Primary Determination of the Composition of Secondary Metabolites in the Wild and Introduced Artemisia martjanovii Krasch: Samples from Yakutia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Reagents
2.3. Extraction
2.4. Liquid Chromatography
2.5. Mass Spectrometry
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Class of Compounds | Identification | Formula | Calculated Mass | Retention Time (min.) | Observed Mass [M-H]− | Observed Mass [M+H]+ | MS/MS Stage 1 Fragmentation | MS/MS Stage 2 Fragmentation | MS/MS Stage 3 Fragmentation | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Flavone | Apigenin [5,7-Dixydroxy-2-(40Hydroxyphenyl)-4H-Chromen-4-One] * | C15H10O5 | 270.2369 | 49.0 | 271 | 225 | 179 | Lonicera henryi [15]; Ribes meyeri [16]; Lonicera japonica [17]; Mexican lupine species [18]; Exocarpium Citri Grandis [19]; Stevia rebaudiana [20]; Propolis [21]; Jatropha [22] | ||
| 2 | Flavone | Trihydroxy(iso)flavone * | C15H10O5 | 270.2369 | 13.0 | 271 | 215 | 173 | Propolis [21] | ||
| 3 | Flavone | Hispidulin | C16H12O6 | 300.2629 | 45.8 | 301 | 282 | 254 | Artemisia argyi [23]; Cirsium japonicum [24]; Mentha [25] | ||
| 4 | Flavone | Trihydroxymethoxyflavone | C16H12O6 | 300.2629 | 30.0 | 301 | 286; 226; 136 | 258; 132 | 189; 162; 135 | Artemisia absinthium [6] | |
| 5 | Flavone | Cirsimaritin [Scrophulein; 4′,5-Dihydroxy-6,7-Dimethoxyflavone] | C17H14O6 | 314.2895 | 36.0 | 315 | 300 | 285; 229 | 257; 229 | Artemisia annua [6]; Ocimum [26]; Rosmarinus officinalis [27] | |
| 6 | Flavone | Salvigenin * | C18H16O6 | 328.3160 | 52.5 | 329 | 296 | 268 | 240; 133 | Dracocephalum palmatum [28]; Ocimum [26] | |
| 7 | Flavone | Jaceosidin [5,7,4′-trihydroxy-6′,5′-dimethoxyflavone] | C17H14O7 | 330.2889 | 42.3 | 329 | 314; 229 | 299 | 271 | Artemisia argyi [23]; Mentha [25] | |
| 8 | Flavone | Dihydroxy-dimethoxyflavone | C17H14O7 | 330.2889 | 26.5 | 329 | 313 | 299 | 270 | Artemisia absinthium [6] | |
| 9 | Flavone | Cirsiliol * | C17H14O7 | 330.2889 | 35.7 | 331 | 316 | 298 | 270 | Ocimum [26]; Juglans mandshurica [29] | |
| 10 | Flavone | 3-Hydroxy-6,7,4′-trimethoxyflavone | C18H16O7 | 344.3154 | 7.8 | 343 | 328 | 313 | 298; 270 | Artemisia annua [30] | |
| 11 | Flavone | Cirsilineol [Eupatrin; Fastigenin; Cirsileneol] * | C18H16O7 | 344.3154 | 9.1 | 345 | 312 | 284; 269 | 269 | Ocimum [26] | |
| 12 | Flavone | Nevadensin * | C18H16O7 | 344.3154 | 41.0 | 345 | 330 | 312 | 284; 135 | Mentha [25]; Ocimum [26] | |
| 13 | Flavone | Penduletin | C18H16O7 | 344.3154 | 40.4 | 343 | 328 | 313 | 298 | Artemisia annua [6] | |
| 14 | Flavone | Eupatilin | C18H16O7 | 344.3154 | 35.8 | 345 | 312 | 284 | 269 | Artemisia argyi [23] | |
| 15 | Flavone | Syringetin * | C17H14O8 | 346.2883 | 24.3 | 347 | 332 | 317 | 289 | C. edulis [31]; Grape [32] | |
| 16 | Flavone | Tetrahydroxy-dimethoxyflavone | C17H14O8 | 346.2883 | 29.0 | 345 | 330 | 315 | 287 | Artemisia absinthium [6] | |
| 17 | Flavone | Gardenin B [Demethyltangeretin] * | C19H18O7 | 358.342 | 44.4 | 359 | 326; 344; 295 | 298; 269 | 283; 269; 227 | Mentha [25]; Ocimum [26]; Actinocarya tibetica [33]; | |
| 18 | Flavone | 3,5 -Dihydroxy -6,7,4′-trimethoxyflavone | C18H16O8 | 360.3148 | 35.8 | 358 | 343 | 328 | 300 | Artemisia annua [30] | |
| 19 | Flavone | Centaureidin [5,7,3′-Trihydroxy-3,6,4′-trimethoxyflavone] | C18H16O8 | 360.3148 | 27.5 | 361 | 328 | 300 | 285 | Artemisia argyi [23] | |
| 20 | Flavone | Dihydroxy-trimethoxyflavone | C18H16O8 | 360.3148 | 30.9 | 361 | 346; 142 | 328; 217 | 300 | Artemisia absinthium [6] | |
| 21 | Flavone | Thymonin [5,6,4′-trihydroxy-7,8,3′-tri-methoxyflavone] * | C18H16O8 | 360.3148 | 32.8 | 361 | 345; 187 | 328; 217 | 300; 164 | Mentha [25,34] | |
| 22 | Flavone | 3,5-Dihydroxy-6,7,3′,4′-tetramethoxyflavone | C19H18O8 | 374.3414 | 37.5 | 373 | 358 | 343 | 328; 300 | Artemisia annua [30] | |
| 23 | Flavone | Casticin [Vitexicarpin; Dihydroxy-tetramethoxyflavone] | C19H18O8 | 374.3414 | 36.5 | 375 | 342 | 313; 151 | 299; 151 | Artemisia annua [6,35]; Artemisia argyi [23] | |
| 24 | Flavone | Chrysoeriol C-hexoside * | C22H22O11 | 462.4036 | 42.2 | 463 | 445; 233 | 427; 229 | 399; 197 | Triticum aestivum L. [36,37] | |
| 25 | Flavone | Chrysoeriol 6-O-hexoside * | C22H22O11 | 462.4036 | 49.0 | 463 | 445; 231 | 427; 287; 229 | 409; 229 | Triticum aestivum L. [38] | |
| 26 | Flavone | Dihydroxy-trimethoxyflavone-O-hexoside | C23H22O13 | 506.413 | 29.4 | 507 | 345 | 312 | 284 | Citrus species [39] | |
| 27 | Flavone | Dihydroxy tetramethoxyflavone hexoside * | C25H28O13 | 536.4820 | 27.3 | 537 | 375 | 342 | 314; 151 | F. pottsii [31] | |
| 28 | Flavone | Acacetin C-glucoside methylmalonylated * | C26H26O13 | 546.4758 | 48.1 | 547 | 529; 327; 231 | 312; 160 | 284 | Mexican lupine species [18] | |
| 29 | Flavonol | Dihydroquercetin (Taxifolin; Taxifoliol) * | C15H12O7 | 304.2516 | 8.5 | 305 | 286; 234; 175; 147 | 240; 199; 148 | 157 | Juglans mandshurica [29]; Glycine soja [40]; millet grains [41] | |
| 30 | Flavonol | Quercetin 3-O-glucoside [Isoquercetin; Isoquercitrin; Hirsutrin] | C21H20O12 | 464.3763 | 50.3 | 465 | 447; 231 | 187 | 145 | Lonicera henryi [15]; Ribes meyeri [16]; Lonicera japonica [17]; Mexican lupine species [18]; Juglans mandshurica [29]; Artemisia annua [30]; Vaccinium myrtillus [42]; Embelia [43] | |
