Quantification and Comparison of Nutritional Components in Oni Walnut (Juglans ailanthifolia Carr.), Hime Walnut (Juglans subcordiformis Dode.), and Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of the Total Lipid Content
2.2. Evaluation of the Fatty Acid Composition
2.3. Measurement of the Total Protein Content
2.4. Measurement of the Amino Acid Content
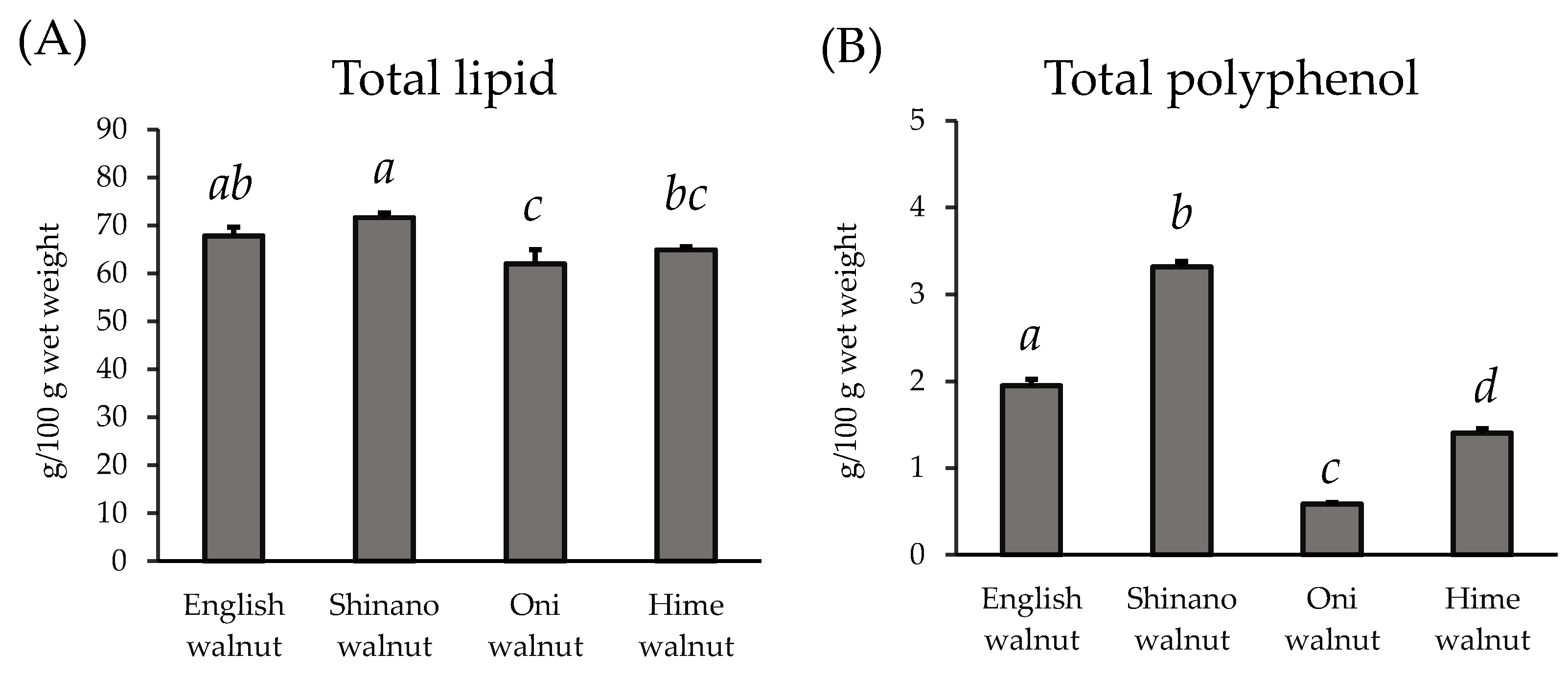
2.5. Measurement of the Total Polyphenol Content
2.6. Measurement of the Mineral Content
2.7. Statistical Analysis
3. Results and Discussion
| Constituent | Walnuts | |||
|---|---|---|---|---|
| English Walnut | Shinano Walnut | Oni Walnut | Hime Walnut | |
| Mean | Mean | Mean | Mean | |
| (Measured Value) | (Measured Value) | (Measured Value) | (Measured Value) | |
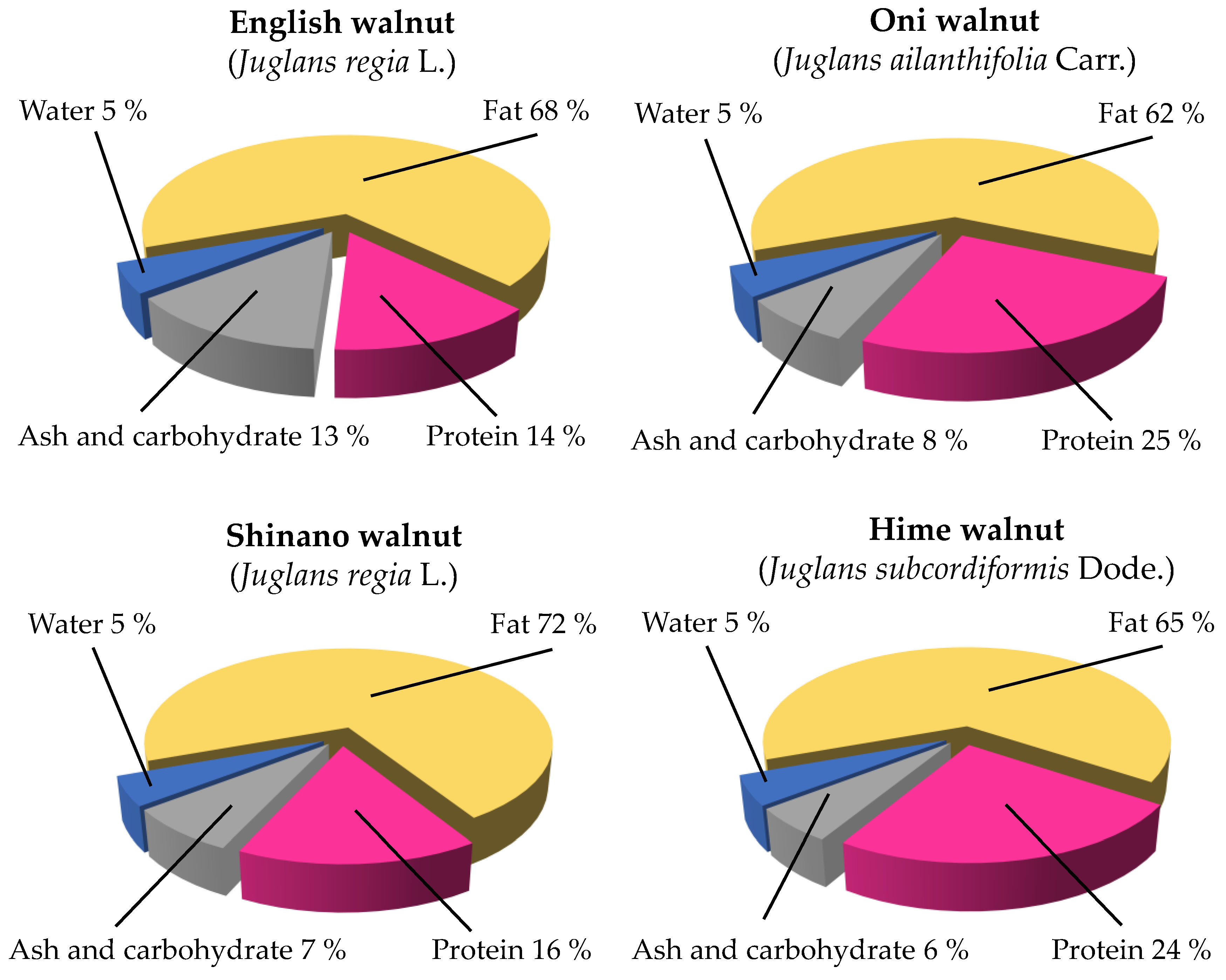
| Total protein | 13.78 | 15.88 | 25.23 | 24.45 |
| (g/100 g wet wt) | (13.75, 13.80) | (15.88, 15.88) | (24.98, 25.59) | (24.51, 24.39) |
| Amino acids | ||||
| (g/100 g wet wt) | ||||
| Alanine | 0.67 † | 0.68 | 1.14 | 1.11 |
| (Ala) | (0.69, 0.67) | (1.14, 1.14) | (1.11, 1.12) | |
| Arginine | 2.28 † | 2.40 | 4.06 | 3.89 |
| (Arg) | (2.40, 2.39) | (4.06, 4.06) | (3.87, 3.92) | |
| Aspartic acid | 1.83 † | 1.58 | 2.70 | 2.62 |
| (Asp) | (1.59, 1.57) | (2.68, 2.71) | (2.59, 2.64) | |
| Cystine | 0.21 † | 0.27 | 0.40 | 0.38 |
| (Cyss) | (0.27, 0.27) | (0.40, 0.40) | (0.38, 0.38) | |
| Glutamic acid | 2.82 † | 3.31 | 5.37 | 5.17 |
| (Glu) | (3.37, 3.24) | (5.32, 5.42) | (5.15, 5.19) | |
| Glycine | 0.82 † | 0.84 | 1.31 | 1.20 |
| (Gly) | (0.85, 0.83) | (1.31, 1.31) | (1.20, 1.21) | |
