Genome-Wide Identification, Evolution, and Expression Analysis of the MAPK Gene Family in Rosaceae Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. MAPK Gene Family Identification in Rosaceae Plants
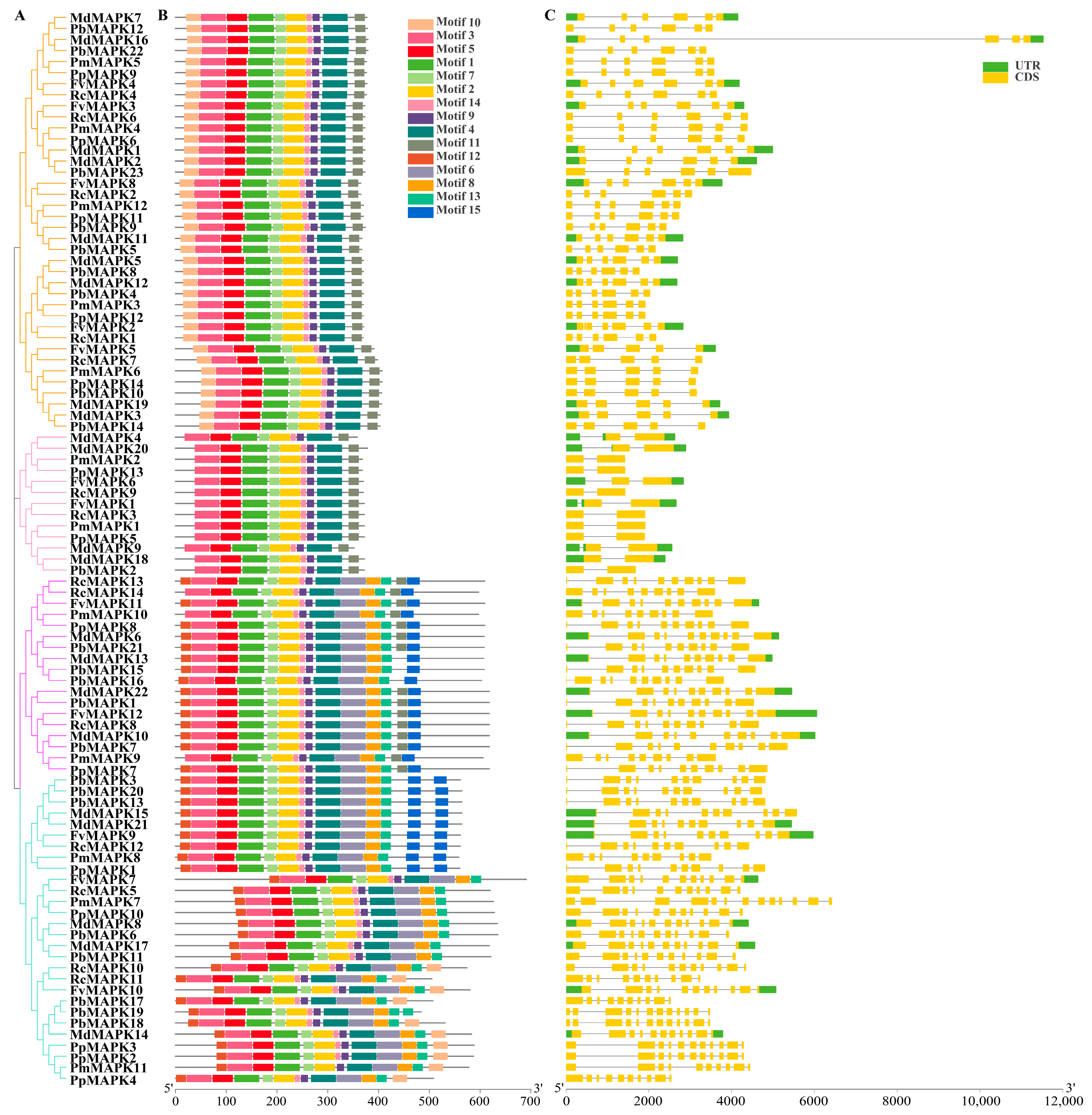
2.2. Gene Structure and Protein Motifs Analyses
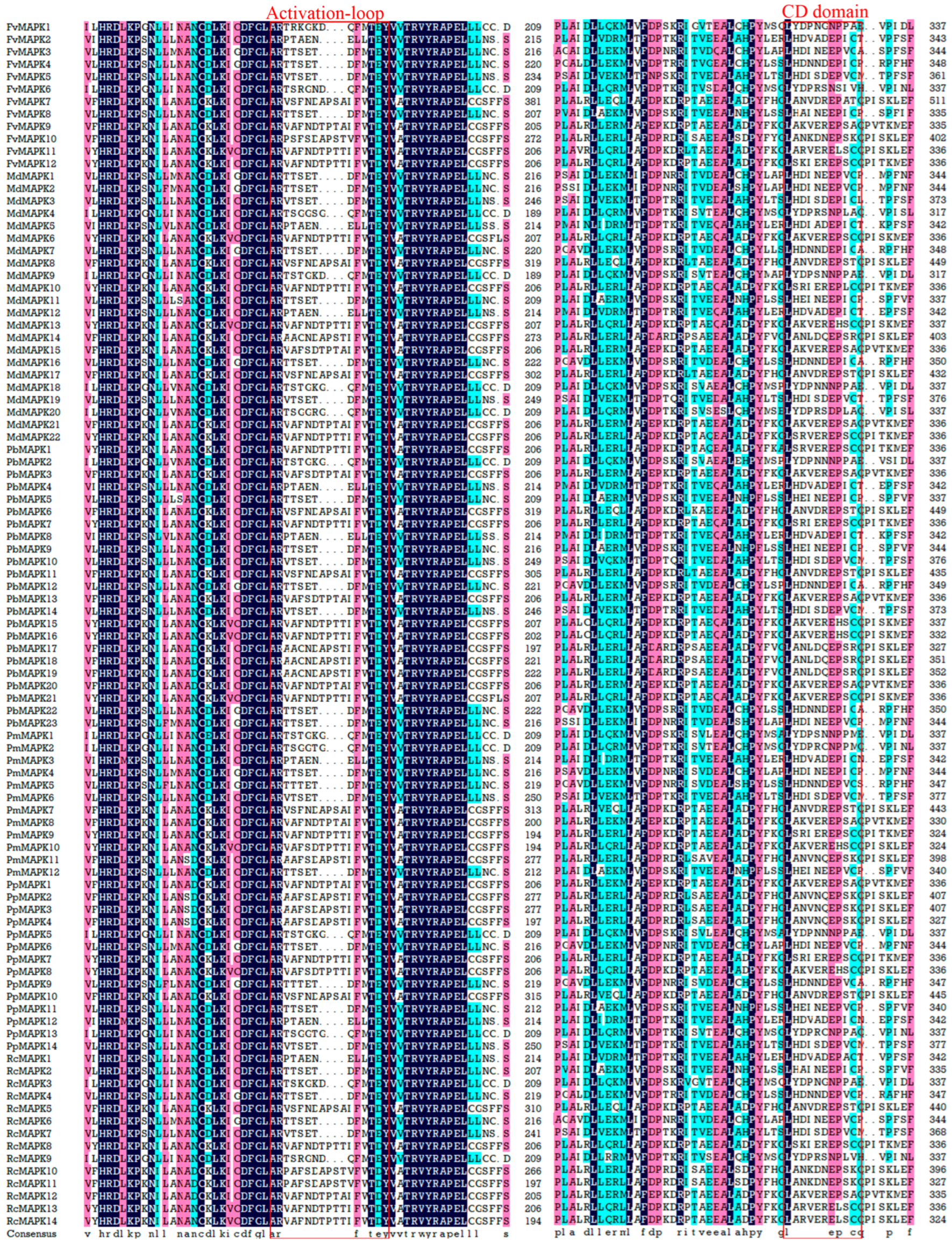
2.3. Phylogenetic Tree Construction and Conservative Analysis
2.4. Gene Duplications and Collinearity Analysis
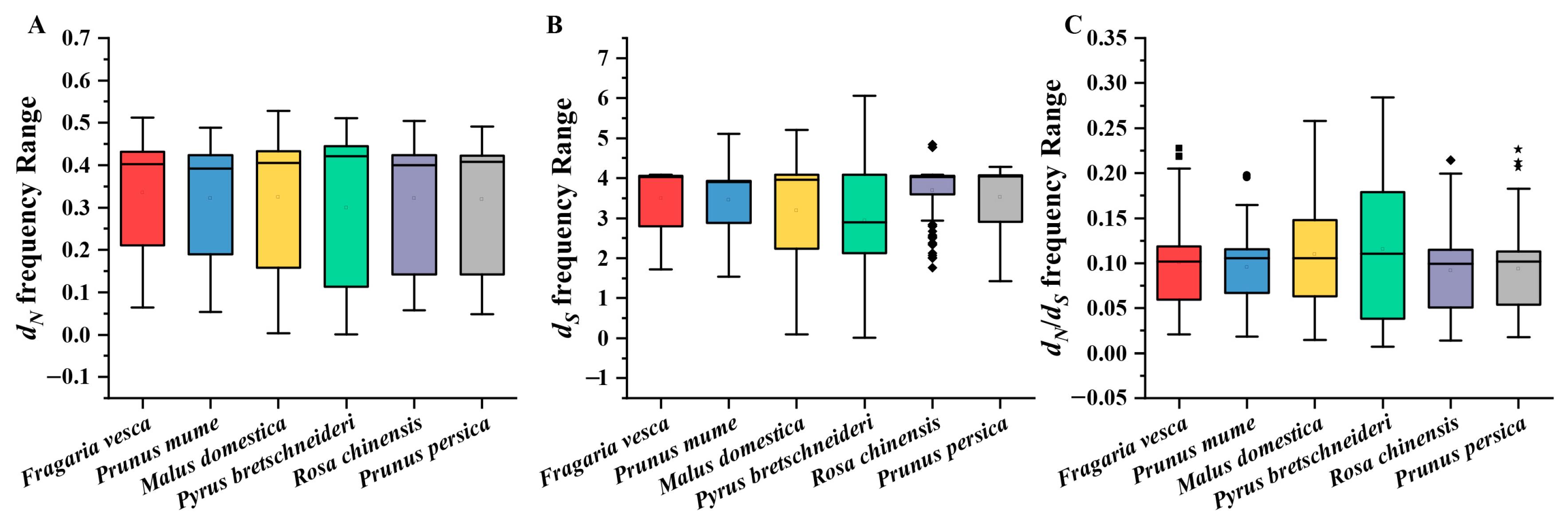
2.5. Selective Pressure Estimation
2.6. Functional Divergence Detection
2.7. Gene Expression Profile of MAPK Genes
3. Results
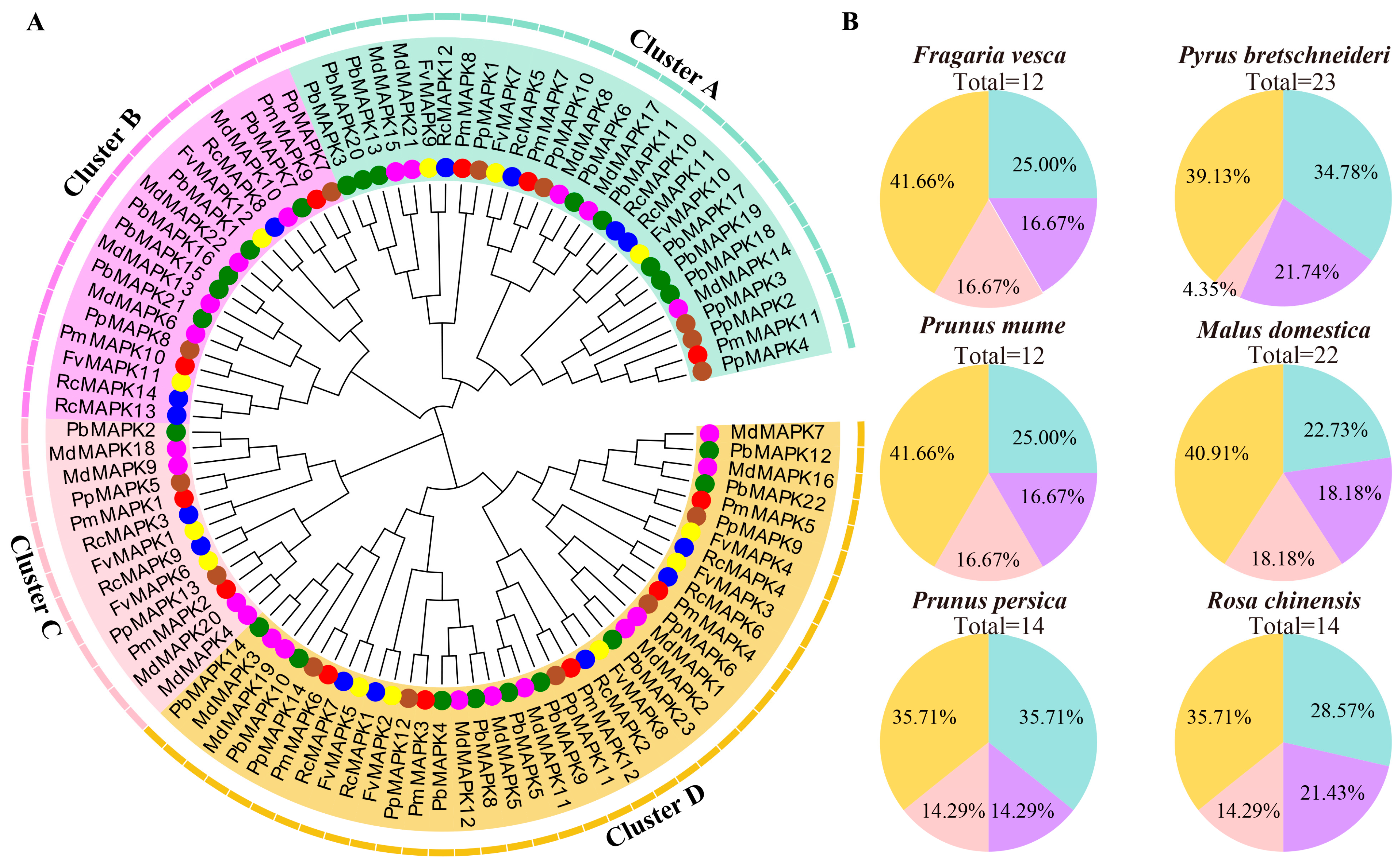
3.1. Identification and Sequence Analyses of the MAPK Gene Family in Rosaceae
3.2. Phylogenetic Tree Construction of MAPK Genes
3.3. Sequence Analyses of the MAPK Gene Family in Rosaceae
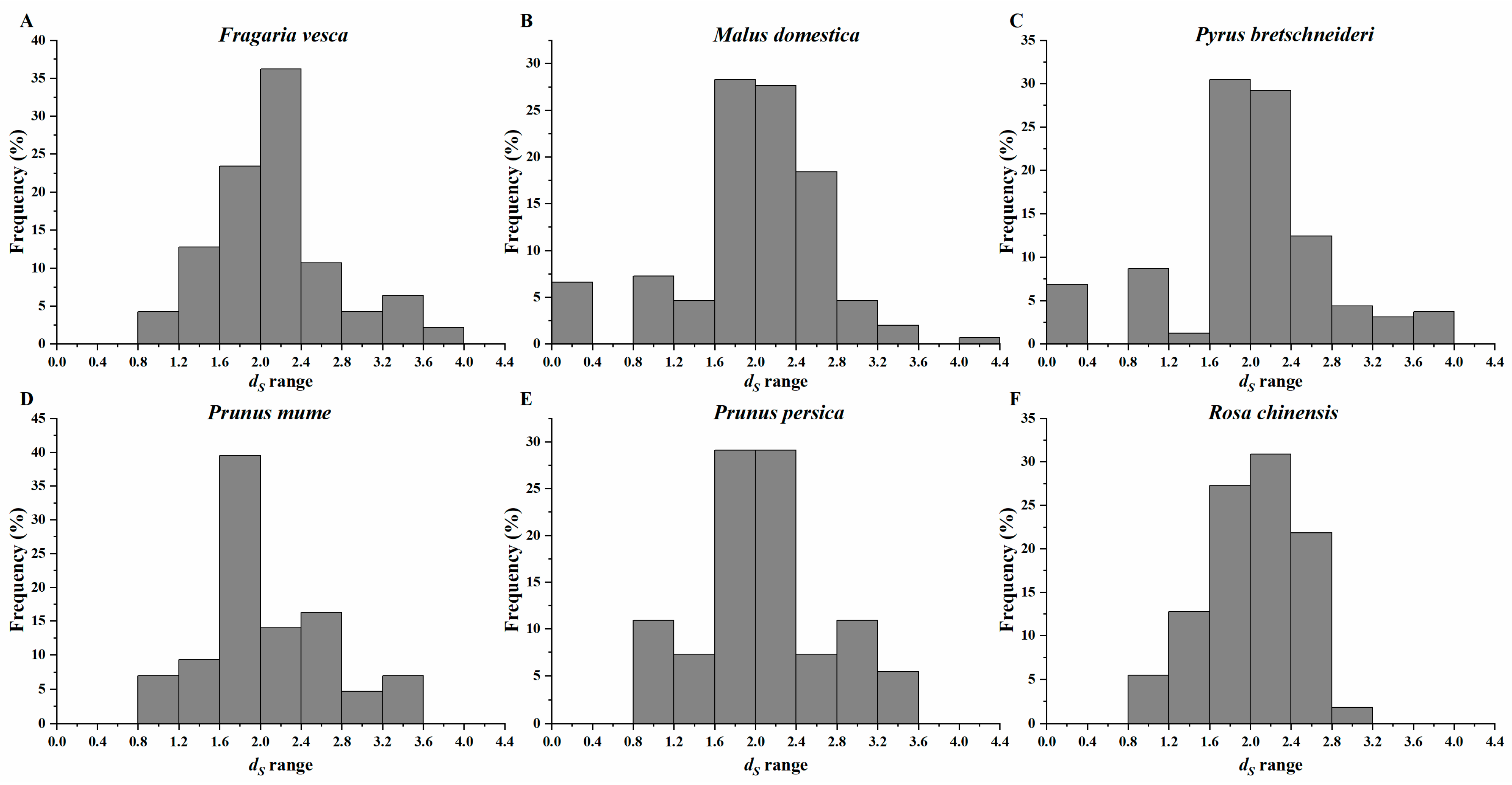
3.4. Collinearity Analysis
3.5. Selective Force Analyses of MAPK Family
3.6. Gene Functional Divergence Analysis
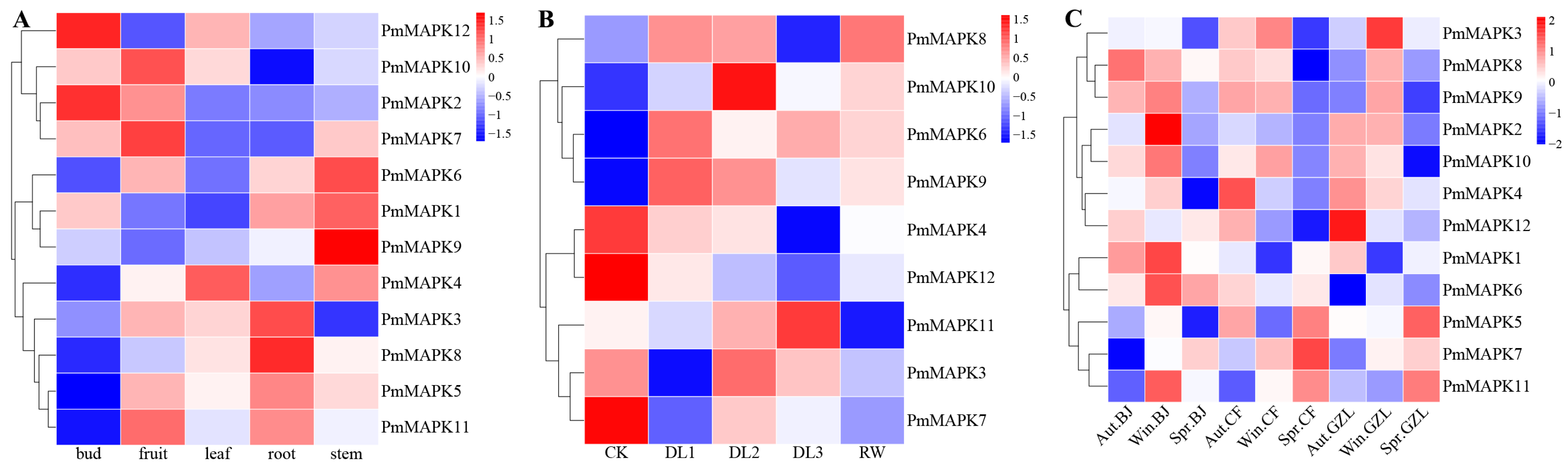
3.7. Gene Expression Patterns Analysis of PmMAPKs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.F.; van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef] [PubMed]
- Romeis, T. Protein kinases in the plant defence response. Curr. Opin. Plant Biol. 2001, 4, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Tena, G.; Asai, T.; Chiu, W.L.; Sheen, J. Plant mitogen-activated protein kinase signaling cascades. Curr. Opin. Plant Biol. 2001, 4, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Group, M. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar] [CrossRef]
- Hamel, L.P.; Nicole, M.C.; Duplessis, S.; Ellis, B.E. Mitogen-activated protein kinase signaling in plant-interacting fungi: Distinct messages from conserved messengers. Plant Cell 2012, 24, 1327–1351. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The Function of MAPK Cascades in Response to Various Stresses in Horticultural Plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Rao, K.P.; Richa, T.; Kumar, K.; Raghuram, B.; Sinha, A.K. In Silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2010, 17, 139–153. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, H.; Hong, H.; Yao, W.; Xie, W.; Xiao, J.; Li, X.; Wang, S. Transcriptome-based analysis of mitogen-activated protein kinase cascades in the rice response to Xanthomonas oryzae infection. Rice 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Reyna, N.S.; Yang, Y. Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Mol. Plant-Microbe Interact. MPMI 2006, 19, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Nicole, M.C.; Hamel, L.P.; Morency, M.J.; Beaudoin, N.; Ellis, B.E.; Seguin, A. MAP-ping genomic organization and organ-specific expression profiles of poplar MAP kinases and MAP kinase kinases. BMC Genom. 2006, 7, 223. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, R.; Luo, X.; Jiang, Z.; Shu, H. Genome-wide identification and expression analysis of MAPK and MAPKK gene family in Malus domestica. Gene 2013, 531, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Kilickaya, O. Mitogen-activated protein kinase cascades in Vitis vinifera. Front. Plant Sci. 2015, 6, 556. [Google Scholar] [CrossRef]
- Zhan, H.; Yue, H.; Zhao, X.; Wang, M.; Song, W.; Nie, X. Genome-Wide Identification and Analysis of MAPK and MAPKK Gene Families in Bread Wheat (Triticum aestivum L.). Genes 2017, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Xue, C.; Fang, H.; Zhao, J.; Liu, M. Genome-wide identification and analysis of MAPK and MAPKK gene family in Chinese jujube (Ziziphus jujuba Mill.). BMC Genom. 2017, 18, 855. [Google Scholar] [CrossRef]
- Neupane, S.; Schweitzer, S.E.; Neupane, A.; Andersen, E.J.; Fennell, A.; Zhou, R.; Nepal, M.P. Identification and Characterization of Mitogen-Activated Protein Kinase (MAPK) Genes in Sunflower (Helianthus annuus L.). Plants 2019, 8, 28. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Xing, Q.; Yue, L.; Qi, H. Genome-wide identification of mitogen-activated protein kinase (MAPK) cascade and expression profiling of CmMAPKs in melon (Cucumis melo L.). PLoS ONE 2020, 15, e0232756. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wang, N.N.; Zhang, J.B.; Zheng, Y.; Li, X.B. Genome-wide identification of the mitogen-activated protein kinase (MAPK) family in cotton (Gossypium hirsutum) reveals GhMPK6 involved in fiber elongation. Plant Mol. Biol. 2020, 103, 391–407. [Google Scholar] [CrossRef]
- Wang, Z.; Wan, Y.; Meng, X.; Zhang, X.; Yao, M.; Miu, W.; Zhu, D.; Yuan, D.; Lu, K.; Li, J.; et al. Genome-Wide Identification and Analysis of MKK and MAPK Gene Families in Brassica Species and Response to Stress in Brassica napus. Int. J. Mol. Sci. 2021, 22, 544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Gu, C.; Jiang, H.; Sun, J.; Li, J. Genome-wide identification of MAPK family genes and their response to abiotic stresses in tea plant (Camellia sinensis). Open Life Sci. 2022, 17, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, S.; Ren, W.; Liu, Y.; Sun, W.; Liu, M.; Lu, J.; Mi, Y.; Ma, W. Genome-Wide Identification of MAPK, MAPKK, and MAPKKK Gene Families in Fagopyrum tataricum and Analysis of Their Expression Patterns Under Abiotic Stress. Front. Genet. 2022, 13, 894048. [Google Scholar] [CrossRef]
- Li, M.; Li, B.; Yang, M.; Wang, L.; Hou, G.; Lin, Y.; Zhang, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Genome-Wide Identification and Expression of MAPK Gene Family in Cultivated Strawberry and Their Involvement in Fruit Developing and Ripening. Int. J. Mol. Sci. 2022, 23, 5201. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, M.; Meng, J.; Miao, R.; Liu, X.; Fan, D.; Lv, W.; Cheng, T.; Zhang, Q.; Sun, L. Genome-Wide Identification of the MAPK and MAPKK Gene Families in Response to Cold Stress in Prunus mume. Int. J. Mol. Sci. 2023, 24, 8829. [Google Scholar] [CrossRef]
- Andreasson, E.; Ellis, B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 2010, 15, 106–113. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Schwarz, E.M.; Lin, A.; Guan, K.; Ulevitch, R.J.; Han, J. Structure-function studies of p38 mitogen-activated protein kinase. Loop 12 influences substrate specificity and autophosphorylation, but not upstream kinase selection. J. Biol. Chem. 1997, 272, 11096–11102. [Google Scholar] [CrossRef]