| 31 | Flavonol | Isorhamnetin 3-O-glucoside | C22H22O12 | 478.4029 | 23.4 | 479 | 317 | 302; 165 | 274; 153 | Artemisia annua [30]; Actinidia valvata [44]; Actinidia polygama [45] | |
| 32 | Flavonol | Mearnsetin-glucoside | C22H22O13 | 494.4023 | 31.3 | 495 | 477; 233 | 459; 244 | 431; 186 | Artemisia annua [30] | |
| 33 | Dihydroxy-flavonol | Tetrahydroxy-dimethoxyflavone-hexoside [Syringetin-hexoside; dimethyl-myricetin-hexoside] * | C23H24O13 | 508.4289 | 24.3 | 509 | 347 | 332 | 317 | Mentha [46]; Pomegranate [47]; Vaccinium macrocarpon [48] | |
| 34 | Flavonol | Isorhamnetin 3-O-(6″-O-rhamnosyl-hexoside) * | C28H32O16 | 624.5441 | 24.2 | 623 | 315; 300 | 300; 255 | 271; 255 | Lonicera henryi [15]; Bee-pollen [49] | |
| 35 | Flavan-3-ol | (Epi)-catechin * | C15H14O6 | 290.2681 | 7.4 | 291 | 272; 216 | 240; 216; 184 | 211; 184; 158 | Glycine soja [40]; millet grains [41]; Vaccinium myrtillus [42]; Vaccinium macrocarpon [50] | |
| 36 | Flavanone | Eriodictyol [3′,4′,5,7-tetrahydroxy-flavanone] | C15H12O6 | 288.2522 | 48.5 | 289 | 271; 191 | 201 | 160 | Artemisia absinthium [6]; Propolis [21]; Jatropha [22]; Rosmarinus officinalis [27]; Juglans mandshurica [29]; Embelia [43] | |
| 37 | Flavanone | (S)-eriodictyol-6-C-beta-D-glucopyranoside * | C21H22O11 | 450.3928 | 26.3 | 451 | 433; 321 | 247; 167 | 231 | Aspalathus linearis [51] | |
| 38 | Anthocyanin | Petunidin * | C16H13O7+ | 317.2702 | 23.4 | 317 | 302 | 274; 153 | 246; 153 | A. cordifolia; C. edulis [31]; Vines [52] | |
| 39 | Hydroxybenzoic acid | Gallic acid | C7H6O5 | 170.1195 | 15.7 | 171 | 152; 138 | 135 | Huolisu Oral Liquid [7]; Ribes meyeri [16]; Juglans mandshurica [29]; Vaccinium macrocarpon [50]; Punica granatum [53]; Actinidia [54] | ||
| 40 | Hydroxycinnamic acid | Caffeic acid [(2E)-3-(3,4-Dihydroxyphenyl)acrylic acid] | C9H8O4 | 180.1574 | 8.7 | 181 | 163; 135 | 145; 121 | 117 | Artemisia argyi [23]; Juglans mandshurica [29]; Soybean leaves [55] | |
| 41 | Methylbenzoic acid | Methylgallic acid [Methyl gallate] * | C8H8O5 | 184.1461 | 39.5 | 185 | 143 | 116 | Grape [32]; Rhus coriaria [56]; Terminalia arjuna [57]; Phyllanthus [58] | ||
| 42 | Trans-cinnamic acid | Ferulic acid | C10H10O4 | 194.184 | 26.2 | 193 | 176 | 132 | Lonicera japonica [17]; Juglans mandshurica [29]; Soybean leaves [55]; Soybean [59]; Ribes nigrum [60]; | ||
| 43 | Hydroxybenzoic acid | Syringic acid [Benzoic acid; Cedar acid] * | C9H10O5 | 198.1727 | 49.8 | 199 | 197; 171; 157; 143 | 142; 129 | Rosa acicularis [8]; Juglans mandshurica [29]; A. cordifolia; G. linguiforme; F. glaucescens [31]; millet grains [41]; Vaccinium macrocarpon [50]; Actinidia [54] | ||
| 44 | Cinnamic acid derivative | cis-3-Caffeoylquinic acid | C16H18O9 | 354.3087 | 6.4 | 353 | 191 | Camellia kucha [9]; Lonicera henryi [15]; Crataegus monogyna [61] | |||
| 45 | Cinnamic acid derivative | Chlorogenic acid [3-O-CaffeoylqChlorogenic acid [3-O-Caffeoylquinic acid]uinic acid] | C16H18O9 | 354.3087 | 16.5 | 353 | 191; 321 | Artemisia annua [6]; Lonicera henryi [15]; Lonicera japonica [17]; Artemisia argyi [23]; Juglans mandshurica [29]; Vaccinium myrtillus [42]; Vaccinium macrocarpon [48,50]; Rhus coriaria [56] | |||
| 46 | Cinnamic acid derivative | Neochlorogenic acid [5-O-Caffeoylquinic acid] | C16H18O9 | 354.3087 | 7.3 | 353 | 191; 321 | 127 | Artemisia annua [6]; Lonicera henryi [15]; Lonicera japonica [17]; Artemisia argyi [23]; Dracocephalum palmatum [28]; Vaccinium myrtillus [42] | ||
| 47 | Caffeic acid derivative | C16H18O9Na | 377.2985 | 6.4 | 377 | 341 | 179 | Embelia [43]; Bougainvillea [62] | |||
| 48 | Phenolic acid | 3,4-O-dicaffeoylquinic acid [Isochlorogenic acid B] | C25H24O12 | 516.4509 | 7.2 | 515 | 353 | 173 | Lonicera henryi [15]; Lonicera japonica [17]; Stevia rebaudiana [20]; Artemisia argyi [23]; Artemisia annua [30] | ||
| 49 | Phenolic acid | Tetramethylellagic acid hexose | C26H34O11 | 522.5416 | 27.1 | 523 | 361 | 346 | 328; 217 | Strawberry [63] | |
| 50 | Phenolic acid | 3,4,5-Tri-O-caffeoylquinic acid | C34H30O15 | 678.5930 | 27.8 | 677 | 515; 353 | 353; 173 | 173 | Lonicera henryi [15]; Artemisia annua [30] | |
| 51 | Stilbene | Resveratrol [trans-Resveratrol; 3,4′,5-Trihydroxystilbene; Stilbentriol] * | C14H12O3 | 228.2433 | 7.5 | 229 | 172 | 158; 144 | Embelia [43]; Grape [32]; A. cordifolia; F. glaucescens; F. herrerae [31]; Radix polygoni multiflori [64] | ||
| 52 | Hydroxycoumarin | Umbelliferone [Skimmetin; Hydragin] * | C9H6O3 | 162.1421 | 9.2 | 163 | 145; 121 | 117 | F. glaucescens [31]; Actinidia [54]; Sanguisorba officinalis [65]; Zostera marina [66] | ||
| 53 | Coumarin | Fraxetin * | C10H8O5 | 208.1675 | 36.0 | 209 | 191 | 117 | Jatropha [22]; Embelia [43]; Actinidia [54] | ||
| 54 | Natural plant coumarin | Tomentin * | C11H10O5 | 222.1941 | 51.1 | 223 | 208 | 178 | 165 | Jatropha [22] | |
| 55 | Dihydrochalcone | Phloretin [Dihydronaringenin; Phloretol] * | C15H14O5 | 274.2687 | 31.3 | 275 | 257; 147 | 239; 187 | 197; 117 | Rosa rugosa [8]; G. linguiforme [31]; Punica granatum [53]; Malus toringoides [67] | |
| 56 | Lignan | Podophyllotoxin [Podofilox; Condylox; Condyline; Podophyllinic acid lactone] * | C22H22O8 | 414.4053 | 49.0 | 415 | 397; 195 | 369; 167 | 351; 179 | Lignans [68] | |
| OTHERS | |||||||||||
| 57 | Amino acid | L-Valine [(S)-2-Amino-Methylbutanoic acid] | C5H11NO2 | 117.1463 | 7.0 | 118 | 116 | Lonicera japonica [17]; Soybean leaves [55]; Vigna unguiculata [69] | |||