| Histidine | 0.39 † | 0.39 | 0.70 | 0.67 |
| (His) | (0.39, 0.39) | (0.70, 0.70) | (0.67. 0.68) | |
| Isoleucine | 0.63 † | 0.60 | 0.99 | 0.96 |
| (Ile) | (0.61, 0.59) | (0.99, 0.99) | (0.96, 0.97) | |
| Leucine | 1.17 † | 1.13 | 1.84 | 1.79 |
| (Leu) | (1.14, 1.12) | (1.84, 1.84) | (1.77, 1.80) | |
| Lysine | 0.42 † | 0.43 | 0.65 | 0.65 |
| (Lys) | (0.44, 0.42) | (0.65, 0.65) | (0.65, 0.64) | |
| Methionine | 0.24 † | 0.25 | 0.52 | 0.50 |
| (Met) | (0.24, 0.25) | (0.52, 0.52) | (0.50, 0.50) | |
| Phenylalanine | 0.71 † | 0.70 | 1.22 | 1.18 |
| (Phe) | (0.70, 0.69) | (1.22, 1.22) | (1.17, 1.19) | |
| Proline | 0.71 † | 0.55 | 0.96 | 0.94 |
| (Pro) | (0.55, 0.55) | (0.96, 0.96) | (0.94, 0.95) | |
| Serine | 0.93 † | 0.85 | 1.38 | 1.32 |
| (Ser) | (0.86, 0.85) | (1.38, 1.38) | (1.32, 1.33) | |
| Threonine | 0.60 † | 0.53 | 0.84 | 0.82 |
| (Thr) | (0.54, 0.53) | (0.84, 0.85) | (0.82, 0.83) | |
| Tyrosine | 0.41 † | 0.52 | 0.85 | 0.81 |
| (Tyr) | (0.52, 0.51) | (0.84, 0.85) | (0.80, 0,82) | |
| Tryptophan | 0.17 † | 0.20 | 0.36 | 0.40 |
| (Trp) | (0.20, 0.19) | (0.36, 0.36) | (0.41, 0.40) | |
| Valine | 0.75 † | 0.72 | 1.25 | 1.22 |
| (Val) | (0.73, 0.71) | (1.26, 1.24) | (1.22, 1.23) | |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gupta, A.; Behl, T.; Panichayupakaranan, P. A review of phytochemistry and pharmacology profile of Juglans regia. Obes. Med. 2019, 16, 100142. [Google Scholar] [CrossRef]
- Lockyer, S.; de la Hunty, A.E.; Steenson, S.; Spiro, A.; Stanner, S.A. Walnut consumption and health outcomes with public health relevance—A systematic review of cohort studies and randomized controlled trials published from 2017 to present. Nutr. Rev. 2023, 81, 26–54. [Google Scholar] [CrossRef]