- Wu, P.; Wang, W.; Li, Y.; Hou, X. Divergent evolutionary patterns of the MAPK cascade genes in Brassica rapa and plant phylogenetics. Hortic. Res. 2017, 4, 17079. [Google Scholar] [CrossRef]
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Tomsho, L.P.; Hu, Y.; Liang, H.; Soltis, P.S.; et al. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef]
- Doczi, R.; Okresz, L.; Romero, A.E.; Paccanaro, A.; Bogre, L. Exploring the evolutionary path of plant MAPK networks. Trends Plant Sci. 2012, 17, 518–525. [Google Scholar] [CrossRef]
- Janitza, P.; Ullrich, K.K.; Quint, M. Toward a comprehensive phylogenetic reconstruction of the evolutionary history of mitogen-activated protein kinases in the plant kingdom. Front. Plant Sci. 2012, 3, 271. [Google Scholar] [CrossRef] [PubMed]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Droillard, M.J.; Boudsocq, M.; Barbier-Brygoo, H.; Lauriere, C. Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of Arabidopsis thaliana: Activation by hypoosmolarity and negative role in hyperosmolarity tolerance. Febs Lett. 2004, 574, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yang, F.; Li, L.; Chen, H.; Chen, S.; Zhang, J. The Arabidopsis transcription factor BRASSINOSTEROID INSENSITIVE1-ETHYL METHANESULFONATE-SUPPRESSOR1 is a direct substrate of MITOGEN-ACTIVATED PROTEIN KINASE6 and regulates immunity. Plant Physiol. 2015, 167, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; De Vleesschauwer, D.; Sharma, M.K.; Ronald, P.C. Recent advances in dissecting stress-regulatory crosstalk in rice. Mol. Plant 2013, 6, 250–260. [Google Scholar] [CrossRef]
- Zhang, G.; Shen, T.; Ren, N.; Jiang, M. Phosphorylation of OsABA2 at Ser197 by OsMPK1 regulates abscisic acid biosynthesis in rice. Biochem. Biophys. Res. Commun. 2022, 586, 68–73. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, H.; Dong, C.; Ji, R.; Cai, L.; Fu, H.; Liu, S. Overexpression of Brassica napus MPK4 enhances resistance to Sclerotinia sclerotiorum in oilseed rape. Mol. Plant-Microbe Interact. MPMI 2009, 22, 235–244. [Google Scholar] [CrossRef]
