long. 4.697433° E; alt. 166 m above sea level). The greenhouse has an area of 198 m
2 and is divided into three compartments dedicated to research, teaching, and demonstration (
Figure 1). The trial was conducted in the research compartment (132 m
2) consisting of measurements 5.5 m wide, 24 m long, and 4.2 and 5.6 m high at the eaves and ridge, respectively, from 29 April 2022 to 19 August (Santhica 27) and 31 August (Félina 32) 2022. Fourteen days after sowing in rockwooll, the seedlings were transplanted into hydroponic Nutrient Film Technique Goponic
® gutters (Agri Logic System, Normandie, New Caledonia, France) (
Figure 2). The nutrient film technique (NFT) involves exposing plant roots to a thin layer of nutrient-enriched water flowing through horizontal gutters, installed with a slope of 1–2% to ensure proper water flow. By keeping the stream shallow, the roots benefit from an increased air exchange surface. This method promotes optimal oxygen availability to the roots, ultimately supporting higher vegetable yields [
37].
The gutters were arranged in groups of 5; the 5-gutter table measured 3 m in length and 1.23 m in width, with 3 to 4 plants arranged per gutter, i.e., 14 plants per culture table, 7 plants from Félina 32, and 7 plants from Santhica 27 with a density of 14 plants per 3.69 m
2 (
Figure 2). Each table was connected to a 100 L basin containing the nutrient solution. The regulation of the parameters of a table was performed automatically via a control computer that adjusted the fertilizers and the pH of the nutrient solution (H2Hydroponics, Nigran, Spain). A treatment based on ethylene supplementation in nutrient solution was applied per table. In addition to the control without ethylene (control), three ethylene doses were studied, D1 (30 µL L