- Hardman, W.E. Diet components can suppress inflammation and reduce cancer risk. Nutr. Res. Pract. 2014, 8, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, M.; Sharma, M. A comprehensive review on ethnobotanical, medicinal and nutritional potential of walnut (Juglans regia L.). Proc. Indian Natl. Sci. Acad. 2022, 88, 601–616. [Google Scholar] [CrossRef]
- Antora, S.A.; Ho, K.V.; Lin, C.H.; Thomas, A.L.; Lovell, S.T.; Krishnaswamy, K. Quantification of vitamins, minerals, and amino acids in Black walnut (Juglans nigra). Front. Nutr. 2022, 9, 936189. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, S.; Wang, R.; Wu, Y.; Zhang, W.; Han, Y.; Tang, F.; Shen, D.; Liu, Y. Analysis and correlationship of chemical components of various walnut (Juglans regia L.) cultivars. J. Food Meas. Charact. 2020, 14, 3605–3614. [Google Scholar] [CrossRef]
- Bernard, A.; Lheureux, F.; Dirlewanger, E. Walnut: Past and future of genetic improvement. Tree Genet. Genomes. 2018, 14, 1. [Google Scholar] [CrossRef]
- Camara, C.R.S.; Schlegel, V. A review on the potential human health benefits of the black walnut: A comparison with the english walnuts and other tree nuts. Int. J. Food Prop. 2016, 19, 2175–2189. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.F.; Guerriero, G.; Cai, G. Agrobiotechnology goes wild: Ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef]
- BaSeFood Site. Available online: http://www.basefood-fp7.eu/www.basefood-fp7.eu/index.html (accessed on 3 September 2023).