- Weng, C.M.; Jun-Xing, L.U.; Wan, H.F.; Wang, S.W.; Wang, Z.; Kun, L.U.; Liang, Y. Over-Expression of BnMAPK1 in Brassica napus Enhances Tolerance to Drought Stress. J. Integr. Agr. 2014, 13, 2407–2415. [Google Scholar] [CrossRef]
- Chen, L.; Sun, H.; Wang, F.; Yue, D.; Shen, X.; Sun, W.; Zhang, X.; Yang, X. Genome-wide identification of MAPK cascade genes reveals the GhMAP3K14-GhMKK11-GhMPK31 pathway is involved in the drought response in cotton. Plant Mol. Biol. 2020, 103, 211–223. [Google Scholar] [CrossRef]
- Edger, P.P.; VanBuren, R.; Colle, M.; Poorten, T.J.; Wai, C.M.; Niederhuth, C.E.; Alger, E.I.; Ou, S.; Acharya, C.B.; Wang, J.; et al. Single-molecule sequencing and optical mapping yields an improved genome of woodland strawberry (Fragaria vesca) with chromosome-scale contiguity. GigaScience 2018, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Daccord, N.; Celton, J.M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, S.; Yao, J.L.; Deng, C.H.; Wang, L.; Su, Y.; Zhang, H.; Zhou, H.; Sun, M.; Li, X.; et al. Chromosome level high-density integrated genetic maps improve the Pyrus bretschneideri ‘DangshanSuli’ v1.0 genome. BMC Genom. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Saint-Oyant, L.H.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakwani, D.; Zhou, N.-N.; Bourke, P.M.; Daccord, N.; Leus, L.; Schulz, D.; et al. A high-quality sequence of Rosa chinensis to elucidate genome structure and ornamental traits. bioRxiv 2018, 10, 254102. [Google Scholar]
- Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; Cattonaro, F.; et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, W.; Sun, L.; Zhao, F.; Huang, B.; Yang, W.; Tao, Y.; Wang, J.; Yuan, Z.; Fan, G.; et al. The genome of Prunus mume. Nat. Commun. 2012, 3, 1318. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, K.; Zhang, T.; Li, L.; Wang, J.; Cheng, T.; Zhang, Q. Characteristics and Expression Analyses of Trehalose-6-Phosphate Synthase Family in Prunus mume Reveal Genes Involved in Trehalose Biosynthesis and Drought Response. Biomolecules 2020, 10, 1358. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Jiang, L. Physiological Changes and Gene Expression Pattern in Response to Low Temperature Stress in Prunus mume. Ph.D. Dissertation, Beijing Forestry University, Beijing, China, 2020. [Google Scholar]
- Yang, Y. Difference Analysis of Drought Tolerance in Cultivars of Prunus mume and Function Study of Genes in Melatonin Biosynthesis. Ph.D. Dissertation, Beijing Forestry University, Beijing, China, 2021. [Google Scholar]
- Wu, Y.; Wu, S.; Wang, X.; Mao, T.; Bao, M.; Zhang, J.; Zhang, J. Genome-wide identification and characterization of the bHLH gene family in an ornamental woody plant Prunus mume. Hortic. Plant J. 2022, 8, 531–544. [Google Scholar] [CrossRef]
- Pellicer, J.; Hidalgo, O.; Dodsworth, S.; Leitch, I.J. Genome Size Diversity and Its Impact on the Evolution of Land Plants. Genes 2018, 9, 88. [Google Scholar] [CrossRef]
- Gu, X.; Zou, Y.; Su, Z.; Huang, W.; Zhou, Z.; Arendsee, Z.; Zeng, Y. An update of DIVERGE software for functional divergence analysis of protein family. Mol. Biol. Evol. 2013, 30, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Liu, X.; Wu, J.; Xu, G.; Zhang, S. Characterization of the MAPK Gene Family and PbrMAPK13 Response to Hormone and Temperature Stresses via Different Expression Pattern in Pyrus × bretschneideri Pollen. J. Am. Soc. Hortic. Sci. Am. Soc. Hortic. Sci. 2017, 142, 163–174. [Google Scholar] [CrossRef]
- Wei, W.; Chai, Z.; Xie, Y.; Gao, K.; Cui, M.; Jiang, Y.; Feng, J. Bioinformatics identification and transcript profile analysis of the mitogen-activated protein kinase gene family in the diploid woodland strawberry Fragaria vesca. PLoS ONE 2017, 12, e0178596. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Arora, P.K.; Mohanta, N.; Parida, P.; Bae, H. Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genom. 2015, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, M.; Wu, Y.; Tian, Y.; Han, Y.; Liu, C.; Hao, J.; Fan, S. Genome-Wide Identification and Expression Analysis of MAPK Gene Family in Lettuce (Lactuca sativa L.) and Functional Analysis of LsMAPK4 in High-Temperature-Induced Bolting. Int. J. Mol. Sci. 2022, 23, 11129. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T.; Jia, Z.H.; Xuan, J.P.; Pan, D.L.; Guo, Z.R.; Zhang, J.Y. Genome-Wide Bioinformatics Analysis of MAPK Gene Family in Kiwifruit (Actinidia chinensis). Int. J. Mol. Sci. 2018, 19, 2510. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef]
- Wu, J.; Liang, X.; Lin, M.; Lan, Y.; Xiang, Y.; Yan, H. Comprehensive analysis of MAPK gene family in Populus trichocarpa and physiological characterization of PtMAPK3-1 in response to MeJA induction. Physiol. Plant 2023, 175, e13869. [Google Scholar] [CrossRef]
- Ali, A.; Chu, N.; Ma, P.; Javed, T.; Zaheer, U.; Huang, M.T.; Fu, H.Y.; Gao, S.J. Genome-wide analysis of mitogen-activated protein (MAP) kinase gene family expression in response to biotic and abiotic stresses in sugarcane. Physiol. Plant 2021, 171, 86–107. [Google Scholar] [CrossRef]
- Kong, X.; Pan, J.; Zhang, D.; Jiang, S.; Cai, G.; Wang, L.; Li, D. Identification of mitogen-activated protein kinase kinase gene family and MKK-MAPK interaction network in maize. Biochem. Biophys. Res. Commun. 2013, 441, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, Q.; Zhang, H.; Zhou, N.; Yan, H.; Jian, H.; Li, S.; Xiang, G.; Tang, K.; Qiu, X. Genome-wide identification and evolutionary analysis of MLO gene family in Rosaceae plants. Hortic. Plant J. 2022, 8, 13. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.-C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.A.; et al. MAP Kinase Cascades Regulate the Cold Response by Modulating ICE1 Protein Stability. Dev. Cell 2017, 43, 618–629.e615. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Pineda, I.G.; Carmona-Salazar, L.; Saucedo-García, M.; Cano-Ramírez, D.; Morales-Cedillo, F.; Peña-Moral, A.; Guevara-García, Á.A.; Sánchez-Nieto, S.; Gavilanes-Ruíz, M. MPK6 Kinase Regulates Plasma Membrane H+-ATPase Activity in Cold Acclimation. Int. J. Mol. Sci. 2021, 22, 6338. [Google Scholar] [CrossRef]