−1), D2 (15 µL L
−1), and D3 (7.5 µL L
−1), for a total of 4 tables. The ethylene dose was added on day 21 to the nutrient solution and kept for 2 weeks in the solution; afterward, the nutrient solution was renewed. The pH was maintained between 5.5 and 6.5, and the EC was 1000 µS/cm during the first 15 days then 1500 µS/cm during the following 15 days and 2000 µS/cm until the harvest. The greenhouse growing conditions (temperature and relative humidity) are described in
Figure 3.
2.4. Field Cultivation
The experimental field trial was carried out at the experimental farm of the University of Liège–Gembloux Agro-Bio Tech located in Gembloux (Walloon Region, Belgium; lat. 50.565473° N, long. 4.704183° E; alt. 162 m above sea level). The plot was divided into sub-plots. There were a total of 24 cultivated sub-plots, 12 for each cultivar studied (Félina 32 and Santhica 27). Each sub-plot corresponds to a cultivated area of 14.76 m
2. The surrounding sub-plots were considered borders and therefore not considered in the study, leaving 3 central sub-plots per cultivar (
Figure 4). The previous crop was ray-grass (
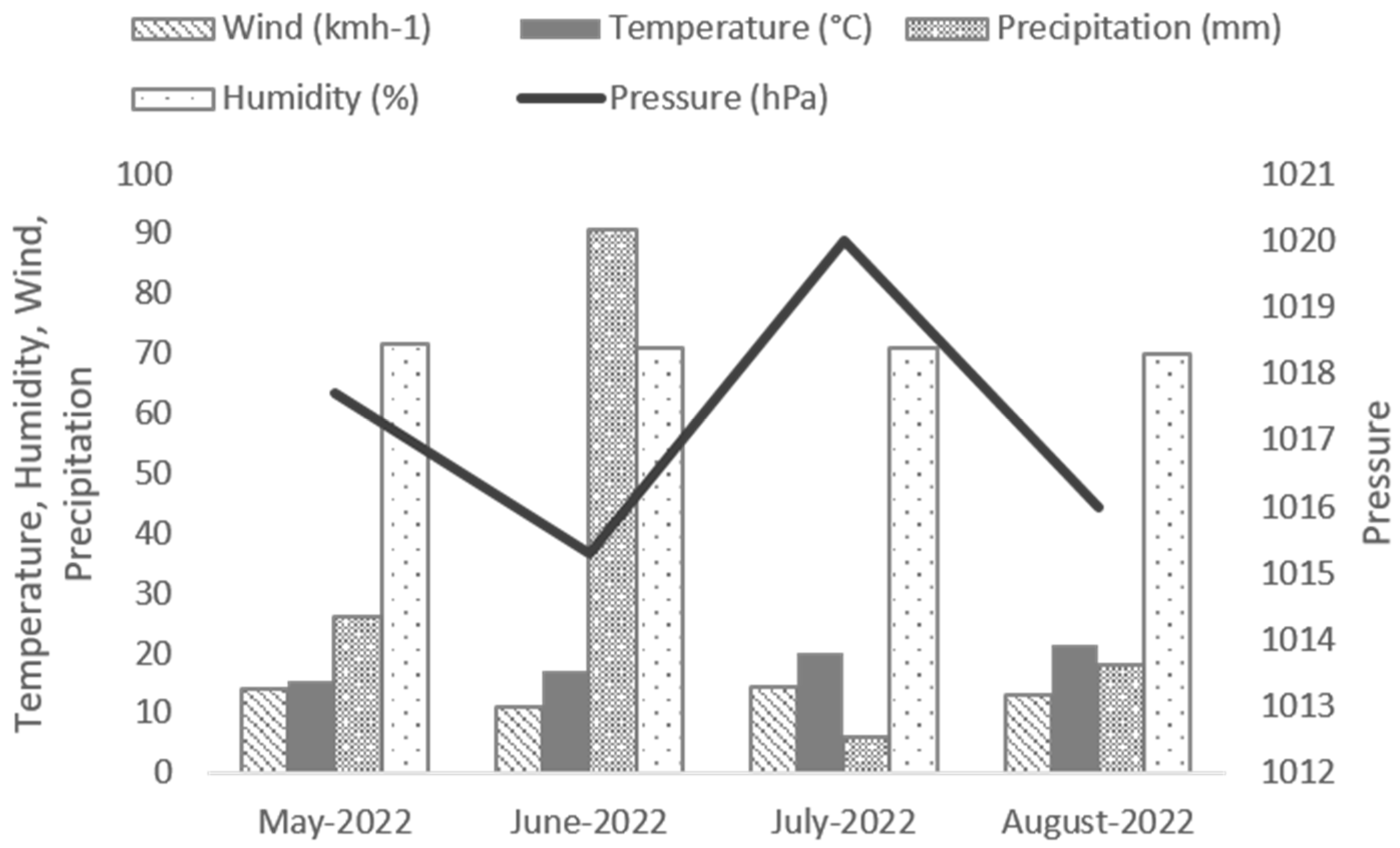
Lolium spp.) in 2020 and 2021. Prior to sowing, the seed bed was prepared using a rotary harrow to a depth of 8 cm. The sowing was carried out on 10 May 2022 with a density of 40 kg/ha. Post-sowing fertilizer was carried out on 15 May 2022 (100 kg/ha nitrogen, N27% amonitrate). No other intervention was necessary during cultivation (watering, weeding, or treatment). The field growing conditions (temperature, relative humidity, wind, precipitation, and air pressure) are described in