- Vu, D.C.; Nguyen, T.H.D.; Ho, T.L. An overview of phytochemicals and potential health-promoting properties of black walnut. RSC Adv. 2020, 10, 33378–33388. [Google Scholar] [CrossRef]
- Hama, J.R.; Omer, R.A.; Rashid, R.S.M.; Mohammad, N.; Thoss, V. The diversity of phenolic compounds along defatted kernel, green husk and leaves of walnut (Juglansregia L.). Anal. Chem. 2016, 6, 1. [Google Scholar]
- Uozumi, M. Walnut dishes and Kurumiaji in Iwate Prefecture. Bull. Morioka Jr. Coll. Iwate Prefect. Univ. 2017, 19, 1–7. [Google Scholar]
- Takahashi, H.; Uozumi, M. Japanese home cooking: Dishes to hand down the next generation Tohoku and Hokkaido branch summary of the results in Iwate prefecture. J. Cook. Sci. Jpn. 2016, 49, 253–257. [Google Scholar]
- Fukasawa, R.; Miyazawa, T.; Abe, C.; Bhaswant, M.; Toda, M. Functional components of walnuts: A review focusing on native and cultivated species. Food Sci. Technol. Res, 2023; in press. [Google Scholar] [CrossRef]
- Makino, T. New Makino’s Illustrated Flora of Japan; The Hokuryoukan Co., Ltd.: Tokyo, Japan, 2017. [Google Scholar]
- Jensen, P.N.; Sorensen, G.; Engelsen, S.B.; Bertelsen, G. Evaluation of quality changes in walnut kernels (Juglans regia L.) by Vis/NIR spectroscopy. J. Agric. Food Chem. 2001, 49, 5790–5796. [Google Scholar] [CrossRef]
- Bakkalbas, E.; Yılmaz, Ö.M.; Javidipour, I.; Artık, N. Effects of packaging materials, storage conditions and vari ety on oxidative stability of shelled walnuts. Food Sci. Technol. 2012, 46, 203–209. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparison of solvents for extraction of walnut oils: Lipid yield, lipid compositions, minor-component content, and antioxidant capacity. Food Sci. Technol. Res. 2019, 110, 346–352. [Google Scholar] [CrossRef]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P.P. Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six walnut (Juglans regia L.) cultivars grown in Portugal. J. Agric. Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef]
- Kafkas, E.; Burgut, A.; Ozcan, H.; Ozcan, A.; Sutyemez, M.; Kafkas, S.; Turemis, N. Fatty acid, total phenol and tocopherol profiles of some walnut cultivars: A comparative study. Food Nutr. Sci. 2017, 8, 1074–1084. [Google Scholar] [CrossRef]
- Jung, S.; Rickerta, D.A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. JAOCS 2003, 80, 12. [Google Scholar] [CrossRef]
- Serrano, S.; Rincon, F.; Garcia-Olmo, J. Cereal protein analysis via Dumas method: Standardization of a micro-method using the EuroVector Elemental Analyser. J. Cereal Sci. 2013, 58, 31–36. [Google Scholar] [CrossRef]
- Wu, S.; Ni, Z.; Wang, R.; Zhao, B.; Han, Y.; Zheng, Y.; Liu, F.; Gong, Y.; Tang, F.; Liu, Y. The effects of cultivar and climate zone on phytochemical components of walnut (Juglans regia L.). Food Energy Secur. 2020, 9, e196. [Google Scholar] [CrossRef]