| 58 | L-Ascorbic acid [Vitamin C] | C6H8O6 | 176.1241 | 7.1 | 177 | 160; 126 | 158; 141; 132 | Potato leaves [70]; Strawberry, Lemon, Papaya [71]; Phoenix dactylifera [72] | |||
| 59 | Aromatic amino acid | Tyrosine [(2S)-2-Amino-3-(4-Hydroxyphnyl)Propanoic acid] * | C9H11NO3 | 181.1885 | 8.1 | 182 | 165; 136 | 147; 123 | 119 | Euphorbia hirta [10]; Hylocereus polyrhizus [11]; Soybean leaves [55]; Vigna unguiculata [69] | |
| 60 | Naphthoquinone | Plumbagin * | C11H8O3 | 188.1794 | 16.8 | 189 | 187; 133 | Juglans mandshurica [29] | |||
| 61 | Propenyl phenol derivative | Methoxyeugenol * | C11H14O3 | 194.2271 | 21.0 | 195 | 177 | 133 | 131 | Ocimum [26] | |
| 62 | Carboxylic acid | 7-Methoxybenzo[d][1,3] dioxole-5-carboxylic acid | C9H8O5 | 196.1568 | 39.5 | 197 | 179 | 151 | 123 | Actinidia [54] | |
| 63 | Benzofuran | Isololiolide * | C11H16O3 | 196.2429 | 39.6 | 197 | 179 | 151; 149 | 123 | Jatropha gossypifolia [22]; Olive leaves [73] | |
| 64 | Essential amino acid | L-Tryptophan [Tryptophan; (S)-Tryptophan] * | C11H12N2O2 | 204.2252 | 16.9 | 205 | 188 | 146 | 144; 118 | Huolisu Oral Liquid [7]; Rosa acicularis [8]; Camellia kucha [9]; Euphorbia hirta [10]; Hylocereus polyrhizus [11]; Rapeseed petals [12] | |
| 65 | Polysaccharides | Glucaric acid [ D-Glucaric acid; Saccharic acid; D-Glutarate] * | C6H10O8 | 210.1388 | 8.7 | 211 | 193; 147 | 147 | 118 | Soybean [59]; Cherimoya, Papaya [71] | |
| 66 | Saturated fatty acid | Hydroxydodecenoic acid * | C12H22O3 | 214.3013 | 39.0 | 215 | 197 | 195 | Jatropha gossypifolia [22] | ||
| 67 | Alpha, omega dicarboxylic acid | Undecanedioic acid * | C11H20O4 | 216.2741 | 50.3 | 217 | 199; 189; 159 | 157; 143 | 143 | Jatropha [22]; G. linguiforme [31] | |
| 68 | Carboxylic acid | Myristoleic acid [Cis-9-Tetradecanoic acid] * | C14H26O2 | 226.3550 | 20.1 | 227 | 209; 165 | 121 | F. glaucescens [31]; Maackia amurensis [74] | ||
| 69 | Sesquiterpenoid | Atractylenolide I * | C15H18O2 | 230.3022 | 27.7 | 231 | 185 | 157 | 142 | Atractylodes macrocephalae rhizoma [13]; Chinese herbal formula Jian-Pi-Yi-Shen pill [14] | |
| 70 | Sesquiterpenoid | Atractylenolide II [Asterolide; 2-Atractylenolide] * | C15H20O2 | 232.3181 | 10.2 | 233 | 215; 205; 187; 145 | 145; 131 | Codonopsis Radix; Atractylodes macrocephalae rhizoma [13]; Chinese herbal formula Jian-Pi-Yi-Shen pill [14] | ||
| 71 | Germacranolide | Costunolide * | C15H20O2 | 232.3181 | 41.4 | 233 | 185 | 143 | 128 | [75] | |
| 72 | Monocarboxylic acid | Artemisinic acid [Artemisic acid; arteannuic acid] | C15H22O2 | 234.3340 | 28.7 | 235 | 216 | 187 | 145 | Artemisia annua [30] | |
| 73 | Hydroxytetradecanoic acid | Hydroxy myristic acid [2S-Hydroxytetradecanoic acid; Alpha-Hydroxy Myristic acid] * | C14H28O3 | 244.3703 | 38.6 | 245 | 228 | 172 | F. pottsii [31] | ||
| 74 | Medium-chain fatty acid | Hydroxy dodecanoic acid * | C12H22O5 | 246.3001 | 26.0 | 247 | 229; 201 | 187 | 159; 145 | F. glaucescens [31] | |
| 75 | Sesquiterpenoid | Santonin [Alpha-Santonin; Semenen; Santoninic anhydride] | C15H18O3 | 246.3016 | 44.1 | 247 | 228 | 200 | Artemisia absinthium [6] | ||
| 76 | Sesquiterpene lactone | Artemisinin C | C15H20O3 | 248.3175 | 20.6 | 249 | 231 | 213 | 171 | Artemisia annua [6] | |
| 77 | Sesquiterpene lactone | Artemannuin B | C15H20O3 | 248.3175 | 25.3 | 247 | 203 | 201 | Artemisia annua [6,30] | ||
| 78 | Sesquiterpenoid | Dihydroarteannuin B | C15H22O3 | 250.3334 | 27.5 | 251 | 232 | 215 | 187 | Artemisia absinthium [6] | |
| 79 | Sesquiterpenoid | Dihydrosantamarin | C15H22O3 | 250.3334 | 39.3 | 249 | 205 | 203; 121 | 121 | Artemisia absinthium [6] | |
| 80 | Sesquiterpenoid | Artemisin | C15H18O4 | 262.3010 | 34.3 | 263 | 245 | 227 | Pubchem | ||
| 81 | Sesquiterpenoid | Pseudosantonin | C15H20O4 | 264.3169 | 26.3 | 265 | 247 | 229 | 201 | Artemisia absinthium [6] | |
| 82 | Sesquiterpenoid | Deoxyartemisinin I | C15H22O4 | 266.3328 | 32.1 | 267 | 249 | 229 | 213 | Artemisia absinthium [6] | |
| 83 | Omega-3-fatty acid | Stearidonic acid [6,9,12,15-Octadecatetraenoic acid; Moroctic acid] * | C18H28O2 | 276.4137 | 18.5 | 277 | 217 | 189; 171 | 161; 134 | Jatropha [22]; G. linguiforme [31]; Rhus coriaria [56]; Salviae Miltiorrhizae [76] | |
| 84 | Sesquiterpenoid | Chrysartemin A | C15H18O5 | 278.3004 | 42.7 | 279 | 163 | 145 | 143 | Artemisia absinthium [6] | |
| 85 | Omega-3-fatty acid | Linolenic acid (Alpha-Linolenic acid; Linolenate) * | C18H30O2 | 278.4296 | 17.1 | 279 | 261 | 234; 111 | 123 | Jatropha [22]; Maackia amurensis [74]; Salviae Miltiorrhizae [76] | |
| 86 | Gamma-lactone | Artabsinolide A | C15H20O5 | 280.3163 | 9.1 | 279 | 247; 235 | 203 | Artemisia absinthium [6] | ||
| 87 | Sesquiterpenoid | Dihydroartemisinin | C15H24O5 | 284.3481 | 7.9 | 285 | 227 | 199 | 130 | Artemisia annua [30] | |
| 88 | Octadecadienoic acid | Linoleic acid (Linolic acid; Telfairic acid) * | C18H32O2 | 280.4455 | 26.5 | 281 | 245 | 228 | 183 | Soybean [59]; Soybean leaves [55]; Jatropha [22]; | |
| 89 | Omega-3-fatty acid | Stearidonic acid methyl ester | C19H30O2 | 290.4403 | 51.6 | 291 | 259; 149 | 241; 161 | 173 | Jatropha [22] | |
| 90 | Diterpenoid naphthoquinone | Tanshinone IIA [Tanshinone II; Tanshinone B] * | C19H18O3 | 294.3444 | 49.5 | 295 | 277; 241 | 161 | 161; 133 | Huolisu Oral Liquid [7]; Chinese herbal formula Jian-Pi-Yi-Shen pill [13]; | |