Figure 5. Santhica 27 plants samples were harvested on August 16, and Félina 32 plants were harvested on September 05.
2.5. Plant Growth Parameters
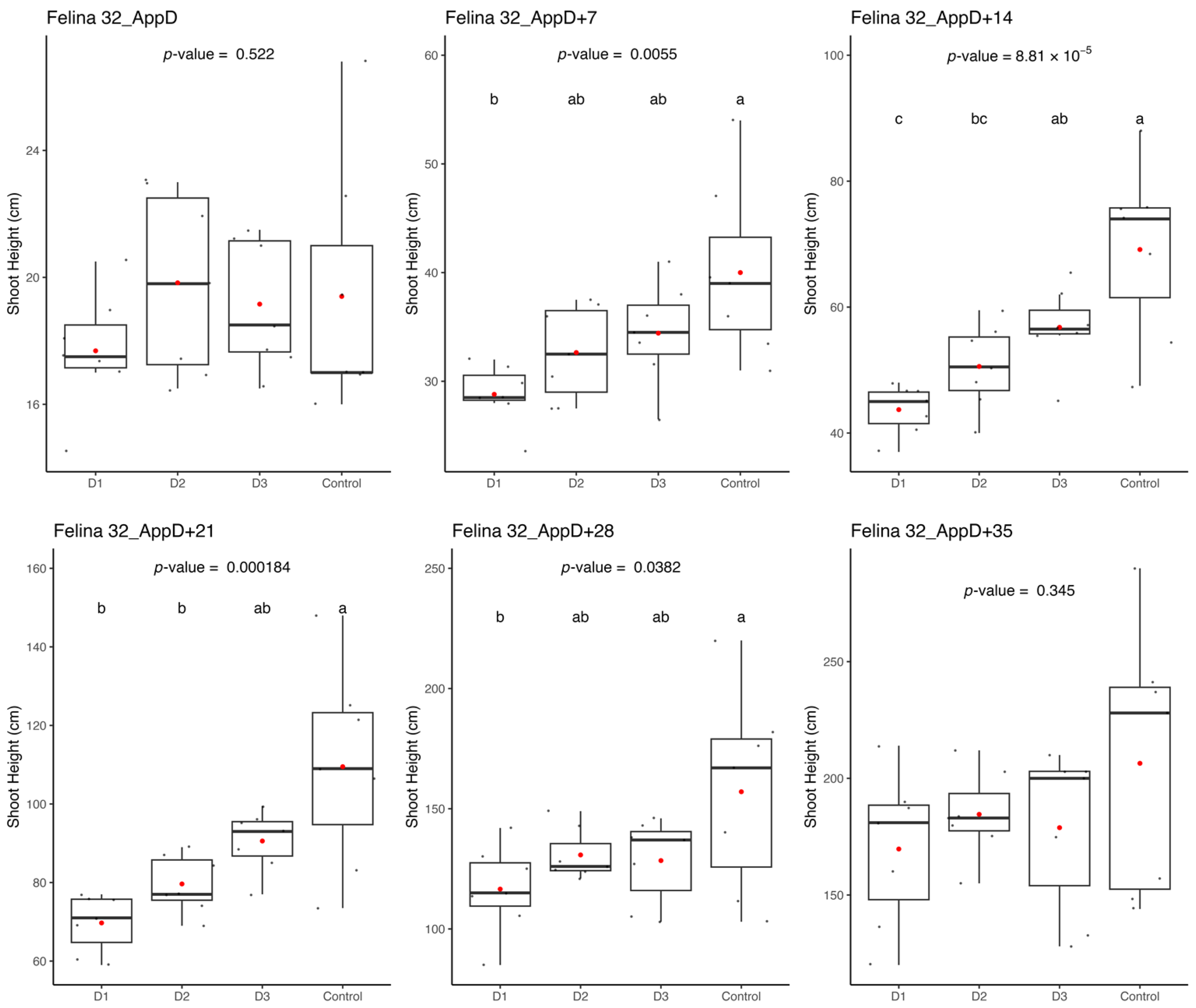
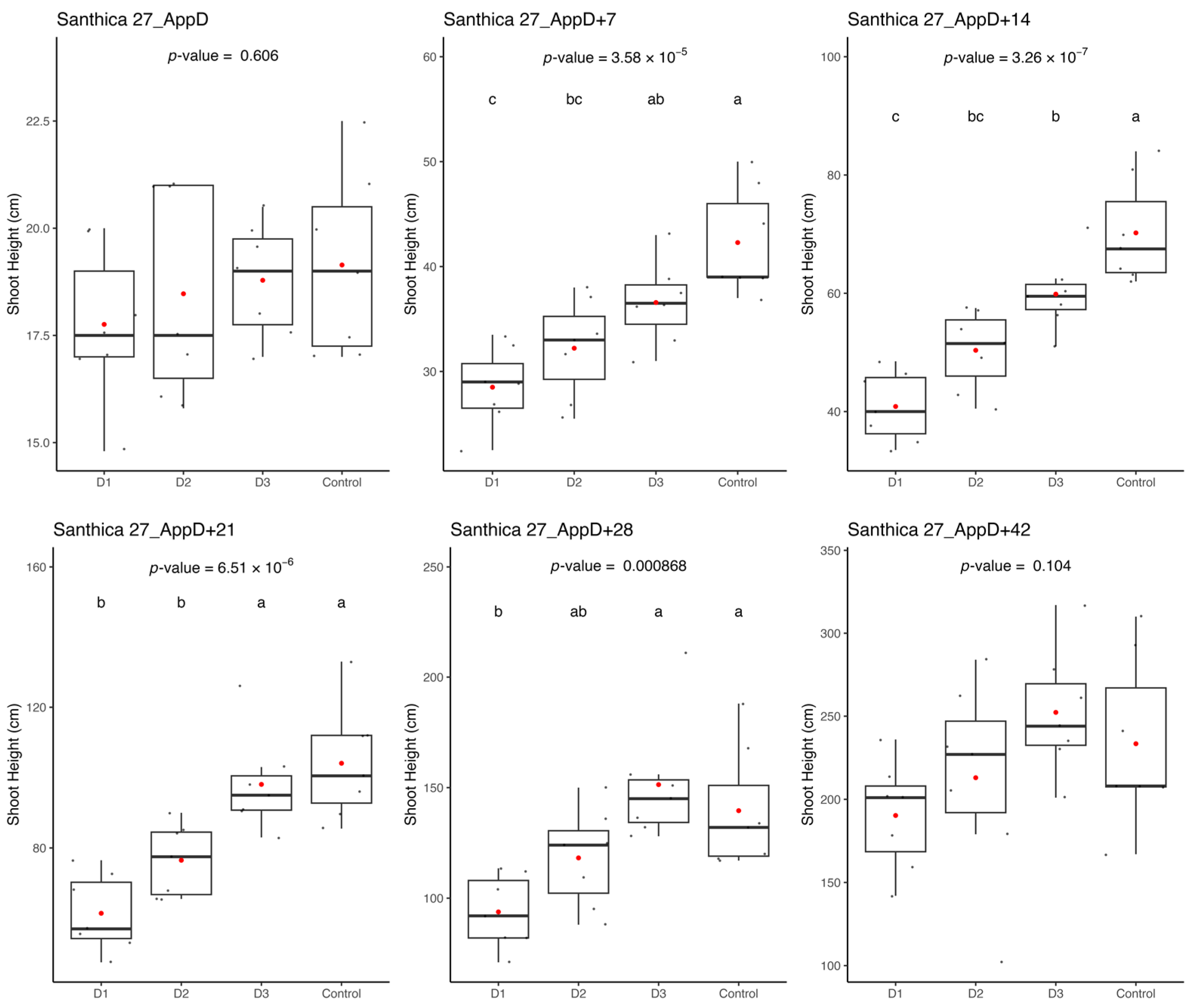
In the greenhouse, at the harvesting date, each plant was sampled. Subsequently, shoot height, stem circumference, the number of nodes, shoot biomass, and flower biomass were assessed. The plant internode was calculated by dividing height by the number of nodes. During ethylene supplementation, several parameters were measured once a week: height, number of nodes, and plant internode. Those parameters were measured from ethylene treatment until harvest.
In the field trial, the sub-plots were subdivided into randomly assessed quadrats of 0.25 m2. In each quadrat, 10 plants were randomly marked on day 41, and agronomic characteristics (shoot height, stem circumference, number of nodes, internode, shoot biomass, and flower biomass) of each plant were determined at the harvesting date.
2.6. Cannabinoid Determination
In the greenhouse trial, the flower biomass per plant was sampled for individual plant cannabinoid determination.
In a field trial, to obtain representative flower biomass and cannabinoid measurements, two quadrats of 0.25 m2 were randomly assessed per sub-plot for a total of 6 quadrats per cultivar and fully harvested for a total harvested surface of 1.5 m2 per cultivar.