- Liu, B.; Liang, J.; Zhao, D.; Wang, K.; Jia, M.; Wang, J. Morphological and compositional analysis of two walnut (Juglans regia L.) cultivars growing in China. Plant Foods Hum. Nutr. 2020, 75, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Pismag, R.Y.; Laudouze, M.; Martinez, M.M. Systematic evaluation of the Folin–Ciocalteu and Fast Blue BB reactions during the analysis of total phenolics in legumes, nuts and plant seeds. Food Funct. 2020, 11, 9868–9880. [Google Scholar] [CrossRef]
- Larrauri, M.; Zunino, M.P.; Zygadlo, J.A.; Grosso, N.R.; Nepote, V. Chemical characterization and antioxidant properties of fractions separated from extract of peanut skin derived from different industrial processes. Ind. Crop. Prod. 2016, 94, 964–971. [Google Scholar] [CrossRef]
- Azevedo, R.; Oliveira, A.R.; Almeida, A.; Gomes, L.R. Determination by ICP-MS of essential and toxic trace elements in gums and carrageenans used as food additives commercially available in the Portuguese market. Foods 2023, 12, 1408. [Google Scholar] [CrossRef] [PubMed]
- Cindric, I.J.; Zeiner, M.; Hlebec, D. Mineral composition of elements in walnuts and walnut oils. Int. J. Environ. Res. Public Health 2018, 15, 2674. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Yu, A.; Kong, X.; Hua, Y. Stable mixed beverage is produced from walnut milk and raw soymilk by homogenization with subsequent heating. Food Sci. Technol. Res. 2014, 20, 583–591. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Desai, S.S.; Mane, V.K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends Food Sci. Technol. 2022, 120, 140–153. [Google Scholar] [CrossRef]
- Abbas, K.A.; Abdelmontaleb, H.S.; Hamdy, S.M.; Aït-Kaddour, A. Physicochemical, functional, fatty acids profile, health lipid indices, microstructure and sensory characteristics of walnut-processed cheeses. Foods 2021, 10, 2274. [Google Scholar] [CrossRef]
- Florowski, T.; Florowska, A.; Chmiel, M.; Adamczak, L.; Pietrzak, D.; Ostrowska, A.; Szymańska, I. Quality aspects of designing prohealth liver sausages enriched with walnut paste. Foods 2022, 11, 3946. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J.H. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- USDA Nuts, Walnuts, Black, Dried. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170186/nutrients (accessed on 6 September 2023).
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The critical role of the branched chain amino acids (BCAAs) catabolism-regulating enzymes, branched-chain aminotransferase (BCAT) and branched-chain and α-keto acid dehydrogenase (BCKD), in human pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef]
- Canfield, C.-A.; Bradshaw, P.C. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Joint WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. In World Health Organ Technical Report Series; WHO: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Nuts, Walnuts, English. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170187/nutrients (accessed on 6 September 2023).