| 91 | Polyunsaturated fatty acid | Alpha-Kamlolenic Acid [18-Hydroxy-9Z,11E,13E-Octadecatrienoic Acid] * | C18H30O3 | 294.4290 | 45.3 | 293 | 275; 171 | 231 | G. linguiforme; F. glaucescens; F. pottsii [31] | ||
| 92 | Essential faty acid | Hydroxy octadecadienoic acid | C18H32O3 | 296.4449 | 47.5 | 295 | 277; 171 | 275 | Artemisia absinthium [6]; Jatropha [22]; A. cordifolia; F. glaucescens; F. herrerae [31] | ||
| 93 | Oxylipin | Trihydroxyoctadecadienoic acid | C18H32O5 | 328.4437 | 32.4 | 327 | 229 | 210 | 209 | Artemisia absinthium [6]; Potato leaves [70] | |
| 94 | Naphthoquinone | 3,3′-di-O-methyl ellagic acid * | C16H10O8 | 330.2458 | 30.9 | 331 | 316 | 298 | 270 | Juglans mandshurica [29]; Terminalia arjuna [57] | |
| 95 | Oxylipin | 13- Trihydroxy-Octadecenoic acid [THODE] * | C18H34O5 | 330.4596 | 33.0 | 329 | 229 | 209 | Jatropha [22]; Phoenix dactylifera [72]; Bituminaria [77]; Broccoli [78] | ||
| 96 | Naphthoquinone | Tri-O-methylellagic acid * | C17H12O8 | 344.2724 | 29.6 | 343 | 328; 300; 247 | 313; 285 | 298; 270 | Terminalia arjuna [57] | |
| 97 | Sesquiterpene lactone | Artemetin [Artemisetin; Erianthin] | C20H20O8 | 388.3680 | 41.4 | 389 | 356; 325 | 313 | 285; 267 | Pubchem | |
| 98 | Naphthoquinone | 1,4,8-Trihydroxy-3-tetralone-methyl formate-4-O-beta-D-glucopyranoside | C18H20O10 | 396.3454 | 8.7 | 397 | 379; 233 | 217 | 159 | Juglans mandshurica [29] | |
| 99 | Anabolic steroid | Vebonol * | C30H44O3 | 452.6686 | 25.6 | 453 | 435; 210 | 226; 336 | 210 | Hylosereus polyrhizus [11]; Rhus coriaria [56] | |
| 100 | Sesquiterpene lactone | Absinthin | C30H40O6 | 496.6350 | 30.8 | 497 | 476; 246 | 228; 172 | 172 | Artemisia absinthium [6] | |
| 101 | Triterpe | 3-O-acetyl-betulinic acid * | C32H50O4 | 498.7370 | 41.4 | 499 | 480; 233 | 462; 231 | 417; 198 | Juglans mandshurica [29] | |
| 102 | Sesquiterpene lactone | Absinthin derivative | C30H38O7 | 510.6185 | 30.3 | 511 | 492; 246 | 474; 246 | 228; 172 | Artemisia absinthium [6] | |
| 103 | Indole sesquiterpene alkaloid | Sespendole * | C33H45NO4 | 519.7147 | 47.3 | 520 | 184 | 125 | Hylosereus polyrhizus [11]; Rhus coriaria [56] | ||
| 104 | Product of chlorophyll degradation | Pheophytin A | C55H74N4O5 | 871.1999 | 52.4 | 872 | 593 | 533 | 461 | Physalis peruviana [79]; Capsicum [80] |
References
- Polyakov, P.P. The genus Artemisia L.—Artemisia. Flora USSR 1961, 26, 425–631. (In Russian) [Google Scholar]
- Red Book of the Republic of Sakha (Yakutia). Vol. 1: Rare and endangered species of plants and fungi. In Red Book of the Republic of Sakha (Yakutia); Danilova, N.S., Ed.; Publishing House “Reart”: Moscow, Russia, 2017; 412p. (In Russian) [Google Scholar]
- Danilova, N.S.; Borisova, S.Z.; Ivanova, N.S. Brief review of the polynyas of Central Yakutia. NEFU Bull. 2022, 4, 13–23. (In Russian) [Google Scholar]
- Red Book of the Krasnoyarsk Territory. Vol. 2: Rare and endangered species of wild plants and fungi. In Red Book of the Krasnoyarsk Territory; Stepanov, N.V., Andreeva, E.B., Antipova, E.M., Eds.; Ministry of Natural Resources and Ecology of the Krasnoyarsk Territory: Krasnoyarsk, Russia, 2012; Volume 2, 572p. (In Russian) [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Wozniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, K.; Wei, L.; Chen, D.; Chen, Q.; Jiao, M.; Li, X.; Huang, J.; Gong, Z.; Kang, N.; et al. The Molecular Mechanism of Antioxidation of Huolisu Oral Liquid Based on Serum Analysis and Network Analysis. Front. Pharma. 2021, 12, 710976. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Bazhenova, B.A.; Zabalueva, Y.Y.; Burkhanova, A.G.; Zakharenko, A.M.; Kupriyanov, A.N.; Sabitov, A.S.; Ercisli, S.; Golokhvast, K.S. Rosa davurica Pall., Rosa rugosa Thumb., and Rosa acicularis Lindl. originating from Far Eastern Russia: Screening of 146 Chemical Constituents in Tree Species of the Genus Rosa. Appl. Sci. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Qin, D.; Wang, Q.; Li, H.; Jiang, X.; Fang, K.; Wang, Q.; Li, B.; Pan, C.; Wu, H. Identification of key metabolites based on non-targeted metabolomics and chemometrics analyses provides insights into bitterness in Kucha [Camellia kucha (Chang et Wang) Chang]. Food Res. Int. 2020, 138, 109789. [Google Scholar] [CrossRef]
- Mekam, P.N.; Martini, S.; Nguefack, J.; Tagliazucchi, D.; Stefani, E. Phenolic compounds profile of water and ethanol extracts of Euphorbia hirta L. leaves showing antioxidant and antifungal properties. S. Afr. J. Bot. 2019, 127, 319–332. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; He, Y.; Shi, M.; Han, X.; Li, W.; Zhang, X.; Wen, X. Metabolic Profiling of Pitaya (Hylocereus polyrhizus) during Fruit Development and Maturation. Molecules 2019, 24, 1114. [Google Scholar] [CrossRef]
- Yin, N.-W.; Wang, S.-X.; Jia, L.-D.; Zhu, M.-C.; Yang, J.; Zhou, B.-J.; Yin, J.-M.; Lu, K.; Wang, R.; Li, J.-N.; et al. Identification and Characterization of Major Constituents in Different-Colored Rapeseed Petals by UPLC−HESI-MS/MS. Agricult. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, P.; Leung, K.W.; Wang, H.; Kong, X.P.; Wang, L.; Dong, T.T.X.; Chen, Y.; Tsim, K.W.K. The Yin-Yang Property of Chinese Medicinal Herbs Relates to Chemical Composition but Not Anti-Oxidative Activity: An Illustration Using Spleen-Meridian Herbs. Front. Pharmacol. 2018, 9, 1304. [Google Scholar] [CrossRef]