To evaluate the impacts of the cropping itinerary on the production of cannabinoids by the plants, a quantification method was internally developed by Celabor (Herve, Belgium) in order to determine the contents of the cannabinoids. UPLC-MS analysis was performed using a Waters Acquity UPLC H-Class system coupled to a Waters PDA detector and a Waters Xevo TQ mass spectrometer (Waters Analytical Instruments Sdn. Bhd., Shah Alam Selangor, Malaysia). The analytical column was a Waters Acquity BEH Shield RP18 (100 mm × 2.1 mm, 1.7 µm) maintained at 40 °C. The mobile phases were ammonium formate buffer (2 mM, pH 2.4) (A) and acetonitrile (B). The flow rate was 0.5 mL/min, and the injection volume was 2 µL. The elution gradient was as follows: 0 min—45% A, 1.5 min—39%, 8 min—39% A, 9 min—0% A, 10 min—0% A, 12 min—45% A, and 15 min—45%. The mass spectrometer was operated in MRM mode with positive ionization for the neutral forms and negative ionization for the acidic forms of the cannabinoids. The capillary voltage was set at 2 kV, and the other parameters were optimized for each compound. The MRM transitions are listed in
Table 1.
Two types of samples were analyzed: hemp’s inflorescence and supercritical fluid extracts. For the inflorescence samples, 100 mg of dried material was extracted with 10 mL of ethanol 99.97%. For the extract samples, 25 mg of material was dissolved in 10 mL of ethanol. In both cases, ultrasound was used to optimize the solubilization step. The prepared samples were filtered through a PTFE filter and diluted to be quantified by an external calibration curve consisting of 10 points ranging from 1 ppb to 1000 ppb in ethanol. The standards used were 1000 ppm solutions from Sigma-Aldrich (St. Louis, MO, USA).
2.7. Supercritical Fluid Extraction of Cannabinoids
The optimal cannabinoid extraction procedure was carried out on the base of a selection work performed during the “Tropical Plant Factory” research program, focusing on the environmental impact of the solvents used and was internally developed by Celabor (Herve, Belgium).
Inflorescence hemp samples were ground to a particle size of 1 mm using a Retsch SM-300 mill (Verder Scientific, Haan, Germany). The ground material was then subjected to supercritical CO2 extraction using a laboratory-scale supercritical extractor (SFE Process) process extractor. The extraction conditions were as follows: 20 g of inflorescence were extracted at a pressure of 250 bar and a temperature of 50 °C. The CO2 flow rate was 50 g/min for 30 min. The total amount of CO2 used for the extraction was 1500 g, resulting in a CO2-to-material ratio of 75. The extracts were collected in glass vessels using acetone to ensure the best recovery of the extract. The acetone was then evaporated in an oven at 45 °C overnight to remove any trace of it and thus calculate the extraction yield.
2.8. Operational Cost Calculation
Throughout the cultivation period in the field and in the greenhouse, the volumes of inputs used for production (water, fertilizer, acid, and base) as well as the human labor time in several categories (sowing, planting, greenhouse maintenance, plant maintenance, irrigation, processing, data encoding, harvesting, post-harvest, laboratory analysis, and field actions) were recorded. With the balance of this information, the human labor time necessary for the 2 cropping itineraries was calculated. By reducing this information to the cost of an employee and the production volumes, the operational cost price was assessed.
2.9. Statistical Analysis
The first objective was to study the effect of the ethylene treatment in terms of agronomic characteristics (shoot height, number of nodes, internode, stem circumference, shoot biomass, and flower biomass). Therefore, the plants were arranged according to a factorial design with 7 replicates in which the ethylene treatments, i.e., one control without ethylene supplementation (Control) vs. three ethylene supplementations (D1, D2, and D3), and two hemp cultivars (Félina 32 and Santhica 27) were considered factors. Prior to this analysis, the homogeneity between the gutters per cultivar (3 plants vs. 4 plants) in each treatment was checked. In order to characterize the ethylene physiological effect on the crop, one-way ANOVA tests were performed on agronomic characteristics (height, number of nodes, and internode) measured once a week from the ethylene treatment until harvest, and in which ethylene treatment was considered a factor (Control, D1, D2, and D3).
The second objective was to measure the agronomics characteristics in a traditional field system. In the field, the plants were arranged according to a factorial design with 10 replicates per sub-plot repeated three times in which the hemp cultivar (Félina 32 and Santhica 27) was considered as a factor. Prior to this analysis, the homogeneity between the three repetitions in each cultivar was checked.
The third objective was to assess the cannabinoid content per unit of surface in all cropping itineraries. For this, THC Total, CBD, CBDA, and CBD Total were measured in sample plants of Félina 32; and THC Total, CBG, CBGA, and CBG Total were measured in sample plants of Santhica 27. The cannabinoid content in each cultivar was analyzed in a factorial design where the cropping itinerary (GH, D1, D2, D3, and Field) was considered a factor. The factorial design consisted of a surface of 1.8 m2 per cropping itinerary in the greenhouse (GH, D1, D2, and D3) and a surface of 1.5 m2 in the field (Field). In the greenhouse, each surface unit corresponds to 7 replicates. In the field, each surface unit corresponds to 6 replicates of 0.25 m2, randomly distributed in 3 sub-plots (two per sub-plot). Prior to this analysis, the homogeneity between the six field replicates was checked.