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Walnut (Juglans regia)-morphology, taxonomy, composition and health benefits. In Fruits Grown in Highland Regions of the Himalayas; Hussain, S.Z., Naseer, B., Qadri, T., Fatima, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 269–281. [Google Scholar]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in aging, health and diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Keefe, J.H.O.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Ho, L.-H.; Rode, R.; Siegel, M.; Reinhardt, F.; Neuhaus, H.E.; Yvin, J.-C.; Pluchon, S.; Hosseini, S.A.; Pommerrenig, B. Potassium application boosts photosynthesis and sorbitol biosynthesis and accelerates cold acclimation of common plantain (Plantago major L.). Plants 2020, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Socha, A.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef]
- Shi, R.; Yu, J.; Chang, X.; Qiao, L.; Liu, X.; Lu, L. Recent advances in research into jasmonate biosynthesis and signaling pathways in agricultural crops and products. Processes 2023, 11, 736. [Google Scholar] [CrossRef]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current use of Fenton reaction in drugs and food. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T. Lipid hydroperoxides in nutrition, health, and diseases. Proc. Jpn. Acad. B Phys. Biol. Sci. 2021, 97, 161–196. [Google Scholar] [CrossRef]
- Miyazawa, T.; Abe, C.; Burdeos, G.C.; Matsumoto, A.; Toda, M. Food antioxidants and aging: Theory, current evidence and perspectives. Nutraceuticals 2022, 2, 181–204. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in health and disease: Gut microbiota, bioaccessibility, and bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Fuentealba, C.; Hernández, I.; Saa, S.; Toledo, L.; Burdiles, P.; Chirinos, R.; Campos, D.; Brown, P.; Pedreschi, R. Colour and in vitro quality attributes of walnuts from different growing conditions correlate with key precursors of primary and secondary metabolism. Food Chem. 2017, 232, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Solar, A.; Jakopic, J.; Miklavc, J.; Stampar, F.; Veberic, R.; Trdan, S. Walnut husk fly substantially affects sensory attributes and phenolic contents of the kernels in common walnut. Sci. Hortic. 2019, 247, 17–26. [Google Scholar] [CrossRef]
- Li, W.; Gao, H.; Fang, X.; Tao, F.; Chen, H.; Mu, H.; Jiang, Y. Accumulation of lipofuscin-like pigments of walnuts (Carya cathayensis) during storage: Potential roles of lipid oxidation and non-enzymatic glycosylation. J. Sci. Food Agric. 2014, 94, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.I.; Sánchez-Morgado, J.R.; García-Parra, J.; Ramírez, R.; Hernández, T.; González-Gómez, D. Comparative study of the nutritional and bioactive compounds content of four walnut (Juglans regia L.) cultivars. J. Food Compos. Anal. 2013, 31, 232–237. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Electronic Files and Web Site. Available online: https://data.worldbank.org/indicator/AG.LND.PRCP.MM (accessed on 15 September 2023).