- Wang, F.; Huang, S.; Chen, Q.; Hu, Z.; Li, Z.; Zheng, P.; Liu, X.; Li, S.; Zhang, S.; Chen, J. Chemical characterisation and quantification of the major constituents in the Chinese herbal formula Jian-Pi-Yi-Shen pill by UPLC-Q-TOF-MS/MS and HPLC-QQQ-MS/MS. Phytochem. Anal. 2020, 31, 915–929. [Google Scholar] [CrossRef]
- Jaiswal, R.; Muller, H.; Muller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, H.; Wang, Q.; Liu, H.; Shen, H.; Xu, W.; Ge, J.; He, D. Rapid qualitative profiling and quantitative analysis of phenolics in Ribes meyeri leaves and their antioxidant and antidiabetic activities by HPLC-QTOF-MS/MS and UHPLC-MS/MS. J. Sep. Sci. 2021, 44, 1404–1420. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1936. [Google Scholar] [CrossRef]
- Wojakowska, A.; Piasecka, A.; Garcia-Lopez, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, L.; Kachlicki, P.; Stobiecki, M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochem 2013, 92, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. UFLC-Q-TOF-MS/MS-Based Screening and Identification of Flavonoids and Derived Metabolites in Human Urine after Oral Administration of Exocarpium Citri Grandis Extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shaari, K. LC–MS metabolomics analysis of Stevia rebaudiana Bertoni leaves cultivated in Malaysia in relation to different developmental stages. Phytochem. Analys. 2021, 33, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Belmehdi, O.; Bouyahya, A.; József, J.E.K.Ő.; Cziáky, Z.; Zengin, G.; Sotkó, G.; El Baaboua, A.; Senhaji, N.S.; Abrini, J. Synergistic interaction between propolis extract, essential oils, and antibiotics against Staphylococcus epidermidis and methicillin resistant Staphylococcus aureus. Int. J. Second Metab. 2021, 8, 195–213. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Sinan, K.I.; Ak, G.; Etienne, O.K.; Sharmeen, J.B.; Brunetti, L.; Leone, S.; Di Simone, S.C.; Recinella, L.; et al. Chemical composition and biological properties of two Jatropha species: Different parts and different extraction methods. Antioxidants 2021, 10, 792. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, D.; Yang, G.; Zheng, Y.; Guo, L. Screening of Anti-Lipase Components of Artemisia argyi Leaves Based on Spectrum-Effect Relationships and HPLC-MS/MS. Front. Pharmacol. 2021, 12, 675396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jia, P.; Zhang, X.; Zhang, Q.; Yang, H.; Shi, H.; Zhang, L. LC–MS/MS determination and pharmacokinetic study of seven flavonoids in rat plasma after oral administration of Cirsium japonicum DC. extract. J. Ethnopharmacol. 2014, 158, 66–75. [Google Scholar] [CrossRef]
- Xu, L.L.; Xu, J.J.; Zhong, K.R.; Shang, Z.P.; Wang, F.; Wang, R.F.; Liu, B. Analysis of non-volatile chemical constituents of Menthae Haplocalycis herba by ultra-high performance liquid chromatography—High resolution mass spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and their interspecies variation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical Profiling of Flavonoids, Phenolic Acids, Terpenoids, and Volatile Fraction of a Rosemary (Rosmarinus officinalis L.) Extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Kim, E.; Kim, S.W.; Zulfugarov, I.S. New glycosides of eriodictyol from Dracocephalum palmatum. Chem. Nat. Compd. 2018, 54, 860–863. [Google Scholar] [CrossRef]
- Huo, J.-H.; Du, X.-W.; Sun, G.-D.; Dong, W.-T.; Wang, W.-M. Identification and characterization of major constituents in Juglans mandshurica using ultra performance liquid chromatography coupled with time-of-flight mass spectrometry (UPLC-ESI-Q-TOF/MS). Chin. J. Nat. Medic. 2018, 16, 0525–0545. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. Pharm. Biomed. Analysis. 2008, 47, 516–525. [Google Scholar] [CrossRef]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae Through LC/MS –Bioassay Guided Approach. J. Chrom. Sci. 2021, 59, 618–626. [Google Scholar] [CrossRef]
- Flamini, R. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols. Hindawi ISRN Spectrosc. 2013, 2013, 813563. [Google Scholar] [CrossRef]
- Singh, B.; Jain, S.K.; Bharate, S.B.; Kushwaha, M.; Vishwakarma, R.A. Simultaneous Quantification of Five Bioactive Flavonoids in High Altitude Plant Actinocarya tibetica by LC-ESI-MS/MS. J. AOAC Int. 2015, 98, 907–912. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Xuan, Z.; Ge, D.; Chen, X.; Zhang, J.; Wang, Q.; Wu, Y.; Liu, B. The phenolic fraction of Mentha Haplocalyx and its constituent linarin ameliorate inflammatory response through inactivation of NF-κB and MAPKs in lipopolysaccharide-induced RAW264. 7 cells. Molecules 2017, 22, 811. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Yang, L.; Li, Y.J.; Zhang, D.; Chen, Y.; Kostecka, P.; Kmonickova, E.; Zidek, Z. Effects of sesquiterpene, flavonoid and coumarin types of compounds from Artemisia annua L. on production of mediators of angiogenesis. Pharmacol. Rep. 2013, 65, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Stallmann, J.; Schweiger, R.; Pons, C.A.; Müller, C. Wheat growth, applied water use efficiency and flag leaf metabolome under continuous and pulsed deficit irrigation. Sci. Rep. 2020, 10, 10112. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Laganà, A. Identification and mass spectrometric characterization of glycosylated flavonoids in Triticum durum plants by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 3143–3158. [Google Scholar] [CrossRef] [PubMed]