All data were processed with the statistical data processing software R Studio version 2023.03.0 and validated with Minitab® 19.2020.1. The significance test of factors using ANOVA was combined with Student’s test with Holm corrections for multiple testing. The results were considered statistically significant when p-values were below 0.05. Cannabinoid data were analyzed using a one-way ANOVA in order to assess the significance of the factor “Cropping Itinerary”. In the greenhouse, data of agronomic measurements (shoot height, number of nodes, internode, stem circumference, shoot biomass, and flower biomass) were analyzed using a two-way ANOVA in order to assess the significance of the interaction between the experimental factors, namely “ethylene treatment” and “cultivar”. In the field, data of agronomic measurements (shoot height, number of nodes, internode, stem circumference, shoot biomass, and flower biomass) were analyzed using a one-way ANOVA in order to assess the significance of the factor “cultivar”. Normal distribution and homogeneous variance were checked, respectively, with Shapiro–Wilk and Levene tests. For the agronomic traits and CBD, CBD Total, and THC Total, the data were transformed with the Johnson transformation (Minitab ®). In this case, estimates were back-transformed for presentation purposes only.
4. Discussion
Greenhouse hydroponic systems promote larger individual plant biomass. In soilless cultivation, the cannabinoid pathway was either inhibited—with a lower cannabinoid content—or unmodified, although agronomic characteristics were significantly modified as compared to the field cropping itinerary. Higher individual plant shoot biomass, inflorescence biomass, stem circumference, and apical height in the soilless greenhouse environment were observed, and various factors can account for those differences. These factors include significantly lower crop density (approximately 30 times lower in the greenhouse), the absence of adverse weather conditions and wind stress, consistent shading against excessive sunlight exposure, and the application of continuous mineral nutrition and water supply through a hydroponic solution set the greenhouse apart from the field. Each of these elements exerts a distinct influence on plant growth and development. Environmental factors influence physiological processes and lead to differences in the phenotype of fresh vegetables and in the production of primary metabolites such as sugars and secondary metabolites [
38]. Greenhouse cultivation offers optimized environmental conditions in terms of light, temperature, humidity, water supply, and fertilization, favoring a high growth rate, but stressful situations in terms of heat and light energy can be present [
38,
39]. For example, the absence of supplementary lighting to natural radiation may induce an inappropriate environment; the covering may also retain UV-B, reducing the synthesis of polyphenols [
39,
40].
Ethephon application in greenhouse hydroponic systems affected hemp growth and architecture for 4–5 weeks, although shading, water management, and aphid stress also influenced overall plant performance. Within the greenhouse setting, the hydroponic gutter allowed for uniformity in the management of crops across growing tables. However, the conditions established for activating the shading system, which was activated for green-leafy crops, may not have been optimally aligned with the needs of the cannabis plant. The short photoperiod has a significant effect on cannabis florogenesis, resulting in a change in shoot architecture that forms a compound racemose inflorescence structure [
41]. It is plausible that the alternating periods of shade produced by the shade cloths had an impact on the plant’s reproductive cycle. Given the high rate of vegetative growth exhibited by the plants, increased water consumption necessitated frequent replenishing of the basins during hot spells. The introduction of tap water led to significant fluctuations in the pH and electrical conductivity (EC) within the nutrient solutions each time they were refilled. To mitigate this effect, larger reservoirs would be more advisable for plants of this size. On an industrial scale, automated filling methods would be imperative.
The results pertaining to the influence of ethephon on the three monitored parameters during cultivation enable quantification of the product’s effects in solution. The effects of different ethephon doses are discernible for up to 4 to 5 weeks after the application of the product, contingent upon the variety. Specifically, the effects intensify during the initial 2 weeks (the duration of product presence in the solution) before gradually diminishing over the subsequent 2 weeks. As discussed in the literature review section, ethephon degrades into ethylene when diluted in water and applied to the plant. Nonetheless, this study reveals a rapid and enduring effect of the product when added to the hydroponic solution. Ethephon treatment has been suggested to induce the formation of more female flowers if applied after primordia formation and should be tested in the future [
32].
While harvest data yields results indicative of ethephon’s influence, some modalities (Dose 2 and Dose 3) exhibit unexpectedly low values for several studied parameters. At the time of harvest, the impact of ethephon dosage had dissipated, yet these two central tables remain conspicuously underperforming. Suboptimal exposure to light may account for this effect. Moreover, a notable aphid outbreak was observed on these tables. Given the additional stress imposed on the plants, the observed variations may, in part, be attributed to these adverse growing conditions.