- Annual Precipitation in Japan. 2022. Available online: https://www.data.jma.go.jp/obd/stats/data/stat/tenko2022_besshi.pdf (accessed on 15 September 2023).
- Average Annual Temperatures for Cities in California. Available online: https://www.currentresults.com/Weather/California/average-annual-city-temperatures.php (accessed on 15 September 2023).
- Statistics Bureau in Japan. Chapter 1: Land and Weather. Available online: https://www.stat.go.jp/data/nenkan/back64/01.html (accessed on 15 September 2023).
- Annual Average Humidity in California. Available online: https://www.currentresults.com/Weather/California/humidity-annual.php (accessed on 15 September 2023).
- Average Annual Sunshine in American Cities. Available online: https://www.currentresults.com/Weather/US/average-annual-sunshine-by-city.php (accessed on 15 September 2023).
- Amaral, J.S.; Alves, M.R.; Seabra, R.M.; Oliveira, B.P.P. Vitamin E composition of walnuts (Juglans regia L.): A 3-year comparative study of different cultivars. J. Agric. Food Chem. 2005, 53, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Cichońska, P.; Ziębicka, A.; Ziarno, M. Properties of rice-based beverages fermented with lactic acid bacteria and propionibacterium. Molecules 2022, 27, 2558. [Google Scholar] [CrossRef]
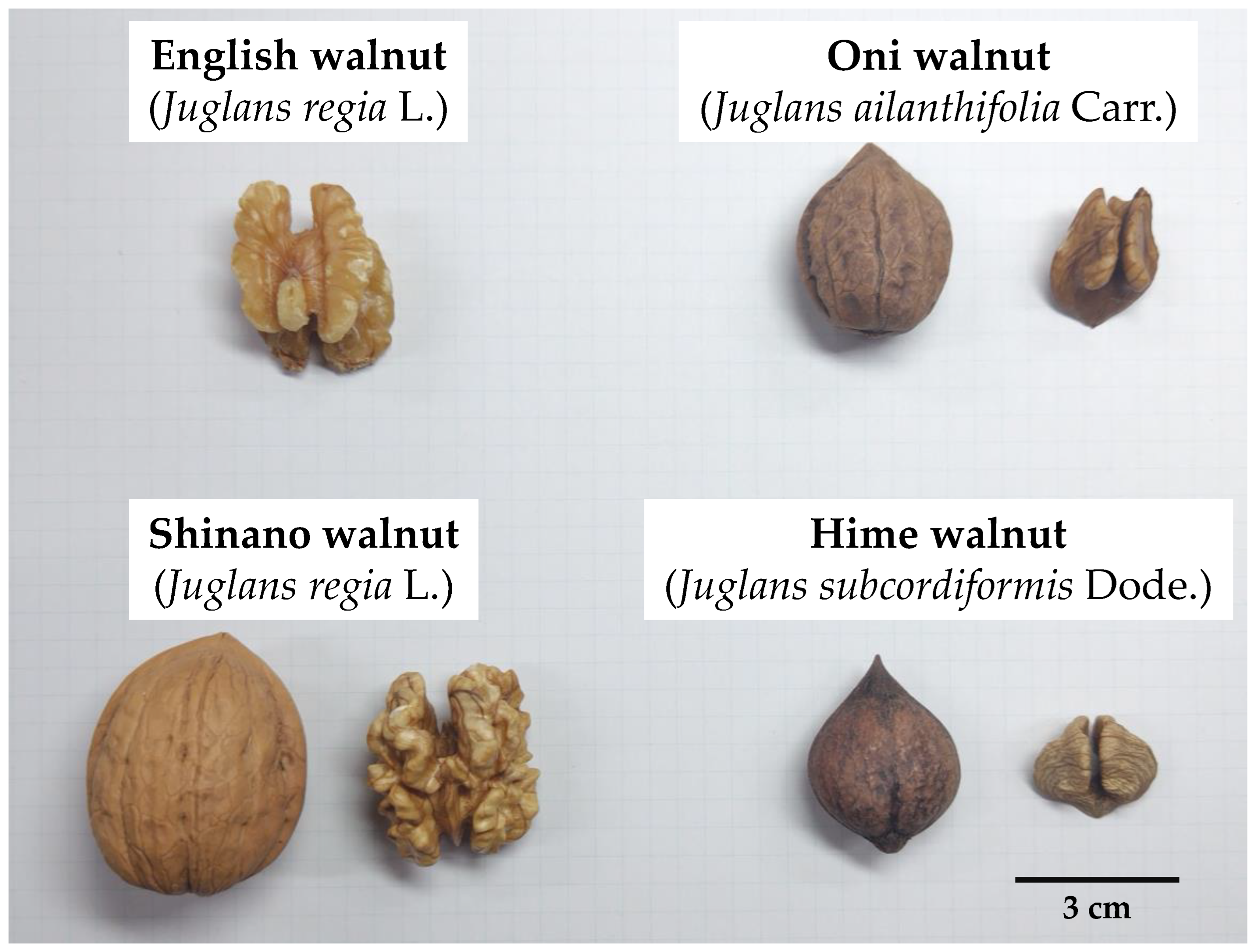
| Species | Botanical Name | Country | State or Prefecture | Color of Pellicle | |
|---|---|---|---|---|---|
| Cultivar | English walnut | Juglans regia L. | United States | California (CA) | Extra Light |
| Shinano walnut | Juglans regia L. | Japan | Nagano | Extra Light | |
| Japanese native walnut | Oni walnut | Juglans ailanthifolia Carr. | Japan | Yamagata | Light Amber |
| Hime walnut | Juglans subcordiformis Dode. | Japan | Yamagata | Light | |
| Constituent | Walnuts | |||||||
|---|---|---|---|---|---|---|---|---|
| English Walnut | Shinano Walnut | Oni Walnut | Hime Walnut | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Fatty acids (%) | ||||||||
| C14:0 | 0.05 | 0.00 | ND | ND | ND | |||
| C16:0 | 5.08 a | 0.01 | 5.06 a | 0.01 | 2.33 b | 0.12 | 2.09 c | 0.02 |
| C18:0 | 2.22 a | 0.02 | 2.84 b | 0.01 | 0.61 c | 0.02 | 0.69 c | 0.01 |
| C20:0 | 0.08 | 0.00 | 0.09 | 0.00 | ND | ND | ||
| Total SFA | 7.42 | 0.02 | 8.10 | 0.00 | 2.99 | 0.15 | 2.84 | 0.04 |
| C16:1 ω7 | 0.06 | 0.02 | ND | 0.05 | 0.03 | ND | ||
| C18:1 ω9 | 12.29 a | 0.06 | 21.76 b | 0.05 | 8.66 c | 0.32 | 14.58 d | 0.06 |
| C20:1 ω9 | 0.25 a | 0.06 | 0.17 b | 0.01 | 0.19 a | 0.02 | 0.22 a | 0.00 |
| C22:1 ω9 | ND | 0.11 | 0.12 | 0.22 | 0.02 | ND | ||
| Total MUFA | 13.18 | 0.71 | 22.09 | 0.12 | 9.13 | 0.28 | 14.89 | 0.05 |
| C18:2 ω6 | 62.66 a | 0.58 | 61.95 a | 0.17 | 74.21 b | 0.32 | 73.17 c | 0.05 |