- Ioset, J.-R.; Urbaniak, B.; Ndjoko-Ioset, K.; Wirth, J.; Martin, F.; Gruissem, W.; Hostettmann, K.; Sautter, C. Flavonoid profiling among wild type and related GM wheat varieties. Plant Mol. Biol. 2007, 65, 645–654. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Tu, H.; Zhou, J.; Liu, X.; Cheng, Y.; Luo, J.; Deng, X.; Zhang, H.; Xu, J. Characterization and Metabolic Diversity of Flavonoids in Citrus Species. Sci. Rep. 2017, 7, 10549. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Wang, J.; Chen, G.; Tao, X.; Xu, S. Metabolomic Analysis Reveals Domestication-Driven Reshaping of Polyphenolic Antioxidants in Soybean Seeds. Antioxidants 2023, 12, 912. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Determination of antioxidant activity in free and hydrolyzed fractions of millet grains and characterization of their phenolic profiles by HPLC-DAD-ESI-MSn. J. Funct. Foods 2011, 3, 144–158. [Google Scholar] [CrossRef]
- Liu, P.; Lindstedt, A.; Markkinen, N.; Sinkkonen, J.; Suomela, J.; Yang, B. Characterization of Metabolite Profiles of Leaves of Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.). J. Agric. Food Chem. 2014, 62, 12015–12026. [Google Scholar] [CrossRef]
- Vijayan, K.P.R.; Raghu, A.V. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019, 52, 653–670. [Google Scholar] [CrossRef]
- Du, Q.-H.; Zhang, Q.-Y.; Han, T.; Jiang, Y.-P.; Peng, C.; Xin, H.-L. Dynamic changes of flavonoids in Actinidia valvata leaves at different growing stages measured by HPLC-MS/MS. Chin. J. Nat. Medic. 2016, 14, 0066–0072. [Google Scholar]
- Syed, A.S.; Jeon, J.-S.; Kim, C.Y. A new diacetylated flavonol triglycoside from the aerial parts of Actinidia polygama. Nat. Prod. Res. 2017, 31, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef]
- Fischer, U.A.; Dettmann, J.S.; Carle, R.; Kammerer, D.R. Impact of processing and storage on the phenolic profiles and contents of pomegranate (Punica granatum L.) juices. Eur. Food Res. Technol. 2011, 233, 797–816. [Google Scholar] [CrossRef]
- Rafsanjany, N.; Senker, J.; Brandt, S.; Dobrindt, U.; Hensel, A. In Vivo Consumption of Cranberry Exerts Ex Vivo Antiadhesive Activity against FimH-Dominated Uropathogenic Escherichia coli: A Combined In Vivo, Ex Vivo, and In Vitro Study of an Extract from Vaccinium macrocarpon. J. Agric. Food Chem. 2015, 63, 8804–8818. [Google Scholar] [CrossRef] [PubMed]
- Mosic, M.; Trifkovic, J.; Vovk, I.; Gasic, U.; Tesic, Z.; Sikoparija, B.; Milojkovic-Opsenica, D. Phenolic Composition Influences the Health-Promoting Potential of Bee-Pollen. Biomolecules 2019, 9, 783. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agr. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- Fantoukh, O.I.; Wang, Y.-H.; Parveen, A.; Hawwal, M.F.; Ali, Z.; Al-Hamoud, G.A.; Chittiboyina, A.G.; Joubert, E.; Viljoen, A.; Khan, I.A. Chemical Fingerprinting Profile and Targeted Quantitative Analysis of Phenolic Compounds from Rooibos Tea (Aspalathus linearis) and Dietary Supplements Using UHPLC-PDA-MS. Separations 2022, 9, 159. [Google Scholar] [CrossRef]
- Fermo, P.; Comite, V.; Sredojevic, M.; Ciric, I.; Gasic, U.; Mutic, J.; Baosic, R.; Tesic, Z. Elemental Analysis and Phenolic Profiles of Selected Italian Wines. Foods 2021, 10, 158. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; Garcia-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly)phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, X.; Li, G.; He, X.; Yu, X.; Yu, X.; Xiao, Q.; Xiang, Z.; Wang, C. Chemical constituents of radix Actinidia chinensis planch by UPLC–QTOF–MS. Biomed. Chromatogr. 2021, 35, e5103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Xu, J.; Liu, X.; Wang, S.; Shi, L. Physiological and metabolomics analyses of young and old leaves from wild and cultivated soybean seedlings under low-nitrogen conditions. BMC Plant Biol. 2019, 19, 389. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arraes-Roman, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, S.; Rathi, B.; Bhrara, K.; Chhikara, B.S. Therapeutic analysis of Terminalia arjuna plant extracts in combinations with different metal nanoparticles. J. Mater. NanoSci. 2015, 2, 1–7. [Google Scholar]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef]
- Ieri, F.; Martini, S.; Innocenti, M.; Mulinacci, N. Phenolic Distribution in Liquid Preparations of Vaccinium myrtillus L. and Vaccinium vitis idaea L. Phytochem. Anal. 2013, 24, 467–475. [Google Scholar] [CrossRef]
- Barros, L.; Duenas, M.; Carvalho, A.M.; Ferreira, I.C.F.R.; Santos-Buelga, C. Characterization of phenolic compounds in flowers of wild medicinal plants from Northeastern Portugal. Food Chem. Toxicol. 2012, 50, 1576–1582. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Abbas, F.A.; Refaat, S.; El-Shafae, A.M.; Fikry, E. UPLC-ESI-MS/MS Profile of The Ethyl Acetate Fraction of Aerial Parts of Bougainvillea ‘Scarlett O’Hara’ Cultivated in Egypt. Egypt. J. Chem. 2021, 64, 22. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMSn. Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef]
- Zhu, Z.-W.; Li, J.; Gao, X.-M.; Amponsem, E.; Kang, L.-Y.; Hu, L.-M.; Zhang, B.-L.; Chang, Y.-X. Simultaneous determination of stilbenes, phenolic acids, flavonoids and anthraquinones in Radix polygoni multiflori by LC–MS/MS. J. Pharmaceut. Biomed. Analys. 2012, 62, 162–166. [Google Scholar] [CrossRef]