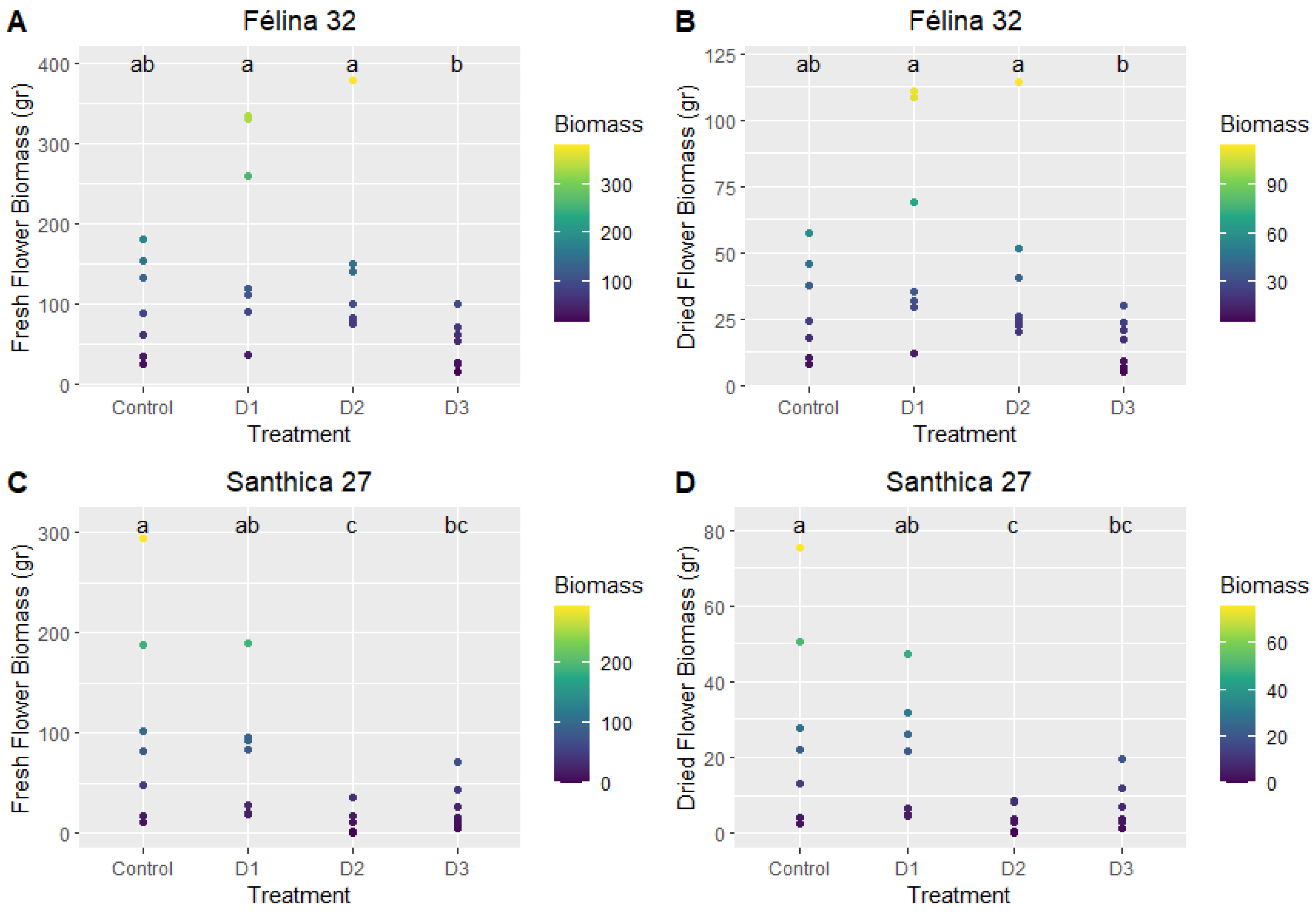
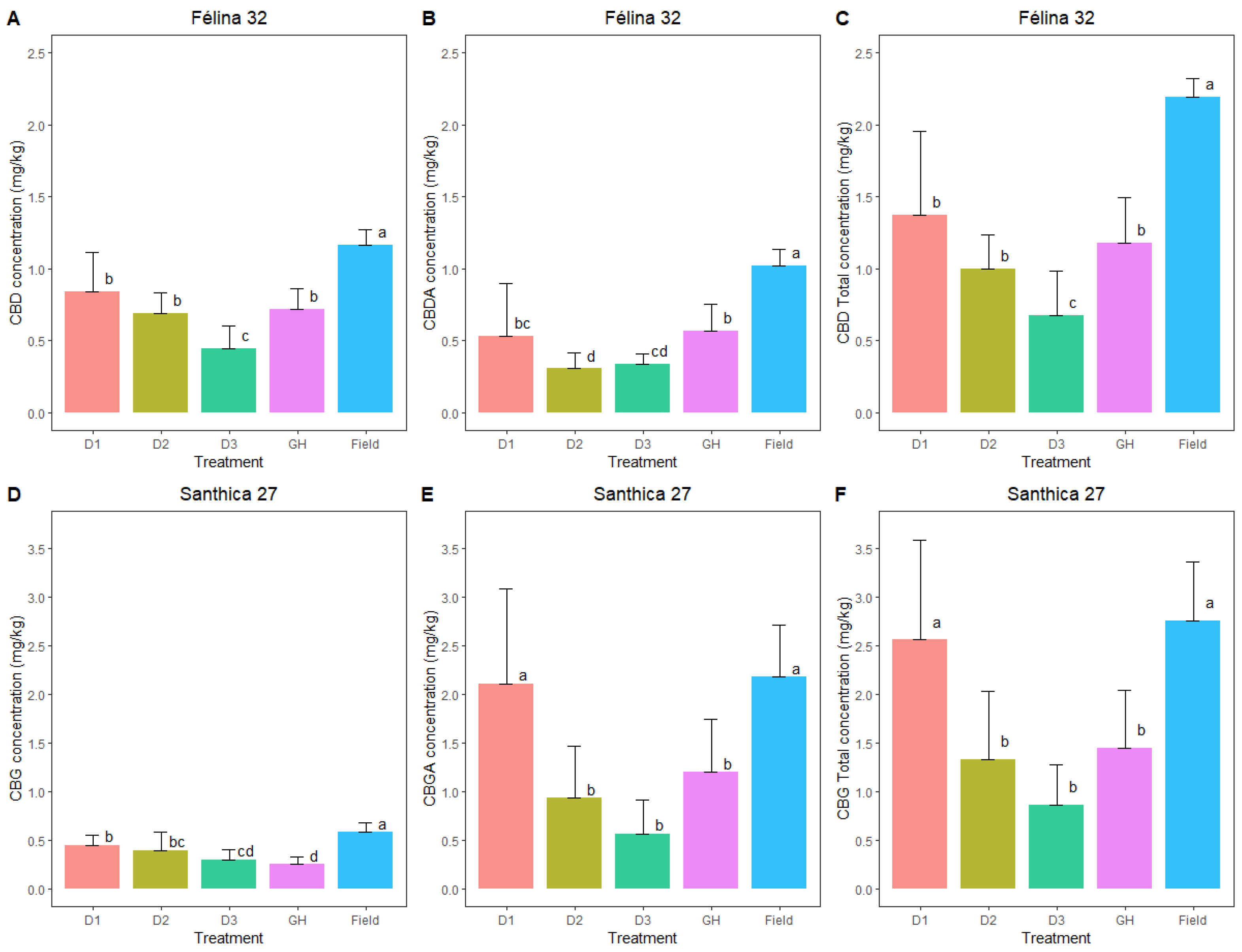
Similar Total CBG levels are achieved with Santhica 27 in the field and with high ethephon doses in the greenhouse, while lower Total CBD levels are observed with Félina 32 in the greenhouse compared to the field.
The flower biomass obtained contains a proportion of seeds. For the Santhica 27 variety, characterized by its high CBG content, high ethephon treatment (D1) was observed to positively influence CBG concentration in the plants, as the total CBG content was increased as compared to the greenhouse control group and lower ethephon doses (D1 and D3). It is plausible that the initial stress induced during cultivation impacts the plant’s subsequent responses and the production of metabolites throughout the cultivation cycle. The dose 1 (D1) aligns with findings from field cultivation, although the environmental factors differed (e.g., wind, rain). Nevertheless, these factors may have exerted a similar influence on CBG concentration in the plants. A comparison of CBG concentrations with flower biomass yields reveals that tables with the lowest concentrations also yield the least floral biomass. The combined stress from ethephon dosages (2 and 3) did not stimulate flower development. Additionally, the presence of aphids, primarily on these tables, exacerbates plant stress, further hindering or preventing optimal flowering.
In the case of the Félina 32 variety, valued for its CBD content, the concentrations achieved are consistent with the literature findings. Ethephon treatment appears to have a negative effect with the most diluted dose, although the effect of ethephon with higher doses seems to be limited as CBD content was not statistically different from the soilless control group. Therefore, the lowered CBD content in the soilless itinerary crops, as compared to field cultivation, seems to be more related to the soilless and greenhouse conditions. The field-grown plants exhibited concentrations nearly 1.5 times higher. Fewer aphids were observed on this variety, yet trends resembled those of the S27 variety. When evaluating production quantities per square meter, the same patterns persist.
Adding supplementary light to the greenhouse could increase the cannabinoid content. CBD and CBG showed higher concentrations under LED light treatments compared to HPS light [