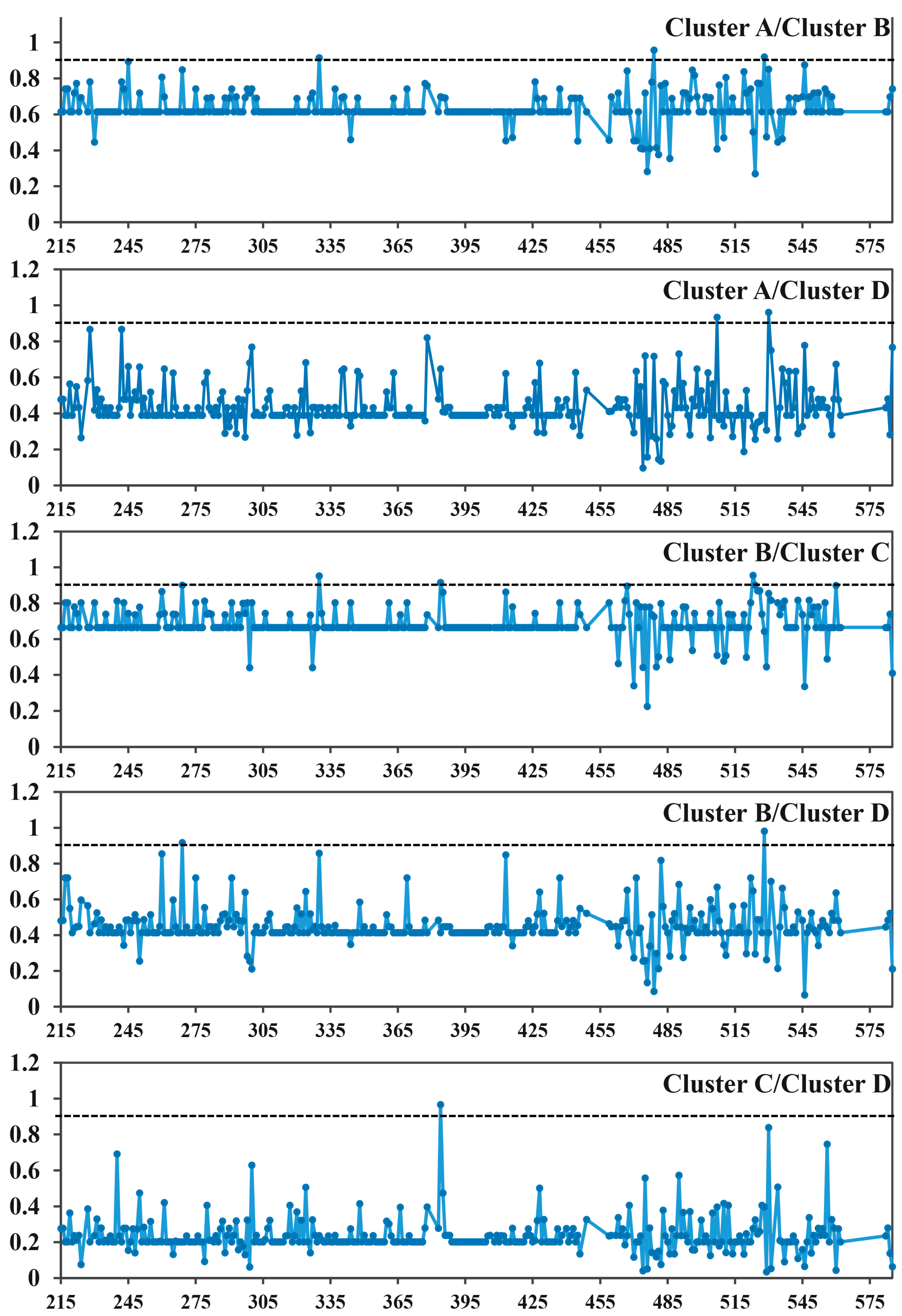
| Pairs | Type I | Type II | |||||
|---|---|---|---|---|---|---|---|
| ϴI ± SE a | LRT | MFE z Score | p-Value | Qk ≥ 0.90 b | ϴII ± SE c | p-Value | |
| A/B | 0.636 ± 0.133 | 22.965 | −6.711 | 0.000 | 330, 497, 528 | 0.032 ± 0.040 | 0.425 |
| A/C | 0.240 ± 0.110 | 4.802 | −2.894 | 0.004 | - | 0.381 ± 0.043 | 0.000 |
| A/D | 0.436 ± 0.093 | 22.199 | −6.444 | 0.000 | 507, 530 | 0.273 ± 0.061 | 0.000 |
| B/C | 0.687 ± 0.127 | 29.222 | −7.328 | 0.000 | 269, 330, 384, 523, 524, 560 | 0.362 ± 0.042 | 0.000 |
| B/D | 0.451 ± 0.099 | 20.673 | −7.160 | 0.000 | 269, 528 | 0.258 ± 0.060 | 0.000 |
| C/D | 0.238 ± 0.071 | 11.335 | −5.758 | 0.000 | 384 | 0.118 ± 0.062 | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Tang, H.; Huang, Y.; Zheng, Y.; Sun, Y.; Wang, Q. Genome-Wide Identification, Evolution, and Expression Analysis of the MAPK Gene Family in Rosaceae Plants. Horticulturae 2023, 9, 1328. https://doi.org/10.3390/horticulturae9121328
Yang Y, Tang H, Huang Y, Zheng Y, Sun Y, Wang Q. Genome-Wide Identification, Evolution, and Expression Analysis of the MAPK Gene Family in Rosaceae Plants. Horticulturae. 2023; 9(12):1328. https://doi.org/10.3390/horticulturae9121328
Chicago/Turabian StyleYang, Yongjuan, Hao Tang, Yuchen Huang, Yanyi Zheng, Yuanyuan Sun, and Qi Wang. 2023. "Genome-Wide Identification, Evolution, and Expression Analysis of the MAPK Gene Family in Rosaceae Plants" Horticulturae 9, no. 12: 1328. https://doi.org/10.3390/horticulturae9121328
APA StyleYang, Y., Tang, H., Huang, Y., Zheng, Y., Sun, Y., & Wang, Q. (2023). Genome-Wide Identification, Evolution, and Expression Analysis of the MAPK Gene Family in Rosaceae Plants. Horticulturae, 9(12), 1328. https://doi.org/10.3390/horticulturae9121328