- Kim, S.; Oh, S.; Noh, H.B.; Ji, S.; Lee, S.H.; Koo, J.M.; Choi, C.W.; Jhun, H.P. In Vitro Antioxidant and Anti-Propionibacterium acnes Activities of Cold Water, Hot Water, and Methanol Extracts, and Their Respective Ethyl Acetate Fractions, from Sanguisorba officinalis L. Roots. Molecules 2018, 23, 3001. [Google Scholar] [CrossRef] [PubMed]
- Razgonova, M.P.; Tekutyeva, L.A.; Podvolotskaya, A.B.; Stepochkina, V.D.; Zakharenko, A.M.; Golokhvast, K.S. Zostera marina L. Supercritical CO2-Extraction and Mass Spectrometric Characterization of Chemical Constituents Recovered from Seagrass. Separations 2022, 9, 182. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Yang, M.; Cao, J.; Khan, A.; Cheng, G. UHPLC-ESI-HRMS/MS analysis on phenolic compositions of different E Se tea extracts and their antioxidant and cytoprotective activities. Food Chem. 2020, 318, 126512. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.A.; Smeds, A.I.; Sjoholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectr. 2008, 43, 97–107. [Google Scholar] [CrossRef]
- Perchuk, I.; Shelenga, T.; Gurkina, M.; Miroshnichenko, E.; Burlyaeva, M. Composition of Primary and Secondary Metabolite Compounds in Seeds and Pods of Asparagus Bean (Vigna unguiculata (L.) Walp.) from China. Molecules 2020, 25, 3778. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, C.; Gomez-Caravaca, A.M.; Guerra-Hernandez, E.; Cerretani, L.; Garcia-Villanova, B.; Verardo, V. Comprehensive metabolite profiling of Solanum tuberosum L. (potato) leaves T by HPLC-ESI-QTOF-MS. Food Res. Int. 2018, 112, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Spinola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Said, R.B.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Suarez Montenegro, Z.J.; Alvarez-Rivera, G.; Mendiola, J.A.; Ibanez, E.; Cifuentes, A. Extraction and Mass Spectrometric Characterization of Terpenes Recovered from Olive Leaves Using a New Adsorbent-Assisted Supercritical CO2 Process. Foods. 2021, 10, 1301. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Cherevach, E.I.; Tekutyeva, L.A.; Fedoreev, S.A.; Mishchenko, N.P.; Tarbeeva, D.V.; Demidova, E.N.; Kirilenko, N.S.; Golokhvast, K.S. Maackia amurensis Rupr. et Maxim.: Supercritical CO2-extraction and Mass Spectrometric Characterization of Chemical Constituents. Molecules 2023, 28, 2026. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Liu, Z.; Zhang, Z. Identification and Simultaneous Determination of Twelve Active Components in the Methanol Extracts of Traditional Medicine Weichang’an Pill by HPLC-DAD-ESI-MS/MS. Iran. J. Pharm. Res. 2013, 12, 15–24. [Google Scholar]
- Yang, S.T.; Wu, X.; Rui, W.; Guo, J.; Feng, Y.F. UPLC/Q-TOF-MS Analysis for Identification of Hydrophilic Phenolics and Lipophilic Diterpenoids from Radix Salviae Miltiorrhizae. Acta Chromatogr. 2015, 27, 711–728. [Google Scholar] [CrossRef]
- Llorent-Martinez, E.J.; Spinola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Industr. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Park, S.K.; Ha, J.S.; Kim, J.M.; Kang, J.Y.; Lee, D.S.; Guo, T.J.; Lee, U.; Kim, D.-O.; Heo, H.J. Antiamnesic Effect of Broccoli (Brassica oleracea var. italica) Leaves on Amyloid Beta (A)1-42-Induced Learning and Memory Impairment. J. Agric. Food. Chem. 2016, 64, 3353–3361. [Google Scholar]
- Etzbach, L.; Pfeiffer, A.; Weber, F.; Schieber, A. Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by HPLC-DADAPCI-MSn. Food Chem. 2018, 245, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Calvete, D.; Guauque-Medina, J.; Villegas-Torres, M.F.; Montoya, G. Analysis of triacylglycerides, carotenoids and capsaicinoids as disposable molecules from Capsicum agroindustry. Hortic. Environ. Biotechnol. 2019, 60, 227–238. [Google Scholar] [CrossRef]
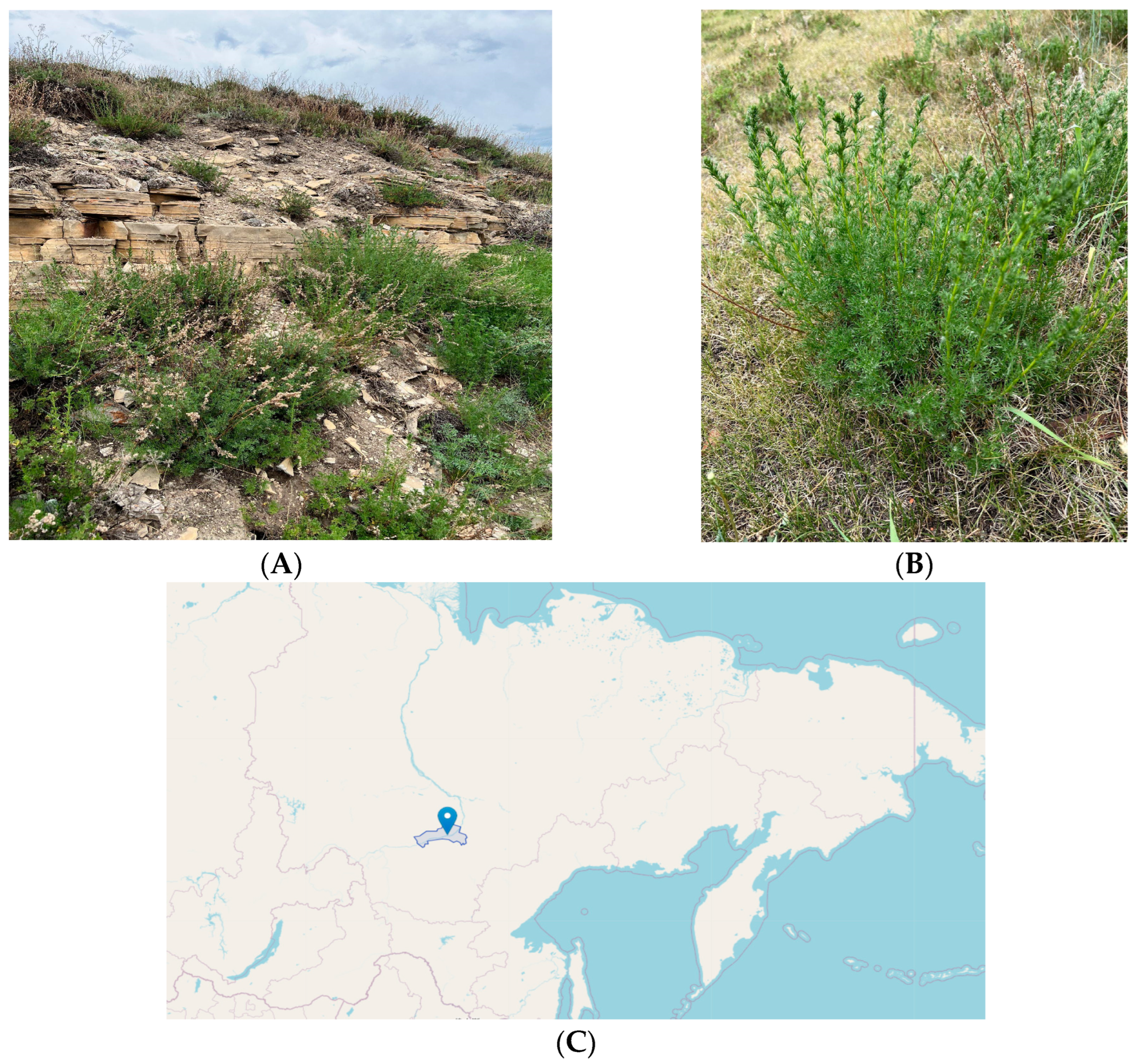
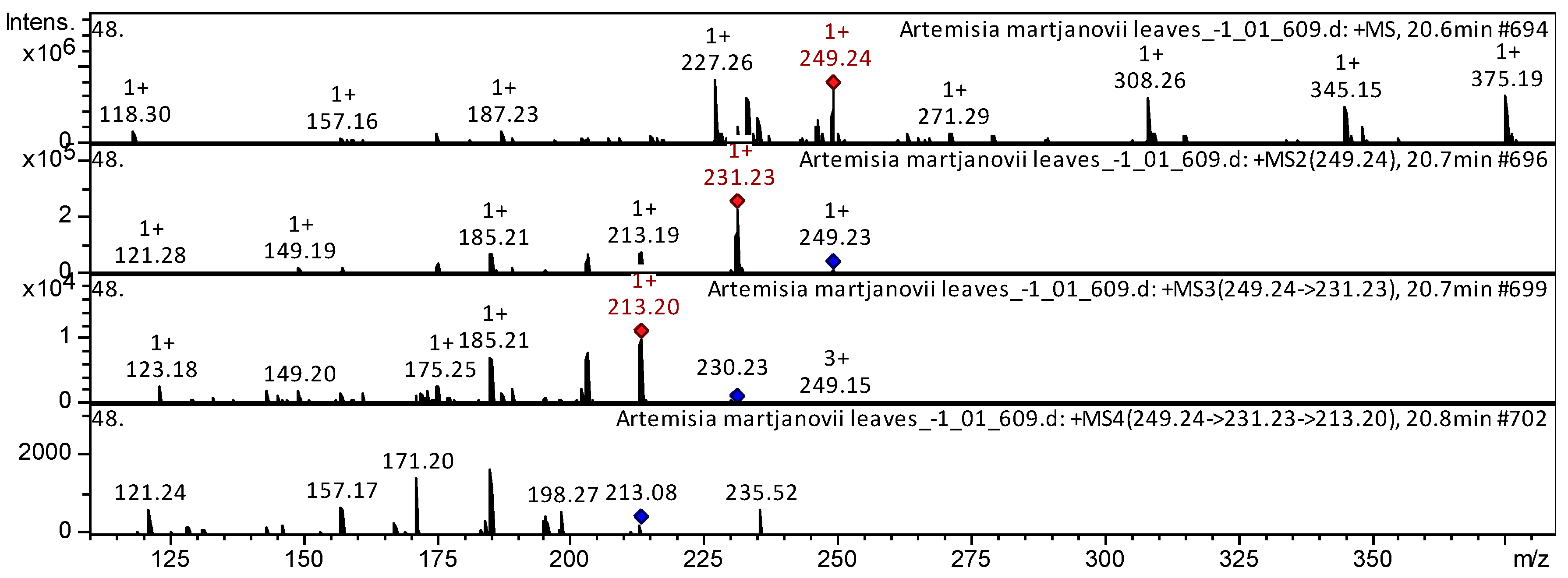
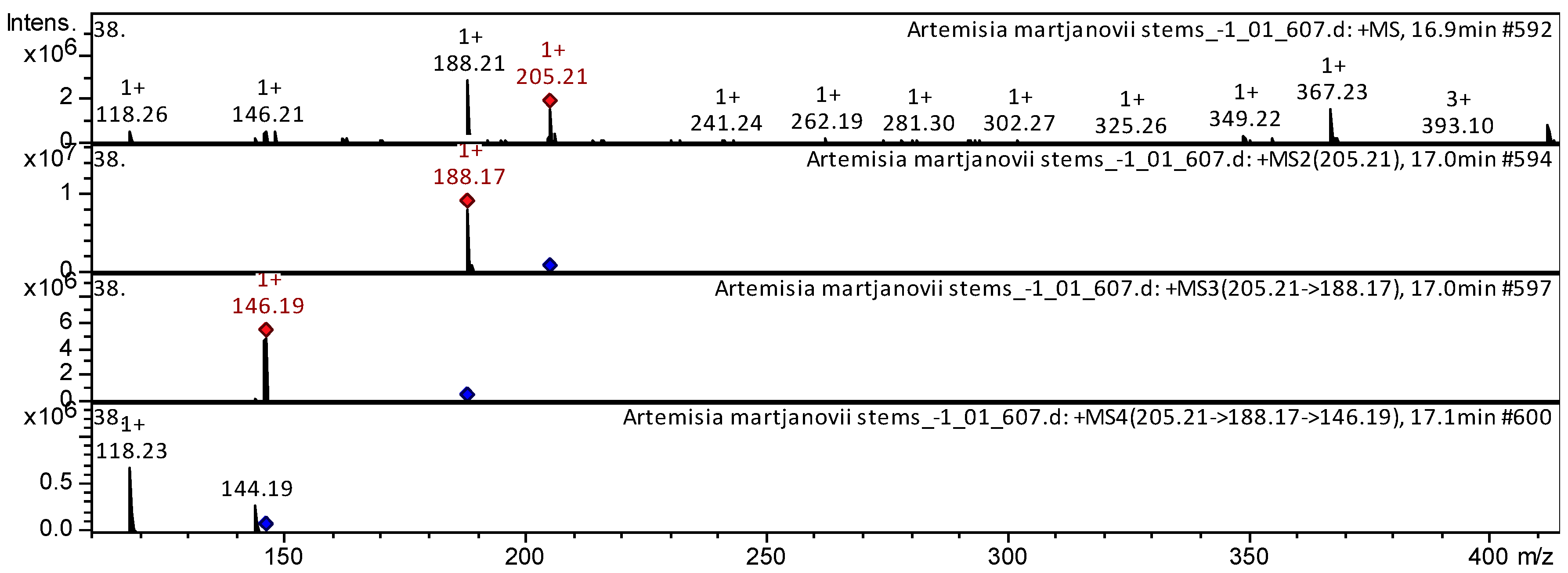
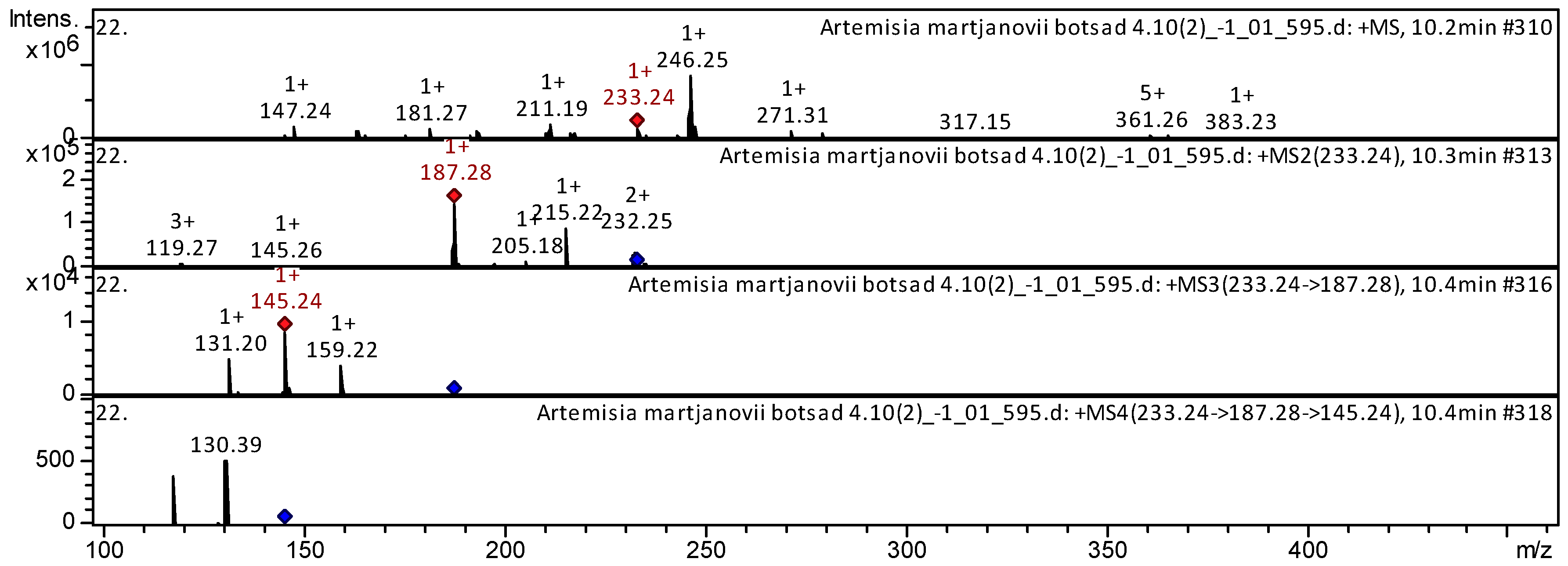
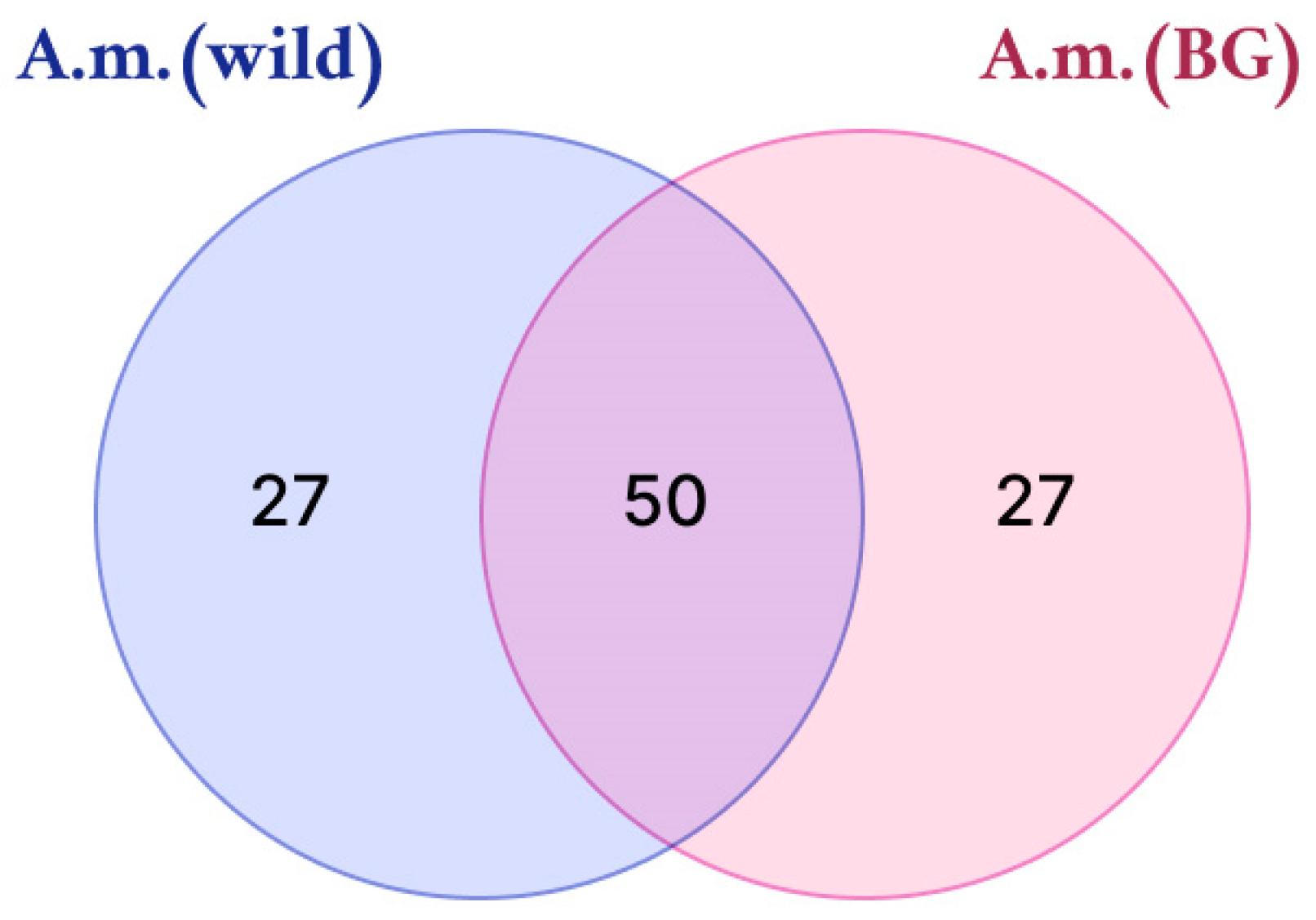
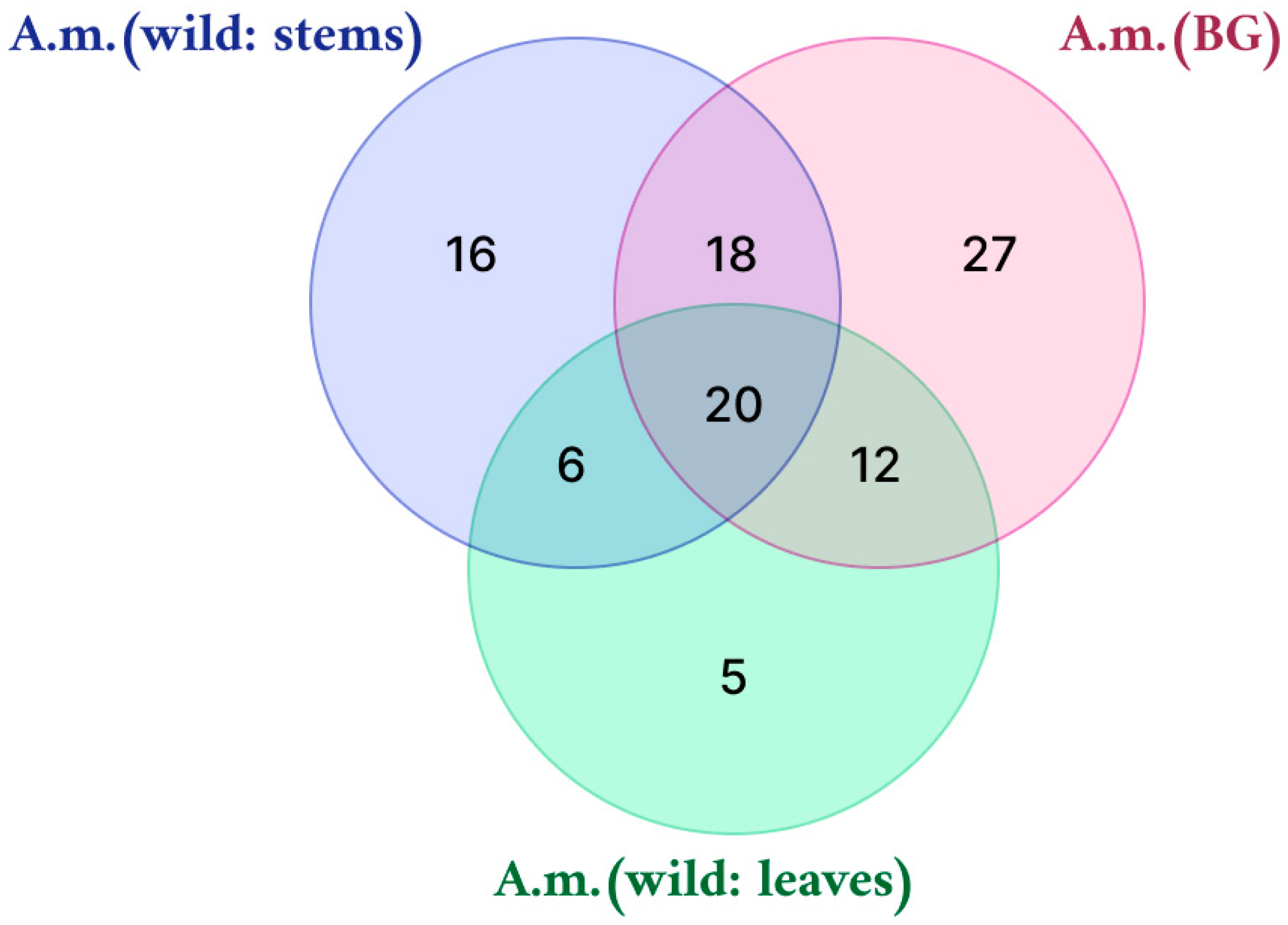
| Sample | Number of Compounds | Compound Names |
|---|---|---|
| A. martjanovii
(wild); A. martjanovii (BG) | 50 | Chrysoeriol C-hexoside; Stearidonic acid methyl ester; Dihydrosantamarin; Undecanedioic acid; Umbelliferone; Trihydroxy(iso)flavone; Deoxyartemisinin I; Petunidin; Syringetin; Vebonol; 3,4-O-dicaffeoylquinic acid; L-Valine; 3-Hydroxy-6,7,4′-trimethoxyflavone; Gardenin B; Dihydroxy tetramethoxyflavone hexoside; Salvigenin; Chrysoeriol 6-O-hexoside; Caffeic acid; Dihydroxy-trimethoxyflavone-O-hexoside; 3,3′-di-O-methyl ellagic acid; Artemisin; Resveratrol; Artemannuin B; Syringic acid; Sespendole; Linolenic acid; Costunolide; Dihydroxy-trimethoxyflavone; Casticin; (Epi)-catechin; Hydroxy myristic acid; Caffeic acid derivative; Hydroxy dodecanoic acid; Pseudosantonin; Dihydroxy-dimethoxyflavone; Centaureidin; Eupatilin; Jaceosidin; Artemetin; Penduletin; Trihydroxymethoxyflavone; Hydroxydodecenoic acid; Myristoleic acid; Atractylenolide I; Artemisinic acid; Atractylenolide II; Artemisinin C; Cirsimaritin; Chrysartemin A; Trihydroxyoctadecadienoic acid |