42]. This up-regulation of secondary metabolites resulted in the up-regulation of isopentenyl diphosphate and dimethylallyl diphosphate [
42].
In the greenhouse, the potential cannabinoid yield increases significantly for both Félina 32 and Santhica 27, with a much larger boost for Santhica 27, but operational costs also rise substantially.
This economic analysis encompasses all stages of hemp production for flower use and quantifies economic prospects on the scale of the rooftop greenhouse. The results indicate considerable variability across different cultivation systems, not directly correlated with the growth regulator utilized. Nonetheless, certain scenarios suggest the potential for achieving relatively low-cost prices. It is pertinent to evaluate economic prospects by linking this research to cannabinoid levels, as explored in the preceding section. Additionally, extrapolating the true investment costs for a larger greenhouse dedicated to tomato production, possibly incorporating horticultural lamps to maximize annual cycles, becomes imperative.
Furthermore, certain labor-intensive tasks within our study, such as post-harvest and irrigation stages, could be automated to curtail production costs. A calculation assessing the time required to recoup investments must be undertaken in this context.
Significantly increased flower biomass in soilless itinerary crops is an interesting agronomic characteristic. The cannabinoid content in greenhouse cultivation systems is identical or lower than in field crops, and this should be investigated and improved to increase the economic viability of the soilless cultivation system. Moreover, a height apical plant is not desirable in the greenhouse and should also be limited. As ethephon showed a clear but limited in time impact, repeated and increased ethephon doses could have a more pronounced impact on the plant’s height and should be tested. This study’s combination of parameters did not result in an optimal cultivation plan. However, by addressing the areas for improvement mentioned above and further refining the use of growth regulators as well as the cannabis strain chosen, we can achieve a more accurate and meaningful economic projection. Without this additional information, any economic extrapolation based on the current results would lack validity.