| C18:3 ω3 | 16.58 a | 0.16 | 7.81 b | 0.05 | 13.60 c | 0.11 | 9.05 d | 0.05 |
| Total PUFA | 79.24 | 0.71 | 69.76 | 0.15 | 87.81 | 0.40 | 82.21 | 0.01 |
| Ratios | ||||||||
| SFA: MUFA: PUFA | 7.42: 13.18: 79.24 | 8.04: 22.22: 69.56 | 2.99: 9.13: 87.81 | 2.84: 14.89: 82.21 | ||||
| ω6/ω3 | 3.78 | 7.93 | 5.45 | 8.09 | ||||
| MUFA/SFA | 1.78 | 2.75 | 3.05 | 5.25 | ||||
| UFA/SFA | 12.45 | 11.43 | 32.42 | 34.24 | ||||
| Constituent | Walnuts | |||||||
|---|---|---|---|---|---|---|---|---|
| English Walnut | Shinano Walnut | Oni Walnut | Hime Walnut | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Minerals | ||||||||
| (mg/100 g wet wt) | ||||||||
| Sodium | 3.85 | 2.13 | 2.52 | 0.40 | 3.79 | 0.89 | 2.82 | 0.66 |
| (Na) | ||||||||
| Magnesium | 154.03 a | 2.68 | 164.59 a | 2.43 | 315.59 b | 12.69 | 319.10 b | 1.86 |
| (Mg) | ||||||||
| Potassium | 413.57 a | 4.39 | 393.27 a | 9.52 | 664.04 b | 100.54 | 675.96 b | 2.82 |
| (K) | ||||||||
| Calcium | 118.53 a | 24.39 | 64.51 b | 1.22 | 83.08 c | 10.20 | 88.74 c | 0.68 |
| (Ca) | ||||||||
| Chromium | 0.16 ab | 0.06 | 0.66 c | 0.09 | 0.35 bd | 0.11 | 0.20 ad | 0.09 |
| (Cr) | ||||||||
| Manganese (Mn) | 2.34 a | 0.04 | 1.50 b | 0.01 | 7.18 c | 0.27 | 7.14 c | 0.07 |
| Iron | 3.85 a | 0.48 | 8.49 b | 0.86 | 8.26 bc | 2.35 | 6.41 abc | 0.72 |
| (Fe) | ||||||||
| Copper | 1.51 a | 0.03 | 1.60 b | 0.03 | 1.66 b | 0.03 | 1.83 c | 0.04 |
| (Cu) | ||||||||
| Zinc | 2.21 a | 0.28 | 1.32 b | 0.02 | 2.41 c | 0.13 | 2.66 c | 0.07 |
| (Zn) | ||||||||
| Selenium | 0.01 ab | 0.00 | 0.01 b | 0.00 | 0.002 a | 0.00 | 0.002 a | 0.00 |
| (Se) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukasawa, R.; Miyazawa, T.; Abe, C.; Bhaswant, M.; Toda, M. Quantification and Comparison of Nutritional Components in Oni Walnut (Juglans ailanthifolia Carr.), Hime Walnut (Juglans subcordiformis Dode.), and Cultivars. Horticulturae 2023, 9, 1221. https://doi.org/10.3390/horticulturae9111221
Fukasawa R, Miyazawa T, Abe C, Bhaswant M, Toda M. Quantification and Comparison of Nutritional Components in Oni Walnut (Juglans ailanthifolia Carr.), Hime Walnut (Juglans subcordiformis Dode.), and Cultivars. Horticulturae. 2023; 9(11):1221. https://doi.org/10.3390/horticulturae9111221
Chicago/Turabian StyleFukasawa, Ritsuko, Taiki Miyazawa, Chizumi Abe, Maharshi Bhaswant, and Masako Toda. 2023. "Quantification and Comparison of Nutritional Components in Oni Walnut (Juglans ailanthifolia Carr.), Hime Walnut (Juglans subcordiformis Dode.), and Cultivars" Horticulturae 9, no. 11: 1221. https://doi.org/10.3390/horticulturae9111221
APA StyleFukasawa, R., Miyazawa, T., Abe, C., Bhaswant, M., & Toda, M. (2023). Quantification and Comparison of Nutritional Components in Oni Walnut (Juglans ailanthifolia Carr.), Hime Walnut (Juglans subcordiformis Dode.), and Cultivars. Horticulturae, 9(11), 1221. https://doi.org/10.3390/horticulturae9111221