| A. martjanovii (wild) | 27 | Phloretin; Tyrosine; Tri-O-methylellagic acid; Isorhamnetin 3-O-(6″-O-rhamnosyl-hexoside); Santonin; L-Ascorbic acid; Tetrahydroxy-dimethoxyflavone; Dihydroartemisinin; Pheophytin A; 13-Trihydroxy-Octadecenoic acid; Podophyllotoxin; Cirsiliol; Nevadensin; Tomentin; Alpha-Kamlolenic Acid; Dihydroquercetin; Gallic acid; Plumbagin; Methoxyeugenol; Chlorogenic acid; Isololiolide; 1,4,8-Trihydroxy-3-tetralone-methyl formate-4-O-β-D-glucopyranoside; 3,5-Dihydroxy-6,7,3′,4′-tetramethoxyflavone; Cirsilineol; L-Tryptophan; Tanshinone IIA; (S)-eriodictyol-6-C-β-D-glucopyranoside |
| A. martjanovii (BG) | 27 | Artabsinolide A; Stearidonic acid; Eriodictyol; Isorhamnetin 3-O-glucoside; Absinthin derivative; Dihydroarteannuin B; Mearnsetin-glucoside; 7-Methoxybenzo[d][1,3] dioxole-5-carboxylic acid; Glucaric acid; Neochlorogenic acid; 3,5-Dihydroxy-6,7,4′-trimethoxyflavone; Ferulic acid; Hispidulin; cis-3-Caffeoylquinic acid; Methylgallic acid; Linoleic acid; Apigenin; Absinthin; Hydroxy octadecadienoic acid; Thymonin; 3,4,5-Tri-O-caffeoylquinic acid; Quercetin 3-O-glucoside; Acacetin C-glucoside methylmalonylated; 3-O-acetyl-betulinic acid; Fraxetin; Tetrahydroxy-dimethoxyflavone-hexoside; Tetramethylellagic acid hexose |
| Sample | Number of Compounds | Number of Compounds Identified for the First Time in Genus Artemisia L. |
|---|---|---|
| A. martjanovii (BG) | 77 | 34 |
| A. martjanovii (wild: leaves) | 43 | 19 |
| A. martjanovii (wild: stems) | 61 | 34 |
| Sample | Total Number of Compounds | Compound Names |
|---|---|---|
| A. martjanovii (wild: stems) | 16 | Phloretin; Tyrosine; 3,4-O-dicaffeoylquinic acid; Isorhamnetin 3-O-(6″-O-rhamnosyl-hexoside); L-Ascorbic acid; Tetrahydroxy-dimethoxyflavone; Dihydroartemisinin; 13- Trihydroxy-Octadecenoic acid; Cirsiliol; Nevadensin; Tomentin; Alpha-Kamlolenic Acid; Gallic acid; Methoxyeugenol; 1,4,8-Trihydroxy-3-tetralone-methyl formate-4-O-β-D-glucopyranoside; L-Tryptophan; (S)-eriodictyol-6-C-β-D-glucopyranoside |
| A. martjanovii (wild: leaves) | 5 | Tri-O-methylellagic acid; Dihydroquercetin; 3,5-Dihydroxy-6,3′,4′-tetramethoxyflavone; Cirsilineol; Tanshinone IIA |
| A. martjanovii (wild: stems+leaves) | 6 | Santonin, Pheophytin A; Podophyllotoxin; Plumbagin; Chlorogenic acid; Isololiolide |
| A. martjanovii (wild: stems + leaves); A. martjanovii (BG) | 20 | Undecanedioic acid; Deoxyartemisinin I; Gardenin B; Salvigenin; Caffeic acid; Artemisin; Dihydroxy-trimethoxyflavone; Casticin; Hydroxy myristic acid; Pseudosantonin; Dihydroxy-dimethoxyflavone; Centaureidin; Eupatilin; Artemetin; Myristoleic acid; Atractylenolide I; Artemisinic acid; Atractylenolide II; Artemisinin C; Cirsimaritin |
| A. martjanovii (wild: stems); A. martjanovii (BG) | 18 | Dihydrosantamarin; Umbelliferone; Trihydroxy(iso)flavone; Petunidin; Syringetin; Vebonol; L-Valine; Dihydroxy tetramethoxyflavone hexoside; Dihydroxy-trimethoxyflavone-O-hexoside; Resveratrol; Sespendole; Linolenic acid; (Epi)-catechin; Caffeic acid derivative; Hydroxy dodecanoic acid; Hydroxydodecenoic acid; Chrysartemin A; Trihydroxyoctadecadienoic acid |
| A. martjanovii (wild: leaves); A. martjanovii (BG) | 12 | Chrysoeriol C-hexoside; Stearidonic acid methyl ester; 4-O-dicaffeoylquinic acid; 3-Hydroxy-6,7,4′-trimethoxyflavone; Chrysoeriol 6-O-hexoside; 3′-di-O-methyl ellagic acid; Artemannuin B; Syringic acid; Costunolide; Jaceosidin; Penduletin; Trihydroxymethoxyflavone |
| A. martjanovii (BG) | 27 | Artabsinolide A; Stearidonic acid; Eriodictyol; Isorhamnetin 3-O-glucoside; Absinthin derivative; Dihydroarteannuin B; Mearnsetin-glucoside; 7-Methoxybenzo[d][13] dioxole-5-carboxylic acid; Glucaric acid; Neochlorogenic acid; 3,5-Dihydroxy-6,7,4′-trimethoxyflavone; Ferulic acid; Hispidulin; cis-3-Caffeoylquinic acid; Methylgallic acid; Linoleic acid; Apigenin; Absinthin; Hydroxy octadecadienoic acid; Thymonin; 4,5-Tri-O-caffeoylquinic acid; Quercetin 3-O-glucoside; Acacetin C-glucoside methylmalonylated; 3-O-acetyl-betulinic acid; Fraxetin; Tetrahydroxy-dimethoxyflavone-hexoside; Tetramethylellagic acid hexose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okhlopkova, Z.M.; Ercisli, S.; Razgonova, M.P.; Ivanova, N.S.; Antonova, E.E.; Egorov, Y.A.; Kucharova, E.V.; Golokhvast, K.S. Primary Determination of the Composition of Secondary Metabolites in the Wild and Introduced Artemisia martjanovii Krasch: Samples from Yakutia. Horticulturae 2023, 9, 1329. https://doi.org/10.3390/horticulturae9121329
Okhlopkova ZM, Ercisli S, Razgonova MP, Ivanova NS, Antonova EE, Egorov YA, Kucharova EV, Golokhvast KS. Primary Determination of the Composition of Secondary Metabolites in the Wild and Introduced Artemisia martjanovii Krasch: Samples from Yakutia. Horticulturae. 2023; 9(12):1329. https://doi.org/10.3390/horticulturae9121329
Chicago/Turabian StyleOkhlopkova, Zhanna M., Sezai Ercisli, Mayya P. Razgonova, Natalia S. Ivanova, Elena E. Antonova, Yury A. Egorov, Elena V. Kucharova, and Kirill S. Golokhvast. 2023. "Primary Determination of the Composition of Secondary Metabolites in the Wild and Introduced Artemisia martjanovii Krasch: Samples from Yakutia" Horticulturae 9, no. 12: 1329. https://doi.org/10.3390/horticulturae9121329
APA StyleOkhlopkova, Z. M., Ercisli, S., Razgonova, M. P., Ivanova, N. S., Antonova, E. E., Egorov, Y. A., Kucharova, E. V., & Golokhvast, K. S. (2023). Primary Determination of the Composition of Secondary Metabolites in the Wild and Introduced Artemisia martjanovii Krasch: Samples from Yakutia. Horticulturae, 9(12), 1329. https://doi.org/10.3390/horticulturae9121329