5. Conclusions
The first objective was to study the effect of the ethylene treatment in terms of agronomic characteristics (shoot height, number of nodes, internode, stem circumference, shoot biomass, flower biomass). The effects of different ethephon doses, applied at the early vegetative stage (day 21), are discernible for up to 4 to 5 weeks after the application of the product. Specifically, the effects intensify during the initial 2 weeks before gradually diminishing over the subsequent 2 weeks. The repetition of an ethephon treatment after the primordia formation stage to induce the formation of more female flowers should be investigated.
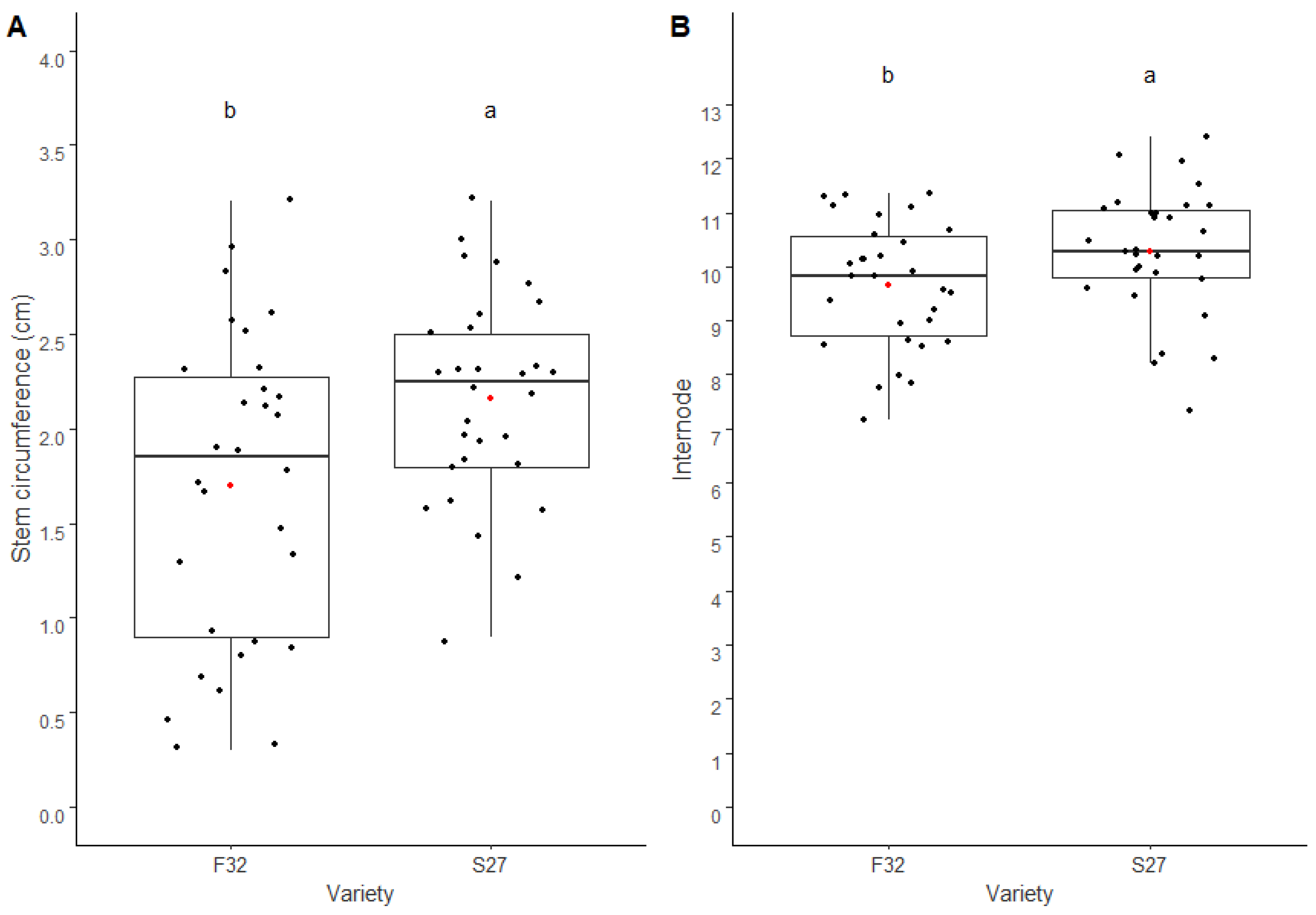
The second objective was to measure the agronomic characteristics in an open-field system. In the field, the plants were cultivated under a traditional density of 40 kg/ha. Almost all the measured agronomic traits, i.e., shoot height, stem circumference, number of nodes, internode, shoot biomass, and flower biomass, were smaller in the field as compared to the greenhouse cropping itinerary, with the exception of stem circumference, which was notably increased in the field. In the field, both varieties showed similar agronomic traits, but Santhica 27 had a mean stem circumference and internode statistically higher than Félina 32.
The third objective was to assess the cannabinoid content per unit of surface in all cropping itineraries. The hydroponic cultivation technique showed an increased potential annual CBD (×4.45) or CBG (×9) yield if several cultivation cycles are realized and when high ethylene doses are applied. However, Operational Expenditures (OpEx) per unit of the dried flower were higher in the greenhouse compared to the field, from 14.9 (Félina 32) to 17.9 (Santhica 27) times higher due to substantially higher expenses in the greenhouse related to energy, operators, irrigation, and fertilizers used. The optimization of the soilless cropping itinerary by adding lighting, developing greenhouse automation, and biomodulator treatment optimization could lead to the achievement of relatively low-cost prices.